Abstract
Purpose
The purpose of this multisite, multioperator, prospective, randomized, controlled clinical trial was to evaluate the 6-month outcomes of diluted formocresol (DFC) compared to gray mineral trioxide aggregate (GMTA) as pulpotomy medicament.
Methods
Determined by a power analysis, 252 molars of 152 children were recruited. The teeth were randomly assigned to receive GMTA or DFC. At the 6-month follow-up, 118 children with 203 treated teeth were evaluated.
Results
Four blinded and calibrated evaluators scored each radiograph for pathologies. Clinical success was similar for DFC (97%) and GMTA (100%), (P<.09). Radiographic success differed significantly (P<.04) for DFC (86%) and GMTA (95%). Pulp canal obliteration was radiographically observed in 25% of the DFC group and in 37% of the GMTA group (P=.07). Dentin bridging was observed in 22% of the GMTA group but was not found in the DFC group (P<.01).
Conclusion
Teeth treated with GMTA showed more favorable radiographic outcomes than DFC at 6 months post-treatment.
Keywords: PULP THERAPY/ENDODONTICS, DENTAL MATERIALS/BIOMATERIALS, DENTIN BRIDGE, PULP CANAL OBLITERATION
Formocresol (FC) has been a popular medicament for vital pulp therapy since its introduction 75 years ago, and is still considered to be the most universally taught and widely used medicament for pulp treatment in primary teeth.1-3 The formocresol pulpotomy, 1:5 dilution or full-strength, continues to be the gold standard for didactic and clinical training of dental students in the United States4 and Canada.5 Formocresol pulpotomy has reported clinical and radiographic success rates, ranging from 55% to 100%.6 Formocresol, however, is associated with potential adverse effects, which have been demonstrated in numerous studies.7
ProRoot mineral trioxide aggregate (MTA) has been used experimentally for a number of years and was given approval for human usage by the FDA in 1998.8 MTA is a powder composed of tricalcium silicate, bismuth oxide, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite, and calcium sulfate dihydrate.9,10 There is no difference between the healing property of MTA and Portland cement as a pulp-capping material.11 ProRoot MTA contains Portland cement clinker 75% w/w, bismuth oxide 20% w/w, and gypsum 5% w/w; these three major components of GMTA are common chemicals, especially bismuth oxide and gypsum which have long been used in dental materials.
There are some trace elements/impurities at the maximum of 0.6% w/w existing in ProRoot MTA, which may include free crystalline silica, calcium oxide, free magnesium oxide, potassium, and sodium sulfate compounds.12 It is a biocompatible material and allows bone regeneration and overgrowth of cementum when used as a root-end filling material.13,14 Its sealing ability is better than amalgam, zinc oxide- eugenol, or Super EBA (Harry J. Bosworth Company, Skokie, Ill) with low cytotoxicity.15,16 It sets via hydration in the presence of moisture to become a colloidal gel with a pH of 12.5. The setting time of the cement is 4 hours, and its compressive strength is 70 MPa, which is comparable to intermediate restorative material (IRM). It has been demonstrated to stimulate cytokine release from bone cells, promoting hard tissue formation.17
The use of MTA also has shown promising short-term results in primary teeth. Most of the recent literature has focused on comparing full-strength FC and gray mineral trioxide aggregate (GMTA) for pulpotomy of primary teeth.2,18-21 In 2006, a meta-analysis was conducted to compare the clinical and radiographic effects of GMTA with full-strength FC when used for pulpotomies of primary molars.22 Six qualified studies,2,18-23 comprising of 381 teeth, met the strict inclusion criteria. Based on the current available evidence, there was a significant difference between full-strength FC and GMTA-treated primary molars. GMTA appears to be superior to full-strength FC for pulpotomy with a higher clinical and radiographic success rate and a lower rate of internal resorption. This preliminary study was designed to clinically and radiographically compare the effect of GMTA to that of DFC as a pulpal medicament in a primary molar pulpotomy.
Methods
The Institutional Review Board at The University of Michigan, Ann Arbor, Michigan, and Mott Children’s Health Center (MCHC), Flint, Michigan, approved the study protocol. The procedure and its possible discomforts, risks, and benefits were fully explained to the parents/legal guardian of the children involved, and their informed consents were obtained prior to investigation. A power analysis indicated that 203 teeth were needed for the study. One hundred sixty-eight American Society of Anesthesiology (ASA) Class I or II 2½- to 10-year-old children (81 girls and 87 boys) with a mean age of 5.6±1.5 years, attending either the Children’s Clinic of the University of Michigan School of Dentistry or MCHC who met the strict inclusion criteria were randomly selected, contributing a total of 270 teeth for treatment in the study. Clinical and radiographic data were collected over a 1-year period.
