Significance
Detecting and responding to light are basic requirements of nearly all life forms. Species from bacteria to man use light to regulate diverse behaviors from acute phototropism and visual processing to seasonal breeding cycles. Here, we describe a previously unidentified form of photoreception in the brain of frog tadpoles. The photoreceptors are preferentially activated by UV light and link ambient light levels to swimming activity. The pathway may be a simple method to optimize lighting conditions for feeding and avoiding predation, or may overlay and reinforce classical circadian systems. Deep-brain photoreception is of broad significance because the proteins involved are phylogenetically conserved.
Keywords: photoreception, locomotion, CPG, opsin 5, cryptochrome
Abstract
Nonvisual photoreceptors are widely distributed in the retina and brain, but their roles in animal behavior remain poorly understood. Here we document a previously unidentified form of deep-brain photoreception in Xenopus laevis frog tadpoles. The isolated nervous system retains sensitivity to light even when devoid of input from classical eye and pineal photoreceptors. These preparations produce regular bouts of rhythmic swimming activity in ambient light but fall silent in the dark. This sensitivity is tuned to short-wavelength UV light; illumination at 400 nm initiates motor activity over a broad range of intensities, whereas longer wavelengths do not cause a response. The photosensitive tissue is located in a small region of caudal diencephalon—this region is necessary to retain responses to illumination, whereas its focal illumination is sufficient to drive them. We present evidence for photoreception via the light-sensitive proteins opsin (OPN)5 and/or cryptochrome 1, because populations of OPN5-positive and cryptochrome-positive cells reside within the caudal diencephalon. This discovery represents a hitherto undescribed vertebrate pathway that links luminance detection to motor output. The pathway provides a simple mechanism for light avoidance and/or may reinforce classical circadian systems.
Animals use spatiotemporally patterned light information to form images using their eyes, whereas slower changes in illumination can be detected by additional photosensitive regions including the pineal organ. Both visual processing and luminance detection depend on specialized opsin proteins, which are widely expressed in the animal kingdom and located in multiple tissues (1, 2). The idea that regions of the brain other than the pineal complex or retina are sensitive to light was proposed over a century ago when von Frisch demonstrated that blinded and pinealectomized European minnows (Phoxinus phoxinus) retained an ability to change color in response to light (3). In addition, it was demonstrated that lesions to the diencephalon removed this response, implying that the periventricular tissue of the brain was directly light-sensitive. Since then, deep-brain photoreception, specifically in the hypothalamus, has been studied extensively in relation to its role in gonadal induction in birds (4–9).
Movement in response to light is potentially as ancient as photosensitivity itself. It is reasonable to assume that cyanobacteria, which have existed for around 2.8 billion years, were some of the first organisms to sense light (10). In vertebrates, the first evidence for extraretinal, extrapineal “photomotor” behavior came from experiments on blinded and pinealectomized lampreys (11, 12). A similar study of blinded, pinealectomized eels (Anguilla anguilla) showed they too responded to illumination of the head with a change in motor behavior (13). In zebrafish, both positive and negative phototaxis occurs (14, 15). The fish will swim away from a bright light and generally prefer dark conditions, but in a dark environment will swim toward a localized region of light. Although the eyes are required for proper orientation toward a light stimulus, a general increase in motor activity upon loss of illumination, termed dark photokinesis, persists in enucleated fish (16). Using genetic manipulations, Fernandes et al. (16) were able to narrow the photosensitive region to a population of melanopsin-positive neurons of the anterior preoptic area. Another light-driven but nonvisual, nonpineal motor behavior displayed by larval zebrafish is the photomotor response [PMR (17)]. The PMR is characterized by low-frequency, high-amplitude coiling and higher-frequency, lower-amplitude swimming behaviors, which are both increased in response to flashes of bright light. The response occurs transiently during development and is mediated by cells within the caudal hindbrain, which are both necessary and sufficient for the behavior (18).
We have studied the effects of ambient light on spontaneously generated fictive locomotion produced by the isolated nervous system of prometamorphic Xenopus laevis larvae (19). This preparation, devoid of visual and pineal afferent inputs, retains photosensitivity; episodes of locomotor activity occur spontaneously in the light, but preparations fall relatively quiescent or completely silent in the dark. The response is found to be tuned to short-wavelength (390–410 nm) UV illumination, and focal illumination experiments reveal that a confined region of caudal diencephalon is required to generate the response. Moreover, immunostaining for OPN5, a known UV-sensitive opsin (8, 9), and cryptochrome 1, a blue-light sensor found in Drosophila (20, 21), reveals cells in this region of the tadpole diencephalon that express proteins with an appropriate spectral sensitivity. Together, these results suggest that Xenopus larvae are equipped with short wavelength-sensitive neurons deep within the brain that directly link environmental luminance to motor output and may underlie a simple light avoidance response and/or potentially overlay classical circadian systems.
Results
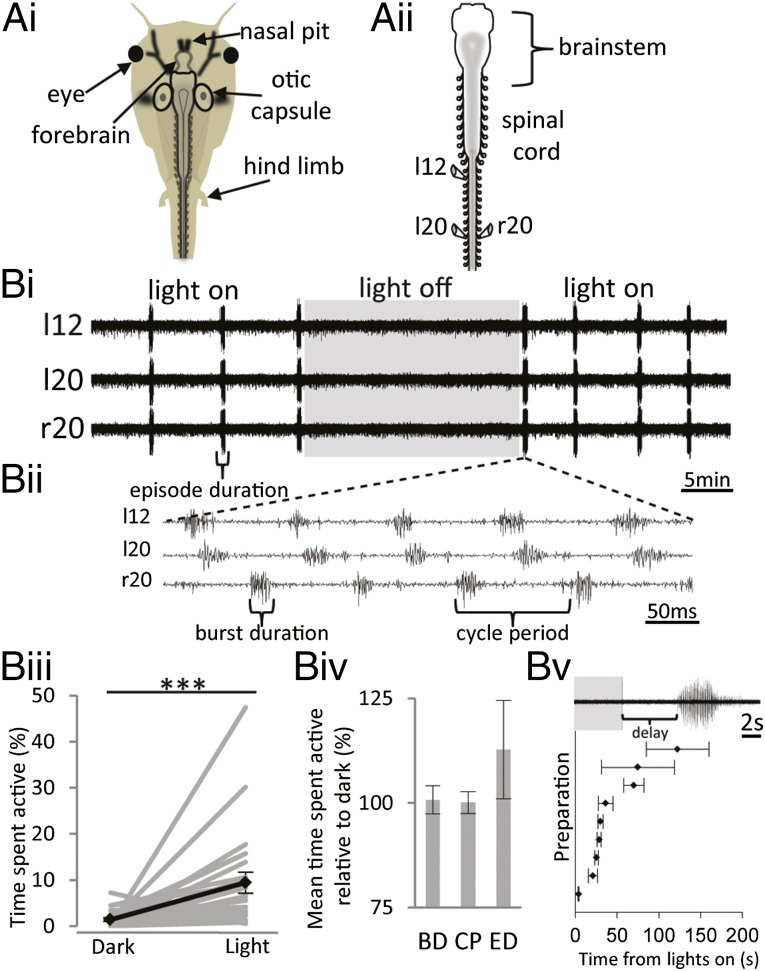
The isolated nervous system of prometamorphic (stage 53–62) X. laevis tadpoles (Fig. 1 A, ii) generates periodic episodes of rhythmic locomotor-like activity (Fig. 1 B, i; n = 23). As previously shown at embryonic and early larval stages of development (22), motor bursts recorded from spinal ventral roots display left–right alternation between opposing sides of the spinal cord and a brief rostro–caudal delay as activity propagates from head to tail (Fig. 1 B, ii). However, instead of requiring sensory stimulation to trigger locomotor activity, episodes at these later larval stages occur spontaneously (19).
