Abstract
It has been demonstrated that the Xenopus oocyte can translate rabbit haemoglobin messenger RNA (mRNA) following microinjection of the message into the cell1. The Xenopus oocyte has since been shown to be capable of translating a variety of messenger RNAs from different species2–4. This system has proved useful in understanding the mechanism of message translation and has also provided information about the translation capability of the Xenopus oocyte5,6. Several other cell types, including HeLa cells and fibroblasts, can also translate exogenous message injected into the cell7,8. However, there have been no reports of injection of mRNA into oocytes or fertilised one-cell ova of mammalian species. Nevertheless, the latter system could be of considerable use in studying the processing of exogenous messages in a mammalian system undergoing development, as well as providing insight into the way the early embryo processes injected messages and the protein products of such messages. We report here the results of injecting message into the fertilised one-cell mouse ovum and show that both mouse and rabbit globin mRNA are translated in this system.
Fertilised one-cell mouse ova were collected following superovulation from hybrid C57 × SJL females on the day of the vaginal plug by techniques described previously9. The ova were collected in Brinster’s medium for ovum culture (BMOC-2, see ref. 9) and the cumulus cells removed with hyaluronidase (300 units ml−1) in phosphate-buffered saline9. After washing to remove cumulus cell debris, the ova were maintained in BMOC-2. Purified mRNA for these experiments was prepared by the method of Gorski et al.10 except that polysomes were dissociated with SDS and applied directly to oligo(dT)-cellulose as described by Krystosek et al.11. For injection, the ova were placed on a depression slide and held with a blunt pipette as shown in Fig. 1. The tip of the injector pipette was filled with a solution of mRNA in water (1 mg ml−1) and a small amount of solution was injected into each ovum. The average volume injected was determined by injecting 3H-inulin into several groups of ova and measuring the radioactivity found in each ovum. On the basis of these measurements, the average volume injected into each ovum was estimated to be 13 pl. Some of the ova underwent lysis during the 15–30 min after removal of the injector pipette and release of the ovum from the holder. The survival rate was 30–40%, and it was found that injection of the ova in medium containing cytochalasin B (5–10 μg ml−1) approximately doubled the chance of survival. Each group of 20–40 injected ova was placed in BMOC-2 containing a mixture of five 3H-labelled amino acids (leucine, lysine, phenylalanine, proline, tyrosine; specific activities 75–120 Ci mmol−1; Amersham Searle) for 15–17 h, during which time approximately one third of the one-cell ova divided into two cells. Generally, about 80% of one-cell ova will cleave during this period, but the high level of radioactivity necessary for labelling decreased the number of ova undergoing cleavage. After labelling, the ova were washed three times in media containing non-radioactive amino acids, placed in a 6 × 60 mm tube and stored at −70 °C. To determine whether globin had been synthesised from the injected message, non-radioactive globin marker was added to the tube, and the proteins were separated by two-dimensional high-resolution electrophoresis12.
Fig. 1.
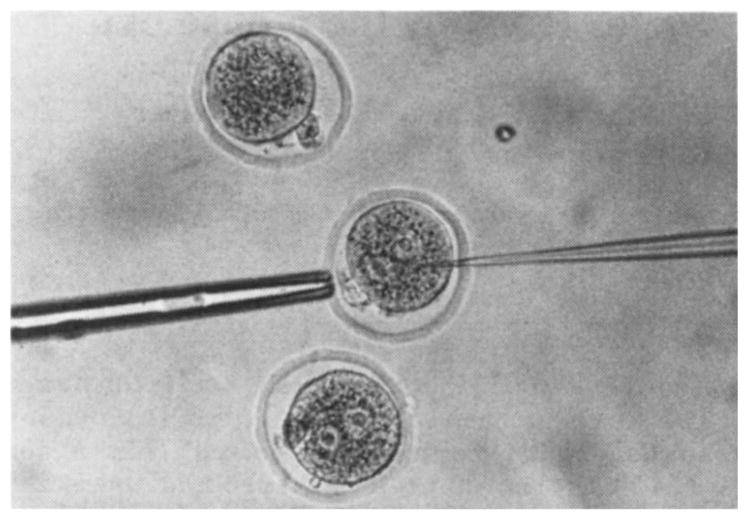
Injection of one-cell mouse ovum with mRNA. The ovum was held with a blunt suction pipette (5 μm i.d., 20 μm o.d.). The o.d. of the injector pipette was 2–3 μm, and the tip was sharpened either by chipping or grinding. The ova were maintained on a depression slide in culture medium under silicone oil. The injection solution drop, from which the injector pipette was filled, was adjacent to the medium containing the ova. The technique was similar to that used for injecting cells into blastocysts15.