All pediatric dental residents, assistants, and faculty involved in the study underwent a calibration session on typodont teeth. Preoperative periapical radiographs of the teeth considered for treatment in the study met the following criteria: proper film density and contrast for radiographic diagnosis; displayed a minimum of 3 mm past the furcation area; taken at a bitewing angle perpendicular to the film to prevent foreshortening/elongation of the tooth; and covering of the furcation with the stainless steel crown (SSC).
Randomization of the medicament used was done by an envelope draw. Only teeth with a clinical diagnosis of reversible pulpitis were included in the study. Teeth with necrotic pulp chambers were excluded. All treated teeth were anesthetized using 2% lidocaine with 1:100,000 epinephrine and isolated with a rubber dam. All teeth exhibiting carious pulp exposure, whose pulp bled upon entering, had the decay removed prior to pulp access were prepared for the placement of a SSC. Initial occlusal access was prepared using a high-speed dental handpiece and a no. 330 carbide bur with water spray. Removal of the coronal pulp was completed using a slow-speed handpiece with a round bur and spoon excavator. In the DFC control group, after hemostasis was achieved with direct pressure, a sterile cotton pellet containing diluted formocresol (Buckley’s Formo Cresol from Sultan Healthcare containing 19% formaldehyde, 35% cresol, 17.5% glycerin) 20% or one fifth strength, was squeeze dried and placed in contact with the amputated pulp for 5 minutes, followed by the placement of IRM.
In the GMTA experimental group, once the hemorrhage was under control using direct pressure of a sterile cotton pellet, the amputated pulp stumps and chamber floor were covered with GMTA paste prepared by mixing MTA powder with sterile saline on a glass slab using a metal spatula. A 3:1 powder/saline ratio was prepared according to the manufacturer’s recommendations to obtain a putty consistency. The mix was delivered to the pulp stumps with an amalgam carrier and condensed lightly with a moistened sterile cotton pellet to ensure a 3- to 4-mm thickness. In both groups, the pulp chamber was filled with IRM, and then a SSC was fitted and cemented using glass ionomer cement (Rely-X 3M ESPE, St. Paul, Minn). The occlusion was checked, and a postprocedure periapical radiograph was taken.
At the 6-month recall visit, the blinded clinical examination was performed by 1 of the 19 operators who were calibrated to the clinical scoring criteria. Periapical radiographs were taken and evaluated for the presence of various pathologies. All radiographs were viewed by 4 blinded, calibrated evaluators; 2 pediatric dentists and 1 endodontist who are full-time faculty members; and 1 pediatric dentist from private practice. The practice experience of the 4 evaluators ranged from 4 to 22 years, with a mean of 15 years. The criteria, based on Zurn and Seale 2008,24 used to score the clinical and radiographic findings are described in Table 1. The scoring system was devised to represent severity of changes but not to label an individual tooth as a “success” or “failure” (ie, as the score gets larger, the pathologies get progressively more invasive and require more frequent follow-up). Data was analyzed using the Statistical Package for Social Sciences v 16.0 (SPSS Inc., Chicago, Ill). The difference between the 2 materials was analyzed using chi-square and Fisher’s exact test. Intra- and inter-rater agreement was measured for the radiographic assessment using Cohen’s kappa test.
Table 1.
CRITERIA FOR CLINICAL AND RADIOGRAPHIC SCORING*
| Clinical score | Definition |
|---|---|
| 1=asymptomatic, 6-month recall |
|
| 2=slight discomfort, short-lived 3-month recall |
|
| 3=minor discomfort, short-lived 1-month recall |
|
| 4=major discomfort, long-lived Extract immediately |
|
|
| |
| Radiographic score | Definition |
|
| |
| 1=no changes present 6-month follow-up |
|
| 2=pathological changes of questionable clinical significance |
|
| 3-month follow-up |
|
| 3=pathological changes present 1-month follow-up |
|
| 4=pathological changes present Extract immediately |
|
Adapted from Zurn and Seale, 2008.