Fig. 1.
Fictive locomotion in prometamorphic X. laevis larvae is sensitive to light. (A, i) Cartoon of stage 56 larva showing the approximate location of the central nervous system. (A, ii) Schematic depicting preparation with glass suction electrodes on ventral motor roots. (B, i) Extracellular record from three ventral motor roots showing spontaneous episodes of fictive locomotion. (B, ii) Expanded time base to show coordination of spontaneous rhythm and various parameters, including burst duration (BD), cycle period (CP), and episode duration (ED). Spontaneous activity is sensitive to ambient light levels. In the light, episodes of activity occur regularly every few minutes, whereas in the dark (gray box) the preparation falls silent. (B, iii) Graph of time spent active in light and dark, expressed as a percentage of total recording period, for 23 larval preparations (light-gray lines). The population mean is shown in black. (B, iv) Other parameters of fictive motor activity are unaffected; BD (n = 18), CP (n = 16), and ED (n = 23) are expressed as mean percentage in light relative to dark. (B, v) Graph of mean latency to motor activity from nine different preparations where at least three transitions between dark and light were recorded. In each example, the latency to activity was measured following 10 min in the dark. (Upper) Example response from a stage 54 larva following 10 min in the dark (gray box). Error bars represent ±SEM; ***P < 0.01.
Despite being devoid of input from all known photoreceptive tissues including the lateral eyes and the pineal complex, the preparations are sensitive to changes in ambient light. When illuminated with a broad-spectrum halogen light source, preparations produced periodic episodes of coordinated locomotor activity (Fig. 1B). However, when placed in the dark (Fig. 1 B, i, gray box), preparations generally fell silent. Data from 23 preparations where there were at least two 15-minute periods alternating between light and dark reveal a significant increase in time spent active, from 1.39 ± 0.40% in the dark to 9.44 ± 2.29% in the light (Fig. 1 B, iii; P < 0.01). This effect relates specifically to the probability of fictive locomotion occurring; other parameters of swimming were unaffected by the changing light conditions. Relative to the value in the dark, the burst duration was 100.72 ± 3.37% (n = 18); the cycle period was 100.12 ± 2.60% (n = 16); and the episode duration was 112.75 ± 11.75% (n = 23). Following a period of darkness (Fig. 1 B, v, Upper, gray box), spontaneous, rhythmic locomotor-like activity was initiated with a short delay. The delay to activation was variable between preparations but was consistent within the same preparation (Fig. 1 B, v). The shortest delay before activation of swimming was 3.94 ± 0.47 s, whereas the longest was 122.43 ± 37.51 s (n = 9). Given the link between light and heat, and knowing that swimming in Xenopus is temperature-sensitive (22), it was important to rule out a thermal contribution to the light sensitivity of these preparations. The experiments were therefore designed to minimize the effect of temperature in two ways: (i) All experiments were carried out in a bath controlled by a Peltier cooler, which maintained the saline at 16.5 ± 0.5 °C; and (ii) the cold light source used generated negligible amounts of heat from the distal end of the fiber optic light pipe, which was positioned ∼10 cm from the recording bath.
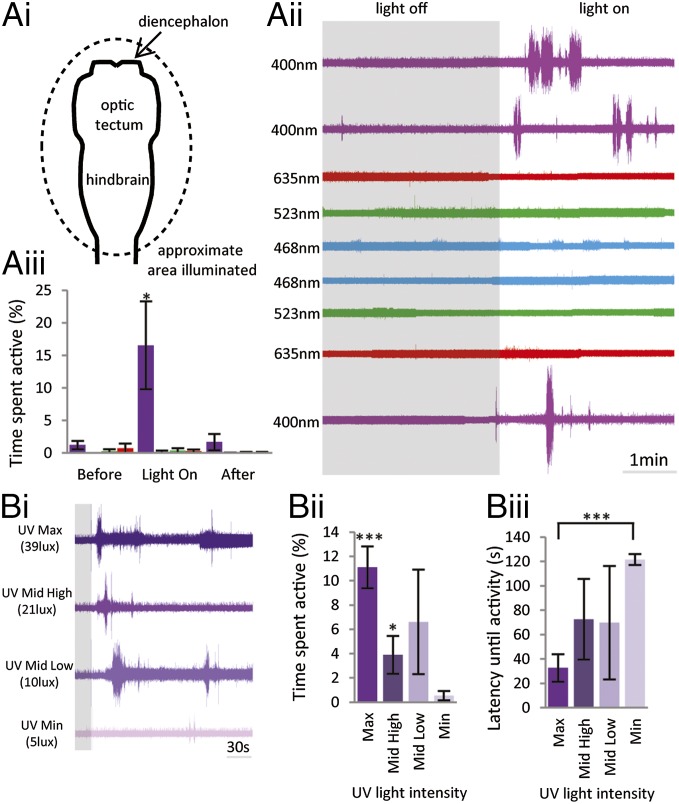
Because classical light sensitivity in the nervous system depends on opsin proteins, which have stereotypical spectral fingerprints (1), a first step in exploring the phototransduction mechanism was to test responsiveness to different wavelengths of light. The halogen light source used in the initial experiments emitted a broad spectrum of white light, so a series of relatively narrow wavelength LEDs were used instead to generate a basic action spectrum of the light sensitivity. Illumination of the nervous system (Fig. 2 A, i) with short-wavelength UV light (390–410 nm; 39 lx) produced a robust locomotor response: The time spent active increased to 16.56 ± 6.76%, compared with 1.24 ± 0.63% before illumination and 1.68 ± 1.26% immediately after the lights-on period (n = 7; P < 0.05; Fig. 2 A, ii and iii, purple). Illumination of the same area with blue (468 nm; 461 lx), green (523 nm; 136 lx), or red (635 nm; 36 lx) light did not increase activity above the value recorded in the dark (Fig. 2 A, ii and iii; color corresponds to wavelength).
Fig. 2.
Photosensitivity is tuned to short wavelengths. (A, i) Schematic depicting brainstem and caudal diencephalon. Approximate area illuminated is shown by the black dotted line. (A, ii) Ventral root trace from a stage 55 larva showing 200 s before and 200 s after a sequence of transitions from dark (gray box) to light. In each case, the preparation was illuminated following 10 min in the dark; the wavelength and intensity of light are shown. (A, iii) Graph displaying average data for time spent active 200 s before, during, and after 200-s illumination for each wavelength (UV, red, green, and blue; n = 7). (B, i) Sequence of responses to different intensities of UV light following 10 min in the dark. (B, ii) Graph showing average data for time spent active during responses to UV light at maximum (Max; 39 lx), medium high (21 lx), medium low (10 lx), and minimum (Min; 5 lx) intensity (n = 4). (B, iii) Graph showing average data for latency to first activity after illumination with UV light of different intensities. Error bars represent ±SEM; ***P < 0.01; *P < 0.05.