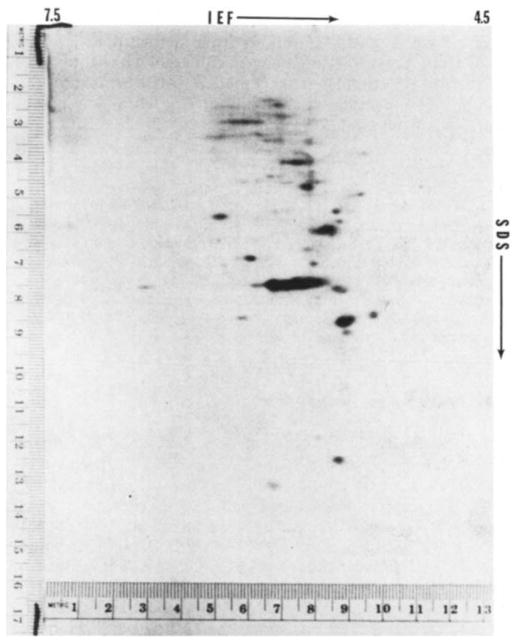
Figure 2 shows a typical fluorograph of the electrophoretic separation of synthesised proteins following injection of ova with mouse globin mRNA. Two major radioactive spots, indicated by arrows, co-migrated with non-radioactive globin marker prepared from mouse blood. Injection of message into mouse oocytes yields a similar pattern (unpublished). The other spots on the fluorograph are endogenous proteins. On control gels from ova not injected with mRNA there are no radioactive spots in the area of globin migration, indicating the absence of endogenous labelled protein in this area that might confuse the interpretation of the fluorographs (Fig. 3). When the non-radioactive markers are separated on isoelectric focusing (IEF) gels and stained for protein, each of the major spots is composed of two closely migrating but clearly distinct bands. This separation, although less apparent in the second dimension, can sometimes be seen in the radioactive spots such as the most basic of those in Fig. 2. No globin marker bands are present in the IEF gel at a more basic position than those shown in Fig. 2. The absence of such bands was confirmed by non-equilibrium pH gradient electrophoresis (unpublished observations); in this procedure the migration is from the acidic end and can be stopped at various positions before reaching the basic end13. Thus, the technique used for the egg proteins is capable of identifying the location of all the globin chains synthesised from the injected message.
Fig. 2.
Fluorograph of two-dimensional electrophoretic separation of proteins from 20 one-cell fertilised mouse ova injected with mouse globin mRNA and incubated in 3H-labelled amino acids. Isoelectric focusing (IEF) was from left (pH 7.5) to right (pH 4.5) and SDS-polyacrylamide electrophoresis was from top to bottom. The technique used was basically that of O’Farrell, as previously described12,16. The IEF was carried out with pH 3–10 ampholines. Marker proteins on the right margin are lactoglobulin (top, MW = 18,400) and methaemoglobin (bottom, MW= 16,000). The two radioactive spots indicated by the arrows coincide with the position of the non-radioactive globin marker protein prepared from mouse blood. α and β indicate the positions of α- and β-chains of globin.
Fig. 3.
