Significance
To prevent a fall in blood glucose during fasting, the counter-regulatory response is activated. An important component of this pathway involves the autonomic nervous system and release of epinephrine from the adrenal gland. This autonomic response is often referred to as a reflex, implying the output is hardwired and inflexible. Here we show the strength of the terminal synapse that controls epinephrine release is actually highly plastic. Fasting leads to a long-lasting increase in synaptic strength by a process that requires neuropeptide Y and Y5 receptors. In the absence of neuropeptide Y, synaptic strengthening is absent, epinephrine release is reduced, and the mice become hypoglycemic. These findings indicate that the response to fasting involves significant autonomic synaptic plasticity.
Keywords: hypoglycemia, autonomic nervous system, synaptic plasticity, adrenal, chromaffin cells
Abstract
During fasting, activation of the counter-regulatory response (CRR) prevents hypoglycemia. A major effector arm is the autonomic nervous system that controls epinephrine release from adrenal chromaffin cells and, consequently, hepatic glucose production. However, whether modulation of autonomic function determines the relative strength of the CRR, and thus the ability to withstand food deprivation and maintain euglycemia, is not known. Here we show that fasting leads to altered transmission at the preganglionic → chromaffin cell synapse. The dominant effect is a presynaptic, long-lasting increase in synaptic strength. Using genetic and pharmacological approaches we show this plasticity requires neuropeptide Y, an adrenal cotransmitter and the activation of adrenal Y5 receptors. Loss of neuropeptide Y prevents a fasting-induced increase in epinephrine release and results in hypoglycemia in vivo. These findings connect plasticity within the sympathetic nervous system to a physiological output and indicate the strength of the final synapse in this descending pathway plays a decisive role in maintaining euglycemia.
Failure to avoid hypoglycemia can lead to dysphoria, ventricular arrhythmia, and even sudden death (1). That these effects are rare and observed only in response to prolonged fasting or severe insulin-induced hypoglycemia is because of the remarkable effectiveness of the counter-regulatory response (CRR). This sensory-motor homeostatic feedback loop detects a fall in blood glucose through central and peripheral receptors and initiates a neuronal, endocrine, and behavioral response that restores euglycemia (2). One of the principal effector arms of the CRR is the sympatho-adrenal branch of the autonomic nervous system. Hypoglycemia elevates sympathetic activity, increasing hepatic glucose production and the release of gluconeogenic substrates while suppressing insulin and potentiating glucagon secretion (3, 4).
During fasting, these autonomic actions are mediated by epinephrine, which enters the systemic circulation after release from adrenal neuroendocrine chromaffin cells and by norepinephrine, secreted directly onto target tissues from postganglionic sympathetic neurons. The importance of sympathetic activity, and in particular circulating epinephrine in the CRR, is illustrated by the poor recovery from insulin-induced hypoglycemia when the release of this hormone is suppressed during hypoglycemia-associated autonomic failure (5, 6), and by recent work showing that deletion of melanocortin 4 receptors from preganglionic sympathetic neurons leads to elevated levels of blood glucose (7).
Given the involvement of the sympathetic nervous system in the CRR, modulation of autonomic activity is thus likely to alter the ability to respond to a hypoglycemic challenge. However, unlike in the CNS, where long-lasting changes in synaptic strength are known to be associated with functional consequences (8–10), whether the output of the autonomic nervous system is regulated in an activity-dependent fashion is largely unexplored. Here we test the consequences of food deprivation on signaling at the excitatory preganglionic → chromaffin cell synapse, the final connection in the descending pathway that drives epinephrine secretion, and find that it displays a long-lasting form of synaptic plasticity. Food deprivation leads to a sustained increase in synaptic strength, an effect that is mediated by neuropeptide Y (NPY), an adrenal cotransmitter, and by the local activation of Y5 receptors. In the absence of NPY, food deprivation results in a loss of synaptic plasticity and leads to a hypoglycemic state that can be reversed by increasing the circulating levels of epinephrine. These findings reveal a critical role for sympatho-adrenal synaptic plasticity in the CRR to hypoglycemia, and suggest that a failure to induce synaptic strengthening may lead to conditions associated with a loss of glycemic control (1, 3). More broadly, this type of sympathetic plasticity could regulate the activity of a growing list of unconventional autonomic targets, including hematopoietic stem cells and bone osteoblasts (11, 12).
Results
Fasting Activates the Sympatho-Adrenal System and Strengthens the Preganglionic → Chromaffin Cell Synapse.
To confirm the involvement of the sympatho-adrenal system in the response to food deprivation, we measured norepinephrine and epinephrine levels in vivo from mice that had been fasted for 24 h and from ad libitum-fed littermate controls. Circulating epinephrine is derived from adrenal chromaffin cells, whereas norepinephrine is mainly contributed by postganglionic sympathetic neurons that innervate peripheral tissues (13).
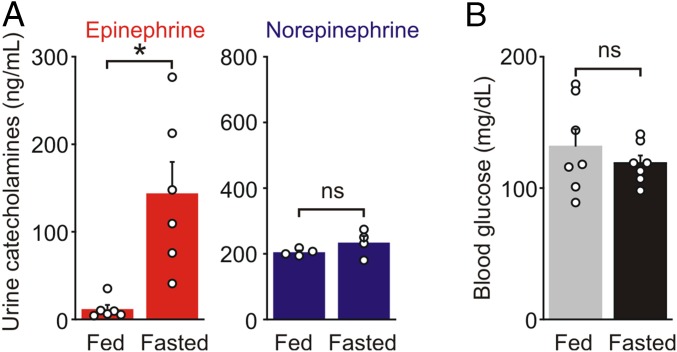
Food deprivation led to a large increase in the urine levels of epinephrine (fed: 11.8 ± 4.8; fasted: 143.9 ± 36.0 ng/mL, n = 6, P = 0.011, paired t test) but not norepinephrine (Fig. 1A), consistent with studies showing that hypoglycemia evokes epinephrine but not norepinephrine release (14, 15). Blood glucose levels did not differ significantly between fed and fasted littermates (fed: 132 ± 13; fasted: 119 ± 6 mg/dL, n = 7, P = 0.423, paired t test) (Fig. 1B), indicating that the CRR could maintain euglycemia even after a substantial period of fasting.
Fig. 1.
Food deprivation activates the sympatho-adrenal system. (A) Food deprivation increased urine epinephrine but did not change urine norepinephrine levels in wild-type mice (mean ± SEM, n = 4–6). (B) Blood glucose levels were not significantly different between fed and fasted littermates (mean ± SEM, n = 7). *P < 0.05; ns, not significant.
What adrenal signaling pathways are involved in sustaining the high level of epinephrine release? A priori this could involve modulation at two sites, presynaptically (via a change in preganglionic → chromaffin cell synaptic strength) or postsynaptically (via catecholamine synthesis or secretion).
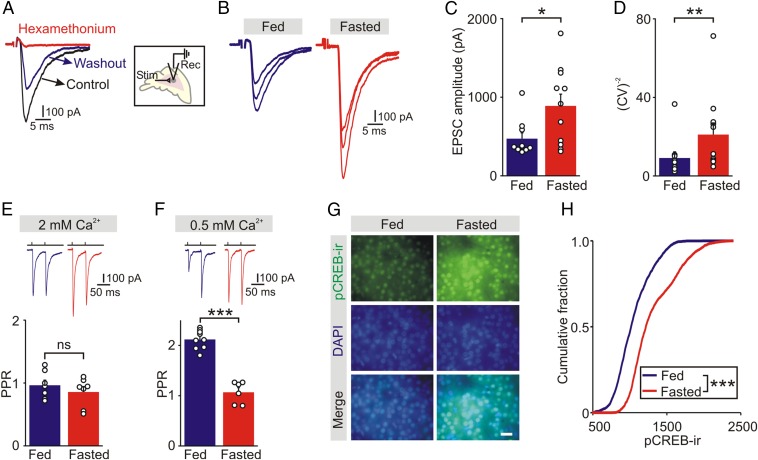
To determine whether a presynaptic mechanism was involved, we quantified cholinergic synaptic strength in fed and fasted mice. Chromaffin cells in adrenal slices were voltage-clamped in the whole-cell configuration and excitatory postsynaptic currents (EPSCs) were evoked by stimulating the preganglionic nerve terminals with a focal electrode. EPSCs were reversibly inhibited by hexamethonium, confirming they were a result of activation of nicotinic acetylcholine receptors (Fig. 2A). The amplitude of the evoked EPSCs was significantly larger in fasted wild-type mice compared with fed littermate controls (fed: 473 ± 73, n = 10; fasted: 890 ± 149 pA, n = 11, P = 0.025, unpaired t test) (Fig. 2 B and C). Food deprivation also led to a significant increase in the coefficient of variation (CV−2) of synaptic current amplitude (Fig. 2D), consistent with a presynaptic modulation of transmitter release probability (16, 17).
Fig. 2.
