Abstract
Purpose
The purpose of this multisite, multioperator, prospective, randomized, controlled clinical trial was to evaluate 2-year outcomes of diluted formocresol (DFC) compared to gray mineral trioxide aggregate (GMTA) as pulpotomy medicaments.
Methods
Following the standard pulpotomy procedure, the pulp stumps of 252 primary molars in 168 healthy children were randomly covered with GMTA or DFC. Pulp chambers were filled with Intermediate Restorative Material (IRM®) and teeth were restored with stainless steel crowns. At each follow-up appointment, the clinical status of the treated tooth was assessed and radiographs were taken. A total of 694 clinical and radiographic evaluations were analyzed.
Results
Gender, study site, arch type, and tooth type did not influence treatment outcome. At the combined 6- to 24-month follow-up, clinical success in the DFC group was no different than for the GMTA group. Radiographically, a significantly lower success rate was found in the DFC group vs the MTA group at all time points (P<.01). Dentin bridge formation was observed at a significantly higher frequency among the GMTA group (P<.01), while internal root resorption was observed at a higher frequency in the DFC group (P<.01).
Conclusion
At the combined 6- to 24-month follow-up, gray mineral trioxide aggregate demonstrated significantly better radiographic outcomes vs diluted formocresol as pulpotomy medicaments.
Keywords: PULPOTOMY, PRIMARY TEETH, CONTROLLED CLINICAL TRIAL, RADIOGRAPHIC IMAGE, LOCALE
The ideal pulpal medicaments should be nontoxic, nonmutagenic, noncarcinogenic, biocompatible, dimensionally stable, bactericidal, harmless to the pulp and surrounding structures, promote healing of the radicular pulp, and not interfere with the physiologic process of root resorption. Formocresol (FC), 1:5 dilution or full-strength, is a mummification agent that is the most widely used medicament for pulp treatment in primary teeth, with reported clinical and radiographic success rates ranging from 70% to 97%.1-6 FC is, however, potentially toxic and caustic and may cause irritation and inflammation upon contact with soft tissues. Although a considerable number of clinical trials and laboratory animal studies have been published on the subject of primary tooth pulpotomy medicaments, a recent Cochrane Review concluded that evidence is lacking to indicate which is the most appropriate technique for pulpotomies in primary teeth.7,8
ProRoot® mineral trioxide aggregate (MTA) was approved by the US Food and Drug Administration in 1998 as a therapeutic endodontic material for humans (Dentsply, Tulsa Dental Products, Tulsa, Okla., USA).9,10 The indications for using MTA mainly include treatments for apexification, root-end fillings, and repair of furcation or strip perforations, thus permitting proper apical sealing and stimulating the formation of periradicular tissues without inflammation.11,12 Given its sealing ability, MTA has been combined with gutta-percha for root canal filling or used alone as a barrier in the internal bleaching of devitalized teeth.12 More recently, in vivo studies have also shown the efficacy of MTA for direct pulp capping and pulpotomies in vital, immature permanent teeth absent signs or symptoms of irreversible pulpitis as well as for pulpotomy treatment of primary teeth.13-16 The success rates of MTA pulpotomies in primary teeth range from 80% to 100% (6-month follow-up) and 97% (4- to 74-month follow-up; Table 1). These reports have demonstrated the ability of MTA, a pulp dressing agent, to induce dentin bridge formation adjacent to the pulp.11,17,18 The limitations associated with those published studies include: lack of adequate sample size; short follow-up times; improper assessment of the long-term outcome; randomization techniques not described; nonblinded evaluators; and restorations other than preformed stainless steel crowns (SSCs).
Table 1.
CLINICAL STUDIES OF MINERAL TRIOXIDE AGGREGATE PRIMARY TOOTH PULPOTOMY*
| Study | Sample size |
Inclusion criteria |
Recall (mos) |
Pulpotomy technique |
Sample size (end of study) |
Clinical success (%) |
Radiographic success (%) |
|---|---|---|---|---|---|---|---|
| Doyle et al.34 | FS: 58 EF-FS: 78 GMTA: 57 FS/GMTA: 77 |
Symptomless, deep caries and vital pulp |
6-38 |
|
FS: 46 EF-FS: 64 GMTA: 47 FS/GMTA: 70 |
FS: 78 EF-FS: 74 GMTA: 100 FS/GMTA: 90 |
FS: 74 IRR: 33 PCO: 28 EF-FS: 52 IRR: 31 PCO: 13 GMTA: 96 IRR: 6 PCO: 43 FS/GMTA: 87 IRR: 17 PCO: 34 |
| Subramaniam et al.20 |
DFC: 20 GMTA: 20 |
Symptomless, carious exposure of vital pulp |
24 | DFC 1 min vs GMTA 2-stage restoration with SSC within 1 week |
DFC: 20 GMTA: 20 |
DFC: 100 GMTA: 100 |
DFC: 85 GMTA: 95 PCO: 25 |
| Sakai et al.35 | PC: 15 GMTA: 15 |
Symptomless, carious molar with vital pulp |
24 | PC vs GMTA All restored with GI |
PC: 9 GMTA: 9 |
PC: 100 GMTA: 100 |
PC: 100 GMTA: 100 |
| Sonmez36 | DFC: 20 FS: 20 CH: 20 GMTA: 20 |
Carious exposure of vital pulp |
6-24 |
|
DFC: 13 FS: 15 CH: 13 GMTA: 15 |
DFC: 85 FS: 100 CH: 92 GMTA: 93 |
DFC: 77 FS: 73 CH: 46 GMTA: 67 |
| Noorollahian21 | DFC: 30 GMTA: 30 |