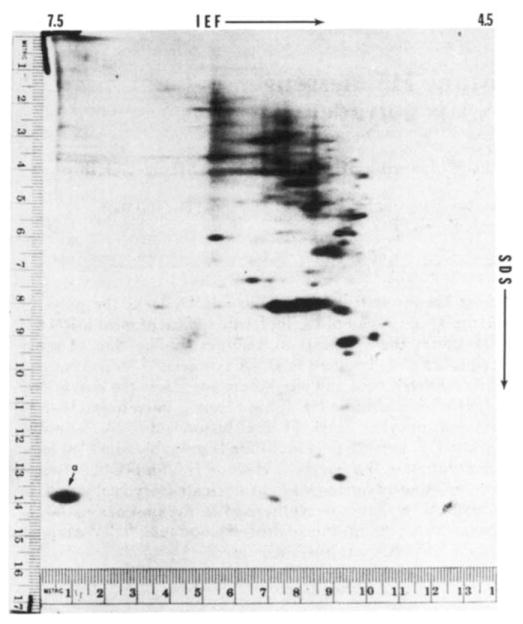
Fluorograph of two-dimensional electrophoretic separation of proteins from 20 one-cell fertilised mouse ova injected with water. Techniques used were similar to those for Fig. 2. No radioactivity appears in the area of globin migration.
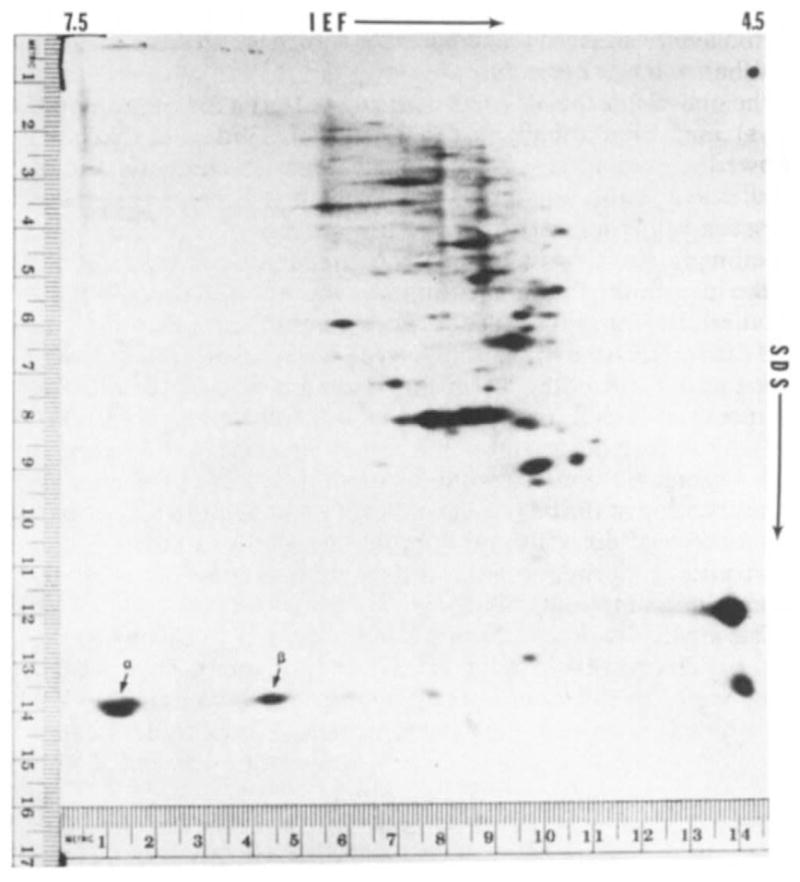
When rabbit mRNA is injected into the ova, the pattern is slightly different. As shown in Fig. 4, only one major globin spot is present. This major spot seems to be composed of two sub-bands, similar to the situation for the mouse globin. However, because of the high level of radioactivity, it generally appears as one spot in the photographs of the fluorograph. By reference to purified globin markers for mouse α- and β-chain and for rabbit α- and β-chain, the large basic spot in Figs 2 and 4 was identified as α-globin for both the mouse and rabbit mRNA. The more acidic spot from the mouse globin mRNA is β-globin. No β-globin spot, which would be located in approximately the same position as the mouse β-globin, can be detected in Fig. 4. However, other studies suggest that a low level of rabbit β-globin synthesis does occur (unpublished observations).
Fig. 4.
Fluorograph of two-dimensional electrophoretic separation of proteins from 20 one-cell fertilised mouse ova injected with rabbit globin mRNA. Techniques used were similar to those for Fig. 2. The single radioactive spot indicated by the arrow coincides with the position of the non-radioactive globin marker protein prepared from rabbit blood. α Indicates the position of the α-chain of globin. No β-chain was detected on the fluorograph.
