Abstract
Tendon injuries are common and heal poorly. Strategies to regenerate or replace injured tendons are challenged by an incomplete understanding of normal tendon development. Our previous study showed that embryonic tendon elastic modulus increases as a function of developmental stage. Inhibition of enzymatic collagen crosslink formation abrogated increases in tendon elastic modulus at late developmental stages, but did not affect increases in elastic modulus of early stage embryonic tendons. Here, we aimed to identify potential contributors to the mechanical properties of these early stage embryonic tendons. We characterized tendon progenitor cells in early stage embryonic tendons, and the influence of actin cytoskeleton disruption on tissue elastic modulus. Cells were closely packed in embryonic tendons, and did not change in density during early development. We observed an organized network of actin filaments that seemed contiguous between adjacent cells. The actin filaments exhibited a crimp pattern with a period and amplitude that matched the crimp of collagen fibers at each developmental stage. Chemical disruption of the actin cytoskeleton decreased tendon tissue elastic modulus, measured by atomic force microscopy. Our results demonstrate that early developmental stage embryonic tendons possess a well organized actin cytoskeleton network that contributes significantly to tendon tissue mechanical properties.
Keywords: tendon, actin cytoskeleton, development, elastic modulus, crimp
Tendons, composed of a dense and hierarchical structure of aligned collagen fibers, transfer forces from muscle to bone. Frequent injury and the poor innate healing ability of tendons have motivated strategies to regenerate, repair, or replace injured tendons. A better understanding of normal embryonic tendon development may improve these approaches.1,2 Efforts to characterize developing tendons have focused primarily on late embryonic and early postnatal stages of development. These studies have examined the contributions of extracellular matrix (ECM) content, quantity, and organization to tissue mechanical properties and structure.3–13 However, we have found that embryonic tendon formation and mechanical property elaboration begin prior to significant mechanical contributions from deposited ECM. There is little information about the factors affecting tissue mechanical properties during these early stages of development.
We recently characterized mechanical and biochemical properties of embryonic chick tendon throughout development.14 Elastic modulus of the calcaneal tendon was measured using force–volume atomic force microscopy (FV-AFM) and found to increase nonlinearly as a function of embryonic developmental stage. Over the same developmental stages, collagen content in tendons was found to increase exponentially. In the same study, chemical inhibition of lysyl oxidase (LOX)-mediated collagen crosslinks significantly reduced tendon elastic modulus at late Hamburger–Hamilton (HH)15 developmental stages (HH 40 and 43).14 In contrast, no change in elastic modulus with LOX inhibition was detected in embryonic tendons of earlier developmental stages (HH 28–37). Notably, we measured total collagen content in HH 28–37 tendons to be no greater than approximately 1% of the tissue dry mass.14 Taken together, we attributed the limited influence of collagen crosslinking on tendon elastic modulus during early stages of tendon development to low collagen content.
These results led us to consider other components of tendons as contributors to tissue mechanical properties during these early stages of tendon formation. We previously found DNA content was far greater than collagen in content, accounting for approximately 5–9% of the tendon dry mass in HH 28–37 embryonic tendons.14 In contrast, adult tendon dry mass is approximately 0.02% DNA16 and 65–80% collagen content.17 Based on this, we are interested in examining how cells contribute to the physical properties of tendon tissue during early developmental stages. For instance, the actin cytoskeleton is an intracellular network of structural filaments that can influence the mechanical properties of an individual cell.18–21 However, how the actin cytoskeleton of cells in a tissue influence the mechanical properties of the tissue has been minimally investigated. Considering the high cell density of embryonic tendon, we propose the actin cytoskeleton of tendon progenitor cells (TPCs) influence the mechanical properties of the tissue.
We hypothesize that the actin cytoskeleton of TPCs physically contributes to the mechanical properties of embryonic tendon at early developmental stages. To test this hypothesis, we characterized cell and actin cytoskeleton organization of early stage (HH 34–37) embryonic chick tendons using histological staining, confocal microscopy, and image analysis. To test the influence of the actin cytoskeleton of TPCs on embryonic tendon mechanical properties, we treated HH 36 embryonic chick whole limb explants with blebbistatin and measured changes in tendon tissue elastic modulus using FV-AFM. We also performed this experiment with embryonic TPCs seeded in alginate gel constructs in vitro. By seeding TPCs at high density in these simplified tissue constructs, we were able to examine the potential for cells to contribute to the mechanical integrity of a construct in the absence of significant ECM. The results of this study provide new insights into the contribution of cells to the mechanical properties of embryonic tendon during early developmental stages.