Results
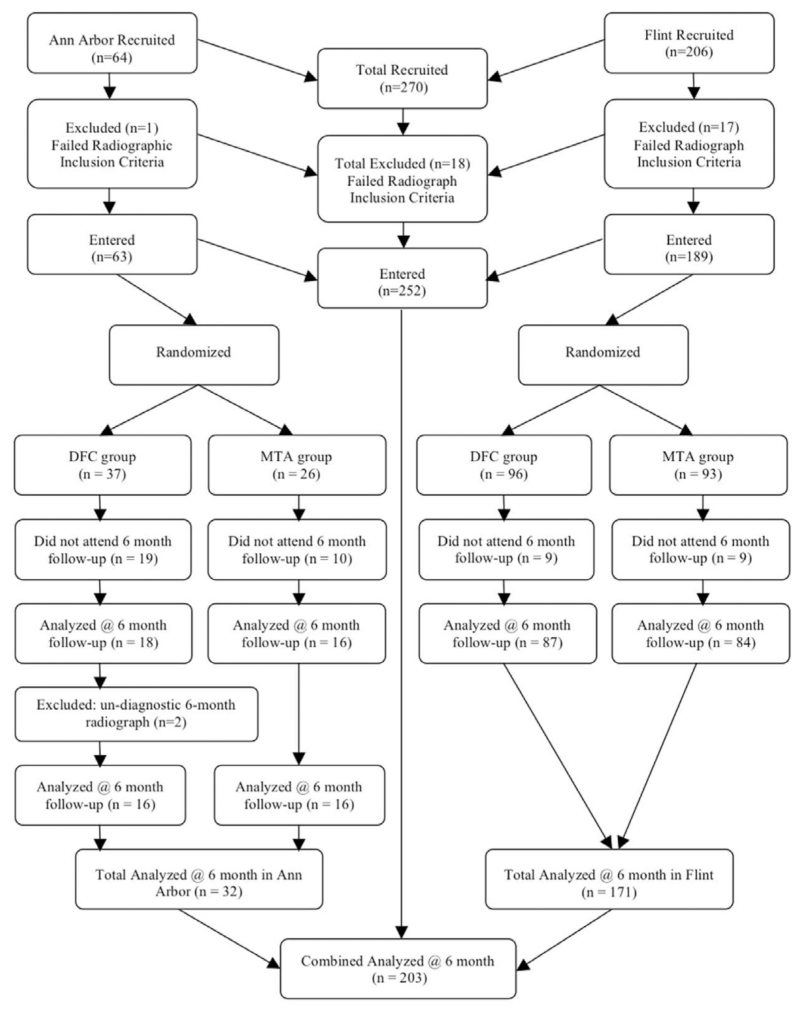
The number of recruited teeth that satisfied the inclusion criteria and entered the study was 252. Overall, 203/252 (81%) teeth were available for the 6-month evaluation, resulting in 19% lost at follow-up (Figure 1). The age of patients at recall, in both genders, ranged from 2½ to 10 years, with a mean of 5.5±1.5 years. A total of 17 operators were involved in treating the 203 teeth recalled between the 2 study sites, although half of the recruited teeth were treated by 3 operators. The intra- and inter-rater agreement for each pathology was measured using Cohen’s kappa (±SD) and found to be at moderate to good agreement range.25 Specifically, intra-rater agreement scores were: no change (NC; 0.57±0.05); dentinal bridging (DB; 0.57±0.11); pulp canal obliteration (PCO; 0.52±0.07); internal root resorption (IRR; 0.54±0.21); external root resorption (ERR; 0.59±0.09); periodontal ligament widening (PDL; 0.49±0.12); and periradicular lesion (PRL; 0.70±0.13). Inter-rater agreement scores were: NC (0.64±0.03); DB (0.65±0.14); PCO (0.63±0.02); IRR (0.47±0.08); ERR (0.52±0.15); PDL (0.57±0.10); and PRL (0.66±0.27). Overall, intra- and inter-rater reliability was good, with kappa values of 0.65±0.10 and 0.64±0.05, respectively.
Figure 1.
Distribution of recruited teeth through study.
The average clinical scores were the same for the DFC and the GMTA. Clinical outcomes of recalled teeth are summarized in Table 2. All teeth in the GMTA group were judged to be clinically successful (100%), and 3 out of 103 teeth in the DFC group were judged to have failed (success rate 97%). No significant difference was found between these 2 groups (P>.05).