Food deprivation increases the strength of the preganglionic → chromaffin cell synapse in wild-type mice. (A) EPSCs recorded in a chromaffin cell evoked by stimulating the preganglionic nerve terminals were blocked in the presence of 100 μM hexamethonium chloride, a cholinergic antagonist, and recovered during washout. (B) Examples of evoked EPSCs recorded in adrenal slices from fed and fasted littermates. (C) Group data show that food deprivation increased the amplitude of evoked EPSCs (mean ± SEM, fed, n = 10 cells from 5 animals; fasted, n = 11 cells from 4 animals). Open symbols show the average EPSC value from each cell. (D) CV−2 of the EPSC amplitude was significantly greater in chromaffin cells from fasted compared with fed mice. (E) PPR of evoked EPSCs were similar in chromaffin cells from fed and fasted mice in ACSF containing 2 mM extracellular calcium (mean ± SEM, fed, n = 7 cells from 5 animals; fasted, n = 7 cells from three animals). (F) The PPR of EPSCs recorded in ACSF containing 0.5 mM extracellular calcium was significantly smaller in chromaffin cells from fasted compared with fed mice (mean ± SEM, fed, n = 9 cells from seven animals; fasted, n = 6 cells from 4 animals). (G) Examples of adrenal slices stained for pCREB from fed and fasted littermates. DAPI was used as a nuclear marker. (Scale bar, 20 μm.) (H) Cumulative intensity distributions of pCREB-ir in chromaffin cells from fed and fasted mice show that food deprivation led to an increase in pCREB-ir (fed: 1,845 cells; fasted: 1,820 cells; n = 3 separate experiments; Kolmogorov–Smirnov test). *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
To examine further the site of the fasting-induced modification, we investigated the influence of food deprivation on the paired-pulse ratio (PPR = EPSC2/EPSC1) of evoked synaptic currents. Low PPR values (synaptic depression) indicate a high synaptic release probability (18, 19). If the effect of fasting was mediated presynaptically, we expected to find a decrease in PPR. However, the PPR recorded in chromaffin cells was not significantly different in fed and fasted mice (fed: 0.96 ± 0.08, n = 7; fasted: 0.86 ± 0.09, n = 7, P = 0.389, unpaired t test) (Fig. 2E). Because a recent study has shown that synapses with a high release probability can have a reduced capacity for inhibition of the PPR (20), so masking a presynaptic mechanism, we lowered the [Ca2+] in the artificial cerebrospinal fluid (ACSF) from 2 mM to 0.5 mM to increase basal PPR values. In this condition, stimulation of the preganglionic input to chromaffin cells revealed a paired-pulse facilitation, and this was absent at synapses in food-deprived mice (fed: 2.12 ± 0.06, n = 9; fasted: 1.07 ± 0.09, n = 6, P < 0.001, unpaired t test) (Fig. 2F). A similar effect on PPR was also found after insulin-induced hypoglycemia, although this did not reach significance (Fig. S1). Thus, the change in both CV−2 and PPR are consistent with the idea that the synaptic potentiation of the preganglionic → chromaffin cell synapse following food deprivation is expressed presynaptically.
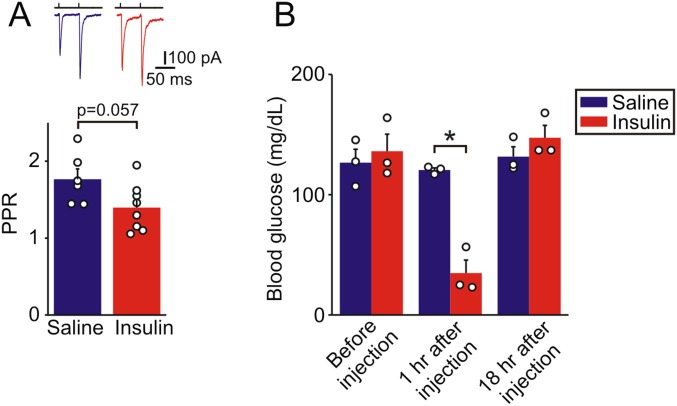
Fig. S1.
Preganglionic → chromaffin cell synaptic strength following insulin-induced hypoglycemia. (A) PPR of evoked synaptic currents recorded in chromaffin cells from mice following insulin-induced hypoglycemia (n = 8 cells from 3 animals) or saline injection (n = 6 cells from 3 animals). Mice were fasted for 3 h then injected with either insulin (2.5 U/kg, i.p.) or vehicle. Food was returned 1 h after injection and mice were killed 18 h after injection. Insulin produced a decrease in PPR, although unlike fasting this did not reach statistical significance (P = 0.057), perhaps because of the transient hypoglycemia. (B) Insulin injection produced a fall in blood glucose levels compared with saline when measured 1 h after injection (121 ± 2 vs. 35 ± 11 mg/dL; mean ± SEM; P = 0.014, unpaired t test), confirming that this protocol did induce significant hypoglycemia. *P < 0.05.
To obtain independent evidence that fasting led to a change in synaptic signaling, we also quantified the postsynaptic level of a marker whose expression is activity-dependent. c-Fos is commonly used but its levels can decay in the presence of ongoing activity (21). However, some forms of synaptic plasticity produce a sustained increase in phosphorylated cyclic AMP-responsive element binding protein (pCREB) (22, 23). Because nicotine can increase pCREB expression in the adrenal medulla (24), we used pCREB-ir as an indirect monitor of neuronal activity. Significantly higher levels of pCREB-ir were found in chromaffin cells from fasted animals (Fig. 2G), and the cumulative intensity distribution of pCREB-ir was shifted to the right after fasting (Fig. 2H). This result is consistent with an increase in chromaffin cell activity and the release of epinephrine during food deprivation (Fig. 1A).
Fasting Does Not Alter the Adrenal Catecholamine Secretory Capacity.
The above results indicate the existence of a form of long-lasting plasticity at the preganglionic → chromaffin cell synapse that was maintained after slice preparation. We next determined whether food deprivation also altered postsynaptic signaling by using carbon fiber amperometry to quantify secretion from single chromaffin cells in vitro from fed and fasted mice.
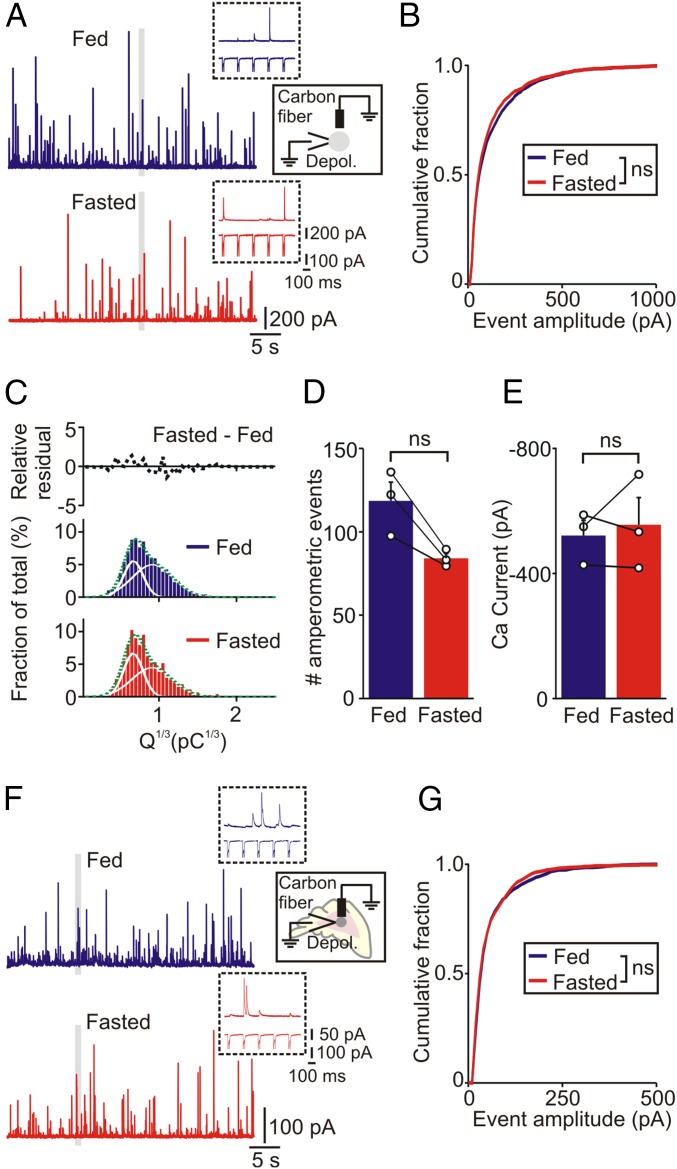
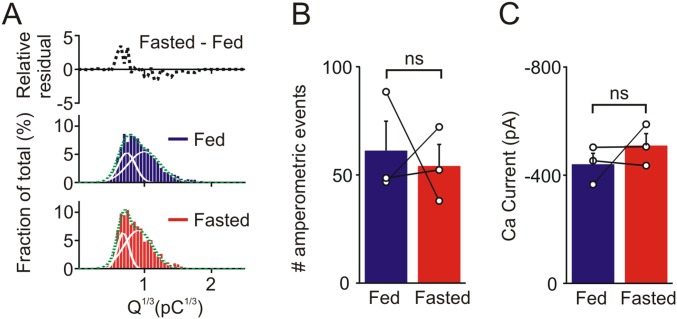
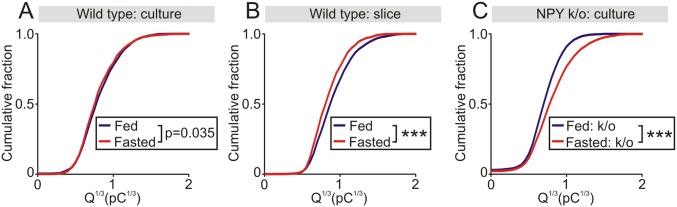
Catecholamine secretion was evoked by a train of voltage-clamp depolarizations (Fig. 3A). The amplitude of all single amperometric events that occurred in response to a train of depolarizations was measured and secretion was quantified as the cumulative amperometric amplitude distribution. Previous studies have shown this approach is a sensitive way to detect changes in release (25, 26). The distribution of amperometric events was not different in the fed and fasted conditions (Fig. 3B). When the cube root of the charge of the amperometric events was plotted against event number the distribution was well fit with the sum of two Gaussian distributions. This finding suggests the presence of two populations of dense core granules, as previously reported (26, 27). Subtraction of the Q1/3 distribution from fed and fasted mice confirmed that food deprivation did not alter the catecholamine secretory capacity in wild-type animals (Fig. 3C, fasted–fed). There was also no significant difference in the number of amperometric events or the amplitude of the depolarization-evoked calcium current between fed and fasted mice (Fig. 3 D and E). Although these experiments indicated that food deprivation did not increase the secretory capacity or deplete the releasable stores, one possibility was that an effect of fasting was lost during cell culture. To address this issue, we quantified catecholamine secretion from chromaffin cells in adrenal slices that were acutely prepared from fed and fasted mice. Chromaffin cells were stimulated with a train of voltage-clamp depolarizations and release was quantified using amperometry (Fig. 3F). There was no difference in the cumulative amplitude distribution after fasting (Fig. 3G). The subtracted Q1/3 distribution revealed a shift toward smaller events but there was no difference in the number of amperometric events or the amplitude of the depolarization-evoked calcium current (Fig. S2). These in situ results closely mimic those seen in vitro. Thus, we conclude that the food deprivation does not increase the secretory capacity in chromaffin cells or deplete the releasable stores of these transmitters.