The intensity of light applied depended upon the specific LED used. Compared with the white light source (∼13,000 lx), UV light elicited a ventral root motor response even at 39 lx (total time spent active increased to 11.09 ± 1.72%, compared with 0.05 ± 0.05% before illumination and 0.30 ± 0.18% immediately after the lights-on period; n = 4; P < 0.01) and 23 lx (total time spent active increased to 3.89 ± 1.56%, compared with 0.19 ± 0.19% before illumination and 0.28 ± 0.28% immediately after the lights-on period; n = 4; P < 0.05) (Fig. 2 B, i and ii). In addition, 2/4 preparations tested showed activity in response to UV light at 10 and 5 lx (Fig. 2 B, i). In comparison, blue, green, and red light failed to cause a response to light at their maximum intensity values of 461, 136, and 36 lx, respectively (Fig. 2 A, ii and iii). This tight spectral tuning is particularly clear comparing the robust UV light responses to the next-shortest wavelength, blue light, which did not elicit a response, even at 10 times the light intensity.
As well as the total time spent active, the intensity of UV light also dictated the latency to onset of the first swimming episode when the illumination is turned on (Fig. 2 B, i and iii). The mean latency to activity was significantly shorter at 39 lx (32.63 ± 11.27 s) than at 5 lx (121.50 ± 4.5 s; n = 4; P < 0.01). This graded response to the illumination intensity could be important behaviorally, allowing the animal to respond appropriately to the relative amount of light in the environment.
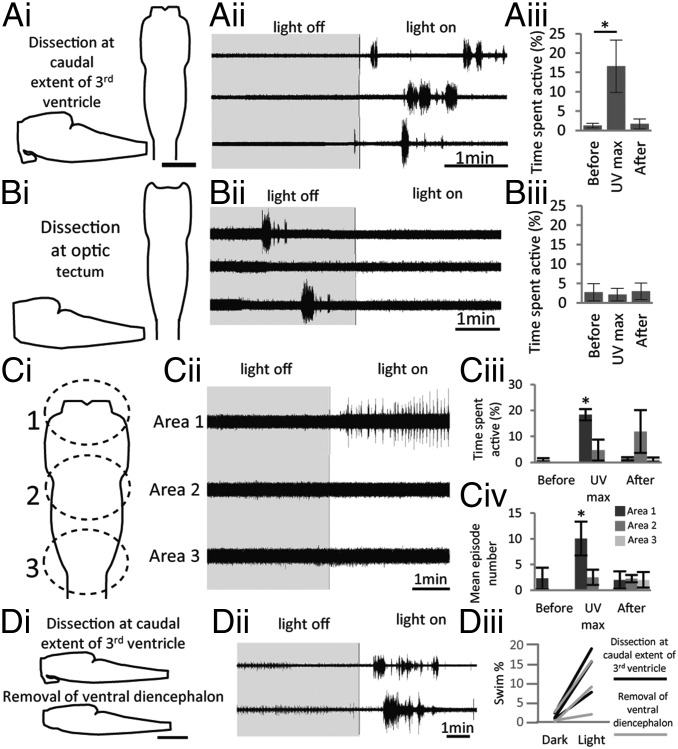
Having established that UV wavelengths produce a maximal response to illumination, the next step was to localize the sensitivity within the isolated nervous system. When light was shone on the spinal cord alone, no response could be elicited at any intensity or wavelength, including broad-spectrum white light. The standard dissection in these experiments involved making a cut level with the caudal extent of the third ventricle (Fig. 3 A, i). Shining UV light on these preparations produced a reliable, robust response (Fig. 3 A, ii and iii; also Fig. 2A). When a more caudal cut was performed, flush with the optic tectum and removing the entire diencephalon (Fig. 3 B, i), the preparations were rendered light-insensitive. In preparations that were spontaneously active (see the episode of activity in the dark period; Fig. 3 B, ii), illumination did not increase locomotor activity. The mean time spent active during the lights-on period was 2.10 ± 1.60%, compared with 2.75 ± 2.23% before illumination and 2.95 ± 2.16% immediately after (Fig. 3 B, iii; n = 4).
Fig. 3.
Photosensitive tissue resides within the caudal diencephalon. (A, i) Schematic of the dissection performed: The forebrain is removed apart from a small portion of diencephalon caudal to the dorsal opening of the third ventricle. Both dorsal and sagittal aspects are depicted. (Scale bar, 200 µm.) (A, ii) Ventral root recording from a stage 54 larva showing three consecutive responses to illumination with UV light (400 nm; 39 lx). (A, iii) Graph of average data comparing time spent active 200 s before, during, and after illumination (n = 7). (B, i) Schematic illustrating the dissection made flush with the optic tectum to completely remove the diencephalon. (B, ii and iii) Equivalent data to A displayed for preparations lacking the diencephalon (n = 4). (C, i) Schematic illustrating the approximate location of focal illumination of three areas of isolated nervous system. (C, ii) Sequence of responses to illumination of these different areas with UV light following 10 min in the dark (gray box). (C, iii and iv) Graphs showing average data for time spent active (C, iii) and mean episode number (C, iv) for illumination of each area; comparison of 200 s before, during, and after illumination are plotted (n = 4). (D, i) Schematic illustrating isolated nervous system before (Upper) and after (Lower) removal of the ventral diencephalon containing the hypothalamus and pituitary. (D, ii) Ventral root recording from a stage 56 larva before (Upper) and after (Lower) the dissection was performed. (D, iii) Graph illustrating data from three different preparations. Swim % is shown both before (black line) and after (gray lines) removal of the ventral diencephalon. Error bars represent ±SEM; *P < 0.05.
In a parallel set of experiments, a smaller-diameter light guide was used to focally illuminate three different areas of the light-sensitive, diencephalon-attached preparation. Illumination of area 1 (Fig. 3 C, i), the rostral extent of the preparation including the caudal diencephalon, produced a significant increase in both the time spent active (Fig. 3 C, iii) and the number of swim episodes (Fig. 3 C, iv; also Fig. 3 C, ii). The time spent active increased to 18.37 ± 2.18%, compared with 0.85 ± 0.80% before illumination and 1.31 ± 0.66% after the lights-on period (Fig. 3 C, iii; n = 4; P < 0.05). The total number of episodes increased to 10.07 ± 3.28, compared with 2.23 ± 2.11 before illumination and 2.00 ± 1.68 after the lights-on period (Fig. 3 C, iv; n = 4; P < 0.05). Illumination of either area 2, the midbrainstem, or area 3, the caudal brainstem, did not elicit an increase in locomotor activity during illumination with UV light—the time spent active during illumination of area 2 was 4.75 ± 4.08%, compared with no activity recorded before illumination and 11.88 ± 8.26% after the lights-on period; during these same conditions the mean number of episodes was zero before illumination, 2.5 ± 1.5 during UV illumination, and 2.25 ± 0.72 after the lights-on period (Fig. 3 C, ii–iv; n = 4). The time spent active was zero both during and before illumination of area 3 and 1.06 ± 0.86% after the lights-on period; the mean number of episodes during this condition was 2.0 ± 1.53 (Fig. 3 C, ii–iv; n = 4). Taken together, these results strongly suggest that the light sensitivity of the isolated tadpole nervous system is dependent on the diencephalic tissue located between the caudal extent of the third ventricle and the optic tectum. To provide further evidence for this hypothesis, we investigated the possible means of phototransduction in the tadpole diencephalon, paying particular attention to the region where the light sensitivity resides.