Methods
Embryonic Tendon Harvest
Fertilized white leghorn chicken eggs (UConn Poultry Farm, Storrs, CT) were incubated at 37°C in a humidified egg incubator. Embryonic chicks were sacrificed at HH 34–37 by decapitation, and the limbs were excised and skinned.
Characterization of TPCs in Embryonic Tendon
Calcaneal tendons were dissected from the limbs, fixed in 4% paraformaldehyde overnight at 4°C, and cryopre-served at −80°C in optimal cutting temperature compound (OCT) (Sakure, Tokyo, Japan). After thawing, whole tendons were rinsed twice in phosphate buffered saline (PBS) to remove the OCT then stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) to detect cell nuclei. Whole tendons were mounted on a glass-bottomed Petri dishes in Hank’s Balanced Salt Solution (HBSS) and imaged on a Leica TCS SP2 inverted confocal microscope (Lecia Microsystems, Buffalo Grove, IL) using a 63X objective. Confocal z-stack images were taken in the midsubstance of tendon fascicles. Spacing between confocal slices in the z-direction was not greater than 2 μm in thickness. Three sets of z-stack images were taken from tendons of three independent animals at HH 34–37. To calculate cell density, cell nuclei were counted by two observers using the counter tool in ImageJ (NIH, Bethesda, MD), and normalized to the volume of the confocal image stack. For plasma membrane staining, freshly dissected embryonic tendons were incubated in a standard cell culture incubator in pre-warmed HBSS containing CellMask Plasma Membrane Stain (0.1% v/v; Invitrogen, Carlsbad, CA) at 37°C for 30 min. After washing in HBSS and fixing in 10% formalin, whole tendons were imaged on the confocal microscope.
Characterization of Actin Filaments and Collagen Fibers
To detect actin filaments, longitudinal tendon cryosections (10 μm-thick) were stained with Alexa Fluro 488-phalloidin (Invitrogen, Carlsbad, CA). Second harmonic generation imaging (SHG) was used to detect collagen fibers, as previously described.14 Images were collected on the confocal microscope. To quantitatively characterize actin filaments and collagen fiber crimp, NeuronJ (ImageJ) was used to trace actin filaments and collagen fibers. From the tracing curve, crimp period and amplitude were calculated using a custom Matlab (Mathworks Inc., Canton, MA) analysis.
TPC-Alginate Gel Constructs
Tendons from 12 embryonic chicks at HH 36 were dissected and pooled. Primary embryonic chick TPCs were isolated from the tendons in type II collagenase using a standard primary cell isolation protocol, as previously described.22 TPCs were seeded at a density of 5 × 107 cells/ml into 3-dimensional gels of 1.5% (w/v) alginate that were functionalized with a 0.03 w/w ratio of RGD to alginate,23 and crosslinked with calcium ions, as previously described.24,25 TPC-encapsulating gel constructs were cultured for 72 h in complete growth medium (Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin).
Blebbistatin Treatment of Embryonic Tendons and TPC-Alginate Gel Constructs
Skinless HH 36 whole limbs or TPC-alginate gel constructs were cultured for 24 h or 5 h, respectively, at 37°C in complete growth medium supplemented with 25 μM blebbistatin (Sigma-Aldrich, St. Louis, MO). Blebbistatin disrupts the actin cytoskeleton by inhibiting rigid crosslinking between nonmuscle myosin II and the actin filaments.26,27,28 Controls (e.g., contralateral limb) were cultured in an identical manner, but treated with the vehicle (0.5% v/v dimethyl sulfoxide; DMSO).
Viability Assay
Following 24 h of explant culture with blebbistatin or vehicle control treatment, tendons (n = 3) were dissected from the limb and immediately placed in warmed HBSS containing LIVE/DEAD stain (Invitrogen, Carlsbad, CA) to detect living and dead cells. Images were collected on the confocal microscope.