Fig. 3.
Food deprivation does not alter the adrenal catecholamine secretory capacity. (A) Amperometric events evoked by a train of voltage clamp depolarizations from chromaffin cells in vitro from fed and fasted mice. Insets are excerpts from the regions indicated by the gray bars in A, showing amperometric events (Upper trace) and corresponding calcium currents (Lower trace) evoked by five depolarizations. (B) Cumulative amplitude distribution of amperometric events from fed and fasted mice (fed: 2,134 events; fasted: 1,513 events; n = 18 cells per condition from three paired experiments). (C) Cube root of event charge (from events plotted in B) fitted with two Gaussian distributions (solid lines). The relative residual distribution is the difference between the fed and fasted histograms. (D) Number of amperometric events evoked by a train of 200 voltage-clamp depolarizations and (E) amplitude of the voltage-dependent calcium current from cells from fed and fasted mice (mean ± SEM, n = 3 paired experiments, 6 cells per treatment in each experiment). (F) Amperometric events evoked by a train of voltage-clamp depolarizations (same protocol as A) from chromaffin cells in adrenal slices from fed and fasted mice. (G) Cumulative amplitude distribution of amperometric events recorded in slices from fed and fasted mice (fed: 1,239 events; fasted: 1,065 events; n = 20 cells per condition from three paired experiments). ns, not significant.
Fig. S2.
Effect of food deprivation on catecholamine secretion in adrenal slices. (A) Cube root of amperometric event charge (from events plotted in Fig. 3G) fitted with two Gaussian distributions (solid lines). The relative residual distribution is the difference between the fed and fasted histograms. (B) Number of amperometric events and (C) amplitude of the voltage-dependent calcium current from cells from fed and fasted mice (mean ± SEM, n = 3 paired experiments, 6–7 cells per treatment in each experiment). ns, not significant.
Fasting Alters the Adrenal Expression of NPY but Not Tyrosine Hydroxylase.
Food deprivation thus leads to an increase in preganglionic → chromaffin cell synaptic strength with no change in postsynaptic secretory capacity. We next sought to determine the mechanism underlying this effect. Because fasting does not globally increase sympathetic output (28) we reasoned that local adrenal signaling pathways were likely to be responsible for synaptic strengthening. NPY is costored and coreleased with the catecholamines in chromaffin cells and multiple Y receptors are expressed in the adrenal medulla (29–31), making it a good candidate to mediate the fasting-induced change. We therefore measured the adrenal levels of NPY together with tyrosine hydroxylase (TH), the enzyme that catalyzes the rate-limiting step in catecholamine synthesis.
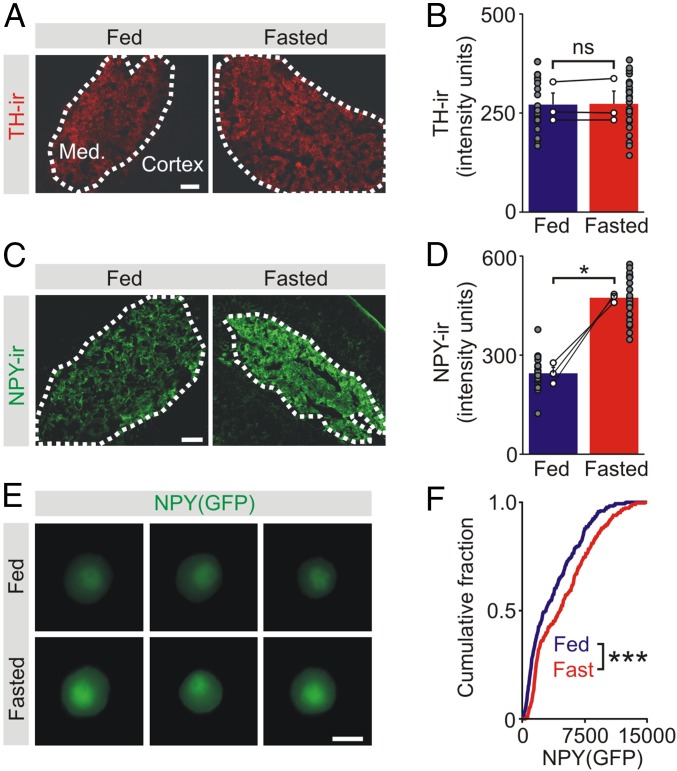
In the adrenal, TH-immunoreactivity (TH-ir) was confined to the catecholaminergic chromaffin cells in the medulla (Fig. 4A). When the levels of TH-ir were quantified between matched pairs of fed and fasted animals, no difference was found (Fig. 4B). In contrast to TH, the level of NPY-ir in the adrenal medulla was significantly higher after fasting (fed: 245 ± 18; fasted: 474 ± 8, n = 3, P = 0.012, paired t test) (Fig. 4 C and D). The increase in NPY-ir could be caused by a reduction in peptide secretion or an increase in synthesis. To distinguish between these possibilities, we used an NPY(GFP) BAC transgenic mouse in which GFP expression is driven by regulatory elements in the NPY gene (32). Therefore, an increase in GFP expression would reflect a transcriptional activation of the NPY gene (or less likely, a decrease in GFP degradation). Importantly, because GFP is present in the cytoplasm not the regulated secretory pathway, a change in GFP expression would be independent of secretory activity. When the levels of GFP were measured from chromaffin cells in vitro, we found that GFP expression was significantly higher in cells isolated from fasted animals (Fig. 4E). Group data confirmed that food deprivation led to a rightward shift in the cumulative intensity distribution (Fig. 4F).
Fig. 4.
Food deprivation increases the adrenal expression of NPY but not TH. (A) TH-ir in adrenal sections from fed and fasted mice. (Scale bar, 100 μm.) (B) Group data (open symbols are mean values from each animal) shows that food deprivation did not alter the levels of TH-ir (mean ± SEM, n = 3 independent experiments). Filled symbols show TH-ir in all analyzed cryosections. (C) NPY-ir in adrenal sections from fed and fasted mice. (Scale bar, 100 μm.) (D) Group data (open symbols) shows that food deprivation increased the levels of NPY-ir (mean ± SEM, n = 3 independent experiments). Filled symbols show NPY-ir in all analyzed cryosections. (E) Examples of GFP expression in chromaffin cells from fed and fasted NPY(GFP) BAC mice. (Scale bar, 10 μm.) (F) Cumulative frequency distributions showing food deprivation led to an increase in GFP expression in chromaffin cells (300 cells for each distribution, n = 3 separate experiments; Kolmogorov–Smirnov test). *P < 0.05, ***P < 0.001; ns, not significant.
NPY Is Required for the Fasting-Induced Increase in Preganglionic → Chromaffin Cell Synaptic Strength.
Given that fasting induced an increase in NPY, we next tested whether its loss would alter synaptic strengthening. As described below, we found the synaptic phenotype in NPY knockout mice was effectively the opposite of what we had observed in wild-type animals.