Table 2.
SIX-MONTH CLINICAL AND RADIOGRAPHIC SCORE OF RECALLED TEETH (n=203)*
| Clinical score |
|||||
|---|---|---|---|---|---|
| Material | 1 (NC) (%) |
2 (poor OH) (%) |
3 (perio) (%) |
4 (PRL) (%) |
Total (%) |
| DFC | 97 (94) | 3 (3) | - | 3 (3) | 103 (51) |
| GMTA | 95 (95) | 5 (5) | - | 0 (0) | 100 (49) |
| Chi-square (2 degrees of freedom)=0.29 | |||||
| Clinical outcome |
|||
|---|---|---|---|
| Material | Success (%) | Failure (%) | Total (%) |
| DFC | 100 (97) | 3 (3) | 103 (51) |
| GMTA | 100 (100) | 0 (0) | 100 (49) |
| Chi-square (1 degree of freedom)=0.09, Fisher’s exact test=0.25 | |||
| Radiographic score |
|||||
|---|---|---|---|---|---|
| Material | 1 (NC) (%) |
2 (DB, PCO, IRR-NP) (%) |
3 (ERR, PDL, IRR-P) (%) |
4 (PRL) (%) |
Total (%) |
| DFC | 58 (56) | 28 (27)† | 14 (14) | 3 (3) | 103 (51) |
| GMTA | 51 (51) | 39 (39)‡ | 10 (10) | 0 (0) | 100 (49) |
| Chi-square (3 degrees of freedom)=0.12 | |||||
| Radiographic outcome |
|||
|---|---|---|---|
| Material | Success (%) | Failure (%)§ | Total (%) |
| DFC | 89(86) | 14(14) | 103(51) |
| GMTA | 95(95) | 5(5) | 100(49) |
| Chi-square (1 degree of freedom)=0.04, Fisher’s exact test=0.05∥ | |||
NC=no change; DB=dentin bridge; PCO=pulp canal obliteration; IRR-NP=internal root resorption-nonperforated form; PDL=periodontal ligament widening; ERR=external resorption; IRR-P=internal root resorption–perforated form; PRL=periradicular lesion.
IRR-NP=5/28 (18%).
IRR-NP=4/39 (10%).
Radiographic failures included: IRR, ERR, IRR-P, and PRL; PDL occurring alone was not considered a failure.
Statistically significant at the P≤.05 level.
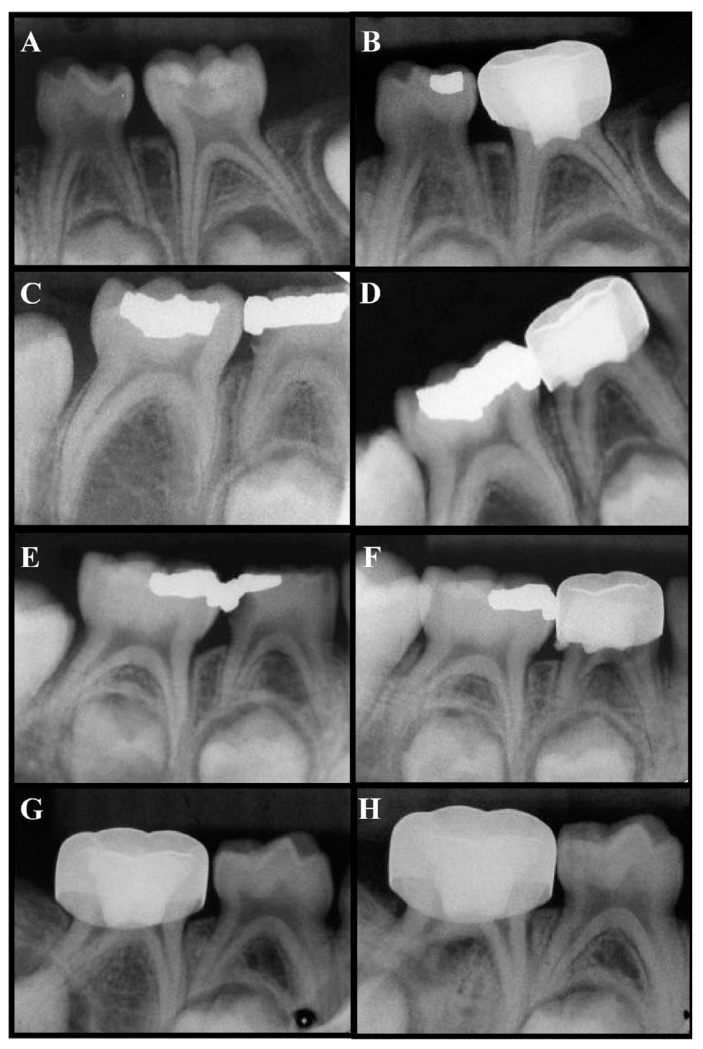
The radiographic pathologies observed include: NC; DB (Figure 2A-B); PCO (Figure 2A-B); IRR/nonperforated form (IRR-NP; Figure 2C-D); PDL; ERR; IRR/perforated form (IRR-P; Figure 2E-F); and PRL (Figure 2G-H). Radiographic failures included: IRR-NP; ERR; IRR-P; and PRL. Widening of PDL alone was not considered a failure. The 2 most common radiographic findings in the DFC-treated teeth were: PCO (25%); and PDL widening (11%). The 2 most common radiographic findings in the GMTA-treated teeth were: PCO (37%); and DB (22%). The only pathology that was significantly different (P<.05) between the 2 treated groups was DB in the GMTA-treated teeth, whereas the DFC-treated teeth did not have any occurrence of DB (Table 3).