Carious exposure of vital pulp |
6-24 | DFC 5 min vs GMTA 2-stage restoration with SSC after 24 hrs |
DFC: 27 GMTA: 29 |
DFC: 100 GMTA: 100 |
DFC: 100 PCO: −22 GMTA: 95 PCO: −5 |
| Moretti et al.22 | CH: 15 DFC: 15 GMTA: 15 |
Carious exposure of vital pulp |
3-24 | DFC 5 min vs CH vs GMTA All restored with GI |
CH: 14 DFC: 15 GMTA: 14 |
CH: 57 DFC: 100 GMTA: 100 |
CH: 36 IR: 43 DFC: 100 GMTA: 100 DB: 29 |
| Aeinehchi et al.37 | FC: 75 GMTA: 51 |
Deep caries | 3-6 | FC 5 min vs GMTA | FC: 57 GMTA: 43 |
FC: 100 GMTA: 100 |
FC: 89 IR: 11 GMTA: 100 |
| Maroto et al.38 | GMTA: 69 | Carious exposure of vital pulp |
42 | GMTA | 26 (11 teeth exfoliated by 42 months) |
GMTA: 100 | GMTA: 99 PCO: 84 DB: 83 |
| Percinoto et al.39 | CH: 45 GMTA: 45 |
Carious exposure of vital pulp |
12 | CH vs GMTA | CH: 45 GMTA: 45 |
CH: 87 GMTA: 96 |
|
| Holan et al.4 | FC: 29 MTA: 33 |
Symptomless, carious exposure of vital pulp |
4-74 | Full-strength FC (5 min) vs MTA |
FC: 24 MTA: 32 |
FC: 83 MTA: 97 (combined success) |
FC: IRR: 21 PCO: 52 MTA: IRR: 6 PCO: 58 |
| Saltzman et al.32 | FC-ZOE: 26 L-GMTA: 26 |
Carious exposure of vital pulp |
3- 15 | Full-strength FC (5 min) + ZOE base vs Diode laser + GMTA |
FC-ZOE: 13 L-GMTA: 7 |
FC-ZOE: 100 L-GMTA: 100 |
FC-ZOE: 89 L-MTA: 73 |
| Maroto et al.40 | GMTA: 20 | Carious exposure of vital pulp |
6 | GMTA | GMTA: 20 | GMTA: 100 | GMTA: 100 DB: 55 PCO: 60 |
| Naik et al.23 | DFC: 23 GMTA: 24 |
Carious exposure of vital pulp |
6 | DFC (5 min) vs GMTA |
DFC: 23 GMTA: 24 |
DFC: 100 GMTA: 100 |
DFC: 100 GMTA: 100 |
| Farsi et al.41 | FC: 60 GMTA: 60 |
Carious exposure of vital pulp |
6-24 | Full-strength FC (5 min) vs GMTA |
FC: 35 MTA: 38 |
FC: 97 GMTA: 100 |
FC: 86 IRR: 14 PCO: 3 GMTA: 100 PCO: 8 |
| Jabbarifar et al.42 | FC: 32 GMTA: 32 |
Symptomless, carious exposure of vital pulp |
6-12 | Full-strength FC (5 min) vs GMTA |
FC: 29 GMTA: 30 |
FC: 91 GMTA: 94 |
FC: 91 IRR: 9 GMTA: 94 IRR: 6 |
| Agamy et al.43 | FC: 24 WMTA: 24 GMTA: 24 |
Carious exposure of vital pulp |
1-12 | Full-strength FC (5 min) vs GMTA |
FC: 20 WMTA: 19 GMTA: 19 |
FC: 90 WMTA: 80 GMTA: 100 |
FC: 90 WMTA: 80 GMTA: 100 PCO: 56 |
| Cuisia et al.44 | FC: 30 GMTA: 30 |
Carious exposure of vital pulp |
6 | Full-strength FC (5 min) vs GMTA |
FC: 30 GMTA: 30 |
FC: 93 GMTA: 97 |
FC: 77 MTA: 93 |
| Eidelman et al.45 | FC: 22 GMTA: 23 |
Carious exposure of vital pulp |
6-30 | Full-strength FC (5 min) vs GMTA |
FC: 15 GMTA: 19 |
FC: 93 GMTA: 100 |
FC: 93 IRR: 7 PCO: 13 GMTA: 100 PCO: 41 |
FC=formocresol.
CH=calcium hydroxide.
EF-FS=eugenol-free ferric sulfate.
IPT=indirect pulp therapy.
PCO=pulp canal obliteration.
PC=Portland cement.
FR=furcation radiolucency.
ZOE=zinc oxide eugenol.
FR=furcation radiolucency.
GMTA=gray mineral trioxide aggregate.
WMTA=white mineral trioxide aggregate.
ER=external root resorption.
DB=dentin bridge formation.
SSC=stainless steel crown.
IRR=internal root resorption.
CM=calcific metamorphosis.
PR=periapical radiolucency.
IR=internal.
FS=ferric sulfate.
AM=amalgam.
GI=glass ionomer.
Compared to formocresol, MTA is a relatively new material still being investigated as a potential agent for pulp therapies in both primary and permanent dentitions.19 Only a few studies were found in the literature comparing diluted formocresol (DFC) to gray MTA.20-24 These studies followed a total of 40 to 60 teeth over a period of 6 to 24 months (Table 1). Considering concerns over potential FC toxicity and mutagenicity, this study was conducted to test an alternative to the current standard pulpotomy technique.
The purpose of this study was to compare the outcomes of diluted formocresol and gray mineral trioxide aggregate (GMTA) in human primary molar pulpotomies based on long-term follow-up of a multioperator, prospective, randomized, controlled clinical trial with adequate sample size, blinded evaluation, proper calibration/training of 3 blinded raters, and statistical analysis.
Methods
This study was reviewed and approved by the Medical School of the University of Michigan, Ann Arbor, Mich., and the Institutional Review Board of Mott Children’s Health Center (MCHC), Flint, Mich. This investigation followed an existing experimental protocol and expanded the subject population established in a preliminary study conducted by Zealand et al.16 All operators and their dental assistants were trained and calibrated for the pulpotomy procedure and follow-up evaluations. Although the pulpotomies were completed by both staff/faculty dentists and pediatric dentistry residents, all resident treatments were evaluated by faculty during treatment.
A power analysis was conducted using a published meta-analysis that compared GMTA and FC for primary molar tooth pulpotomies.24 A minimum sample of 250 teeth was set to ensure that an adequate sample size was collected and recalled to show validity and 80% power, assuming 20% attrition. The target subject population was a total of 200 ASA I and II healthy 2.5- to 10-year-old children. Patients were selected to be included in the study based on a list of selection and exclusion criteria (Table 2).
Table 2.