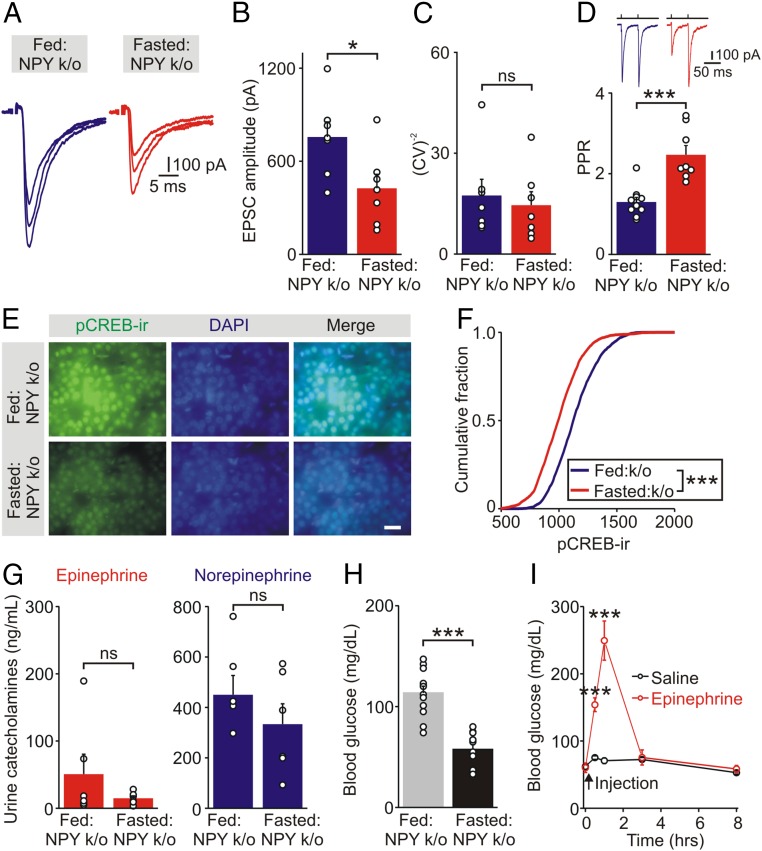
Thus, food deprivation did not increase synaptic transmission in NPY knockout mice, but significantly reduced the EPSC amplitude (fed: 756 ± 97; fasted: 426 ± 91 pA, n = 7, P = 0.029, unpaired t test) (Fig. 5 A and B). There was no difference in the CV−2 of EPSC amplitudes between fed and fasted mice (Fig. 5C). Food deprivation significantly increased the PPR in chromaffin cells in NPY knockout mice (fed: 1.30 ± 0.12, n = 10; fasted: 2.47 ± 0.23, n = 8, P < 0.001, unpaired t test) (Fig. 5D), suggesting that the fasting-induced decrease in synaptic efficacy in NPY knockout mice was presynaptically mediated and a result of altered release of ACh.
Fig. 5.
Food deprivation-induced synaptic strengthening and epinephrine secretion are both absent in NPY knockout mice. (A) EPSCs recorded in chromaffin cells in adrenal slices from fed and fasted NPY knockout mice. (B) Group data show that food deprivation reduced the amplitude of EPSCs in NPY knockout mice (mean ± SEM, n = 7 cells from 3 animals in each group). Open symbols show the average EPSC value from each cell. (C) CV−2 of the EPSC amplitude was not significantly different between fed and fasted NPY knockout mice. (D) Food deprivation significantly increased the PPR of evoked EPSCs in NPY knockout mice recorded in ACSF containing 0.5 mM extracellular calcium (mean ± SEM, fed, n = 10 cells from 4 animals; fasted, n = 8 cells from 6 animals). (E) pCREB-ir in adrenal sections from fed and fasted NPY knockout mice. (Scale bar 20 μm.) (F) Cumulative intensity distributions of pCREB-ir show that food deprivation led to a decrease in pCREB-ir in NPY knockout mice (fed: 1,798 cells; fasted: 2,140 cells; n = 3 separate experiments; Kolmogorov–Smirnov test). (G) Urine levels of epinephrine (mean ± SEM, n = 6) and norepinephrine (n = 6) were not different between fed and fasted NPY knockout mice. (H) Food deprivation resulted in hypoglycemia in NPY knockout mice (mean ± SEM, n = 12). (I) Blood glucose levels in fasted (16 h) NPY knockout mice that received either epinephrine (2 mg/kg, i.p.) or saline injection. Blood glucose levels were monitored immediately before (0 h) and 0.5, 1, 3, 8 h after injection (mean ± SEM, n = 5; one-way ANOVA). *P < 0.05, ***P < 0.001; ns, not significant.
Finally, quantification of pCPEB-ir in adrenal slices showed that the levels were lower in fasted NPY knockout mice compared with fed littermates and there was a corresponding leftward shift in the cumulative intensity distribution after food deprivation (Fig. 5 E and F). Thus, fasting leads to a long-lasting increase in the strength of the preganglionic → chromaffin cell synapse and this effect requires NPY.
NPY Is Required for Fasting-Induced Epinephrine Release and the Maintenance of Euglycemia.
Because loss of NPY prevented the fasting-induced change in preganglionic → chromaffin cell synaptic strength, we next asked whether its absence also altered epinephrine release in vivo by quantifying urinary catecholamine levels in fed and fasted NPY knockout mice. Fasting did not increase epinephrine release in NPY knockout mice (Fig. 5G, Left). The urine norepinephrine levels were also not elevated after food deprivation (Fig. 5G, Right). Consistent with the low levels of epinephrine and loss of the counter regulatory response, NPY knockout mice were hypoglycemic after food deprivation (fed: 114 ± 7; fasted: 58 ± 5 mg/dL, n = 12, P < 0.001, paired t test) (Fig. 5H). However, intraperitoneal injection of epinephrine significantly increased blood glucose levels in fasted NPY knockout mice (Fig. 5I). The hypoglycemia in the absence of NPY was therefore likely a result of the loss of epinephrine secretion rather than a downstream change in the counter regulatory response (e.g., in the hepatic ability to release glucose). Thus, the loss of NPY prevents fasting-induced sympatho-adrenal synaptic plasticity, epinephrine release, and leads to hypoglycemia.
NPY Covertly Regulates Adrenal Catecholamine Secretory Capacity.
The preceding experiments indicated that NPY knockout mice have a defect in fasting-induced synaptic strengthening (Fig. 5), an effect that is likely to be mediated presynaptically. We next investigated whether NPY also regulated the postsynaptic ability to secrete catecholamines.
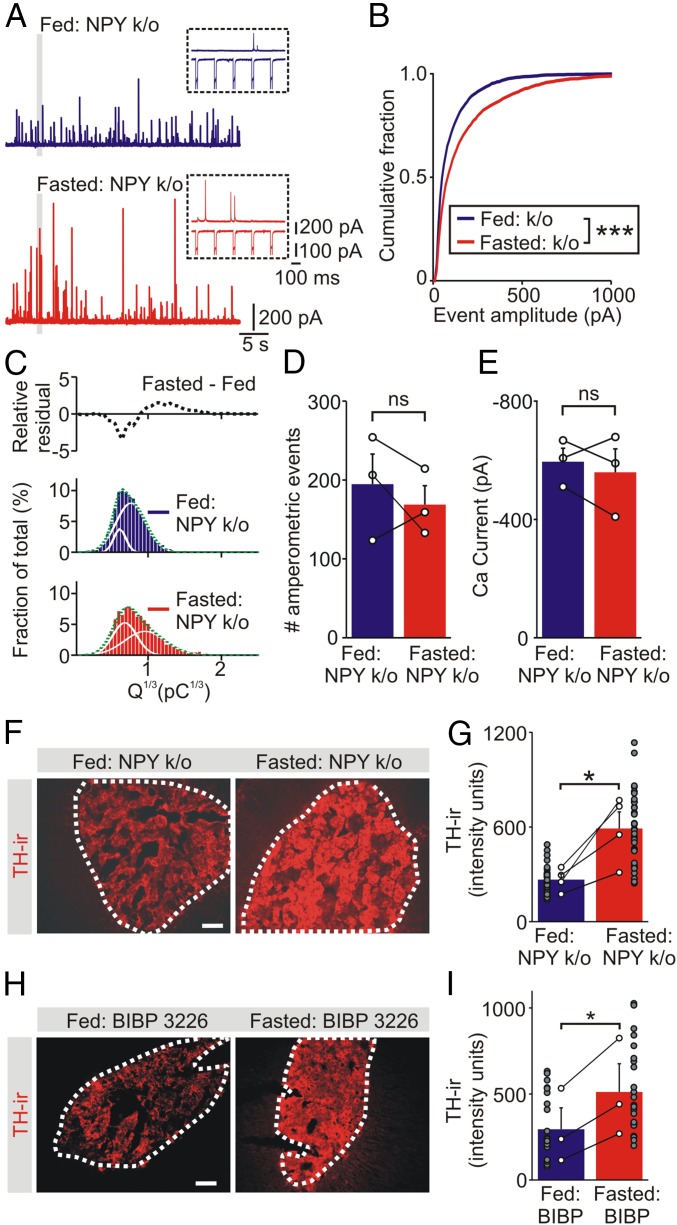
Using carbon fiber amperometry, catecholamine secretion was quantified from isolated chromaffin cells from NPY knockout mice (Fig. 6A). In these animals, food restriction was associated with an increase in secretory capacity, as seen by the large rightward shift in the cumulative amplitude distribution (Fig. 6B). This effect was also observed in the cube root of the amperometric charge (Fig. 6C). No difference was found in the number of amperometric events or the integral of the evoked calcium currents, suggesting that the increase in secretory capacity was downstream of calcium influx (Fig. 6 D and E). From these experiments we conclude that NPY also inhibits a food deprivation-induced increase in adrenal secretory capacity. Because the cumulative amplitude distribution curves from fed and fasted wild-type mice are identical (Fig. 3), this indicates that the inhibitory effect of NPY is usually masked.