Figure 2.
Radiographic changes observed at 6-month follow-up. (A) Preoperative view and (B) 6-month postoperative view of GMTA-treated tooth K showing dentin bridge and pulp canal obliteration (mesial and distal canal). (C) Preoperative view and (D) 6-month postoperative view of diluted formocresol (DFC)-treated tooth S showing internal root resorption-nonperforated form (IRR-NP) in distal canal. (E) Preoperative view and (F) 6-month postoperative view of DFC-treated tooth S showing IRR-P (perforated form). (G) Preoperative view and (H) 6-month postoperative view of DFC-treated tooth T showing a periradicular lesion.
Table 3.
SIX-MONTH RADIOGRAPHIC OUTCOME OF RECALLED TEETH: DISTRIBUTION AND FREQUENCY OF RADIOGRAPHIC CHANGES OF DFC AND GMTA PULPOTOMIES*
| Radiographic outcome |
Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||||||||||
|
|
|||||||||||||||||
| Radiographic changes |
NC | DB | PCO | IRR-NP | PDL | ERR | IRR-P | PRL | |||||||||
| Treatment | DFC | GTMA | DFC | GTMA | DFC | GMTA | DFC | GMTA | DFC | GMTA | DFC | GMTA | DFC | GMTA | DFC | GMTA | |
| Combined number (n) Frequency (%) |
54(52) | 52(52) | 0(0) | 22(22) | 26(25) | 37(37) | 5(5) | 4(4) | 11(11) | 9(9) | 6(6) | 1(1) | 1(1) | 0(0) | 3(3) | 0(0) | 231(100) |
| Chi-square/ Fisher’s exact tests |
0.95/1.0 | <0.01†/<0.01† | 0.07/0.10 | 0.77/1.0 | 0.69/0.82 | 0.06/0.12 | 0.32/1.0 | 0.09/0.50 | |||||||||
NC=no change; DB=dentin bridge; PCO=pulp canal obliteration; IRR-NP=internal root resorption-nonperforated form; PDL=periodontal ligament widening; ERR=external resorption; IRR-P=internal root resorption–perforated form; PRL=periradicular lesion.
Statistically significant at the P≤.05 level.
In the DFC group, 27% of the teeth scored a 2, 18% of which involved IRR-NP. In the GMTA group, 39% of the teeth scored a 2 and only 10% of those teeth involved IRR-NP. The average radiographic scores were equal for the DFC (1.6) and the GMTA (1.6) groups, indicating the same number of radiographic changes over the 6-month evaluation period. In the DFC group, most scores came from IRR-NP and PCO. In the GMTA group, most scores came from DB and PCO. These changes were not significant in the 6-month period (P>.05). Radiographic success in the GMTA group (95%) was judged to be higher than in the DFC group (86%), which was statistically different (P<.05).