Second Harmonic Generation Imaging (SHG)
Following blebbistatin or vehicle control treatment, tendons (n = 3) were dissected from the limb and cryopreserved in OCT. SHG images were collected on the confocal microscope, as previously described.14
FV-AFM of Embryonic Tendon Tissue and TPC-Alginate Gel Constructs
Following 24 h of explant culture, blebbistatin-treated (or vehicle control) tendons (n = 7) were dissected from the limb, immediately placed in HBSS and indented with a 5 μm-radius spherical probe and cantilever (Bruker, Santa Barbara, CA) using a Dimension 3100 AFM (Bruker), as previously described.14 To determine elastic modulus, the raw force volume data were processed using a custom Visual Basic module in Excel (Microsoft) to calculate the slope of the linear region of the force-displacement curve. These slopes were converted to elastic modulus using a spherical model of Hertzian indentation mechanics.29 The elastic moduli of the TPC-alginate gel constructs (n = 6) were measured with FV-AFM in an identical manner.
Statistical Analysis
For comparisons between tendons from different developmental stages (HH 34–37) a one-way ANOVA was used followed by a Tukey’s Post hoc test and evaluated for significance using p < 0.05 in Prism statistical software (GraphPad Inc., San Diego, CA). Changes in elastic modulus following blebbistatin treatment are reported as the mean ± standard deviation, and differences were evaluated for significance with Student’s t-tests using p < 0.05.
RESULTS
Embryonic TPC Density
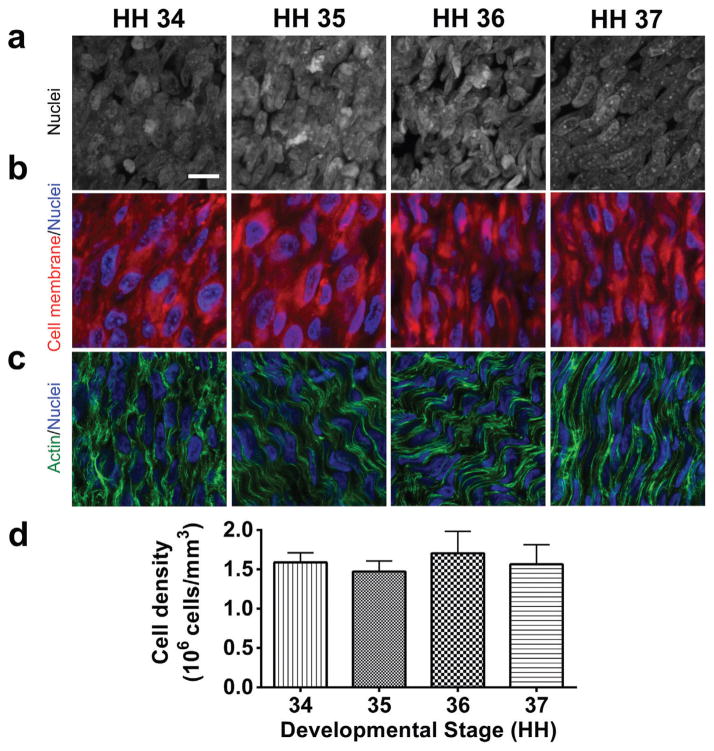
Embryonic chick calcaneal tendons from HH 34–37 contain densely packed cells, as represented by cell nuclei detected with DAPI in the 2-dimensional projections of the 3-dimensional z-stack confocal images (Fig. 1a). TPCs in the embryonic tendons were also detected with CellMask Plasma Membrane stain, which showed tendon cells to be densely packed (Fig. 1b). Phalloidin and DAPI staining were used to detect actin filament organization relative to nuclei. Actin filaments were aligned in parallel, and appeared to extend over multiple cell lengths along the tendon long axis in HH 35 and later tendons (Fig. 1c). Cell density was quantified by counting the number of cell nuclei and that were then normalized to the volume of the confocal z-stack. Cell density in tendons averaged 1.58 × 106 cells/mm3 from HH 34–37, with no significant differences detected between developmental stages (p > 0.05) (Fig. 1d).
Figure 1.