Fig. 6.
Food deprivation increases the adrenal secretory capacity in NPY knockout mice. (A) Amperometric events evoked by a train of voltage clamp depolarizations from chromaffin cells from fed and fasted NPY knockout mice. (Right) Excerpts from the regions indicated by the gray bars showing the response to five depolarizing steps. (Upper) Amperometric recording. (Lower) ICa. (B) Cumulative amplitude distribution of amperometric events from fed and fasted NPY knockout animals (fed: 3,710 events; fasted: 3,172 events; n = 17 cells per condition from 3 paired experiments). (C) Frequency distribution of the cube root of the amperometric spike charge from fed and fasted NPY knockout mice. The relative residual is the difference between the fed and fasted histograms. (D) Amperometric event number and (E) amplitude of the voltage-dependent calcium current from the control and experimental cells (mean ± SEM, n = 3 paired experiments, 6 to 7 cells per treatment in each experiment). (F) Examples of TH-ir in adrenal sections from fed and fasted NPY knockout (k/o) mice. (G) Group data (open symbols are mean values from each animal) shows that food deprivation led to an increase in the level of TH-ir (mean ± SEM, n = 4 independent experiments). Filled symbols show TH-ir in all analyzed cryosections. (H) TH-ir in adrenal sections from a pair of fed and fasted mice injected with BIBP3226 (Y1 receptor antagonist). (I) Group data (open symbols) shows that fasting significantly increased the level of TH-ir in BIBP3226-injected animals (mean ± SEM, n = 3 independent experiments). Filled symbols show TH-ir in all analyzed cryosections. (Scale bars, 100 μm.) *P < 0.05, ***P < 0.001; ns, not significant.
We have recently shown that NPY inhibits the adrenal expression of TH and this effect is mediated by Y1 receptors (26). Thus, the increase in secretory capacity in the NPY knockout mice could be because of a change in TH expression. To test this idea, we used two approaches. First, the expression of TH was examined in fed and fasted NPY knockout animals. In these mice, food restriction was now associated with a large increase in the adrenal expression of TH compared with control animals (fed: 267 ± 36; fasted: 591 ± 105, n = 4, P = 0.029, paired t test) (Fig. 6 F and G). Second, animals were injected intraperitoneally with the Y1 antagonist, BIBP3226, immediately before the onset of food deprivation. Control animals that were allowed access to food were also injected with BIBP3226. As shown in Fig. 6 H and I, the levels of TH-ir were significantly higher in the fasted animals (fed: 295 ± 124; fasted: 512 ± 164, n = 3, P = 0.033, paired t test). In contrast the level of TH-ir in the BIBP3226-injected control animals was not different from untreated animals (compare with Fig. 4 A and B) (P > 0.5). Thus, one role of the fasting-induced increase in NPY is to tonically suppress TH expression.
Fasting-Induced Synaptic Strengthening Requires Activation of Adrenal Y5 Receptors.
From the experiments so far, we concluded that fasting has two antagonistic effects on adrenal function: first, a strengthening of the preganglionic → chromaffin cell synapse; second, an inhibition of catecholamine secretory capacity. Because food deprivation results in a robust increase in epinephrine release in vivo (Fig. 1), the first of these effects must predominate.
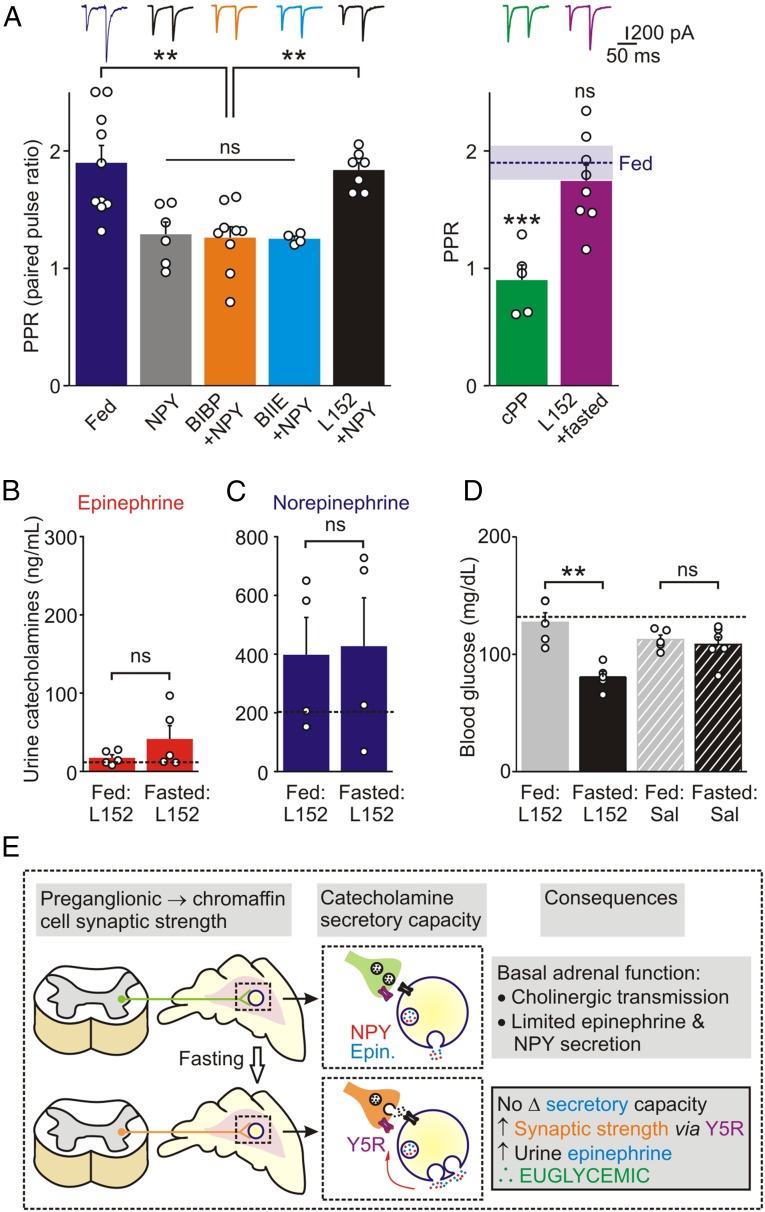
In a final set of experiments, we wanted to determine whether the NPY-dependent signaling pathway that led to synaptic strengthening was located within the adrenal. We incubated slices from fed mice with NPY (1 µM for 3–6 h) and then quantified synaptic transmission. Under these conditions, the PPR was significantly lower than that of fed animals (Fig. 7A). Y1, Y2, Y4, and Y5 receptor mRNAs have been identified in the mouse sympathetic nervous system (26, 30). Because the physiological agonist for the Y4 receptor is pancreatic polypeptide, which is not synthesized by mouse chromaffin cells (26), either Y1, Y2, or Y5 receptors could mediate the effect of NPY. To identify which receptors were responsible, we incubated adrenal slices from fed animals in NPY plus Y1, Y2, or Y5 antagonists (BIBP3226, BIIE0246, and L152,806, respectively). Only the Y5 antagonist prevented the NPY-induced change in the PPR (Fig. 7A). To confirm the involvement of Y5 receptors, we incubated adrenal slices in the selective Y5 receptor agonist [cPP1–7,NPY19–23,Ala31,Aib32,Gln34]-hPancreatic Polypeptide (cPP, 1 µM, 3–6 h). EPSCs recorded from chromaffin cells in these slices had a significantly smaller PPR compared with control cells from fed mice (Fig. 7A), mimicking the effects seen with both fasting and NPY incubation.
Fig. 7.
Y5 receptor activation regulates synaptic strength and epinephrine secretion during food deprivation. (A) PPR of EPSC’s in chromaffin cells in adrenal slices from fed mice (n = 10 cells from 5 animals) or in slices incubated in NPY (1 μM, n = 6 cells from 5 animals) or in NPY (1 μM) plus BIBP3226 (1 μM, Y1 antagonist, n = 9 cells from 4 animals); NPY plus BIIE0246 (100 nM, Y2 antagonist, n = 4 cells from 2 animals) or NPY plus L152,804 (5 μM, Y5 antagonist, n = 7 cells from 6 animals). Only the Y5 antagonist prevented the change in PPR induced by NPY (one-way ANOVA). Incubation of slices in the Y5 agonist cPP (1 μM, n = 5 cells from 5 animals) mimicked the actions of NPY as indicated by a significant reduction in PPR compared with fed mice. The fasting-induced decrease in the PPR was reversed when adrenal slices were incubated in L152,804 (5 μM, n = 8 cells from 3 animals), indicating that constitutive activation of adrenal Y5 receptors is required for fasting-induced synaptic plasticity. The PPR (mean ± SEM) in fed mice is shown as a horizontal bar (data from the Left). (B) Food deprivation did not increase urine levels of epinephrine (n = 5) or (C) norepinephrine (n = 4) in L152,804 (Y5 antagonist) injected wild-type mice. The black dashed lines in B and C show mean values of urine catecholamines in fed wild-type mice (data from Fig. 1A). (D) Blood glucose levels were significantly decreased following food deprivation in mice injected with L152,804 (n = 5) compared with fed mice (n = 5). Injection of vehicle did not alter blood glucose levels in fed or fasted mice (n = 5 and 6, respectively). Black dashed line shows the mean value of blood glucose levels in control wild type mice (data from Fig. 1B). Values are mean ± SEM; **P < 0.01, ***P < 0.001; ns, not significant. (E) Working model of the experimental results. Food deprivation strengthens the preganglionic → chromaffin cell synapse via a presynaptic mechanism involving Y5 receptors (also shown in a presynaptic location for simplicity). Following local release NPY may act in an autocrine (shown) or paracrine manner (because of the acinar-like distribution of chromaffin cells around local blood vessels). Although fasting does not alter the catecholamine secretory capacity, the NPY-dependent synaptic strengthening is required for epinephrine release during food deprivation. Epinephrine contributes to the maintenance of euglycemia by increasing hepatic glucose production. In NPY knockout mice, synaptic strengthening is absent, epinephrine release does not occur and consequently the animals are hypoglycemic.