PATIENT SELECTION CRITERIA
| Inclusion criteria |
| ASA I and II children between 2.5 and 10 years old. |
| At least 1 primary molar, with no reported symptoms or reversible pulpitis, that has vital carious pulp exposures and whose pulp bled upon entering the pulp chamber. |
| Teeth in which hemostasis could be achieved within 5 minutes with direct pressure via a dry sterile cotton pellet, prior to medicament/material placement. |
| No clinical symptoms or evidence of pulp degeneration, such as excessive bleeding from the root canal, history of swelling, clinical mobility, spontaneous pain, or sinus tracts. |
| No radiographic signs of internal or external root resorption, inter-radicular and/or periapical bone destruction, furcal radiolucency, or periodontal ligament space widening. |
| No more than one third physiologic root resorption. |
| Teeth had not previously been pulpally treated. |
| Teeth that were deemed to be restorable with posterior stainless steel crowns. |
| Patients who were able to cooperate for periapical radiographs. |
| Patients whose parents were committed to returning for at least 1 recall visit 6 months after the pulpotomy was completed. |
| Exclusion criteria |
| A primary molar with symptoms and signs consistent with irreversible pulpitis. |
| Lacking pretreatment or immediate post pulpotomy radiographs. |
| Lacking radiographs of diagnostic quality. |
| Premature loss of restoration. |
| Crowns with inadequately sealed margin or inappropriate seating. |
| Subjects who failed to return for follow-up. |
All subjects who participated in this study were patients of record at 1 of 2 facilities: the Department of Orthodontics and Pediatric Dentistry, School of Dentistry, University of Michigan; and the Pediatric Dentistry Clinic of MCHC. The study sample included patients who presented between March 1, 2007 and February 29, 2008 having at least 1 carious primary molar requiring vital pulp treatment in the operating room or clinical setting. Vital pulpotomy was performed and definitive restoration was placed following the standard protocol (Zealand et al.)16
Under local anesthesia and rubber dam isolation, teeth were briefly prepped to have all caries removed and pulp exposure encountered. Using a no. 330 carbide bur in a high-speed handpiece with water spray, the pulp chamber was opened. Next, the coronal pulp was removed with a no. 6 round bur in a slow-speed handpiece and spoon excavator. In the DFC group, hemostasis was achieved with direct pressure of a sterile cotton pellet. A sterile cotton pellet moistened with diluted formocresol (20% or one-fifth strength; Buckley’s Formocresol, Sulton Healthcare, Englewood, N.J., USA), was squeeze dried and placed in contact with the surface of the pulp stumps for 5 minutes.
In the experimental group, hemorrhage was also achieved with direct pressure of a sterile cotton pellet. The GMTA paste was prepared by mixing 0.2 gm of powder with sterile saline to the consistency of a paste, which was placed directly onto the surface of the pulpal stumps and the chamber floor to a uniform 3 to 4 mm thickness. In both groups, the pulp chamber was then filled with IRM®; next, a SSC was fitted and cemented using glass ionomer cement (Rely-X 3M/ESPE, St. Paul, Minn., USA). The occlusion was checked, and a postprocedure periapical radiograph was taken. Clinical and radiographic outcomes were evaluated and scored based on the criteria established in Zealand et al.,16 and data were collected over a 2-year period.
Clinical success was defined as teeth that remained unchanged or within normal limits from treatment date for physiological mobility, minor/short-lasting percussion or chewing sensitivity, gingival inflammation from poor oral hygiene, and the absence of pathology, including: nonphysiological mobility greater than 2 mm; gingival inflammation not due to poor oral hygiene; periodontal pocket formation; spontaneous pain; sinus tract presence; and premature tooth loss due to pathology.
All periapical radiographs were digitized using 16-bit gray-scale and 600 dpi resolution. Evaluation tables were organized using spreadsheet software (Excel, Microsoft Inc, Redmond, Wash., USA) that contained the following information: patient numbero.; tooth numbero.; tooth treated; evaluation pathoses; and hyperlinks to pre- and post-treatment and 6-, 12-, 18-, and 24-month scanned radiographic images. Radiographic images could be viewed sequentially by recall month in an Explorer window (Microsoft Inc) by selecting multiple hyperlinked radiographic images and toggling between them.
Raters evaluated each follow-up radiograph for the presence of: unremarkable findings (NC); internal root resorption (IRR/IRR-P; nonperforated/perforated); external root resorption (ERR); dentin bridge formation (DB); pulp canal obliteration/calcific metamorphosis (PCO); furcal/periradicular radiolucencies (PRL); widening of the periodontal ligament space (PDL); periapical bone destruction; and physiological root resorption. Radiograph evaluations were retrospectively applied to missed recalls (eg, if a subject missed the 6-month recall, but presented for the 12-month recall, the 12-month evaluation was applied to both the 6- and 12-month evaluations). Teeth were considered radiographic failures at the time of extraction, but were not counted at future follow-up time points.
All radiographs were viewed by 3 blinded evaluators: 2 pediatric dentists and 1 endodontist who were full-time faculty members. The practice experience of the 3 raters ranged from 7 to 23 years, with a mean of 15 years. Before radiographic evaluation, the 3 raters were familiarized with rating worksheets and trained in scoring. Training was performed on 50 radiographs that were representative of the full range of pathoses seen at follow-up. All 3 raters examined and scored each 6-, 12-, 18-, and 24-month follow-up radiograph independently. The observers were blinded to the outcome of the other rater’s examination. A final consensus session was held after independent evaluation to discuss discrepancies in scoring among the 3 evaluators.
Radiographic success was defined as teeth that remained unchanged or within normal limits from treatment date or demonstrated dentin bridge formation, pulp canal obliteration, and the absence of: pathologic nonperforated and perforated internal resorption; external resorption; and inter-radicular or periapical bone destruction. PDL widening was not considered a failure in the absence of other concurrent pathoses.
Statistical analysis
Descriptive statistics were calculated for all study variables, and data was analyzed using the Statistical Package for Social Sciences v 17.0 (SPSS Inc, Chicago, Ill., USA). Clinical and radiographic success and failures and overall successes and failures were tested with each variable having a sample size of at least 5 using a 2-tailed, chi-square test with Yates’ correction where appropriate. Fisher’s exact test (2-tailed) was used to compare the treatment response of DFC and GMTA. Statistical significance was determined at P≤.05. Intra-rater and inter-rater reliability among the raters and with the consensus was calculated using Cohen’s kappa test.