HH 34–37 embryonic tendons have high cell density throughout early stages of tendon development, as seen by (a) representative 2-dimensional projections of z-stack confocal images of DAPI-stained cell nuclei in whole mount embryonic tendons, and (b) CellMask Plasma Membrane staining (red) for cell membranes, and DAPI staining (blue) for cell nuclei. (c) Phalloidin (green) and DAPI (blue) staining showed organized actin filaments formed an apparent contiguous network spanning adjacent cells (bar, 10 μm). (d) Cell density quantified with image analysis was constant between stages.
Actin Filament and Collagen Fiber Organization
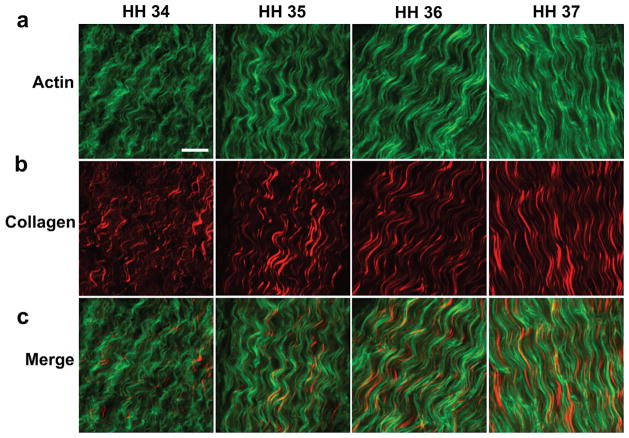
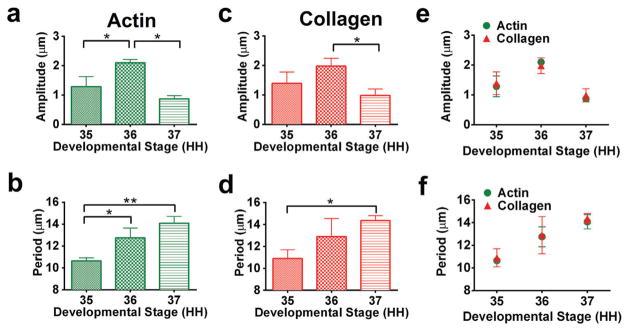
Actin filaments and collagen fibers were detected with phalloidin staining and SHG, respectively. A crimp pattern was observed in both the actin filaments and collagen fibers at each developmental stage (Fig. 2). The crimp amplitude and period for both actin filaments and collagen fibers were quantified as a function of stage, beginning at HH 35. Actin filament crimp amplitude at HH 36 was increased compared to HH 35 and HH 37 (p < 0.05; Fig. 3a). Actin filament crimp period increased from HH 35 to 36 and from HH 35 to 37 (p < 0.05; Fig. 3b). Between HH 35 and 37, collagen crimp amplitude decreased (p < 0.05; Fig. 3c). Collagen crimp period increased from HH 35 to 37 (p < 0.05; Fig. 3d). The quantitative characteristics of the crimp pattern were remarkably similar between the actin filaments and collagen fibers. There were no significant differences between actin filament crimp and collagen fiber crimp at each developmental stage, for both amplitude and period (p > 0.05; Fig. 3e,f).
Figure 2.
A highly organized crimp pattern was observed in (a) phalloidin-stained actin filaments (green) and (b) SHG-detected collagen fibers (red) of embryonic tendons during early developmental stages. (c) Merged images of actin and collagen show similar crimp morphology (bar, 10 μm).
Figure 3.
Actin filament crimp (a) amplitude and (b) period were calculated using image analysis of HH 35–37 phalloidin-stained tendons. Collagen fiber crimp (c) amplitude and (d) period were calculated using image analysis of HH 35–37 SHG-imaged tendons. Actin filament crimp and collagen fiber crimp changed as a function of stage (*p < 0.05, **p < 0.01). No differences were detected between actin filament crimp and collagen fiber crimp at each developmental stage, for (e) amplitude and (f) period (p > 0.05).