Theoretically, the fasting-induced, long-lasting synaptic modulation could be because of an acute activation of Y5 receptors or may require ongoing receptor signaling. To distinguish between these possibilities, we tested whether the effect of fasting could be reversed with a Y5 antagonist. The PPR of evoked EPSC’s was quantified in slices prepared from fasted mice and subsequently incubated in L152,806 for 3–6 h. Following this treatment the PPR was not significantly different from that observed at synapses from fed mice (Fig. 7A). We therefore conclude that chronic activation of adrenal Y5 receptors is both necessary and sufficient for synaptic plasticity during food deprivation.
Given that blocking Y5 receptors in the isolated slice reversed the fasting-induced change on the PPR, in vivo administration of a Y5 antagonist to wild-type mice should mimic the NPY knockout phenotype. We injected L152,804 before removing food from the experimental animals. Control animals received an injection of L152,804 and had free access to food. As predicted, injection of L152,804 prevented the fasting-induced increase in urine epinephrine levels that was normally seen in wild-type mice (Fig. 7B). Fasting did not change urine norepinephrine levels (Fig. 7C). Furthermore, mice injected with L152,804 had significantly decreased blood glucose levels after food deprivation (fed: 127 ± 8; fasted: 81 ± 5 mg/dL, n = 5, P = 0.002, unpaired t test) (Fig. 7D), whereas mice that received a saline injection were euglycemic after fasting (fed: 113 ± 4; fasted 108 ± 6 mg/dL, n = 5–6, P = 0.59, unpaired t test) (Fig. 7D). In sum, these results indicate that during fasting, activation of adrenal Y5 receptors leads to a long-lasting, presynaptic increase in the strength of the preganglionic → chromaffin cell synapse, thus driving epinephrine secretion and the maintenance of euglycemia.
Discussion
During fasting, it is critical that the CNS receives a steady supply of glucose. If the levels fall below well-defined thresholds a CRR is activated that restores euglycemia. One of the main effector branches of this response is a descending multineuronal signaling pathway that involves hypothalamic and brainstem nuclei, and which culminates in the release of the adrenal hormone, epinephrine (2, 4). We find that the very last synapse in this pathway, the preganglionic → chromaffin cell synapse, exhibits a marked degree of plasticity. Fasting leads to an increase in synaptic strength and the maintenance of euglycemia, but when this plasticity is absent, an increase in epinephrine release does not occur and the mice are hypoglycemic (Fig. 7E). Our study reveals, to our knowledge for the first time, that synaptic plasticity in the sympatho-adrenal system plays a critical role in determining the effectiveness of the response to food deprivation.
The type of autonomic plasticity we describe requires release of NPY, activation of Y5 receptors [which may be presynaptic (33, 34), given the effect of a Y5 agonist on PPR], and an increase in the strength of the cholinergic preganglionic → chromaffin cell synapse. Because chromaffin cells synthesize NPY as a cotransmitter and can secrete this peptide in an activity-dependent manner (26, 29, 31), both the induction and expression of plasticity appear to be localized to the adrenal medulla. Induction likely involves a postsynaptic component (an increase in NPY release), whereas expression is mediated presynaptically (an increase in ACh release as assessed by a change in PPR).
Recent studies have led to the understanding that fasting can also produce lasting changes in the CNS pathways that are involved in food intake. This phenomenon has been termed metabolic memory (35), an allusion to the features that it has in common with other types of long-lasting plasticity, such as long-term potentiation. Such plasticity is mediated via a mechanism that is distinct from the one we identified in the adrenal. Thus, fasting leads to a sustained increase in the activity of hypothalamic AgRP/NPY neurons through mechanisms that involve an increase in intrinsic excitability and a strengthening of the glutamatergic excitatory drive onto these cells (20, 36, 37). These peptidergic interoceptive neurons play an important role in the regulation of food intake and energy metabolism (38).
Although there are redundant control mechanisms that maintain glucose homeostasis (3), a rise in circulating epinephrine is a key event in the response to food deprivation (28, 39). This effect involves epinephrine release from the adrenal medulla, which increases hepatic glucose production (40), inhibits insulin release from pancreatic β-cells (41), and stimulates glucagon release from pancreatic α-cells (42). Taken together, these findings support the idea that the NPY-dependent rise in circulating epinephrine contributes to the increase in glucose production that is needed to maintain euglycemia during food deprivation.
Because the net effect of fasting is elevated epinephrine release, the Y5-mediated increase in preganglionic → chromaffin cell synaptic strength appears to play a decisive role. However, NPY has multiple actions in the adrenal, including a Y1-dependent inhibition of TH expression (26). The latter effect is associated with an inhibition of catecholamine secretory capacity and only became evident when catecholamine secretion was measured from isolated chromaffin cells. Because food deprivation is associated with epinephrine release (and thus presumably an increased need for catecholamine synthesis), what is the purpose of covertly suppressing TH expression? The explanation may lie in the temporal role of epinephrine in the fasting response. This hormone increases both glycogenolysis and gluconeogenesis (43) and mice that have been fasted for 18 h show a large decline in liver glycogen and fat stores (44). Thus, the beneficial actions of epinephrine may wane as the duration of the fast increases, making an elevation in catecholamine synthesis unnecessary. During food deprivation there is a widespread decrease in sympathetic output to most tissues, with the exception of the adrenal medulla and some white adipose tissue stores (28, 45, 46). Because the net effect of sympathetic activity is catabolic, this sympathetic inhibition is likely to be an energy-conserving mechanism (45). Thus, there is clearly a cost to sustained sympathetic activity.
The synaptic plasticity that we have identified in the adrenal may be involved in other conditions that involve robust sympathetic activation (11). For example, the baroreceptor reflex activates preganglionic neurons innervating norepinephrine-secreting chromaffin cells (47). The modulation may also have implications for the dysregulation of the sympathoadrenal system that occurs during hypoglycemia-associated autonomic failure. In this condition, recurrent hypoglycemic events evoke progressively less and less epinephrine release, increasing the risk of severe hypoglycemia. Although the cause is not known, there is evidence for both central and peripheral defects (1, 5, 48). Because the phenomenon is activity-dependent, it is likely to involve the synaptic plasticity we have described here.
Finally, this type of sympathetic modulation appears to be homosynaptic because it is regulated by NPY, a cotransmitter in chromaffin cells. This may be an efficient way to strengthen only those synapses that are activated during hypoglycemia, a form of positive feedback. A bistable, positive feedback loop is thought to control the activity of the hypothalamic AgRP/NPY neurons in response to fasting (20). Although the adrenal is not involved in controlling food intake, it suggests that this type of mechanism may be characteristic of neuronal networks that are intermittently activated such as those involved in metabolic regulation.
Materials and Methods
Animals and Food Deprivation.
C57BL/6J wild-type mice, NPY(GFP) mice (Npy-hrGFP BAC) (32), and 129S-NPYtm1Rpa/J NPY knockout mice (49) were purchased from The Jackson Laboratory. All experiments were performed using male mice [postnatal day (P) 24 to P50] that received ad libitum access to food unless otherwise specified. In the majority of experiments involving food deprivation, animals were individually housed for 3–5 d, then food was removed from the home cage for 24 h before the mice were killed; water was freely available. Control animals were left undisturbed. Many protocols (amperometric and immunohistochemical) used age-matched littermates and experiments were performed in parallel. Data points from these paired animals are connected by lines in each figure. To block Y1 or Y5 receptors in vivo, mice received an intraperitoneal injection of BIBP3226 (1 mg/kg; American Peptide), or L152,804 (10 mg/kg; Tocris Biosciences). After injection, food pellets were removed from the cage. A cohort of NPY knockout mice was injected with epinephrine (2 mg/kg, i.p.) after fasting for 16 h. Control animals were injected with the same volume of vehicle. Food deprivation lasted for 24 h in all other experiments. All experiments involving animals were approved by the Animal Care and Use Committee at Louisiana State University Health Sciences Center.
Blood Glucose Measurements.