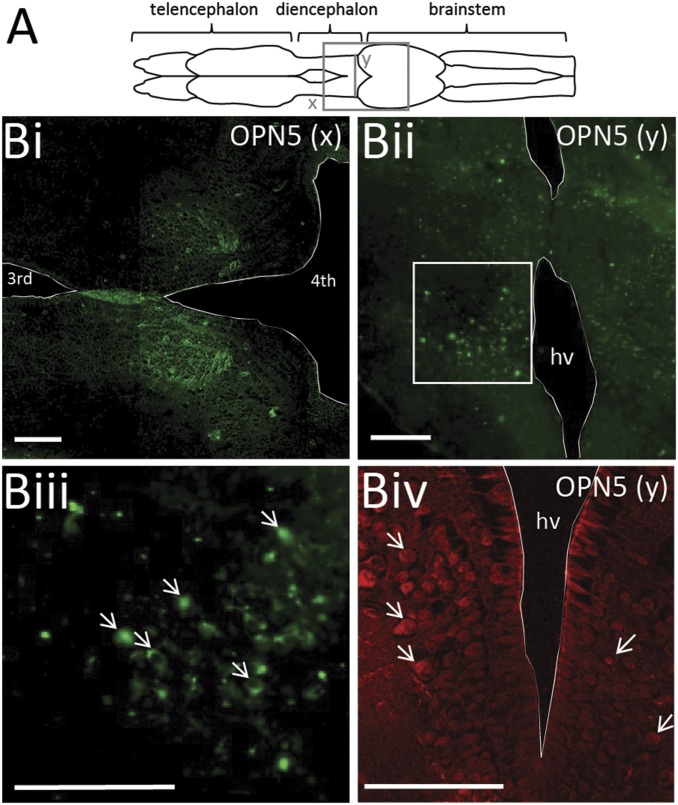
Because most known phototransduction in the vertebrate nervous system is mediated by light-sensitive opsin proteins, the next step was to try and locate opsin-positive neurons within the tadpole caudal diencephalon. Evidence for a UV-specific opsin (OPN5) mediating seasonal reproduction in the quail (8, 9) rendered this protein a good candidate. OPN5 is found within the periventricular organ (PVO) of the quail hypothalamus, close to the sensitive region in our experiments. Moreover, its peak sensitivity of 420 nm is similar to the spectrally tuned response in the tadpole nervous system. Immunofluorescent labeling of OPN5-positive neurons was therefore performed. Both longitudinal (Fig. 4 B, i) and coronal (Fig. 4 B, ii–iv) slices through the tadpole brain (Fig. 4A; n = 13) revealed a bilateral cluster of OPN5-positive neurons within the candidate light-sensing region of the caudal diencephalon (see Fig. S1 for negative controls). The neurons had an average diameter of 8.28 ± 0.73 μm (measuring only clearly defined somata; n = 30 neurons; n = 3 animals). The cluster was located at the level of the hypothalamic ventricle and extended ∼150 μm laterally from the ventricle and spanned a dorso–ventral region of ∼200 μm. This organization places a population of potentially light-sensitive OPN5-positive neurons in the region of the tadpole brain that mediates the photomotor response. Furthermore, the fact that OPN5 is particularly sensitive to short-wavelength UV light matches the spectral sensitivity of the light-triggered locomotor behavior.
Fig. 4.
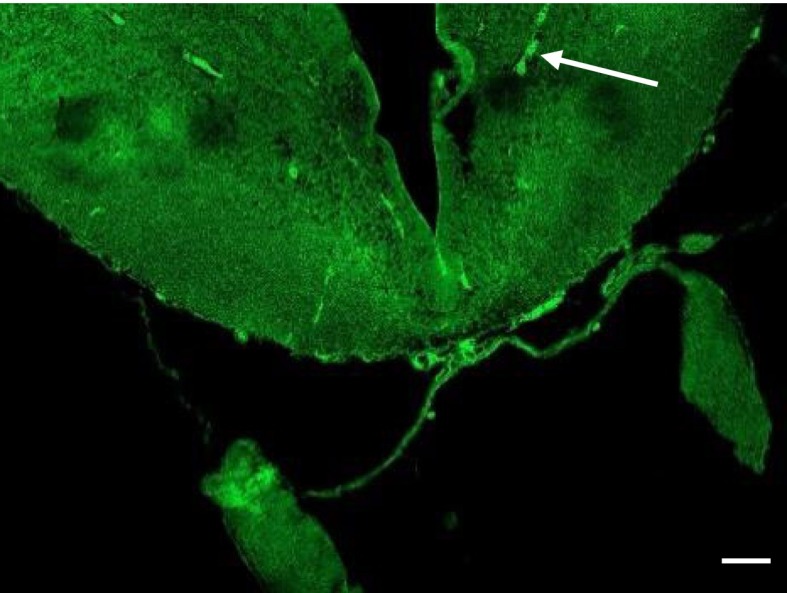
UV-sensitive proteins are located within the tadpole caudal diencephalon. (A) Schematic of a Xenopus tadpole brain showing the approximate position of sections taken for imaging. (B) OPN5-positive neurons within the caudal diencephalon of a stage 55 tadpole. (B, ii) A cluster of neurons is located in the ventral half of the diencephalon in proximity to the hypothalamic ventricle (hv); expanded view of the boxed area (B, iii) and a second more ventral image from a different preparation (B, iv). Examples of neuron cell bodies at arrows in B, iii and B, iv. (Scale bars, 100 μm.)
Fig. S1.
Controls for secondary antibodies. Negative controls lacking primary antibody. (i) Texas red secondary. (ii) FITC secondary. (Scale bar, 100 μm.)
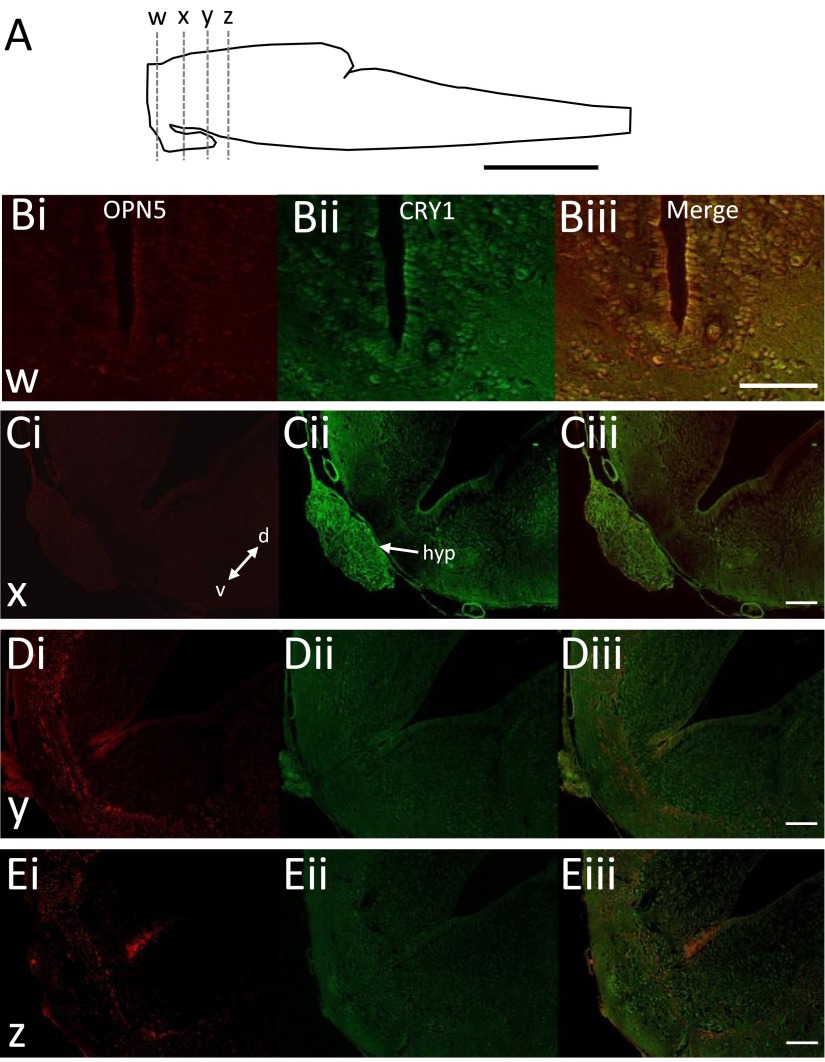
In addition to OPN5, cryptochrome proteins have been reported as blue-light sensors (20, 21) with a spectral sensitivity that also closely matches the wavelengths responsible for the light activation of fictive swimming. To assess the possible contribution of cryptochrome proteins 1 and 2 (CRY1 and CRY2), we performed immunohistochemistry on isolated larval CNSs and report widespread, protein-specific expression. CRY2 expression is abundant only in nonneuronal cells (presumptive microvasculature; Fig. S2; n = 3) but is not regionally restricted, with sporadic staining throughout the brainstem and spinal cord. Thus, CRY2 is highly unlikely to be involved in the increases in fictive swimming induced by light. CRY1 expression, on the other hand, was distinctly different from CRY2. Within the isolated nervous system there was a low level of labeling that was widely distributed, including the OPN5-positive region of the diencephalon (Fig. S3 A and B; n = 8). In addition, we found intense CRY1 labeling in ventrally and caudally located diencephalic structures including the hypothalamus and pituitary, located ventral to the brainstem proper (Fig. 3 D, i and Fig. S3 A and C, ii), suggesting that CRY1 could be responsible for or contribute to the light sensitivity we describe.