Discussion
The outcome of GMTA pulpotomy, with all 100 (100%) molars being clinically successful compared to 100 out of 103 (97%) in the control group after a 6-month follow-up, was not statistically different. The 3 clinical failures in the DFC group involved: 1 primary maxillary first molar; 1 primary mandibular first molar; and 1 primary mandibular second molar. Other studies have reported 90% to 100% clinical success rates with FC after 12-months.3 In the present study, DFC and GMTA had similar efficacy and corroborated the findings of other reports.
The radiographic outcome in this study revealed that 95 of 100 (95%) molars in the experimental group were deemed successful, while only 89 of 103 (86%) of the DFC group were radiographically successful at the 6-month follow-up. This difference was statistically significant. Other studies have reported radiographic success rates of 93% to 100% with GMTA after 12-months,18-21,23,25-27 while lower radiographic success rates of 77% to 100% with DFC after 12-months.18-21,28-30
Dentin bridge formation can be detected in calcium hydroxide and GMTA-treated teeth, which supports the suggestion that both materials have a similar mechanism of action regarding bridging.11,32-34 PCO or calcific metamorphosis is a common radiographic finding in pulpotomized teeth treated with FC34 and DFC.36 PCO occurs as a result of odontoblastic activity and suggests that the affected tooth has retained some degree of vitality23,35—therefore, PCO is not regarded as a failure. The difference in PCO between the 2 groups was not found to be statistically significant.
Internal resorption in primary teeth is associated with persisted inflammation. Because of the thinness of the primary molar roots, if internal resorption can be seen radiographically, a perforation usually exits.37 Internal resorption is a common radiographic finding in pulpotomized teeth. In the present study, it was regarded as a radiographic failure. A wide range of frequencies have been reported in teeth treated with both FC (7-40%)2,20,23,32 and DFC (1-15%)26,37 In this study, the DFC group showed 5 of 103 (5%) cases with internal resorption, which is in the range reported in the literature. The GMTA group showed 4 of 100 (4%) cases with IRR, which also has been reported in 6% of teeth treated with GMTA over a 12- to 38-month period.2,19 The difference in IRR-NP between the 2 groups was not found to be statistically significant. It is reasonable to mention that, regardless of the material employed, a periodic clinical and radiographic evaluation of teeth subjected to pulp treatment must be emphasized.
Interpretation of radiographs of primary teeth is always complicated by the presence of the succedaneous tooth and surrounding follicle. Widening of the PDL spaces occurred in 11% (11/103) of DFC-treated teeth and 9% (9/100) of the GMTA-treated teeth without clinical symptoms. More specifically, PDL widening in conjunction with ERR occurred in 3% (3/103) of teeth in the DFC group and 1/100 (1%) of teeth in the GMTA group. The differences between the 2 groups were not statistically significant.
Six percent (6/103) of DFC-treated teeth presented with external root resorption without any clinical signs and symptoms, and only 1% (1/100) in the GMTA group showed ERR. One percent (1/103) of DFC-treated teeth and 0% in the GMTA group presented IRR-P without any clinical signs and symptoms (P>.05). Although ERR and IRR-P were considered radiographic failures, clinically it may not justify an immediate extraction. Instead, a 1-month follow-up exam is warranted. Three percent (3/103) of DFC-treated teeth clinically presented with abscesses and radiographically presented with radiolucencies in the furcation or PRL. None of the teeth treated in the GMTA group presented with PRL. This type of radiographic pathology indicates immediate extraction, regardless of the clinical signs and symptoms experienced by the patient.
It was speculated that a pulpotomy’s success may be influenced by 4 variables: arch type (maxilla vs mandible); molar type (first molars vs second molars); gender (male vs female); and locale of treatment (clinic vs operating room). The only variable that showed a statistically significant difference was the treatment location. In the clinic, there was a statistically significant difference in success rates between DFC-treated teeth (82%) and GMTA-treated teeth (94%). Interestingly, none of the teeth treated in the operating room under general anesthesia failed, regardless of the pulpal medicament. This could potentially be explained by the fact that cleaner conditions are easier to achieve in the operating room environment, as patient behavior is not a factor. Furthermore, there is a tendency to be more aggressive in the operating room when it comes to pulp therapy than in the clinic. That is, a tooth that may undergo indirect pulp therapy in the clinic is more likely to receive a pulpotomy in the operating room.