Results
Demographic characteristics
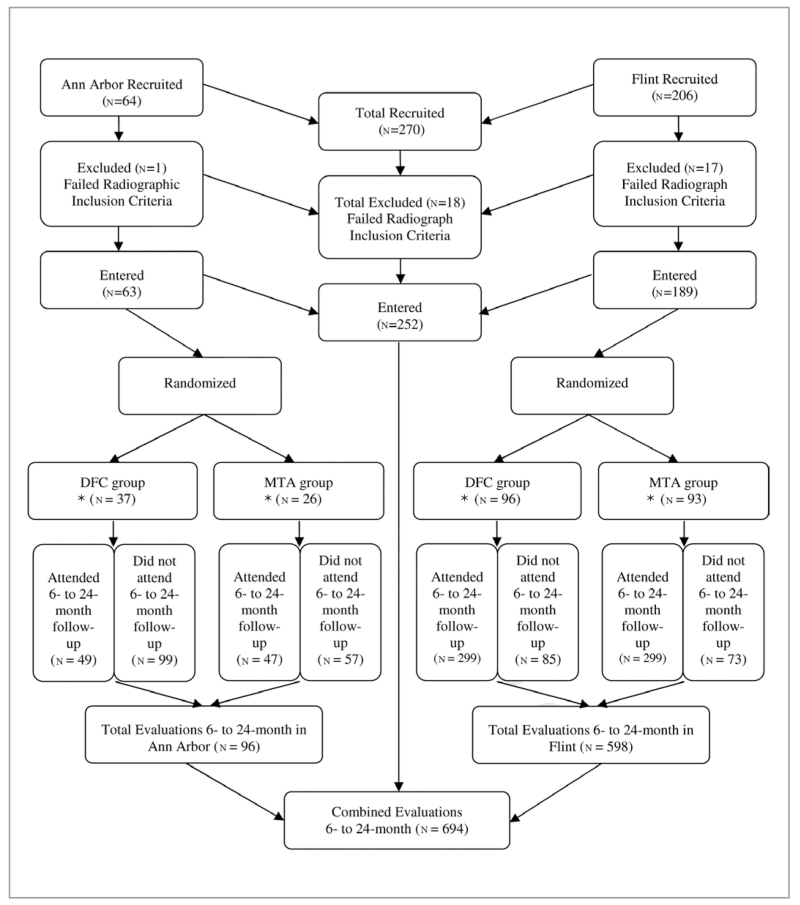
A total of 168 children, 81 females (~48%) and 87 males (~52%), with 252 teeth were included in the 6- to 24-month follow-up period. Females contributed 340 (~49%) evaluations, and males accounted for 354 (~51%) evaluations. Overall, 222/252 (88%) teeth from 152 children generated 694 radiographic and clinical evaluations for analyses, with an average of 12% lost at follow-up. Over the course of this 24-month study, the mean age of the recruited subjects was 5.4±1.4 years old, with a range of 2.5 to 10 years old. The distribution of recruited teeth in this study is shown in Figure 1.
Figure 1.
Distribution of recruited and recalled teeth at 6 to 24 months.
* n= Number of observations.
A total of 29 operators provided 694 evaluations between the 2 study sites, although over half of evaluations were conducted by 6 operators. Most teeth (~81%) were treated in the clinic setting. Overall, mandibular molars (~61%) were more frequently recruited than maxillary molars (~39%). The most frequently treated tooth was the mandibular second molar (~32%).
Three raters independently evaluated 50 periapical radiographs twice, 2 weeks apart, in a training/calibration session and a validation session. The mean inter-rater agreement of each pathology, between each rater and the group consensus, was fair to good (the average κ=0.33±0.11 to 0.59±0.10) from 6 to 24 months. The results from paired t tests indicated that the clinical variables did not influence the kappa values between each rater and consensus (P=.97), and there was no significant difference between rater and consensus regarding pathologic changes of teeth evaluated in the maxillary and mandibular arches.
Clinical findings
The average clinical scores were higher for the DFC group (1.1) than the GMTA group (1.0), indicating more clinical changes over the period of evaluation. These changes were not significant (P>.05). All teeth in the GMTA group were judged to be clinically successful (100%), and 4 teeth (2 maxillary first molars; 2 mandibular first molars) out of 348 teeth in the DFC group were judged to have failed from 6 to 24 months (~99%). At all follow-up time points as well as the combined 6- to 24-month time point, the clinical success rates of the DFC and the GMTA groups did not show statistically significant differences. Tables 3 and 4 present the clinical scores and success rates at the 6- to 24-month time points.
Table 3.
CLINICAL SCORE OF RECALLED TEETH AT 6, 12, 18, AND 24 MONTHS*
| Time (mos) |
Material | Clinical score |
Total n (%) |
|||
|---|---|---|---|---|---|---|
| 1 (NC) n (%) |
2 (poor OH) n (%) |
3 (perio) n (%) |
4 (abscess) n (%) |
|||
| 6 (n=222) | DFC | 110 (96) | 2 (2) | 0 (0) | 2 (2) | 114 (51) |
| GMTA | 104 (96) | 4 (4) | 0 (0) | 0 (0) | 108 (49) | |
| 12 (n=186) | DFC | 88 (98) | 2 (2) | 0 (0) | 0 (0) | 90 (48) |
| GMTA | 93 (97) | 3 (3) | 0 (0) | 0 (0) | 96 (52) | |
| 18 (n=155) | DFC | 73 (94) | 4 (5) | 0 (0) | 1 (1) | 78 (51) |
| GMTA | 75 (97) | 2 (3) | 0 (0) | 0 (0) | 77 (49) | |
| 24 (n=131) | DFC | 60 (91) | 5 (8) | 0 (0) | 1 (2) | 66 (51) |
| GMTA | 62 (95) | 3 (5) | 0 (0) | 0 (0) | 65 (49) | |
| Combined (n=694) |
DFC | 331 (95) | 13 (4) | 0 (0) | 4 (1) | 348 (51) |
| GMTA | 334 (97) | 12 (4) | 0 (0) | 0 (0) | 346 (49) | |
NC=no change; poor OH=poor oral hygiene as defined by plaque index; perio=significant periodontal changes, such as swelling, erythematous, or bleeding gingivae; abscess=accumulation of tissue fluid mixed with pus in the periodontal complex; DFC=diluted formocresol; GMTA=gray mineral trioxide aggregate; only score 4=abscess was considered a failure.
Table 4.
CLINICAL SUCCESS RATES AT 6, 12, 18, AND 24 MONTHS*
| Time (mos) | Material n (%) |
Outcome |
Total n (%) |
|
|---|---|---|---|---|
| Success n (%) |
Failure n (%) |
|||
| 6 (n=222) | DFC | 112 (98) | 2 (2) | 114 (51) |
| GMTA | 108 (100) | 0 (0) | 108 (49) | |
| FET=0.49 | ||||
| 12 (n=186) | DFC | 90 (100) | 0 (0) | 90 (48) |
| GMTA | 96 (100) | 0 (0) | 96 (52) | |
| FET† | ||||
| 18 (n=155) | DFC | 77 (99) | 1 (1) | 78 (50) |
| GMTA | 77 (100) | 0 (0) | 77 (50) | |
| FET=1.0 | ||||
| 24 (n=131) | DFC | 65 (98) | 1 (2) | 66 (50) |
| GMTA | 65 (100) | 0 (0) | 65 (50) | |
| FET=1.0 | ||||
| Combined (n=694) |
DFC | 344 (99) | 4 (1) | 348 (50) |
| GMTA | 346 (100) | 0 (0) | 346 (50) | |
| FET=0.12 | ||||
FET=Fisher’s exact test, 2-tailed; DFC=diluted formocresol; GMTA=gray mineral trioxide aggregate.
