Abstract
Nephrogenic diabetes insipidus (NDI) is characterized by production of very large quantities of dilute urine due to an inability of the kidney to respond to vasopressin. Congenital NDI results from mutations in the type 2 vasopressin receptor (V2R) in ∼90% of families. These patients do not have mutations in aquaporin-2 (AQP2) or urea transporter UT-A1 (UT-A1). We tested adenosine monophosphate kinase (AMPK) since it is known to phosphorylate another vasopressin-sensitive transporter, NKCC2 (Na-K-2Cl cotransporter). We found AMPK expressed in rat inner medulla (IM). AMPK directly phosphorylated AQP2 and UT-A1 in vitro. Metformin, an AMPK activator, increased phosphorylation of both AQP2 and UT-A1 in rat inner medullary collecting ducts (IMCDs). Metformin increased the apical plasma membrane accumulation of AQP2, but not UT-A1, in rat IM. Metformin increased both osmotic water permeability and urea permeability in perfused rat terminal IMCDs. These findings suggest that metformin increases osmotic water permeability by increasing AQP2 accumulation in the apical plasma membrane but increases urea permeability by activating UT-A1 already present in the membrane. Lastly, metformin increased urine osmolality in mice lacking a V2R, a mouse model of congenital NDI. We conclude that AMPK activation by metformin mimics many of the mechanisms by which vasopressin increases urine-concentrating ability. These findings suggest that metformin may be a novel therapeutic option for congenital NDI due to V2R mutations.
Keywords: diabetes insipidus, kinases, vasopressin, urine concentration, AMPK
nephrogenic diabetes insipidus (NDI) is a disease characterized by the production of very large quantities of dilute urine due to an inability of the kidney to respond to vasopressin. Normally, vasopressin binds to the type 2 vasopressin receptor (V2R) and activates protein kinase A (PKA), which increases both water and urea transport by increasing the phosphorylation and apical plasma membrane accumulation of aquaporin-2 (AQP2) and urea transporter-A1 (UT-A1) (reviewed in Refs. 5, 7, 13, 24). Thus, an inability to respond to vasopressin would prevent both water and urea reabsorption, and result in a urine-concentrating defect due to end-organ (kidney) insensitivity to vasopressin, i.e., NDI.
In the US, ∼41,000 people are diagnosed with NDI each year (2, 22). NDI can result from either congenital or acquired etiologies. Congenital NDI results from mutations in the V2R in 90% of families (in which the mutation is known), and from mutations in AQP2 in most of the other 10% of families (18, 22). Patients with the most severe forms of congenital NDI can produce up to 20 l of urine per day, and must drink 20 l of water per day to avoid dehydration (2). Acquired NDI can result from multiple causes, but the most common is chronic use of lithium, a medication that interferes with cAMP signaling (1, 6, 29), and which is used to treat bipolar disorders (27). In patients with congenital NDI due to V2R mutations or taking lithium, there are no mutations in either the AQP2 or UT-A1 proteins, suggesting that if it were possible to phosphorylate these proteins, one could increase their function, independent of vasopressin or cAMP. If so, one might be able to treat, or at least lessen the severity of, the NDI. Therefore, we looked for a kinase that could phosphorylate both AQP2 and UT-A1 independent of vasopressin.
We considered adenosine monophosphate kinase (AMPK) since it can be stimulated by osmotic stress and hypoxia (21). AMPK has never been studied in inner medulla, which is normally hypertonic and hypoxic. In outer medulla, AMPK regulates the Na-K-2Cl cotransporter, NKCC2, through phosphorylation (8–10). This is important since increases in NKCC2 lead to a hypertonic medulla, which is needed to generate the osmotic gradient for water reabsorption. If AMPK also phosphorylated AQP2 and UT-A1, it would recapitulate the action of vasopressin on three proteins that are critical to the production of concentrated urine.
AMPK can be activated by metformin, a medication that is widely used to treat type 2 diabetes mellitus and polycystic ovary syndrome in people. Thus, the purpose of the present study was to determine whether metformin is able to improve water and urea transport, and urine-concentrating ability.
METHODS
Animals.