Blebbistatin Treatment of Embryonic Tendons
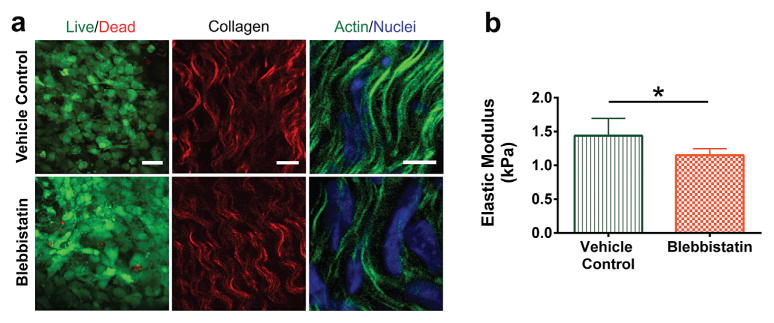
HH 36 whole limb explants were cultured with blebbistatin or vehicle control for 24 h. Blebbistatin treatment did not affect cell viability, demonstrated with Live/Dead staining (Fig. 4a). Collagen fiber organization, detected with SHG, did not appear to be altered with blebbistatin treatment as compared to vehicle controls. Blebbistatin treatment appeared to disrupt actin cytoskeleton of TPCs in tendon (Fig. 4a). The average elastic moduli of vehicle control- and blebbistatin-treated HH 36 embryonic tendons were 1.4 ± 0.3 kPa and 1.1 ± 0.2 kPa, respectively. Blebbistatin treatment reduced the elastic modulus of HH 36 embryonic tendons by 21.4% (n = 7; p = 0.02; Fig. 4b), compared to vehicle controls.
Figure 4.
HH 36 embryonic limb explants were cultured in vitro for 24 h with blebbistatin (25 μM) or vehicle control (0.5% DMSO). (a) LIVE/DEAD staining of live (green) and dead (red) cells (left panel; bar, 25 μm), SHG detection of collagen fibers (red) (middle panel; bar, 10 μm), and phalloidin-stained actin (green) and DAPI-stained nuclei (blue) (right panel; bar, 5 μm), for vehicle control- and blebbistatin-treated tendons. Cell viability and collagen fiber organization did not appear to be altered by blebbistatin treatment. Blebbistatin disrupted the actin cytoskeleton. (b) Blebbistatin treatment decreased tendon elastic modulus, measured using FV-AFM (p = 0.02).
Blebbistatin Treatment of TPC-Alginate Gel Constructs
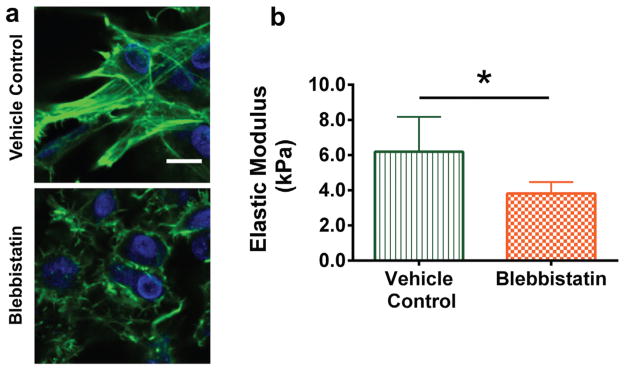
To further investigate the effect of actin cytoskeleton on tissue mechanical properties in a more controlled environment, primary HH 36 TPCs were seeded at high density in alginate gels and cultured for 72 h. Prior to blebbistatin treatment, the TPCs appeared to be in contact with each other. After 5 h of blebbistatin treatment, the actin cytoskeleton of the TPCs appeared disrupted (Fig. 5a). The average elastic moduli of vehicle control- and blebbistatin-treated TPC-alginate gel constructs were 6.2 ± 2.0 kPa and 3.8 ± 0.6 kPa, respectively. Blebbistatin treatment reduced the elastic modulus of TPC-alginate gel constructs by 38.7% (n = 6; p = 0.02; Fig. 5b), compared to vehicle controls.
Figure 5.
HH 36 embryonic TPCs were encapsulated at 5 × 107 cells/ml in alginate gels, cultured for 72 h and treated with blebbistatin (25 μM) or vehicle control (0.5% DMSO) for 5 h. (a) Phalloidin-stained actin (green) and DAPI-stained nuclei (blue) of TPCs within alginate gels show that blebbistatin treatment disrupted the actin cytoskeleton compared to vehicle controls (bar, 10 μm). (b) Blebbistatin treatment decreased TPC-alginate gel construct elastic modulus, measured using FV-AFM (p = 0.02).