Blood samples were collected from the tail vein of animals that were fasted for 24 h and from ad libitum fed littermates. Glucose levels were measured with a portable blood glucose meter (Optium EZ).
Measurement of Urine Catecholamine Levels.
Mice were gently handled to trigger urination while suspended over a layer of cling wrap. Urine was collected within 10 s of handling, acidified volumetrically 1:1 with 0.01 N HCl and stored at −80 °C. Epinephrine and norepinephrine levels were quantified using commercial ELISA kits (RM Diagnostics and Abnova), which included an initial boronate-based extraction step to bind cis-diols and remove metanephrine and normetanephrine [metabolites of epinephrine and norepinephrine (50) that are usually present in higher levels than the free catecholamines]. All catecholamine samples were measured in duplicate.
In Situ Slice Electrophysiology Recordings.
Adrenal glands were obtained from mice (P30–P40) that were killed with an overdose of pentobarbital (150 mg/kg). Glands were embedded in 3.5% (wt/vol) 2-hydroxyethylagarose and the agarose block was transferred to ice-cold slicing solution (81.2 mM NaCl, 2.4 mM KCl, 0.5 mM CaCl2, 6.7 mM MgCl2, 1.4 mM NaH2PO4, 23.4 mM NaHCO3, 23.3 mM glucose, and 69.9 mM sucrose, pH 7.4, saturated with 95% O2/5% CO2). Slices (350 µm) were cut using a Leica VT1000 vibrating microslicer and transferred to a storage chamber maintained at 37 °C containing ACSF (125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 25 mM glucose, pH 7.4, saturated with 95% O2/5% CO2) for 100 min before recording. Slices were superfused with ACSF and recordings were made at 31–33 °C. The cholinergic nerve terminals of preganglionic neurons were stimulated using a focal stimulating electrode made from a patch pipette (3–6 MΩ) that was filled with ACSF and placed in the adrenal medulla, 20–100 µm from the recording electrode. Presynaptic stimulation (1-ms duration) was generated using a Master-8/Iso-flex stimulator (AMPI) that was triggered using Clampex9 software. Patch pipettes (5–7 MΩ) filled with a cesium-based internal solution [120 mM Cs acetate, 10 mM EGTA-Cs, 0.4 mM MgCl2, 1 mM CaCl2, 1.5 mM Na2ATP, 0.4 mM Na2GTP, 2.5 mM MgATP, 5 mM lidocaine N-ethyl bromide (QX-314), 5 mM tetraethylammonium chloride, and 10 mM Hepes, pH 7.25, with CsOH] were used to voltage clamp chromaffin cells in the whole-cell configuration. The holding potential used for voltage-clamp recordings was −60 mV and recordings were made using a Multiclamp 700A amplifier. Series resistance was compensated by 80% and monitored throughout the experiment. If the value changed >20%, the recorded cell was excluded from analysis. EPSCs were evoked by stimulating the preganglionic input at 0.1 Hz and were distinguished by their all-or-none response to presynaptic stimulation and fast kinetics. Mean EPSC amplitude (21–100 events) was calculated for each cell. The reciprocal of the squared CV of the synaptic response amplitude was quantified as
An increase in this parameter is often interpreted as an increase in quantal content due to a presynaptically mediated change in transmitter release (51, 52). To assess short-term synaptic plasticity, the presynaptic input was stimulated with two depolarizations separated by a 50-ms interval and the protocol was repeated at 0.1 Hz. In these experiments, the calcium concentration in the ACSF was reduced from 2 mM to 0.5 mM, except for in Fig. 2E. The PPR of EPSCs (10–90 events per cell) was calculated by dividing the amplitude of the second EPSC by that of the first (PPR = EPSC2/EPSC1). For experiments involving the application of Y receptor agonists and antagonists, adrenal slices were incubated in ACSF containing 0.5 mM Ca2+ with NPY (1 µM; American Peptide Company), [cPP1–7,NPY19–23,Ala31,Aib32,Gln34]-hPancreatic Polypeptide (cPP, 1 µM; Tocris Biosciences), or L152,804 (5 µM; Tocris Biosciences) for 3–6 h after slicing. To assess the effect of Y receptor activation on PPR, adrenal slices were pretreated with BIBP3226 (1 µM), BIIE0246 (100 nM), or L152,804 (5 µM) at room temperature. After 30-min exposure, slices were incubated with NPY (1 µM) in the continued presence of antagonist for 3–6 h.
In Vitro and in Situ Amperometric Recordings.
For in vitro amperometry recordings, littermate paired experimental and control animals were killed by decapitation and chromaffin cells were isolated, as previously described (26). In brief, the adrenal medulla was digested with 1 mg/mL collagenase type IA and 6 mg/mL BSA in HBSS (5.3 mM KCl, 0.44 mM KH2PO4, 4 mM NaHCO3, 138 mM NaCl, 0.3 mM Na2HPO4·7H2O, 5.5 mM glucose, and 20 mM Hepes, pH 7.25, with NaOH) for 15 min at 37 °C. Tissue chunks were then transferred to HBSS containing 1 mg/mL trypsin and 6 mg/mL BSA for 30 min at 37 °C, followed by mechanical dispersion using a fire-polished Pasteur pipette. Cells were plated in culture medium (DMEM/10% FCS) on dishes coated with poly-d-lysine and used for recording within 20 h after isolation. Chromaffin cells were superfused with extracellular solution (135 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 11 mM glucose, pH 7.3, with NaOH) at 31–33 °C. Amperometric and voltage-clamp recordings were made as previously described using a Multiclamp 700A amplifier (26, 31). Chromaffin cells (5–9 pF) were voltage-clamped in the whole-cell configuration with glass electrodes filled with pipette solution (120 mM Cs acetate, 15 mM CsCl, 5 mM NaCl, 10 mM Hepes, 4 mM MgATP, 0.3 mM NaGTP, and 1 mM cAMP, pH 7.2, with CsOH). Secretion was evoked with a train of 200 depolarizing steps (−80 mV to 0 mV for 20 ms) at 5 Hz. Catecholamine secretion was quantified from the clamped cell using carbon fiber amperometric electrodes that were insulated with Sylgard and held at +700 mV. To record secretory events, the amperometric electrode was advanced until it lightly touched the cell membrane. After each recording, fouling of the electrode was minimized by repetitively switching the holding potential between 700 mV and −700 mV for ∼2 min. Amperometric and membrane currents were sampled at 5 kHz and filtered at 2 kHz. Amperometric spike parameters were acquired and analyzed using Clampfit 9 (Molecular Devices). Events >10 pA were detected using the threshold search method, and stimulation artifacts were removed by subtraction of a blank trace. Only amperometric spikes with a rapid rising phase (<5 ms) were selected to minimize the inclusion of exocytotic events that occurred distantly from the carbon fiber and all overlapping spikes were excluded. A change in secretory capacity was then quantified as a shift in the cumulative spike amplitude distribution. When spike charge was measured, either similar results were obtained or the effect seen in the amplitude distribution was more marked in the charge distribution (Fig. S3). Histograms of the cube root of the amperometric charge (Q1/3) were fit using OriginPro 7. Chromaffin cells were isolated from paired fed and fasted mice and interleaved recordings were made. All in vitro amperometric experiments were performed blind with respect to the experimental treatment until the analysis was complete. In situ amperometric recordings were made from chromaffin cells in adrenal slices, acutely prepared as described above, from littermate paired experimental and control animals. Chromaffin cells (7–15 pF) were voltage clamped in the whole-cell configuration using pipettes filled with the same internal solution used for in vitro recordings. Slices were superfused with ACSF and recordings were made at 31–33 °C. Cells were stimulated with the same train of voltage-clamp depolarizations as used for in vitro recordings and catecholamine secretion was monitored using a carbon fiber electrode gently pressed against the recorded cell.
Fig. S3.
Effect of food deprivation on catecholamine secretion from chromaffin cells in vitro and in situ. (A) Cumulative charge distribution of amperometric events from chromaffin cells in vitro from fed and fasted mice (from the same events used to generate Fig. 3B). (B) Cumulative charge distribution of amperometric events from cells in adrenal slices (Fig. 3G). (C) Cumulative charge distribution of amperometric events from cells in vitro from NPY knockout mice (Fig. 6B). ***P < 0.001.
Immunohistochemistry.
Mice were killed by decapitation, the adrenals were removed, dissected free of adhering tissue, and then fixed overnight at room temperature in 4% PFA in PBS. To examine adrenal TH- and NPY-ir, cryosections were made and processed as described previously (26). In brief, glands were snap-frozen in 2-methylbutane on dry ice then embedded in cryomatrix and 30-µm cryosections were collected on glass slides, then refixed in 4% PFA for 20 min. Sections were subsequently permeabilized in 0.3% Triton X-100 in PBS for 15 min, and incubated in 3% H2O2 before being placed in blocking reagent (PerkinElmer) for 30 min. Sections were incubated in primary antibody (rabbit anti-NPY; 1:10,000; #T-4070; Peninsula Labs; sheep anti-TH; 1:100; #AB1542; Millipore; rabbit anti-TH; 1:100; AB152; Millipore) at 4 °C overnight. Sections were washed with PBS containing 0.05% Tween 20 and incubated in secondary antibody (donkey anti-rabbit HRP or donkey anti-sheep HRP; 1:500; Jackson ImmunoResearch) for 30 min at room temperature, washed with PBS containing 0.05% Tween 20, incubated in tyramide signal amplification (TSA)–FITC or TSA–Cy3 (PerkinElmer) for 10 min, washed with PBS containing 0.05% Tween 20, rinsed with distilled water, and mounted in Vectashield.