There is no difference.
Radiographic findings
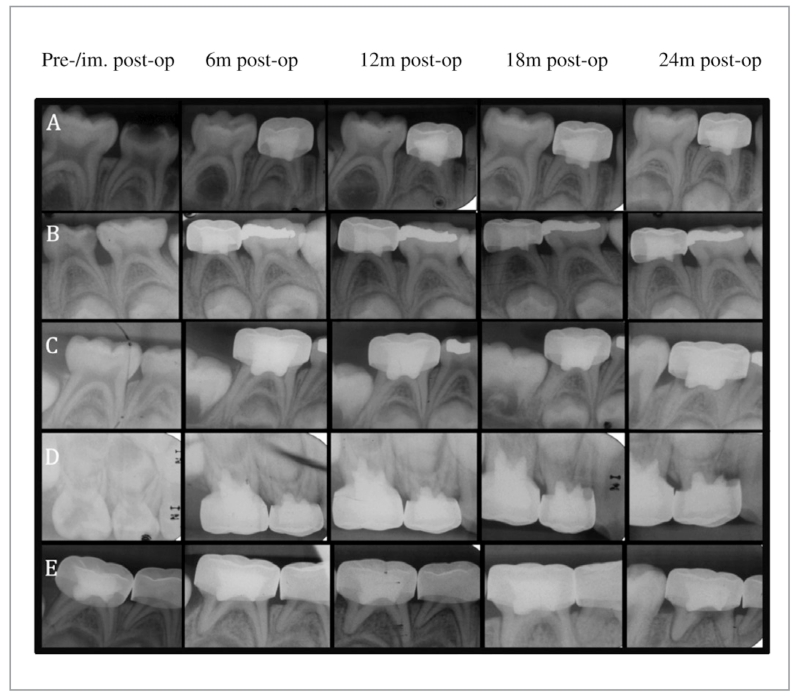
The 4 most common radiographic findings in the DFC-treated teeth were: PCO (~15%); IRR (~9%); PDL (~4%); and PRL (~3%). The 4 most common radiographic findings in the GMTA-treated teeth were: DB (~17%); PCO (15%); PDL (~6%); and IRR (~3%). Radiographs demonstrating the most representative pathoses are presented in Figure 2. Dentin bridge formation was observed at a significantly higher frequency among the GMTA-treated molars at all follow-up time points (P<.01), while IRR was observed at a higher frequency in the DFC-treated molars at 12-, 18-, and 24-month follow-up time points (P<.01). Based on 6- to 24-month radiographic results, there was a significantly lower number of teeth scored as NC, DB, and PCO (P<.01), but higher numbers of IRR, ERR, PDL, and PRL (P<.01) in the DFC-treated teeth vs the GMTA group (Tables 5 and 6).
Figure 2.
Representative periapical radiographs depicting outcomes of pulpotomies: (A) Gray mineral trioxide aggregate-treated tooth (no. S) showing progressive dentin bridge formation and pulpal canal obliteration (mesial and distal canal); (B) Diluted formocresol-treated tooth (no. L) showing progressive pulp canal obliteration (mesial and distal canal); (C) Diluted formocresol-treated tooth (no. T) showing progressive internal root resorption (distal canal); (D) Diluted formocresol-treated tooth (no. B) showing progressive internal root resorption-perforated to external root resorption (mesial canal); and (E) Diluted formocresol-treated tooth (no. T) showing progressive widening of the periodontal ligament space and a periradicular lesion (mesial and distal canal).
Table 5.
RADIOGRAPHIC SCORE AT 6, 12, 18, AND 24 MONTHS*
| Time (mos) | Material | Radiographic score |
Total n (%) |
|||
|---|---|---|---|---|---|---|
| 1 (NC) n (%) |
2 (DB, PCO, IRR) n (%) |
3 (ERR, PDL, IRR-P) n (%) |
4 (PRL) n (%) |
|||
| 6 (n=222) | DFC | 75 (66) | 27 (24) | 8 (7) | 4 (4) | 114 (51) |
| GMTA | 70 (65) | 30 (28) | 8 (7) | 0 (0) | 108 (49) | |
| 12 (n=186) | DFC | 38 (42) | 45 (50) | 6 (7) | 1 (1) | 90 (48) |
| GMTA | 50 (52) | 39 (41) | 7 (7) | 0 (0) | 96 (52) | |
| 18 (n=155) | DFC | 20 (26) | 48 (62) | 8 (10) | 2 (3) | 78 (50) |
| GMTA | 30 (39) | 44 (57) | 3 (4) | 0 (0) | 77 (50) | |
| 24 (n=131) | DFC | 16 (24) | 40 (61) | 5 (8) | 5 (8) | 66 (50) |
| GMTA | 27 (42) | 36 (55) | 2 (3) | 0 (0) | 65 (50) | |
| Combined (n=694) |
DFC | 149 (43) | 160 (46) | 27 (8) | 12 (3) | 348 (50) |
| GMTA | 177 (51) | 149 (43) | 20 (6) | 0 (0) | 346 (50) | |
NC=no change; DB=dentin bridge formation; PCO=pulpal canal obliteration; IRR=internal root resorption; PDL=periodontal ligament space; ERR=external root resorption; IRR-P=internal root resorption–perforated form; PRL=periradicular lesion; DFC=diluted formocresol; GMTA=gray mineral trioxide aggregate; radiographic failures included: IRR, ERR, IRR-P, and PRL. PDL occurring alone was not considered a failure.
Table 6.
RADIOGRAPHIC SUCCESS RATE AT 6, 12, 18, AND 24 MONTHS*
| Time (mos) | Material n (%) |
Outcome |
Total n (%) |
|
|---|---|---|---|---|
| Success n (%) |
Failure n (%) |
|||
| 6 (n=222) | DFC | 97 (85) | 17 (15) | 114 (51) |
| GMTA | 103 (95) | 5 (5) | 108 (49) | |
| FET=0.01 † | ||||
| 12 (n=186) | DFC | 73 (81) | 17 (19) | 90 (48) |
| GMTA | 89(93) | 7 (7) | 96 (52) | |
| FET=0.03 † | ||||
| 18 (n=155) | DFC | 61 (78) | 17 (22) | 78 (50) |
| GMTA | 73 (95) | 4 (5) | 77 (50) | |
| FET <001 † | ||||
| 24 (n=131) | DFC | 50 (76) | 16 (24) | 66 (50) |
| GMTA | 62 (95) | 3 (5) | 65 (50) | |
| FET <0.01 † | ||||
| Combined (n=694) |
DFC | 281 (81) | 67 (19) | 348 (50) |
| GMTA | 327 (95) | 19 (5) | 346 (50) | |
| FET <0.01 † | ||||
DFC=diluted formocresol; GMTA=gray mineral trioxide aggregate; df=degree of freedom.