DISCUSSION
A major challenge for strategies aimed at tendon healing, repair, and replacement is a limited understanding of how embryonic tendons develop normally. We recently demonstrated collagen crosslinking to be a significant mechanism of mechanical property elaboration in middle-to-late developmental stage embryonic tendons.14 In contrast, the elastic moduli of earlier stage (HH 37 and earlier) tendons were not affected by inhibition of collagen crosslinks. In fact, we previously reported that DNA content accounts for approximately 5–9% of the tendon dry mass in HH 28–37 tendons, which is more than the collagen content (less than 1.0% of the tendon dry mass).14 Based on these findings, the current study aimed to understand how cells physically influence the mechanical properties of embryonic tendon at early stages of tissue development. We characterized cell density and organization, and cellular contributions to mechanical properties of HH 34–37 embryonic tendons. We chose HH 34 as the earliest stage to study, because the calcaneal tendon becomes a distinctly defined structure by HH 34, with localized expression of α-scleraxis and α-tenascin.30 We chose HH 37 as the latest stage to study, because our previous study demonstrated that after HH 37 collagen crosslinking becomes a significant contributor to the mechanical properties of developing tendon.14 Here, we found HH 34–37 TPCs to be present in high density, in apparent contact, and to contain actin filaments with crimp morphology. Similar to the surrounding collagen fibers, the crimp morphology of the actin filaments changed during development. We also demonstrated the actin filaments of TPCs to have a direct influence on early stage tendon mechanical properties.
Our histological observations and measured decrease in elastic modulus of blebbistatin-treated tissues suggest the presence of a crosslinked network of cells within early embryonic tendon. Considering adult tendon is relatively acellular,16,17 the observed cell density we quantified in HH 34–37 tendons is extraordinarily high and similar between stages. A remarkable finding is that the actin filaments appear contiguous between adjacent cells in the embryonic tendons along the tendon long axis (Fig. 1c, 2). Beginning at HH 35, an apparent actin filament network was observed in the form of an organized crimp pattern, extending over multiple cell lengths. Disrupting the actin cytoskeleton of TPCs in HH 36 tendon with blebbistatin treatment resulted in a 21.4% decrease in tissue elastic modulus, suggesting the TPC actin cytoskeleton contributes directly to early stage embryonic tendon mechanical properties. Furthermore, the density of TPCs is constant between these early stages, suggesting the apparent actin filament network, spanning multiple cells, is a critical structural component of the tissue.
A prior study in adult tendons found the actin cytoskeleton of adult tendon cells follows the crimp pattern of collagen fibers.31 Despite the low amounts of collagen in embryonic tendon compared to adult, we found that not only do the collagen fibers exhibit a crimp pattern, but also that actin filaments of TPCs have crimp that mimics that of collagen fibers. Using polarized light microscopy, a prior study found crimp in the collagen fibers of HH 41 embryonic chick tendons, but did not examine the morphology of actin filaments.32 We found here that actin filament and collagen fiber crimp period and amplitude were quantitatively identical at each developmental stage starting from HH 35. In a study using transmission electron microscopy, actin filaments in embryonic tendon cells were found to align with collagen fibrils within fibripositors, cell plasma membrane structures involved in collagen fibril secretion.33 Organization of actin filaments in relation to collagen fibrils was reported at the length-scale of fibripositors (~1 μm). Our findings demonstrate that the parallelism they observe at the fibripositor scale is present over multiple cell lengths and manifests as a matched actin filament and collagen fiber crimp. Others used an in vitro fibrin gel and a computer model to study how tendon cell contraction may play a role in collagen crimp formation.34 The actin cytoskeleton is a component of the cell’s contractile machinery, but the role the actin cytoskeleton plays in formation of crimp during tendon development is yet unknown. Here, we found actin filaments to form a network that has a period and amplitude that match collagen crimp, but how this develops is unknown. These findings provide the motivation for future studies to investigate mechanisms of crimp formation in intracellular and extracellular structures of embryonic tendon.