Fig. S2.
CRY2 is found ubiquitously but is localized within nonneuronal tissue. Immunohistochemistry showing widespread CRY2 expression, which is most intense in blood vessels (arrow). (Scale bar, 100 μm.)
Fig. S3.
OPN5 and CRY1 are expressed throughout the nervous system. (A) Schematic of a Xenopus tadpole brain showing the approximate position of sections taken for imaging. (B–E) Immunohistochemistry showing OPN5 and CRY1 expression within the ventral half of the preparation at three distinct anatomical levels moving caudally from the region of light sensitivity. d, dorsal; v, ventral. (C, ii) CRY1 is strongly expressed within the hypothalamus (hyp), the region surgically removed in Fig. 3D, without loss of light sensitivity. [Scale bars, 200 μm (A) and 100 μm (B–E).]
To test this idea further, we first recorded photic activation of swimming in control isolated CNSs (Fig. 3 D, i and ii, Upper). Next, we surgically removed the ventral diencephalon to excise the structures with the strongest CRY1 expression (but retaining the OPN5 neurons) and then reassessed the photic responsiveness of the preparation. In each case, a robust lights-on response was still recorded from spinal ventral roots (Fig. 3 D, i and ii, Lower and iii; n = 3). Therefore, although this intensely labeled CRY1 population does not appear to be necessary for the photoreceptive response, we cannot rule out the possibility that CRY1 is sufficient to play a role via the lower-intensity expression observed in the dorsal portion of the diencephalon. In addition, it remains unknown precisely how the putative deep-brain photoreceptors couple to the locomotor central pattern generator (CPG).
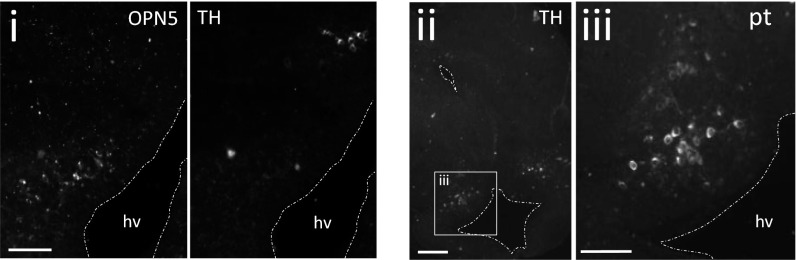
In the zebrafish hypothalamus the nonretinal opsin, melanopsin (OPN4), is coexpressed with tyrosine hydroxylase (TH) within A-11–type dopaminergic neurons and, although their function is unknown, it is presumed they may be important for light-mediated locomotor responses (16). We found no evidence that OPN5 was located within dopaminergic neurons (Fig. S4). However, we did identify a cluster of dopaminergic neurons in the same region of the hypothalamus, located just dorsal to the OPN5-positive somata. These TH-positive neurons are the rostral-most members of a population of dopaminergic neurons contiguous with the dopaminergic neurons of the posterior tuberculum (PT), found more caudally in the hypothalamus (Fig. S4).
Fig. S4.
OPN5 is not found within dopaminergic neurons within the light-sensitive region of the diencephalon. The OPN5 neurons in the caudal diencephalon are in close proximity to the rostral extent of the dopaminergic neurons of the posterior tuberculum. OPN5-positive neurons (i, Left) and TH-positive neurons (i, Right) are found in close proximity to each other within the caudal diencephalon. The TH-positive neurons are the rostral-most members of the dopaminergic neurons of the posterior tuberculum (pt) shown more caudally in the same animal (ii; magnification in iii). (Scale bars, 100 μm.)
Discussion
We have demonstrated that the isolated nervous system of prometamorphic Xenopus frog tadpoles is sensitive to light via a mechanism that does not involve the classical photoreceptive tissues of the eyes or pineal gland. This photosensitivity has been localized to a small region of the caudal diencephalon and shown to be tuned to short-wavelength UV light. Two main candidates with appropriate spectral sensitivity to function as the phototransducers in the lights-on response are OPN5 and CRY1. Both OPN5 and CRY1 are expressed in a region that broadly matches the light-sensitive part of the isolated CNS. At this stage, we cannot completely rule out a contribution from CRY1, which is strongly expressed in the caudal diencephalon that lies ventral to the brainstem. However, in support of OPN5’s important involvement, surgical removal of the region with the strongest CRY1 expression, leaving the periventricular OPN5 neurons intact, does not eliminate light sensitivity. Nevertheless, although we cannot differentiate unambiguously between the contributions of OPN5 and CRY1 to the locomotor response, strong CRY1 expression in the hypothalamus and pituitary suggests that it may play a role in slower, hormonal and/or diurnal changes in tadpole behavior. Future approaches to tease apart the respective roles of CRY1 and OPN5 in photic control of behavior could involve loss-of-function experiments following knockdown of the genes for these proteins, for example using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CAS9) system. However, this approach is beyond the scope of the present study and would best be tackled in a genetically more tractable model animal such as Xenopus tropicalis.
The discovery of neurons within this light-sensitive region of the tadpole brain expressing the UV-specific opsin OPN5 strongly implies this protein is an important mediator of phototransduction. Because photosensitivity in vertebrates is thought to originate from periventricular neurons of the diencephalon, it seems plausible that this mechanism is phylogenetically conserved and may represent a light-detecting component present in the brain of a primitive aquatic protovertebrate (23). An important facet of these experiments is that light sensitivity only links directly to the probability of occurrence of spontaneous locomotor activity. Upon illumination, the isolated nervous system produced regular episodes of fictive locomotion, whereas in the dark the preparations were generally silent. There were no differences between the coordination or basic parameters of the locomotor rhythm in the different light conditions, suggesting that the photic system of the brain controls merely how likely the animal is to swim.