This study’s results show that GMTA has the potential to become a replacement for DFC in primary molar pulpotomies. The list price of a ProRoot MTA refill kit, as of August 2010, is $251.50. In this study, 0.2 gm of MTA was used to seal off the canal orifices following the pulpotomy procedure. Each refill kit containing 5 1-gm packets was sufficient for 25 pulpotomies. Therefore, the estimated cost of MTA per pulpotomy is approximately $10. Even though the sample size was adequate, it is still premature to draw definitive conclusions, as the follow-up period is short. This study provided a scientific basis for a large-scale, ongoing investigation that includes multicenter, large cohort, long-term follow-ups, split-mouth design, and histological examination on treated teeth.
Conclusions
Based on this study’s results, the following conclusions can be made:
Overall, the combined clinical and radiographic success rates at the 6-month follow-up were 95% for gray mineral trioxide aggregate pulpotomies and 86% for diluted formocresol pulpotomies.
Variables such as arch type, molar type, and gender did not appear to influence pulpotomy success over a 6-month period.
Locale of treatment (clinic vs operating room) seemed to influence pulpotomy success, regardless of material used. A significant difference in success rates was found in the operating room compared to a clinic environment.
Acknowlegments
We would like to thank the supporting staff at the Children’s Clinic, University of Michigan School of Dentistry and Mott Children’s Health Center, Flint, Mich., for their contributions to this study. This study was supported by Michigan Institute for Clinical & Health Research and Delta Dental Foundation of Michigan.
Footnotes
The authors declare no conflict of interest.
References
- 1.Seale NS, Glickman GN. Contemporary perspectives on vital pulp therapy: Views from the endodontists and pediatric dentists. Pediatr Dent. 2008;30:261–7. [PubMed] [Google Scholar]
- 2.Milnes AR. Is formocresol obsolete? A fresh look at the evidence concerning safety issues. Pediatr Dent. 2008;30:237–46. [PubMed] [Google Scholar]
- 3.Fuks AB, Papagiannoulis L. Pulpotomy in primary teeth: Review of the literature according to standardized criteria. Eur Arch Paediatr Dent. 2006;7:64–71. doi: 10.1007/BF03320817. discussion 72. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatric Dentistry Clinical Affairs Committee Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;29:163–7. [Google Scholar]
- 5.Casas MJ, Kenny DJ, Judd PL, Johnston DH. Do we still need formocresol in pediatric dentistry? J Can Dent Assoc. 2005;71:749–51. [PubMed] [Google Scholar]
- 6.Loh A, O’Hoy P, Tran X, Charles R, Hughes A, Kubo K, et al. Evidence-based assessment: Evaluation of the formocresol versus ferric sulfate primary molar pulpotomy. Pediatr Dent. 2004;26:401–9. [PubMed] [Google Scholar]
- 7.Ranly D, Garcia-Godoy F. Current and potential pulp therapies for primary and young permanent teeth. J Dent. 2000;28:153–61. doi: 10.1016/s0300-5712(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz R, Mauger M, Clement D, Walker W. Mineral trioxide aggregate: A new material for endodontics. J Am Dent Assoc. 1999;130:967–75. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- 9.Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32:193–7. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pitt Ford TR. The constitutes of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Holland R, de Souza V, Nery MJ, et al. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. Braz Dent J. 2001;12:3–8. [PubMed] [Google Scholar]
- 12.Greenburg J. ProRoot MTA (mineral trioxide aggregate) material safety data sheet. Available at: “ www.dentsply.co.uk/Products/Endodontics/ReTreatment-Repair/Proroot-MTA.aspx”. 2002 Feb 1; www.dentsply.co.uk/Products/Endodontics/ReTreatment-Repair/Proroot-MTA.aspx