All animal protocols and procedures were approved by the Emory University Animal Care and Use Committee. Sprague-Dawley rats weighting ∼200–300 g were used for all rat experiments. Some rats received a daily oral dietary supplement containing 800 mg/kg metformin for 2 wk before euthanasia. Rats were housed in metabolic cages for collection of 24-h urine samples. Urine osmolality was determined using a vapor pressure osmometer (Wescor, Logan, UT). Urine electrolyte contents were determined using an EasyLyte analyzer by Medica. For immunohistochemistry studies, rats were deeply anesthetized with isoflurane and their kidneys were perfusion fixed in vivo, and then the rats were euthanized by cardioectomy. Rats that provided tissue for ex vivo treatment were euthanized by decapitation. Following euthanasia, kidneys were dissected into inner medullary (IM) tip (most distal 40%), IM base (remainder of IM to the IM/outer medullary border), outer medulla (OM), and cortex (CTX).
Mice that were genetically modified to allow conditional knockout of the V2R were obtained from Dr. Jurgen Wess at the National Institutes of Health. Knockout was initiated by feeding tamoxifen as previously described (17). Successful knockout was determined when urine osmolality reached 50–150 mosmol/kgH2O. Mice were euthanized by cervical dislocation.
Western blotting.
Proteins (20 μg/lane) were size separated by SDS-PAGE, then electroblotted to polyvinylidene difluoride (PVDF) membranes, and Western blotting was performed as described previously (12, 15). Primary antibodies included AQP2 and UT-A1 [prepared in our lab (11, 20)], AMPKα and pAMPKα (Cell Signaling, Danvers, MA), and pSer256-AQP2 (Biorbyt, Burlington, NC). Alexa Fluor 680-linked anti-rabbit IgG (Molecular Probes, Eugene, OR) was used as secondary antibody. Blots were visualized using infrared detection with the Licor Odyssey protein analysis system. Total protein staining of the blots was used to determine protein loading and used to normalize Western blot bands.
Immunoprecipitation of radiolabeled transporters.
Rat IM tissue was divided into small (2 mm3) pieces and metabolically labeled with 32P as previously described (30). Following 32P loading, the tissue pieces were further incubated with 800 μM Metformin prepared in phosphate-free DMEM for 30 min. Control tissue was incubated with an equal volume of medium without metformin. Tissue was homogenized as described (15, 30), then incubated overnight with the polyclonal anti-UT-A1 or anti-AQP2 at 4°C with gentle mixing, and then precipitated with protein A-agarose. Laemmli-SDS-PAGE sample buffer was added to the pelleted beads, samples were boiled, and proteins were size-separated on SDS-polyacrylamide gels and analyzed by autoradiography and Western blot.
IMCD suspension and biotinylation.
Inner medullas were digested with collagenase, hyaluronidase, and DNase, for 60 min at 37°C as previously described (3). Suspensions were treated with 800 μM metformin or vehicle for 30 min, and then tubules were incubated with biotin for 60 min at 4°C. After being washed, tubules were solubilized in ice-cold lysis buffer containing 2% NP-40. Biotinylated proteins were collected on streptavidin beads overnight at 4°C (3).
In vitro phosphorylation assay.
Recombinant AQP2 or UT-A1 proteins were synthesized using the TNT T7-coupled rabbit reticulocyte lysate system according to the manufacturer's (Promega) instructions and using the following two constructs: pcDNA3-AQP2 or pcDNA3-UT-A1. Recombinant AQP2 and UT-A1 proteins were purified by immunoprecipitation and collection on protein A sepharose beads. Beads were washed 3 times with 1 ml of lysis buffer [25 mM Tris (pH 7.5), 150 mM NaCl, 50 mM NaF, 0.5 mM EDTA (pH 8.0), 0.5% Triton X-100, 5 mM β-glycerophosphate, 5% glycerol, 1 mM DTT, 1 mM PMSF, 1 mM Na3VO4] followed by a wash in kinase buffer [20 mM Tris·HCl (pH 7.5), 5 mM β-glycerolphosphate, 0.2 mM Na3VO4, 0.5 mM DTT]. Beads were next incubated in 50 μl of kinase buffer containing 300 μM ATP, 0.5 mM AMP, and 5 μCi of γ-32P-labeled ATP (Perkin Elmer) in the presence or absence of 5 μg of active AMPK (α 1, β 1, γ 1 subunits; SignalChem). After 20 min at 30°C, Laemmli sample buffer was added, and samples were separated by SDS-PAGE, transferred to a PVDF membrane, and exposed to X-ray film. The membrane was subsequently analyzed by Western blotting.