This deep-brain light sensitivity could function as a simple mechanism to maintain the tadpole in an optimal photic environment. It could, for example, help avoid exposure to UV radiation from the sun, which can cause DNA damage and is a remarkably well conserved trait found even in bacteria (24). In addition, it may help to avoid the brightest-lit areas of the environment, where detection by predators is likely to occur. This form of light-avoidance strategy is found in many fish species, where it is thought to confer a specific advantage in the face of aerial predation (25). In embryonic Xenopus tadpoles, light avoidance is achieved by a pineal-driven motor response that causes upward swimming in response to shadows cast in the water (26, 27). Although this behavior is sufficient to maintain the relatively dormant embryos in an optimum environment for survival, the addition or predominance of other light-sensitive systems during development may aid survival in highly active, free-feeding larvae. Another, nonmutually exclusive, possibility is that the deep-brain light sensitivity could overlay classical circadian control mechanisms, which regulate behavior in response to predictable diurnal fluctuations in the environment. Given the tuning of this response to short wavelengths, it may be appropriate to detect subtle changes in the lighting conditions in an aquatic environment, where the influence of longer wavelengths is filtered out by the water.
An important next step will be to determine which neuronal pathway links the photoreceptive neurons to the activation of the motor system. The expression of the UV/blue light-sensitive proteins OPN5 and CRY1 was located in relatively close proximity to a set of dopaminergic neurons potentially related to the A-11–type population, known to project to the spinal cord and control motor output in other species (28). However, it is also plausible that OPN5- and/or CRY-positive neurons activate other supraspinal centers involved in vertebrate locomotion, such as the mesencephalic locomotor region (MLR) in the midbrain and/or reticulospinal nuclei in the hind brain (29), for example. Both of these possibilities could be involved simultaneously, as dopaminergic neurons within the PT of the lamprey project to and excite the MLR directly (30).
In zebrafish, the photoreceptors underlying dark photokinesis have been localized to the anterior preoptic area, and they transduce light via the photopigment melanopsin (16). The photosensitivity we report in Xenopus is not mediated by the equivalent region of the brain because the preoptic area has been removed in these light-sensitive preparations. However, melanopsin was also found more caudally in zebrafish, in neurons of the PT (16), an area that is present in the light-sensitive Xenopus preparations. This finding is particularly relevant because the cells in question were A-11–type dopaminergic neurons that comprise a diencephalospinal population implicated in motor control (28). However, they are unlikely to mediate the phototransduction we document here. First, the original work that identified melanopsin as a photopigment was carried out in Xenopus and, although it was found in both the preoptic and suprachiasmatic nuclei, there is no evidence for it being present in the caudal hypothalamus (31). Second, because the photomotor behavior in Xenopus is tuned to short-wavelength UV light, it does not correspond to the profile of a melanopsin-mediated response, which peaks around 480 nm (1, 32, 33).
Alternatively, OPN5 is a UV-specific opsin that has recently been shown to be a component of the photoperiodic response in quail (8, 9). OPN5 was located within the quail PVO, a caudal hypothalamic structure also present in the photosensitive tadpole preparation. Moreover, the PVO cells of other species have been shown to contain dopamine, noradrenaline, and/or serotonin (34), all known modulators of locomotion in Xenopus (35–37). An interesting example is the three-spined stickleback, which has large dopaminergic neurons in the PVO forming a contiguous group with the dopaminergic neurons of the PT (38). This more caudal group is thought to be homologous to the dopaminergic neurons of the mammalian zona incerta, which makes up the subthalamic diencephalic locomotor region, an area important in the supraspinal control of locomotion (39, 40).
What is the behavioral significance of this novel photomotor response in Xenopus tadpoles? The lighting conditions were at physiological levels for a species native to ponds in South Africa: The broad-spectrum, white light was around 13,000 lx, within the range of intensities experienced during the day although not in direct sunlight [10,000–25,000 lx (41)]; the brightest LED (blue; 468 nm) was ∼460 lx and therefore similar to the light intensity experienced at sunrise or sunset; and the UV LED (390–410 nm) that elicited the maximal response to light only emitted 39 lx, and occasionally elicited a response as low as 5 lx.
Deep-brain photoreception may promote light-avoidance behavior by increasing locomotor activity relative to light intensity, and thus increasing the likelihood of navigating to and settling in dimly lit areas. A role for deep-brain photoreception in negative phototaxis has been shown in eels (13); however, the generalized increase in locomotor activity seen in the isolated Xenopus nervous system is more similar to the dark photokinesis behavior displayed by larval zebrafish (16). In the eel, deep-brain photoreception was also shown to mediate photoentrainment to a circadian cycle of increased nocturnal activity (13). Although there is no evidence for circadian variation in activity during larval life, adult Xenopus are nocturnal (42). Tadpoles of the American toad (Bufo americanus) display increased activity and feeding during the day and are generally inactive overnight (43). In tadpoles of X. laevis, we propose that deep-brain photoreception serves the dual purpose of reducing exposure to the damaging influences of both predation and UV on the one hand and automatically adjusting energetically expensive bouts of locomotor activity to diurnal changes in light intensity on the other.
Materials and Methods
Animals and Husbandry.
Experiments were performed on a range of prometamorphic stages of the South African clawed frog X. laevis. Animals were obtained as described previously (19) from an in-house breeding colony. All procedures conformed to UK Animals (Scientific Procedures) Act 1986 and the European Community Council directive of November 24, 1986 (86/609/EEC) and have been approved by the University of St Andrews Animal Welfare Ethics Committee (AWEC).
Extracellular Electrophysiology Apparatus.
After removal of the forebrain apart from the caudal-most portion of the diencephalon under Ethyl 3-aminobenzoate methanesulfonate (MS-222; Sigma-Aldrich) anesthesia (19), the remaining nervous system was dissected free of the carcass, apart from the caudal-most portion of the tail, which was left attached to verify the preparation was capable of normal motor output. Recording conditions were as described previously (19).
Light Sources.
For experiments where the lighting conditions were manipulated, the recording apparatus was housed in a modified Faraday cage covered with aluminum foil and blackout cloth. The light level in the cage during lights-off was negligible (0 lx). Experiments with white light were performed with a standard halogen cold-light source (Olympus; Highlight 2000), which emitted broad-spectrum light at ∼13,000 lx (low-voltage halogen projection lamp; 14.5 V, 90 W; Phillips). When investigating the spectral sensitivity of the preparations, a series of LEDs were used (RS Components; all catalog numbers are provided). The specifications were as follows: blue LED (466-3532), peak λ was 468 nm, brightness was 15,000 millicandela (mcd) or 461 lx; green (671-6852), 523 nm, 21,000 mcd (136 lx); red (496-6178), 635 nm, 16,000 mcd (36 lx); UV (713-5043), 400 nm (39 lx).
Immunohistochemistry.