- 13.Torabinejad M, Pitt Ford T, McKendry D, Abedi H, Miller D, Kariyawasam S. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225–9. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 14.Sluyk S, Moon P, Hartwell G. Evaluation of setting properties and retention characteristics of MTA when used as a furcation perforation repair material. J Endod. 1998;24:768–71. doi: 10.1016/S0099-2399(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 15.Fogel HM, Peikoff MD. Microleakage of root-end filling materials. J Endod. 2001;27:456–8. doi: 10.1097/00004770-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Weldon JK, Pashley DH, Loushine RJ, Weller RN, Kimbrough WF. Sealing ability of mineral trioxide aggregate and super-EBA when used as furcation repair materials: A longitudinal study. J Endod. 2002;28:467–70. doi: 10.1097/00004770-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998;24:543–7. doi: 10.1016/S0099-2399(98)80074-5. [DOI] [PubMed] [Google Scholar]
- 18.Agamy HA, Bakry NS, Mounir MM, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent. 2004;26:302–9. [PubMed] [Google Scholar]
- 19.Jabbarifar SE, Khademi AA, Ghasemi D. Success rate of formocresol versus mineral trioxide aggregate in human primary molar tooth. J Res Med Sci. 2004;6:55–8. [Google Scholar]
- 20.Farsi N, Alamoudi N, Balto K, Mushayt A. Success of mineral trioxide aggregate in pulpotomized primary molars. J Clin Pediatr Dent. 2005;29:307–11. doi: 10.17796/jcpd.29.4.n80t77w625118k73. [DOI] [PubMed] [Google Scholar]
- 21.Naik S, Hegde A. Mineral trioxide aggregate as a pulpotomy agent in primary molars: An in vivo study. J Indian Soc Pedod Prev Dent. 2005;23:13–6. doi: 10.4103/0970-4388.16020. [DOI] [PubMed] [Google Scholar]
- 22.Peng L, Ye L, Tan H, Zhou X. Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e40–4. doi: 10.1016/j.tripleo.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs formocresol in pulpotomized primary molars: A preliminary report. Pediatr Dent. 2001;23:15–8. [PubMed] [Google Scholar]
- 24.Zurn D, Seale NS. Light-cured calcium hydroxide vs formocresol in human primary molar pulpotomies: A randomized controlled trial. Pediatr Dent. 2008;30:34–41. [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 26.Maroto M, Barberia E, Planellis P, Garcia Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent. 2005;18:151–4. [PubMed] [Google Scholar]
- 27.Aeinehchi M, Dadvand S, Fayazi S, Bayat-Movahed S. Randomized controlled trial of mineral trioxide aggregate and formocresol for the pulpotomy in primary molar teeth. Int Endod J. 2007;40:261–7. doi: 10.1111/j.1365-2591.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- 28.Noorollahian H. Comparison of mineral trioxide aggregate and formocresol as pulp medicaments for pulpotomies in primary molars. Br Dent J. 2008;204(11):E20. doi: 10.1038/sj.bdj.2008.319. [DOI] [PubMed] [Google Scholar]
- 29.Markovic D, Zivojinovic V, Vucetic M. Evaluation of three pulpotomy medicaments in primary teeth. Pediatr Dent. 2005;3:133–8. [PubMed] [Google Scholar]
- 30.Huth KC, Paschos E, Hajek-Al-Khatar N, et al. Effectiveness of 4 pulpotomy techniques: Randomized controlled trial. J Dent Res. 2005;84:1144–8. doi: 10.1177/154405910508401210. [DOI] [PubMed] [Google Scholar]
- 31.Ibricevic H, Al-Jame Q. Ferric sulphate and formocresol in pulpotomy of primary molars: Long-term follow-up study. Eur J Paediatr Dent. 2003;4(1):28–32. [PubMed] [Google Scholar]
- 32.Moretti ABS, Oliveira TM, Sakai VT, Santos CF, Machado MAAM, Abdo RCC. Mineral trioxide aggregate pulpotomy of a primary second molar in a patient with agenesis of the permanent successor. Int Endod J. 2007;40:738–45. doi: 10.1111/j.1365-2591.2007.01274.x. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003;29:324–33. doi: 10.1097/00004770-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Chacko V, Kukirose S. Human pulpal response to mineral trioxide aggregate (MTA): A histologic study. J Clin Pediatr Dent. 2006;30:203–10. doi: 10.17796/jcpd.30.3.38h13g5p84651652. [DOI] [PubMed] [Google Scholar]
- 35.Willard RM. Radiographic changes following formocresol pulpotomy in primary molars. J Dent Child. 1976;43:414–5. [PubMed] [Google Scholar]
- 36.Fuks AB, Bimstein E. Clinical evaluation of diluted formocresol pulpotomies in primary teeth of schoolchildren. Pediatr Dent. 1981;3:321–4. [PubMed] [Google Scholar]
- 37.Camp JH. Diagnosis dilemmas in vital pulp therapy: Treatment for the toothache is changing, especially in young, immature teeth. J Endod. 2008;34(suppl 7):S6–12. doi: 10.1016/j.joen.2008.03.020. [DOI] [PubMed] [Google Scholar]