Statistically significant at the FET ≤.05 level.
In the DFC group, 46% of the teeth scored a 2, and approximately 32% of those involved IRR. In the GMTA group, approximately 43% of the teeth scored a 2, and only 5% of those teeth involved IRR. The average radiographic scores were higher for the DFC group (1.7) than the GMTA group (1.5), indicating more radiographic changes over the period of evaluation. These changes were found to be significant between the 2 groups (P≤.05). In the GMTA group, 327/346 (~95%) molars were judged as radiographic successes, while only 281/348 (~81%) of the DFC group were radiographically successful at a combined 6- to 24-month follow-up. Specifically, at the 6-month follow-up, 103/108 (~95%) molars of the MTA group were radiographically successful, while only 97/114 (~85%) molars of the DFC group were radiographically successful. At the 12-month follow-up, 89/96 (~93%) molars of the MTA group were radiographically successful, while only 73/90 (~81%) molars of the DFC group demonstrated radiographic success. The 18-month radiographic evaluation showed that 73/77 (~95%) molars of the MTA group were radiographically successful, while only 61/78 (~78%) molars of the DFC group were radiographically successful. Finally, at the 24-month follow-up 62/65 (~95%) molars of the MTA group were radiographically successful, while only 50/66 (~76%) molars of the DFC group demonstrated radiographic success.
Arch type, molar type, gender, and locale of treatment, based on radiographic evaluations, did not influence the success of either the DFC or GMTA treatment, with one exception (Table 7). At the combined 6- to 24-month follow-up, there was significantly higher radiographic success of the DFC teeth treated in the clinic vs the operating room (P≤.05), while such a difference was not observed in the GMTA group.
Table 7.
INFLUENCE OF ARCH TYPE, MOLAR TYPE, GENDER, AND LOCALE OF TREATMENT ON OUTCOMES OBSERVED AT COMBINED 6 TO 24 MONTHS
| Arch type | Material | Outcome |
Total n (%) |
|
|---|---|---|---|---|
| Success n (%) |
Failure n (%) |
|||
| Maxilla | DFC | 103 (82) | 22 (18) | 125 (48) |
| MTA | 131 (98) | 3 (2) | 134 (52) | |
| Mandible | DFC | 178 (80) | 45 (20) | 223 (51) |
| MTA | 196 (92) | 16 (8) | 212 (49) | |
|
| ||||
| Maxilla DFC vs GMTA: FET <0.01† | ||||
| Mandible DFC vs GMTA: FET <0.01† | ||||
| DFC maxilla vs mandible: chi-square (1 df)=0.66, FET=0.67 | ||||
| GMTA maxilla vs mandible: FET=0.05 | ||||
|
| ||||
| First | DFC | 152 (84) | 28 (16) | 180 (56) |
| MTA | 136 (96) | 6 (4) | 142 (44) | |
| Second | DFC | 129 (77) | 39 (23) | 168 (45) |
| MTA | 191 (94) | 13 (6) | 204 (55) | |
|
| ||||
| First molar DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| Second molar DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| DFC first vs second: chi-square (1 df)=0.09, FET=0.08 | ||||
| GMTA first vs second: chi-square (1 df)=0.53, FET=0.48 | ||||
|
| ||||
| Female | DFC | 132 (80) | 32 (20) | 164 (48) |
| GMTA | 170 (97) | 6 (3) | 176 (52) | |
| Male | DFC | 149 (81) | 35 (19) | 184 (52) |
| GMTA | 157 (92) | 13 (8) | 170 (48) | |
|
| ||||
| Female DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| Male DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| DFC female vs male: chi-square (1 df)=0.91, FET=0.1 | ||||
| GMTA female vs male: chi-square (1 df)=0.14, FET=0.10 | ||||
|
| ||||
| Clinic | DFC | 230 (84) | 45 (16) | 275 (49) |
| MTA | 271 (95) | 14 (5) | 285 (51) | |
| Operating room | DFC | 51 (70) | 22 (30) | 73 (54) |
| MTA | 56 (92) | 5 (8) | 61 (46) | |
|
| ||||
| Clinic DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| Operating room DFC vs GMTA: chi-square (1 df) <0.01, † FET <0.01† | ||||
| DFC clinic vs operating room: chi-square (1 df) <0.01, † FET <0.01† | ||||
| GMTA clinic vs operating room: chi-square (1 df) <0.48, FET <0.35 | ||||
Chi-square=Pearson, 2-tailed; 1 df=1 degree of freedom; FET=Fisher’s exact test, 2-tailed; DFC=diluted formocresol; GMTA=gray mineral trioxide aggregate.
Statistically significant at the P<.05 level.
Discussion
GMTA was used in this study because it is a material with documented success in many endodontic procedures and was selected as the experimental group in our pilot study.16 Several in vitro and in vivo studies have shown that GMTA prevents microleakage, is biocompatible, and promotes regeneration of the original tissues when it is placed in contact with the dental pulp or periradicular tissues.12 Diluted formocresol was selected as the control group, since it is still considered the gold standard in primary tooth pulp therapy, in spite of its reported adverse properties.26-29 Until recent studies were carried out with MTA, none of the products proposed as alternatives to FC had shown greater efficacy or better clinical outcomes for pulpotomy treatment in primary teeth.30-33
Based on this study’s results, clinical success rates differ by only approximately 1% when comparing the GMTA group to the control group at a combined 6- to 24-month follow-up. This difference is not statistically significant. A larger number of observations may be needed to allow the detection of a statistically significant difference between the treatment groups.
Radiographic success in the GMTA group (~95%) was judged to be significantly higher than in the DFC group (~81%) from 6 to 24 months. During all recall periods, this difference was found to be statistically significant. It is interesting to note that GMTA’s radiographic success rate persisted around 94% from 6 months to 24 months, while the DFC group’s success rate continually declined from approximately 85% at 6 months to 81% at 12 months to 78% at 18 months to 76% at 24 months. This finding may support that chronic inflammation of the radicular pulp following DFC treatment promotes tissue degeneration, which may surface as radiographic failure at a later time. The similar clinical and radiographic success rates in the current study compared with previous literature reports validate the training/calibration of the evaluators and the study itself.
One interesting finding in the analysis of teeth treated by locale was that the overall success rate of DFC-treated teeth in the operating room was only approximately 70% (51/73) at the combined 6- to 24-month follow-up. This success rate is similar to the lowest reported DFC success rate in a 24-month follow-up period. The failure rate was significantly higher for DFC-treated molars in the operating room (~30%) than in the clinic (~16%) at the combined 6- to 24-month follow-up (P≤.05).