Tadpole brains were harvested from animals at stage 55. The tissue was fixed overnight at 4 °C in FAA fixative [50% (vol/vol) ethanol; 10% (vol/vol) 37–40% formaldehyde; 5% (vol/vol) acetic acid in dH2O], dehydrated through a graded alcohol series, and cleared in chloroform before wax embedding. Sections were cut at 8 μm on a rotary microtome and then mounted on electrically charged slides. Sections were deparaffinized in xylene, rehydrated through a graded alcohol series, and washed in phosphate-buffered saline with Triton X-100 (PBS-T). High-temperature antigen retrieval was performed in 0.1 M citrate buffer. Ten percent horse serum in PBS-T was used to block nonspecific antibody binding, and then the primary antibody (1:1,000 rabbit anti-OPN5; 1:1,000 rabbit anti-CRY1; or 1:500 anti-CRY2; all Aviva Systems Biology) was introduced and left overnight at 4 °C before detection with the secondary antibody (1:200 FITC–anti-rabbit; Vector Labs). Verification of the OPN5 antibody in Xenopus was carried out (Figs. S5 and S6); cryptochrome antibodies were commercially verified as able to cross-react with Xenopus proteins (Fig. S7). For double labeling, the previous two steps were repeated with a second set of antibodies (1:1,000 mouse anti-TH, Sigma-Aldrich and 1:200 TRITC–anti-mouse, Vector Labs; or 1:1,000 rabbit anti-OPN5 and 1:200 Texas red–anti-rabbit, Vector Labs). Following a final wash in PBS-T (5 × 5 min), the sections were mounted in Citifluor and the coverslip was sealed with ethyl acetate. Following immunohistochemistry, images were obtained on a Zeiss Axio Imager Ax10 at 40× magnification. Neuronal measurements, as well as tiling and stitching of individual images to create larger composites, were performed using ZEN Imaging Pro (v10; Zeiss) software.
Fig. S5.
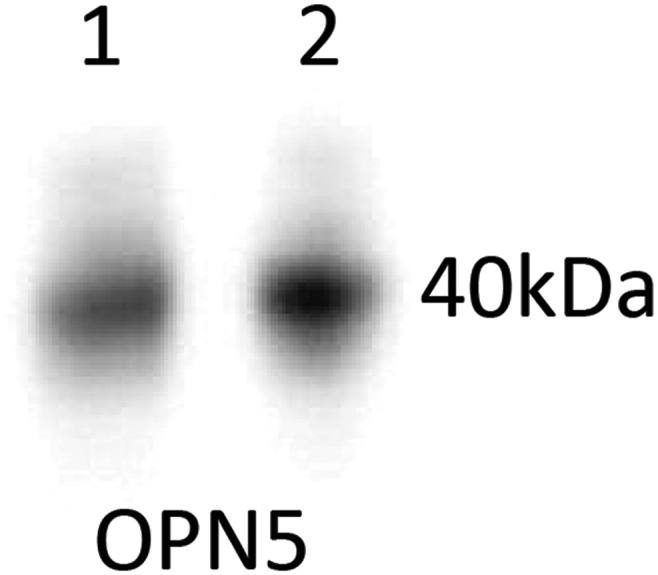
Western blot for OPN5 detection in X. laevis protein samples. Stage 51 (lane 1) and adult (lane 2) Xenopus protein samples were run on 4–12% polyacrylamide gels and transferred to nitrocellulose membrane for Western blotting to detect OPN5 using the antibody described above at 1:5,000. Positive bands at ∼40 kDa were obtained for both samples. This finding revealed that the antibody specifically detected a protein of the correct size for Xenopus OPN5.
Fig. S6.
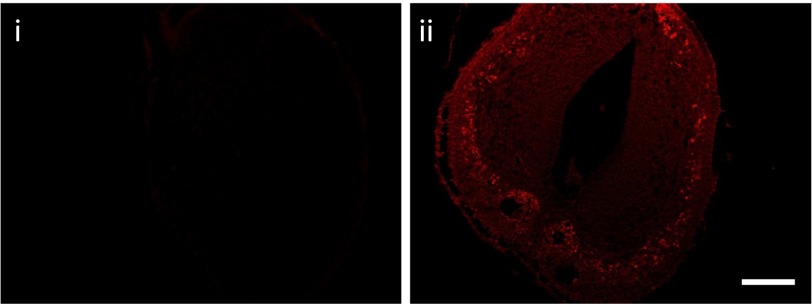
Positive immunoreactivity for OPN5 in the Xenopus nervous system. OPN5-positive immunoreactivity was observed in specific regions of the nervous system, including the cells in the spinal cord (ii). A control section stained with preabsorbed OPN5 is also shown (i). (Scale bar, 150 μm.)
Fig. S7.
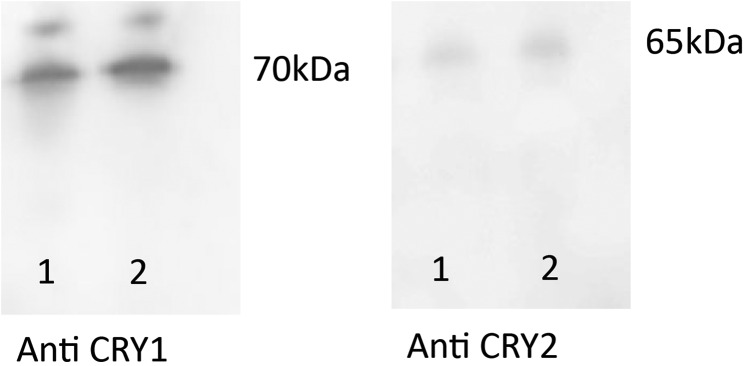
Western blots for the detection of CRY1 and CRY2 in Xenopus samples. Stage 51 (lane 1) and adult (lane 2) Xenopus protein samples were run on 4–12% polyacrylamide gels and transferred to nitrocellulose membrane for Western blotting to detect CRY1 or CRY2 using a commercially sourced antibody (Aviva Systems Biology; 1:5,000 for CRY1 and 1:1,000 for CRY2). Positive bands at ∼70 and 65 kDa were obtained for both samples. This result revealed that the antibody specifically detected proteins of the correct size for Xenopus cryptochromes.
Data Acquisition and Statistical Analysis.
Extracellular signals were amplified using differential AC amplifiers (A-M Systems; model 1700; low cutoff, 300 Hz; high cutoff, 500 Hz), digitized using a 1401 analog-to-digital acquisition system (CED; Cambridge Electronic Design), and stored and processed on a PC using Spike2 (CED) software (sampling rate 8–10 kHz). Electrophysiological data were analyzed using DataView software (v8.62; courtesy of W.J. Heitler, University of St Andrews), and all raw data were imported into Excel (Microsoft). Statistical analysis was performed in SPSS (IBM, v21). For comparison of average data, either a paired t test or a repeated-measures ANOVA with Bonferroni post hoc corrections was used. Error bars represent ±SEM. Due to large interpreparation variation, data were sometimes normalized to the value in control (100%) for a more thorough comparison.
SI Materials and Methods
Optimization and Verification of OPN5 Antibody for Xenopus laevis.
Rabbit anti-OPN5 antibody was purchased from Aviva Systems Biology (ARP59824_P050). The immunopeptide sequence that the antibody had been raised against had 92% computational homology to the X. laevis OPN5 sequence, but confirmation of cross-reactivity with Xenopus tissue had not been carried out.
Dot blotting revealed that positive reactivity occurred between the antibody and Xenopus protein at dilutions between 1:500 and 1:10,000, but that this interaction could be blocked by preabsorption of the antibody with a blocking peptide (AAPP45983; Aviva Systems Biology). Western blotting (Fig. S5) was used to confirm that the antibody detected protein of the appropriate size for Xenopus OPN5.
Because the biochemical studies indicated that this antibody was detecting a protein of the correct size for Xenopus OPN5 and that the interaction could be blocked in the presence of an OPN5 blocking peptide, the antibody was next optimized for use in histological samples. Specific staining was obtained in distinct regions of the nervous system, and again the immunoreactivity was lost in the presence of OPN5 blocking peptide (Fig. S6). These data confirm that the antibody is able to detect OPN5 in the Xenopus nervous system.
Optimization and Verification of CRY1 and CRY2 Antibody for X. laevis.