Blebbistatin treatment reduced elastic modulus of embryonic tendon without affecting cell viability, collagen fiber organization and fiber density. Another study found that 24 h of cytochalasin D treatment to disrupt actin polymerization in adult tendon cells resulted in increased matrix metalloproteinase (MMP)-1 gene expression.35 In our experiment, it is possible that embryonic TPCs produced MMPs in response to 24 h of blebbistatin treatment. MMPs could degrade the native ECM and contribute to the reduction in elastic modulus we observed in the embryonic tendon. To minimize potential side effects, such as MMP degradation or unanticipated activity by other cells in the whole limb explants, we also performed this experiment with TPCs seeded at high density in RGD-functionalized alginate gels. Using this simplified tissue construct, only 5 h of blebbistatin treatment was sufficient to disrupt the actin filaments of encapsulated TPCs in the alginate gel, likely due to greater diffusion rates than through native tissue. Importantly, the shorter treatment time and the lack of MMP cleavage sites in alginate reduced possible unexpected effects, such as MMP degradation of ECM, which could affect the elastic modulus of a collagenous tissue. The reduction in elastic modulus of TPC-alginate gel constructs with blebbistatin treatment corroborated our findings in embryonic tendon tissue. Collectively, these results suggest an intact network of actin filaments that is contiguous throughout adjacent TPCs plays an important role in measureable mechanical properties of developing embryonic tendon. Interestingly, another study found that embryonic frog neural tube tissue stiffness requires an intact actin cytoskeleton.36 Taken together, it appears that TPCs play a significant structural role in the mechanical properties of embryonic tendon during early development.
The actin cytoskeleton is a component of the cell’s contractile machinery that could significantly influence the mechanical properties of individual TPCs. Though not yet studied in TPCs, disrupting the actin cytoskeleton with blebbistatin decreases the elastic modulus of various individual cells,19,21 which may have contributed to our results. In another study, treating NIH 3T3 fibroblasts with blebbistatin to disrupt cell contraction reduced the local elastic modulus of a 2-dimensional fibrin gel substrate when measured 10–15 μm from the cell’s edge.21 In our study, it is possible that decreased elastic modulus of individual TPCs as well as diminished cell contractile forces contributed to the reduction in elastic modulus of blebbistatin-treated embryonic tendons and TPC-alginate gel constructs. These potential contributions deserve further study.
As tendon develops, the tissue transitions from a highly cellular structure to a mechanically strong tissue that is primarily collagen in dry weight. Charting a roadmap for this transition will be important to understanding how functional tendon forms. This study demonstrates the actin cytoskeleton of TPCs forms an apparent contiguous network across multiple cells, and contributes directly to the mechanical properties of whole tendon tissue during early embryonic tendon development. Other studies have shown that the actin cytoskeleton is also a mechanotransducer and involved in signaling.35,37 Future studies are needed to understand multiple roles the actin cytoskeleton may play in tenogenic TPC behavior and tendon formation. Furthermore, though actin is a major cytoskeletal protein, other cytoskeletal components should also be considered for potential contributions to tissue mechanics.38 Additionally, though we previously found no correlation between embryonic tendon elastic modulus and total glycosaminoglycan content,14 specific ECM components other than collagen could also influence tissue mechanical properties, and should be investigated. A more thorough understanding of the mechanisms driving embryonic tendon development may ultimately lead to effective regeneration strategies.
Acknowledgments
We thank Matteo Stoppato, Ph.D. for critically reading this manuscript. We also gratefully acknowledge funding support by the NIH Training in Education and Critical Research Skills (K12GM074869) postdoctoral program (to N.R.S.) and NSF CAREER Award CMMI 1254720 (to C.K.K.).
Footnotes
Conflict of interest: None.