Tubule perfusion.
Terminal IMCDs from Sprague-Dawley rats (50–100 g) were collected, mounted on glass pipettes, and perfused as described previously (23, 25). Osmotic water permeability (Pf) was measured using raffinose as a volume marker (16). Raffinose (5 mM) is added to both the perfusate and bath. An osmotic gradient was imposed across the tubule by adding NaCl to increase bath osmolality. To measure basal osmotic water permeability, 3 collections were made 45 min after the tubules were warmed to 37°C. Next, metformin (400 μM) was added to the bath solution. After a 20-min equilibration period, 3 collections were made. Collected solutions were assayed for raffinose content by ultramicrofluorometry (16). Pf was calculated as previously described (25).
Urea permeability (Purea) was measured by adding 5 mM urea to the bath and 5 mM creatinine to the perfusate to create a 5-mM bath-to-lumen urea gradient without any osmotic gradient as described previously (23, 25, 28). To measure basal urea permeability, 3 collections were made 45 min after the tubules were warmed to 37°C. Next, metformin (400 μM) was added to the bath solution. After a 20-min equilibration period, 3 collections were made. Collected solutions were assayed for urea content by ultramicrofluorometry. Urea flux was calculated as described previously (25).
Immunohistochemistry.
Rats were subjected to perfusion and paraformaldehyde fixation, and paraffin embedding as described previously (4). Paraffin sections of 4-μm thickness were dewaxed and hydrated as previously described (14). Sections were incubated overnight at 4°C with anti-AQP2. Slides were washed free of primary antibody and then incubated for 2 h in peroxidase-conjugated secondary antibody (donkey anti-rabbit IgG). DAB (diaminobenzidine) and 35% H2O2 were added to detect peroxidase activity. Nuclei were stained with Mayers Hematoxylin. Stained sections were visualized using a bright field on an Olympus IX71 inverted microscope.
Statistics.
All data are presented as means ± SE. To test two groups, we used a Student's t-test. To test more than two groups, we used an ANOVA, followed by Fisher's least significant difference (protected t-test) (26) to determine which groups are significantly different. The criterion for statistical significance is P < 0.05.
RESULTS
AMPK phosphorylates AQP2 and UT-A1.
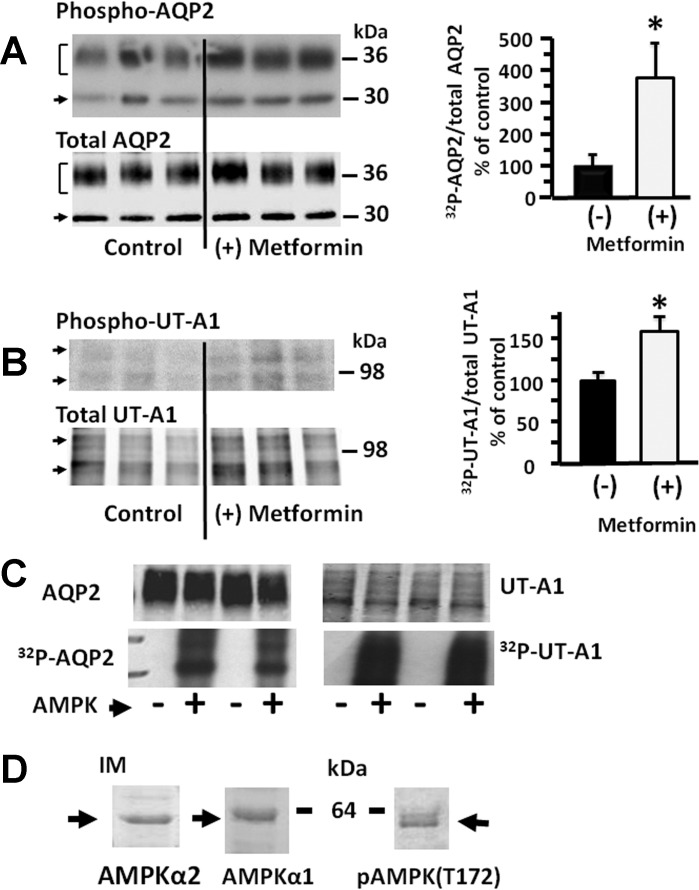