This result may be due to incorrect assessment of the pulpal status in the operating room, where diagnosis is often derived without subjects reporting their symptoms. It could also potentially be explained by the severity of the decay and the complexity of microflora associated with subjects needing full-mouth rehabilitation. Furthermore, patients treated in an operating room setting tend to be younger. Dental pulp maturation and regeneration ability, as well as the anatomy of furcation and accessory canals, may differ as primary teeth age. We speculate that, in a younger tooth, a caustic medicament may have greater adverse impact on the pulp and its surrounding tissues than a medicament that provides a good seal and prevents microleakage.
The inclusion of 252 teeth in 152 children may have introduced bias into the results, as the same pulpotomy technique may be used more than once in the same child. Consequently, the pulpotomy outcome may be influenced by host factors that were not controlled for in the present study. The statistical analysis applied in this study considered all observations of equal importance and did not consider that observations from follow-ups conducted at various time points of the same tooth may not be completely independent. In addition, the ability of healing and regeneration of dental pulp may differ among 2.5- to 10-year-olds. We recognize these factors as limitations of the current study design. To overcome these potential biases, increasing the subject population, limiting the statistics to 1 treated tooth per child, randomly selecting the tooth, and narrowing the age range of the subjects may be necessary in future studies.
There were occasions when findings of a later recall were used to deduce those of the earlier but missed recall. Such deduction was applied primarily to radiographically successful cases, since doing otherwise may lead to the interpretation of failed pulpotomies at an earlier stage.
This study is only a 24-month follow-up examination. As histological analyses were not conducted, the results should be considered with caution. Tissue responses to pulpotomy medicament will provide the most definitive evidence to guide clinical practice. A continuous evaluation of the teeth in this study at time points of 36 months and conducting survival and histologic analyses will provide additional evidence as to whether GMTA is a biocompatible pulpal medicament that can achieve a success comparable to DFC, the current gold standard in pediatric dentistry.
Conclusions
Based on this study’s results and the combined 6- to 24-month follow-up, the following conclusions can be made:
Variables such as arch type, molar type, gender, and locale of treatment did not appear to influence pulpotomy success of the gray mineral trioxide aggregate group vs the diluted formocresol group at the combined 6- to 24-month follow-up.
Dentin bridge formation was observed at a significantly higher frequency among the GMTA-treated molars; while internal root resorption was observed at a higher frequency in the DFC-treated molars.
The success rate is significantly higher for DFC-treated molars in the clinic than in the operating room at the combined 6- to 24-month follow-up.
The GMTA group demonstrated significantly better radiographic outcomes vs the DFC group. Both pulpal medicaments, however, presented comparable clinical outcomes in a period of 2 years.
Acknowledgments
The authors wish to thank the staff at the Children’s Clinic at the School of Dentistry, University of Michigan, Ann Arbor, Mich., and at Mott Children’s Health Center, Flint, Mich., for their contributions. All statistical analyses were conducted under the guidance of senior consultant Kathy B. Welch from The University of Michigan’s Center for Statistical Consultation and Research. This study was supported by the Michigan Institute for Clinical and Health Research (UL1RR024986), Ann Arbor, and Mott Children’s Health Center and Hurley Medical Center, Flint, Mich.
References
- 1.Straffon LH, Han SS. Effects of varying concentrations of formocresol on RNA synthesis of connective tissues in sponge implants. Oral Surg Oral Med Oral Pathol. 1970;29:915–25. doi: 10.1016/0030-4220(70)90448-2. [DOI] [PubMed] [Google Scholar]
- 2.Avram DC, Pulver F. Pulpotomy medicaments for vital primary teeth: Surveys to determine use and attitudes in pediatric dental practice and in dental schools throughout the world. J Dent Child. 1989;56:426–34. [PubMed] [Google Scholar]
- 3.Fuks AB, Eidelman E. Ferric sulfate and formocresol. Pediatr Dent. 2005;27:97. [PubMed] [Google Scholar]
- 4.Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005;27:129–36. [PubMed] [Google Scholar]
- 5.Primosch RE, Glomb TA, Jerrell RG. Primary tooth pulp therapy as taught in predoctoral pediatric dental programs in the United States. Pediatr Dent. 1997;19:118–22. [PubMed] [Google Scholar]
- 6.Vij R, Coll JA, Shelton P, Farooq NS. Caries control and other variables associated with the success of primary molar vital pulp therapy. Pediatr Dent. 2004;26:214–20. [PubMed] [Google Scholar]
- 7.Fuks AB. Pulp therapy for the primary and young permanent dentitions. Dent Clin North Am. 2000;3:571–96. vii. [PubMed] [Google Scholar]
- 8.Nadin G, Goel BR, Yeung CA, Glenny AM. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev. 2003;1:CD003220. doi: 10.1002/14651858.CD003220. [DOI] [PubMed] [Google Scholar]
- 9.Dentsply Endodontics . ProRoot MTA(Mineral Trioxide Aggregate) Root Canal Repair Material. Material safety data sheet. MSDS by Tulsa Dental Specialties, Dentsply International; Tulsa, Okla., USA: 2001. [Google Scholar]
- 10.Schwartz RS, Mauger M, Clement DJ, Walker WA., III Mineral trioxide aggregate: A new material for endodontics. J Am Dent Assoc. 1999;130:967–75. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- 11.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 13.El-Meligy OA, Avery DR. Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis) Pediatr Dent. 2006;28:399–404. [PubMed] [Google Scholar]
- 14.Fuks AB, Papagiannoulis L. Pulpotomy in primary teeth: Review of the literature according to standardized criteria. Eur Arch Paediatr Dent. 2006;7:64–71. doi: 10.1007/BF03320817. discussion 72. [DOI] [PubMed] [Google Scholar]
- 15.Ng FK, Messer LB. Mineral trioxide aggregate as a pulpotomy medicament: An evidence-based assessment. Eur Arch Paediatr Dent. 2008;9:58–73. doi: 10.1007/BF03262612. [DOI] [PubMed] [Google Scholar]