AUTHOR CONTRIBUTIONS
NRS, FvF, and JEM performed experiments. NRS, ZLT, and JJT analyzed the data. This study was conceived, designed and coordinated by NRS and CKK. NRS, LAH, and CKK wrote the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Schiele NR, Marturano JE, Kuo CK. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr Opin Biotechnol. 2013;24:834–840. doi: 10.1016/j.copbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass ZA, Schiele NR, Kuo CK. Informing tendon tissue engineering with embryonic development. J Biomech. 2014;47:1964–1968. doi: 10.1016/j.jbiomech.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oryan A, Shoushtari AH. Histology and ultrastructure of the developing superficial digital flexor tendon in rabbits. Anat Histol Embryol. 2008;37:134–140. doi: 10.1111/j.1439-0264.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 4.Ansorge HL, Adams S, Birk DE, et al. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng. 2011;39:1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C-F, Aschbacher-Smith L, Barthelery NJ, et al. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A. 2011;18:598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn. 2008;237:1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meller R, Schiborra F, Brandes G, et al. Postnatal maturation of tendon, cruciate ligament, meniscus and articular cartilage: A histological study in sheep. Ann Anat. 2009;191:575–585. doi: 10.1016/j.aanat.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Provenzano PP, Vanderby R., Jr Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Birk DE, Zycband EI, Woodruff S, et al. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 11.McBride DJ, Hahn RA, Silver FH. Morphological characterization of tendon development during chick embryogenesis - measurement of birefringence retardation. Int J Biol Macromol. 1985;7:71–76. [Google Scholar]
- 12.McBride DJ, Trelstad RL, Silver FH. Structural and mechanical assessment of developing chick tendon. Int J Biol Macromol. 1988;10:194–200. [Google Scholar]
- 13.Ros MA, Rivero FB, Hinchliffe JR, et al. Immunohistological and ultrastructural study of the developing tendons of the avian foot. Anat Embryol (Berl) 1995;192:483–496. doi: 10.1007/BF00187179. [DOI] [PubMed] [Google Scholar]
- 14.Marturano JE, Arena JD, Schiller ZA, et al. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci USA. 2013;110:6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamburger V, Hamilton HL. A Series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 16.Hammer N, Huster D, Fritsch S, et al. Do cells contribute to tendon and ligament biomechanics. PLoS One. 2014;9:e105037. doi: 10.1371/journal.pone.0105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 18.Qi J, Fox AM, Alexopoulos LG, et al. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol. 2006;101:189–195. doi: 10.1152/japplphysiol.01128.2005. [DOI] [PubMed] [Google Scholar]
- 19.Martens JC, Radmacher M. Softening of the actin cytoskeleton by inhibition of myosin II. Pflugers Arch. 2008;456:95–100. doi: 10.1007/s00424-007-0419-8. [DOI] [PubMed] [Google Scholar]
- 20.Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004;22:131–139. doi: 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 21.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014;47:214–222. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drury JL, Boontheekul T, Mooney DJ. Cellular cross-linking of peptide modified hydrogels. J Biomech Eng. 2005;127:220–228. doi: 10.1115/1.1865194. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CK, Ma PX. Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro. J Biomed Mater Res A. 2008;84:899–907. doi: 10.1002/jbm.a.31375. [DOI] [PubMed] [Google Scholar]
- 25.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 26.Limouze J, Straight AF, Mitchison T, et al. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell M. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs M, Toth J, Hetenyi C, et al. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 28.Schiller ZA, Schiele NR, Sims JK, et al. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res Ther. 2013;4:79. doi: 10.1186/scrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- 30.Roddy KA, Nowlan NC, Prendergast PJ, et al. 3D representation of the developing chick knee joint: a novel approach integrating multiple components. J Anat. 2009;214:374–387. doi: 10.1111/j.1469-7580.2008.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 32.Shah JS, Palacios E, Palacios L. Development of crimp morphology and cellular changes in chick tendons. Dev Biol. 1982;94:499–504. doi: 10.1016/0012-1606(82)90366-9. [DOI] [PubMed] [Google Scholar]
- 33.Canty EG, Starborg T, Lu Y, et al. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem. 2006;281:38592–38598. doi: 10.1074/jbc.M607581200. [DOI] [PubMed] [Google Scholar]
- 34.Herchenhan A, Kalson NS, Holmes DF, et al. Tenocyte contraction induces crimp formation in tendon-like tissue. Biomech Model Mechanobiol. 2012;11:449–459. doi: 10.1007/s10237-011-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnoczky SP, Tian T, Lavagnino M, et al. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu B, Song G, Ju Y, et al. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2011;227:2722–2729. doi: 10.1002/jcp.23016. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Kim HY, Wang JH, et al. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010;137:2785–2794. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]