IM tissue was incubated with 800 μM metformin and either AQP2 or UT-A1 was immunoprecipitated and analyzed for protein phosphorylation levels following 32P metabolic labeling of the IM from control rats. Control rats received identical treatment but with vehicle without metformin added. Metformin significantly increased the phosphorylation of both AQP2 (control: 100 ± 29%, metformin-stimulated: 384 ± 96%, n = 5, P < 0.05, Fig. 1A) and UT-A1 (control: 100 ± 10%, metformin-stimulated: 158 ± 18%, n = 6, P < 0.05, Fig. 1B). Metformin can affect multiple targets, and activated AMPK was not assessed in the IM tissue, so to determine whether the increase in phosphorylation can be directly catalyzed by AMPK, in vitro phosphorylation was performed using AQP2 or UT-A1 constructs. Both transporters were phosphorylated by AMPK (Fig. 1C). To determine whether AMPK is located in the kidney IM where it can phosphorylate AQP2 and UT-A1, Western blots of rat IM tissue lysates were probed for AMPKα1, AMPKα2, and phosphoAMPK. Both isoforms and phosphorylated AMPK are present in the IM (Fig. 1D).
Fig. 1.
Ex vivo stimulation of adenosine monophosphate kinase (AMPK) with metformin increases the phosphorylation of aquaporin-2 (AQP2; A) and urea transporter-A1 (UT-A1; B) in inner medullary tissue. A: representative autoradiograph showing phosphorylated proteins. B: representative Western blot showing total protein in each sample. The bar graphs show the ratio of the band densities as a % of control. Bars, means ± SE, n = 5 for AQP2 and n = 8 for UT-A1, *P < 0.05. C: in vitro phosphorylation of recombinant AQP2 and UT-A1 without (−) and with (+) AMPK and 32P-ATP. Top: Western blot; Bottom: autoradiograph; duplicate determinations. D: Western blots of AMPKα2 (left), AMPKα1 (middle), and phospho-AMPK (right) in rat inner medulla (IM).
AMPK increases water transport.
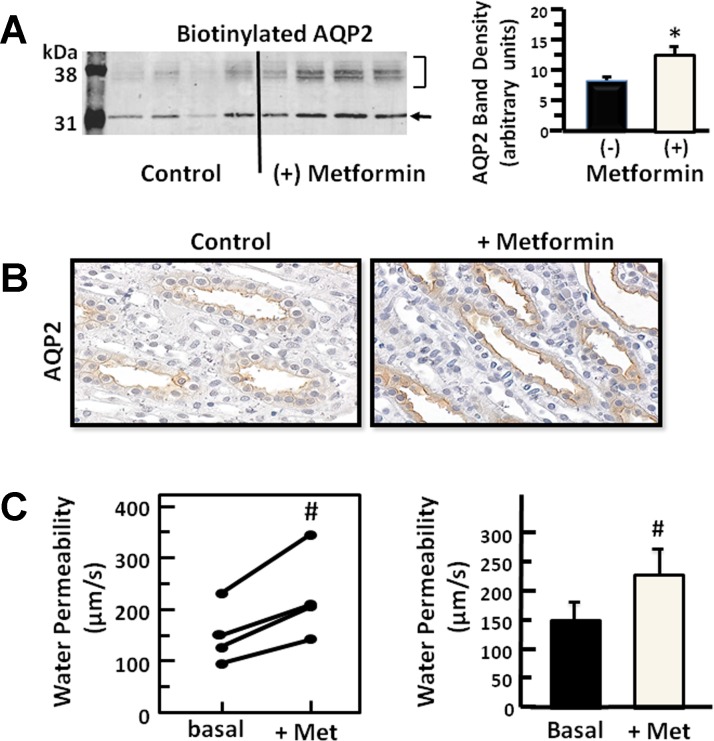
To determine whether AMPK stimulation alters the plasma membrane accumulation of AQP2, IMCD suspensions were stimulated with metformin, and then biotinylated to isolate membrane-associated proteins. The membrane amount of AQP2 was significantly increased (control: 100 ± 13%, metformin-stimulated: 176 ± 26%, n = 8, P < 0.05, Fig. 2A) with AMPK stimulation. Although histochemical analysis is largely not quantitative, injection of the rat with metformin followed by immunohistochemistry of perfusion-fixed kidney sections showed membrane localization consistent with the biotinylation results (Fig. 2B). To determine whether AMPK stimulation increased AQP2 water channel activity, the Pf of isolated perfused terminal IMCDs was measured. Pf significantly increased 51% from 149 ± 30 to 226 ± 43 μm/s in response to stimulation with metformin (n = 4, Fig. 2C).