When ova were injected with either mouse or rabbit message at the one-cell stage, cultivated in vitro to the blastocyst stage (5 days) and then labelled with 3H-amino acids, fluorographs showed no indication of globin synthesis. In fact, preliminary studies suggest that globin synthesis is not detectable 48 h after message injection. However, some globin is synthesised when labelling begins 24 h after message injection. A precise half life of the injected message can only be determined following more detailed investigation to identify the saturating level of message and the lag time before the message is active. However, it is clear that the mRNA half life in mouse ova is considerably shorter than that in Xenopus oocytes, where globin message has been shown to retain its activity for 2 weeks or longer14. Clearly, the cell type containing the message profoundly influences its stability. These studies do not indicate whether the message is degraded or is converted to a non-translatable form. Although the former possibility seems the more likely, the presence of masked message in the embryo makes the latter an intriguing possibility.
These observations on the translation of globin message by the mouse ovum identify an important new system in which to study the activity of mRNA and the synthetic products of the message. Although having some similarities to the Xenopus oocyte, the system shows several differences. For instance, the mouse cells are mammalian rather than amphibian and are actively dividing rather than dormant or static as is the Xenopus oocyte. The unique characteristics of each system may provide different answers to the same question. The large difference found between the two cell types in the stability of injected globin message is one example. Another is the preponderance of synthesis of α- relative to β-chains in the mouse ovum; the relative synthesis is just the opposite in the Xenopus oocyte. The injection of exogenous messages into the mouse ovum should help in understanding message stability and activity in the developing mammalian embryo, as well as providing a means of studying translational activity and specific protein stability in the embryo.
Acknowledgments
Messenger RNA was supplied by Dr J. B. Lingrel, and purified globin markers by Dr Raymond Popp. This work was supported by NIH grants HD 08539, HD 12384 and NSF grant PCM 78-22931. H.Y.C. is a trainee on HD 00239.
References
- 1.Gurdon JB, Lane CD, Woodland HR, Marbaix G. Nature. 1971;233:177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- 2.Gurdon JB. 6th Karolinska Symp on Research Methods in Reproductive. Endocrinology. 1973:225–243. [Google Scholar]
- 3.Colman A, Morser J. Cell. 1979;17:517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- 4.Mous J, Peeters B, Van Bellegem H, Rombauts W. Eur J Biochem. 1979;94:393–400. doi: 10.1111/j.1432-1033.1979.tb12906.x. [DOI] [PubMed] [Google Scholar]
- 5.Laskey RA, Mills AD, Gurdon JB, Partington GA. Cell. 1977;11:345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- 6.Asselbergs FAM, Van Venrooij WJ, Bloemendal H. Eur J Biochem. 1979;94:249–254. doi: 10.1111/j.1432-1033.1979.tb12892.x. [DOI] [PubMed] [Google Scholar]
- 7.Stacey DW, Allfrey VG. Cell. 1976;9:725–732. doi: 10.1016/0092-8674(76)90136-7. [DOI] [PubMed] [Google Scholar]
- 8.Stacey DW, Allfrey FG, Hanafusa H. Proc natn Acad Sci USA. 1977;74:1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinster RL. In: Growth, Nutrition and metabolism of Cells in Culture. Rothblat G, Cristofalo V, editors. Vol. 2. Academic; New York: 1972. pp. 251–286. [Google Scholar]
- 10.Gorski J, Morrison MR, Merkel CG, Lingrel JB. J molec Biol. 1974;86:363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- 11.Krystosek A, Cawthon ML, Kabat D. J biol Chem. 1975;250:6077–6084. [PubMed] [Google Scholar]
- 12.O’Farrell PH. J biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 13.O’Farrell PZ, Goodman HM, O’Farrell PH. Cell. 1977;12:1133–1142. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 14.Gurdon JB, Lingrel JB, Marbaix G. J molec Biol. 1973;80:539–551. doi: 10.1016/0022-2836(73)90421-x. [DOI] [PubMed] [Google Scholar]
- 15.Brinster RL. J exp Med. 1974;140:1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinster RL, Brunner S, Joseph X, Levey IL. J biol Chem. 1979;254:1927–1931. [PubMed] [Google Scholar]