In contrast to the anti-OPN5 antibody, the antibodies for both CRY proteins were commercially available, having already been verified for use in X. laevis. To check the specificity of the antibodies, protein extracted from whole Xenopus brain was run on a Western blot and probed with the anti-CRY1 or anti-CRY2 antibody and the size of the observed bands was compared with a molecular mass standard. Thus, bands of ∼70 and 65 kDa were observed for CRY1 and CRY2, respectively, which is comparable to the predicted molecular masses of 70 and 64 kDa, respectively.
Acknowledgments
We thank the University of St Andrews for support, and Laurence Picton for help with the figures. S.P.C. was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) studentship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.M.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515516113/-/DCSupplemental.
References
- 1.Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: Melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2849–2865. doi: 10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernald RD. Evolution of eyes. Curr Opin Neurobiol. 2000;10(4):444–450. doi: 10.1016/s0959-4388(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 3.von Frisch K. Beiträge zur Physiologie der Pigmentzellen in der Fischhaut. Gesamte Physiol Menschen Tiere. 1911;138(7):319–387. [Google Scholar]
- 4.Benoit J. Stimulation par la lumiere artificielle du developpement testiculaire chez des canards aveugles par section du nerf optique. C R Seances Soc Biol Fil. 1935;120:133–136. [Google Scholar]
- 5.Menaker M, Keatts H. Extraretinal light perception in the sparrow. II. Photoperiodic stimulation of testis growth. Proc Natl Acad Sci USA. 1968;60(1):146–151. doi: 10.1073/pnas.60.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siopes TD, Wilson WO. Extraocular modification of photoreception in intact and pinealectomized coturnix. Poult Sci. 1974;53(6):2035–2041. doi: 10.3382/ps.0532035. [DOI] [PubMed] [Google Scholar]
- 7.Foster RG, Follett BK, Lythgoe JN. Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature. 1985;313(5997):50–52. doi: 10.1038/313050a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakane Y, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci USA. 2010;107(34):15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakane Y, Shimmura T, Abe H, Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr Biol. 2014;24(13):R596–R597. doi: 10.1016/j.cub.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Olson JM. Photosynthesis in the Archean era. Photosynth Res. 2006;88(2):109–117. doi: 10.1007/s11120-006-9040-5. [DOI] [PubMed] [Google Scholar]
- 11.Young JZ. The photoreceptors of lampreys I. Light-sensitive fibres in the lateral line nerves. J Exp Biol. 1935;12:229–238. [Google Scholar]
- 12.Young JZ. The photoreceptors of lampreys II. The functions of the pineal complex. J Exp Biol. 1935;12:254–270. [Google Scholar]
- 13.van Veen T, Hartwig HG, Müller K. Light-dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L.) J Comp Physiol. 1976;111(2):209–219. [Google Scholar]
- 14.Serra EL, Medalha CC, Mattioli R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res. 1999;32(12):1551–1553. doi: 10.1590/s0100-879x1999001200016. [DOI] [PubMed] [Google Scholar]
- 15.Burgess HA, Schoch H, Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr Biol. 2010;20(4):381–386. doi: 10.1016/j.cub.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes AM, et al. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol. 2012;22(21):2042–2047. doi: 10.1016/j.cub.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokel D, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6(3):231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokel D, et al. Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J Neurosci. 2013;33(9):3834–3843. doi: 10.1523/JNEUROSCI.3689-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combes D, Merrywest SD, Simmers J, Sillar KT. Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis. J Physiol. 2004;559(Pt 1):17–24. doi: 10.1113/jphysiol.2004.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogle K, Parson K, Dahm N, Holmes T. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331(6023):1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanVickle-Chavez SJ, Van Gelder RN. Action spectrum of Drosophila cryptochrome. J Biol Chem. 2007;282(14):10561–10566. doi: 10.1074/jbc.M609314200. [DOI] [PubMed] [Google Scholar]
- 22.Sillar KT, Robertson RM. Thermal activation of escape swimming in post-hatching Xenopus laevis frog larvae. J Exp Biol. 2009;212(Pt 15):2356–2364. doi: 10.1242/jeb.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigh B, et al. Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol Histopathol. 2002;17(2):555–590. doi: 10.14670/HH-17.555. [DOI] [PubMed] [Google Scholar]
- 24.Ng WO, Grossman AR, Bhaya D. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J Bacteriol. 2003;185(5):1599–1607. doi: 10.1128/JB.185.5.1599-1607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark CW, Levy DA. Diel vertical migrations by juvenile sockeye salmon and the antipredation window. Am Nat. 1988;131(2):271–290. [Google Scholar]
- 26.Foster RG, Roberts A. The pineal eye in Xenopus laevis embryos and larvae: A photoreceptor with a direct excitatory effect on behaviour. J Comp Physiol. 1982;145(3):413–419. [Google Scholar]
- 27.Jamieson D, Roberts A. Responses of young Xenopus laevis tadpoles to light dimming: Possible roles for the pineal eye. J Exp Biol. 2000;203(Pt 12):1857–1867. doi: 10.1242/jeb.203.12.1857. [DOI] [PubMed] [Google Scholar]
- 28.Clemens S, Rye D, Hochman S. Restless legs syndrome: Revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67(1):125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 29.Jordan LM. Initiation of locomotion in mammals. Ann N Y Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 30.Ryczko D, et al. Forebrain dopamine neurons project down to a brainstem region controlling locomotion. Proc Natl Acad Sci USA. 2013;110(34):E3235–E3242. doi: 10.1073/pnas.1301125110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95(1):340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 33.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigh B, Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc Res Tech. 1998;41(1):57–83. doi: 10.1002/(SICI)1097-0029(19980401)41:1<57::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Sillar KT, Wedderburn JF, Simmers AJ. Modulation of swimming rhythmicity by 5-hydroxytryptamine during post-embryonic development in Xenopus laevis. Proc Biol Sci. 1992;250(1328):107–114. doi: 10.1098/rspb.1992.0137. [DOI] [PubMed] [Google Scholar]
- 36.McDearmid JR, Scrymgeour-Wedderburn JF, Sillar KT. Aminergic modulation of glycine release in a spinal network controlling swimming in Xenopus laevis. J Physiol. 1997;503(1):111–117. doi: 10.1111/j.1469-7793.1997.111bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol. 2012;107(8):2250–2259. doi: 10.1152/jn.00366.2011. [DOI] [PubMed] [Google Scholar]
- 38.Ekström P, Honkanen T, Borg B. Development of tyrosine hydroxylase-, dopamine- and dopamine beta-hydroxylase-immunoreactive neurons in a teleost, the three-spined stickleback. J Chem Neuroanat. 1992;5(6):481–501. doi: 10.1016/0891-0618(92)90004-a. [DOI] [PubMed] [Google Scholar]
- 39.Parker SM, Sinnamon HM. Forward locomotion elicited by electrical stimulation in the diencephalon and mesencephalon of the awake rat. Physiol Behav. 1983;31(5):581–587. [PubMed] [Google Scholar]
- 40.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452(1–2):273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 41.Clark RN. Visual Astronomy of the Deep Sky. Sky; Cambridge, MA: 1990. [Google Scholar]
- 42.Casterlin M, Reynolds W. Diel activity and thermoregulatory behavior of a fully aquatic frog: Xenopus laevis. Hydrobiologia. 1980;75(2):247–254. [Google Scholar]
- 43.Beiswenger RE. Diel patterns of aggregative behaviour in tadpoles of Bufo americanus, in relation to light and temperature. Ecology. 1977;58(1):98–108. [Google Scholar]