Fig. 2.
Stimulation of AMPK results in increased membrane association of AQP2. A: representative Western blot of AQP2 in the biotinylated protein fraction from rat inner medulla treated for 30 min with 400 μM metformin. The bar graph shows the average band density from n = 8 animals per condition from 2 experiments. Bar = means ± SE, *P < 0.05. B: immunohistochemistry of AQP2 in kidneys of rats treated with metformin for 30 min before paraformaldehyde fixation of the kidneys as described in methods. Left: untreated control rat kidney; right: metformin-treated rat kidney (×400 magnification). C: osmotic water permeability measured by perfusion of isolated inner medullary collecting ducts (IMCDs) in response to stimulation of AMPK with 400 μM metformin. Each line represents a separate tubule from a separate animal. Bars, means ± SE, n = 4, #P < 0.05.
AMPK increases urea transport.
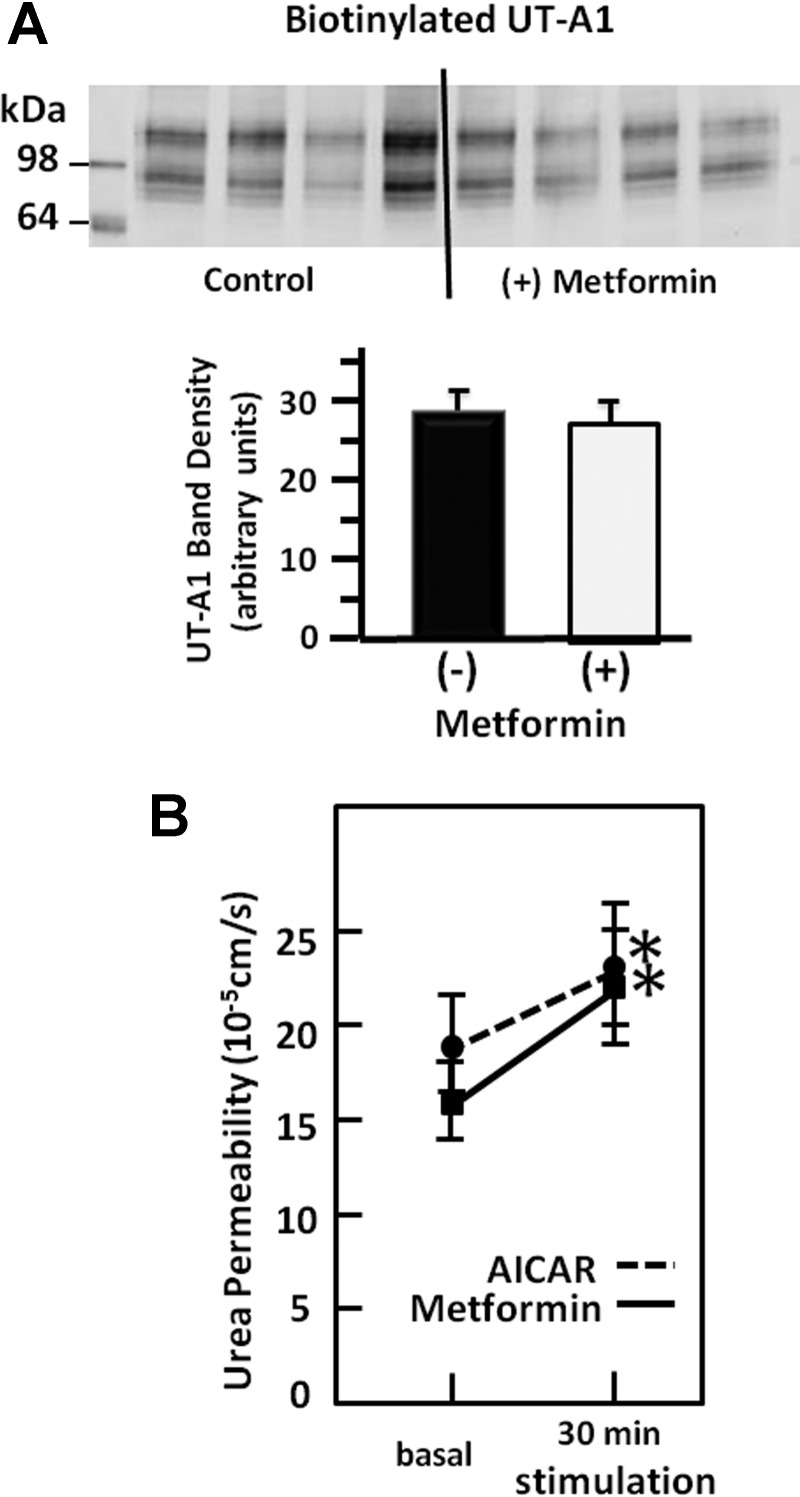
To determine whether AMPK stimulation alters the plasma membrane accumulation of UT-A1, IMCD suspensions were stimulated with metformin, and then biotinylated to isolate membrane proteins. The membrane amount of UT-A1 was unchanged by AMPK stimulation (n = 9, Fig. 3A). However, the Purea of isolated perfused terminal IMCDs was significantly increased by stimulation with metformin by 38%, from 16 ± 2 to 22 ± 3 × 10−5 cm/s, and by a second AMPK activator, AICAR (3 mM), by 21% from 19 ± 2 to 23 ± 3 × 10−5 cm/s (Fig. 3B).