- 16.Zealand CM, Briskie DM, Botero TM, Boynton JR, Hu JC. Comparing gray mineral trioxide aggregate and diluted formocresol in pulpotomized human primary molars. Pediatr Dent. 2010;32:393–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Maroto M, Barberia E, Planells P, Garcia Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent. 2005;18:151–4. [PubMed] [Google Scholar]
- 18.Pitt Ford TA, Dorn SO, Kariyawasam SP. Effect of various zinc oxide materials as root-end fillings on healing after replantation. Int Endod J. 1995;28:273–8. doi: 10.1111/j.1365-2591.1995.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA. Comparison of bioactive glass, mineral trioxide aggregate, ferric sulfate, and formocresol as pulpotomy agents in rat molars. Dent Traumatol. 2003;19:314–20. doi: 10.1046/j.1600-9657.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 20.Subramaniam P, Konde S, Mathew S, Sugnani S. Mineral trioxide aggregate as a pulp capping agent for primary teeth pulpotomy: 2-year follow-up study. J Clin Pediatr Dent. 2009;33:311–4. doi: 10.17796/jcpd.33.4.r83r38423x58h38w. [DOI] [PubMed] [Google Scholar]
- 21.Noorollahian H. Comparison of mineral trioxide aggregate and formocresol as pulp medicaments for pulpotomies in primary molars. Br Dent J. 2008;204:E20. doi: 10.1038/sj.bdj.2008.319. [DOI] [PubMed] [Google Scholar]
- 22.Moretti AB, Sakai VT, Oliveira TM, et al. The effectiveness of mineral trioxide aggregate, calcium hydroxide, and formocresol for pulpotomies in primary teeth. Int Endod J. 2008;41:547–55. doi: 10.1111/j.1365-2591.2008.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Naik S, Hegde AM. Mineral trioxide aggregate as a pulpotomy agent in primary molars: An in vivo study. J Indian Soc Pedod Prev Dent. 2005;23:13–6. doi: 10.4103/0970-4388.16020. [DOI] [PubMed] [Google Scholar]
- 24.Erdem AP, Guven Y, Balli B, et al. Success rates of mineral trioxide aggregate, ferric sulfate, and formocresol pulpotomies: A 24-month study. Pediatr Dent. 2011;33:165–70. [PubMed] [Google Scholar]
- 25.Peng L, Ye L, Tan H, Zhou X. Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e40–e44. doi: 10.1016/j.tripleo.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach C, Moutschen-Dahmen M, Moutschen J. Genetic and cytogenetical effects of formaldehyde and related compounds. Mutat Res. 1977;39:317–61. doi: 10.1016/0165-1110(77)90011-2. [DOI] [PubMed] [Google Scholar]
- 27.King SR, McWhorter AG, Seale NS. Concentration of formocresol used by pediatric dentists in primary tooth pulpotomy. Pediatr Dent. 2002;24:157–9. [PubMed] [Google Scholar]
- 28.Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynolds KE. Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc. 1978;96:805–13. doi: 10.14219/jada.archive.1978.0187. [DOI] [PubMed] [Google Scholar]
- 29.Sun HW, Feigal RJ, Messer HH. Cytotoxicity of glutaraldehyde and formaldehyde in relation to time of exposure and concentration. Pediatr Dent. 1990;12:303–7. [PubMed] [Google Scholar]
- 30.El-Meligy O, Abdalla M, El-Baraway S, El-Tekya M, Dean JA. Histological evaluation of electrosurgery and formocresol pulpotomy techniques in primary teeth in dogs. J Clin Pediatr Dent. 2001;26:81–5. doi: 10.17796/jcpd.26.1.w2243176tj661n8p. [DOI] [PubMed] [Google Scholar]
- 31.Elliott RD, Roberts MW, Burkes J, Phillips C. Evaluation of the carbon dioxide laser on vital human primary pulp tissue. Pediatr Dent. 1999;21:327–31. [PubMed] [Google Scholar]
- 32.Saltzman B, Sigal M, Clokie C, Rukavina J, Titley K, Kulkarni GV. Assessment of a novel alternative to conventional formocresol-zinc oxide eugenol pulpotomy for the treatment of pulpally involved human primary teeth: Diode laser-mineral trioxide aggregate pulpotomy. Int J Paediatr Dent. 2005;15:437–47. doi: 10.1111/j.1365-263X.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 33.Waterhouse PJ, Nunn JH, Whitworth JM. Primary molar vital pulp theory. Br Dent J. 2000;188:417. [PubMed] [Google Scholar]
- 34.Doyle TL, Casas MJ, Kenny DJ, Judd PL. Mineral trioxide aggregate produces superior outcomes in vital primary molar pulpotomy. Pediatr Dent. 2010;32:41–7. [PubMed] [Google Scholar]
- 35.Sakai VT, Moretti AB, Oliveira TM, et al. Pulpotomy of human primary molars with MTA and Portland cement: A randomized controlled trial. Br Dent J. 2009;207:E5. doi: 10.1038/sj.bdj.2009.665. discussion 128-9. [DOI] [PubMed] [Google Scholar]
- 36.Sonmez D, Sari S, Cetinbas T. A comparison of four pulpotomy techniques in primary molars: A long-term follow-up. J Endod. 2008;34:950–5. doi: 10.1016/j.joen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Aeinehchi M, Dadvand S, Fayazi S, Bayat-Movahed S. Randomized controlled trial of mineral trioxide aggregate and formocresol for pulpotomy in primary molar teeth. Int Endod J. 2007;40:261–7. doi: 10.1111/j.1365-2591.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- 38.Maroto M, Barberia E, Vera V, Garcia-Godoy F. Dentin bridge formation after white mineral trioxide aggregate (white MTA) pulpotomies in primary molars. Am J Dent. 2006;19:75–9. [PubMed] [Google Scholar]
- 39.Percinoto C, de Castro AM, Pinto LM. Clinical and radiographic evaluation of pulpotomies employing calcium hydroxide and trioxide mineral aggregate. Gen Dent. 2006;54:258–61. [PubMed] [Google Scholar]
- 40.Maroto M, Barberia E, Planells P, Garcia Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent. 2005;18:151–4. [PubMed] [Google Scholar]
- 41.Farsi N, Alamoudi N, Balto K, Mushayt A. Success of mineral trioxide aggregate in pulpotomized primary molars. J Clin Pediatr Dent. 2005;29:307–11. doi: 10.17796/jcpd.29.4.n80t77w625118k73. [DOI] [PubMed] [Google Scholar]
- 42.Jabbarifar SK, Ghasemi D. Success rate of formocresol versus mineral trioxide aggregate in a human primary molar tooth. J Res Med Sci. 2004;6:55–8. [Google Scholar]
- 43.Agamy HA, Bakry NS, Mounir MM, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent. 2004;26:302–9. [PubMed] [Google Scholar]
- 44.Cuisia ZMR, Schneider P, Dummet C. A study of mineral trioxide aggregate pulpotomies in primary molars (abstract) Pediatr Dent. 2001;23:168. [Google Scholar]
- 45.Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs formocresol in pulpotomized primary molars: A preliminary report. Pediatr Dent. 2001;23:15–8. [PubMed] [Google Scholar]