Fig. 3.
Stimulation of AMPK does not increase membrane association of UT-A1 but does increase urea permeability. A: representative Western blot of UT-A1 in the biotinylated protein fraction from rat inner medulla treated for 30 min with 800 μM metformin. The bar graph shows the average band density from n = 9 animals per condition from 2 experiments. Bar = means ± SE. Bars were not statistically different. B: urea permeability in rat isolated perfused IMCD. Response to AMPK stimulation with metformin (squares, solid) or AICAR (circles, dashed). *P < 0.05.
AMPK increases urine concentration.
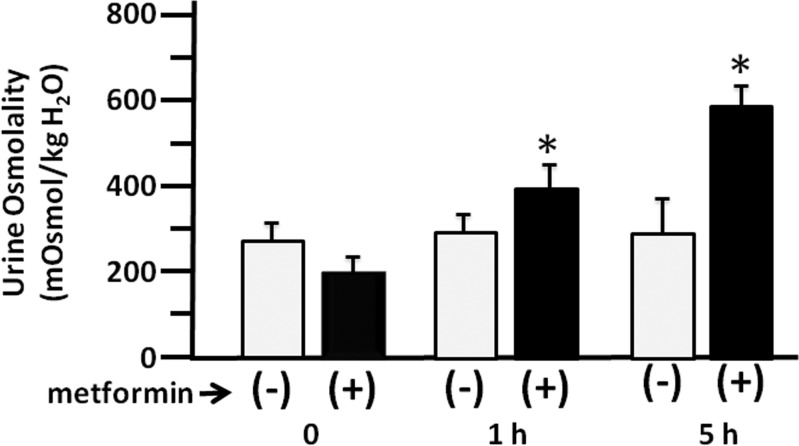
To determine whether stimulation of AMPK could increase urine concentration in the absence of vasopressin, we used an inducible V2R knockout mouse (17). The mice were fed tamoxifen to promote knockdown of the V2R. When urine osmolality reached 200 mosmol/kgH2O or below, the mice were placed in metabolic cages and some were given a single injection of 600 mg/kg metformin to stimulate AMPK. Urine was collected after 0, 1, and 5 h. Metformin significantly increased urine osmolality in the metformin-treated V2R knockout mice at both 1 and 5 h (n = 4, Fig. 4).
Fig. 4.
Stimulation of AMPK improves urine concentration in the absence of the vasopressin receptor. Urine osmolality is shown for 1-h urine samples collected from V2R knockout mice without (open bars) and with (filled bars) 600 mg/kg metformin treatment for 0, 1, and 5 h. Bars: means ± SE, n = 4, *P < 0.05.
DISCUSSION
The ability to produce concentrated or dilute urine results from the integrated function of several key transport proteins in the renal medulla (24). The Na-K-2Cl cotransporter, NKCC2, in the outer medulla is important since increases in NKCC2 lead to a hypertonic medulla, which is needed to generate the osmotic gradient for water reabsorption. The aquaporins, especially AQP2, is important since it increases water permeability of the collecting duct apical plasma membrane in response to vasopressin, facilitating water reabsorption in the presence of an osmotic gradient. The urea transporters, especially UT-A1, are crucial for movement of urea into the interstitial area surrounding the collecting ducts. UT-A1 increases the Purea of the IMCD apical plasma membrane in response to vasopressin, which is needed to augment the osmotic gradient for water reabsorption in the inner medulla. Mice lacking NKCC2, AQP2, or UT-A1/UT-A3 all have a urine-concentrating defect due to their inability to reabsorb NaCl, water, and urea (24). Thus, we hypothesized that a potential therapy for NDI would involve a kinase that could phosphorylate and activate all of the major proteins responsible for urine concentration: NKCC2, AQP2, and UT-A1; independent of the V2R-mediated effects of vasopressin.
AMPK was a logical candidate since it is known to phosphorylate NKCC2 (8–10). AMPK is stimulated by osmotic stress and hypoxia (21), but it has never been studied in the inner medulla, which is normally hypertonic and hypoxic. However, AMPK does regulate several renal transport proteins in the cortex and outer medulla (9, 21). AMPK is a heterotrimeric kinase with one catalytic (α) and two regulatory subunits (β and γ) (19). There are two AMPKα subunits: α1 and α2. We found that both α isoforms are expressed in the inner medulla.
Our current findings show that AMPK phosphorylates AQP2 and UT-A1, both in rat inner medulla and in vitro. The in vitro data indicate that AMPK can directly phosphorylate both AQP2 and UT-A1. We also found that metformin, an AMPK activator, increases both Pf and Purea in perfused rat terminal IMCDs. Interestingly, the mechanism by which AMPK activation increases in the Pf vs. Purea seems to differ. AMPK increases AQP2 phosphorylation and the apical plasma membrane accumulation of AQP2 and is likely to be the mechanism for the increase in Pf. AMPK also increases UT-A1 phosphorylation but we did not detect an increase in the apical plasma membrane accumulation, suggesting that AMPK may be increasing Purea by phosphorylating (activating) UT-A1 that is already present in the membrane. Thus, AMPK activation mimics many of the mechanisms by which vasopressin increases urine-concentrating ability (24).
To be useful therapeutically, an AMPK activator, such as metformin, must increase urine osmolality. Our current findings show that metformin increases urine osmolality in mice lacking a V2R, a mouse model of congenital NDI (17). Metformin is currently used to treat type 2 diabetes mellitus and polycystic ovary disease, indicating that it can be safely given to patients, including those who do not have diabetes mellitus. Our current findings suggest that metformin may be a novel therapeutic option for congenital NDI due to V2R mutations. Future studies involving chronic metformin administration will be necessary to evaluate whether the effect of metformin is sustained, and if it is, could be a useful therapy for NDI. Since metformin is commercially available for human use, it may be possible to translate our current findings to human studies expeditiously.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK41707, R01-DK89828-S1, and R25-DK101390.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.K. and J.M.S. conception and design of research; J.D.K., Y.W., M.A.B., L.M.L., J.A.R., and J.M.S. analyzed data; J.D.K., M.A.B., and J.M.S. interpreted results of experiments; J.D.K., L.M.L., and J.A.R. prepared figures; J.D.K. drafted manuscript; J.D.K. and J.M.S. edited and revised manuscript; J.D.K. and J.M.S. approved final version of manuscript; Y.W., M.A.B., P.A.M., L.M.L., and J.A.R. performed experiments.
REFERENCES
- 1.Anger MS, Shanley P, Mansour J, Berl T. Effects of lithium on cAMP generation in cultured rat inner medullary collecting duct cells. Kidney Int 37: 1211–1218, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis 13: 96–104, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Blessing NW, Blount MA, Sands JM, Martin CF, Klein JD. Urea transporters UT-A1 and UT-A3 accumulate in the plasma membrane in response to increased hypertonicity. Am J Physiol Renal Physiol 295: F1336–F1341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Brown D, Fenton RA. The cell biology of vasopressin action. In: Brenner and Rector's The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, and Brenner BM. Philadelphia: Elsevier, 2011, p. 353–383. [Google Scholar]
- 6.Cogan E, Svoboda M, Abramow M. Mechanisms of lithium-vasopressin interaction in rabbit cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1080–F1087, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Fenton RA, Praetorius J. Molecular physiology of the medullary collecting duct. Compr Physiol 1: 1031–1056, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Kim DU, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol 285: F303–F309, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Klein JD, Blount MA, Fröhlich O, Denson C, Tan X, Sim J, Martin CF, Sands JM. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol 298: F935–F940, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein JD, Blount MA, Sands JM. Urea transport in the kidney. Compr Physiol 1: 699–729, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Klein JD, Murrell BP, Tucker S, Kim YH, Sands JM. Urea transporter UT-A1 and aquaporin-2 proteins decrease in response to angiotensin II or norepinephrine-induced acute hypertension. Am J Physiol Renal Physiol 291: F952–F959, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM. Glucocorticoids mediate a decrease in AVP-regulated urea transporter in diabetic rat inner medulla. Am J Physiol Renal Physiol 273: F949–F953, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamenko M, Dhande I, Tomilin V, Zaika O, Boukelmoune N, Zhu Y, Gonzalez-Garay ML, Pochynyuk O, Doris PA. Defective store-operated calcium entry causes partial nephrogenic diabetes insipidus. J Am Soc Nephrol Epub ahead of print, doi: 10.1681/ASN.2014121200. [DOI] [PMC free article] [PubMed]
- 19.Moffat C, Ellen Harper M. Metabolic functions of AMPK: aspects of structure and of natural mutations in the regulatory gamma subunits. IUBMB Life 62: 739–745, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM. Glucocorticoids downregulate the vasopressin-regulated urea transporter in rat terminal inner medullary collecting ducts. J Am Soc Nephrol 8: 517–523, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Pastor-Soler NM, Hallows KR. AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens 21: 523–533, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Sands JM, Bichet DG. Nephrogenic diabetes insipidus. Ann Intern Med 144: 186–194, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Sands JM, Knepper MA. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest 79: 138–147, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Brenner and Rector's The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, and Brenner BM. Philadelphia: Elsevier, 2011, p. 326–352. [Google Scholar]
- 25.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol Renal Fluid Electrolyte Physiol 253: F823–F832, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State University Press, 1980. [Google Scholar]
- 27.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liedtke CM, Klein JD, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol 299: F1401–F1406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaki M, Kusano E, Tetsuka T, Takeda S, Homma S, Murayama N, Asano Y. Cellular mechanism of lithium-induced nephrogenic diabetes insipidus in rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F505–F511, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. [DOI] [PubMed] [Google Scholar]