For pCREB staining, mice were terminally anesthetized with ketamine/xylazine (100/10 mg/kg) and perfused transcardially with 30 mL ice-cold 4% PFA in PBS. Adrenal glands were removed and cryoprotected in PBS/30% sucrose overnight and 50-µm sections were cut using a vibratome (Leica VT1000). Adrenal sections were permeabilized in 0.3% Triton-X100 in PBS for 2 h, then transferred to 50 mM glycine (in PBS) for 30 min and incubated with blocking solution (5% IgG-free BSA and 0.05% Triton X-100 in PBS) for 30 min. Sections were incubated in primary antibody (rabbit antiphospho-CREB; 1:500; #06–519; Upstate Biotechnology) at 4 °C overnight. Sections were subsequently washed with PBS and incubated with secondary antibody (donkey anti-rabbit Alexa 488; 1:200; Invitrogen) for 90 min at room temperature. To label nuclei, sections were incubated in 1 µg/mL DAPI (Biotium) for 15 min, and then the sections were washed and mounted in Vectashield.
In Vitro NPY(GFP) Quantification.
Chromaffin cells from fed and fasted NPY(GFP) mice were isolated in vitro as described above and plated on poly-d-lysine coated coverslips. Cells were allowed to attach for 1 h, then fixed in 4% PFA, washed with PBS, and mounted in Vectashield.
Image Analysis.
Images were obtained using a Nikon TE2000U microscope, 10× and 60× oil immersion (1.4 numerical aperture) objectives, and a Retiga 1300 monochrome camera. Image-Pro Plus 5.1 (Media Cybernetics), NIS-Elements AR 3.10 (Nikon Instruments), Origin Pro7, and Excel, were used for data analysis. For chromaffin cells in vitro, images of single cells were taken at the level of the nucleus to maintain a consistent plane of focus between experiments. A circular area of interest (AOI) was used to select a single cell, and the mean pixel intensity was quantified.
To measure the level of TH- and NPY-ir in adrenal sections, an AOI comprising the entire medulla minus any cell-free areas was selected and the mean pixel intensity was calculated. Images were background subtracted using AOIs taken from the adrenal cortex, and 8–10 sections from each animal were measured. In each figure, open circles indicate the mean value from each animal, and black lines link the littermate control and food deprived mice (i.e., matched pairs) from each independent experiment. Statistical analysis was performed on the group data (open circles).
To quantify the level of pCREB-ir in adrenal slices, the nucleus of an individual cell was first selected in the DAPI channel, outlined with a circular AOI, and the mean fluorescence intensity of pCREB-ir in the green channel was determined. In all staining experiments, slides were blinded until the analysis was complete.
Statistical Tests.
Two-tailed unpaired Student’s t test was used when comparing the means of two groups, except when comparing paired control and experimental groups when the paired Student’s t test was used. Comparisons between three or more groups were made with a general linear model ANOVA (post hoc Tukey’s paired comparison). The Kolmogorov–Smirnov test was used for analyzing cumulative fraction datasets. In each histogram, the n value is indicated in the legend and by the number of open circles. This corresponds to the number of animals (in vivo, amperometric, and immunohistochemical experiments) or the number of recorded cells (electrophysiological experiments) in each dataset. Statistical tests were performed on the group data (open circles) in all cases. Data were considered to be significantly different if P < 0.05.
Acknowledgments
We thank Drs. June Liu and Yunbing Ma for critically reading the manuscript. This work was supported by National Institutes of Health Grants DK080441 and DK098134 (to M.D.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5766.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517275113/-/DCSupplemental.
References
- 1.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362–372. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 2.Watts AG, Donovan CM. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31(1):32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer PE. Glucose counterregulation: Prevention and correction of hypoglycemia in humans. Am J Physiol. 1993;264(2 Pt 1):E149–E155. doi: 10.1152/ajpendo.1993.264.2.E149. [DOI] [PubMed] [Google Scholar]
- 4.Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: Making ends meet. Front Syst Neurosci. 2014;8:236. doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivitz WI, et al. Effect of acute and antecedent hypoglycemia on sympathetic neural activity and catecholamine responsiveness in normal rats. Diabetes. 2001;50(5):1119–1125. doi: 10.2337/diabetes.50.5.1119. [DOI] [PubMed] [Google Scholar]
- 6.Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes. 2011;60(2):602–606. doi: 10.2337/db10-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund ED, et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17(7):911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabavi S, et al. Engineering a memory with LTD and LTP. Nature. 2014;511(7509):348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487(7406):183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86(2):360–373. doi: 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 14.Scheurink A, Ritter S. Sympathoadrenal responses to glucoprivation and lipoprivation in rats. Physiol Behav. 1993;53(5):995–1000. doi: 10.1016/0031-9384(93)90279-o. [DOI] [PubMed] [Google Scholar]
- 15.Vollmer RR, Balcita JJ, Sved AF, Edwards DJ. Adrenal epinephrine and norepinephrine release to hypoglycemia measured by microdialysis in conscious rats. Am J Physiol. 1997;273(5 Pt 2):R1758–R1763. doi: 10.1152/ajpregu.1997.273.5.R1758. [DOI] [PubMed] [Google Scholar]
- 16.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994;14(1):310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachamp PM, Liu Y, Liu SJ. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1alpha signaling. J Neurosci. 2009;29(2):381–392. doi: 10.1523/JNEUROSCI.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18(6):995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 19.Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23(1):159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146(6):992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nankova BB, Rivkin M, Kelz M, Nestler EJ, Sabban EL. Fos-related antigen 2: Potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J Neurosci. 2000;20(15):5647–5653. doi: 10.1523/JNEUROSCI.20-15-05647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz S, Siemer H, Krug M, Höllt V. Direct evidence for biphasic cAMP responsive element-binding protein phosphorylation during long-term potentiation in the rat dentate gyrus in vivo. J Neurosci. 1999;19(13):5683–5692. doi: 10.1523/JNEUROSCI.19-13-05683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed T, Frey JU, Korz V. Long-term effects of brief acute stress on cellular signaling and hippocampal LTP. J Neurosci. 2006;26(15):3951–3958. doi: 10.1523/JNEUROSCI.4901-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiremagalur B, Sabban EL. Nicotine elicits changes in expression of adrenal catecholamine biosynthetic enzymes, neuropeptide Y and immediate early genes by injection but not continuous administration. Brain Res Mol Brain Res. 1995;32(1):109–115. doi: 10.1016/0169-328x(95)00068-4. [DOI] [PubMed] [Google Scholar]
- 25.Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31(5):819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Wang M, Whim MD. Neuropeptide Y gates a stress-induced, long-lasting plasticity in the sympathetic nervous system. J Neurosci. 2013;33(31):12705–12717. doi: 10.1523/JNEUROSCI.3132-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabner CP, Price SD, Lysakowski A, Fox AP. Mouse chromaffin cells have two populations of dense core vesicles. J Neurophysiol. 2005;94(3):2093–2104. doi: 10.1152/jn.00316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247(1 Pt 1):E35–E40. doi: 10.1152/ajpendo.1984.247.1.E35. [DOI] [PubMed] [Google Scholar]
- 29.Henion PD, Landis SC. Asynchronous appearance and topographic segregation of neuropeptide-containing cells in the developing rat adrenal medulla. J Neurosci. 1990;10(9):2886–2896. doi: 10.1523/JNEUROSCI.10-09-02886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavadas C, et al. Deletion of the neuropeptide Y (NPY) Y1 receptor gene reveals a regulatory role of NPY on catecholamine synthesis and secretion. Proc Natl Acad Sci USA. 2006;103(27):10497–10502. doi: 10.1073/pnas.0600913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whim MD. Near simultaneous release of classical and peptide cotransmitters from chromaffin cells. J Neurosci. 2006;26(24):6637–6642. doi: 10.1523/JNEUROSCI.5100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29(14):4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho MW, Beck-Sickinger AG, Colmers WF. Neuropeptide Y(5) receptors reduce synaptic excitation in proximal subiculum, but not epileptiform activity in rat hippocampal slices. J Neurophysiol. 2000;83(2):723–734. doi: 10.1152/jn.2000.83.2.723. [DOI] [PubMed] [Google Scholar]
- 34.Dubois CJ, Ramamoorthy P, Whim MD, Liu SJ. Activation of NPY type 5 receptors induces a long-lasting increase in spontaneous GABA release from cerebellar inhibitory interneurons. J Neurophysiol. 2012;107(6):1655–1665. doi: 10.1152/jn.00755.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spanswick DC, Simonds SE, Cowley MA. Transmitter time: Synaptic plasticity and metabolic memory in the hypothalamus. Cell Metab. 2012;15(3):275–276. doi: 10.1016/j.cmet.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146(3):1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73(3):511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternson SM. Hypothalamic survival circuits: Blueprints for purposive behaviors. Neuron. 2013;77(5):810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landsberg L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26(4-6):497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu CA, et al. Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest. 1997;99(5):1044–1056. doi: 10.1172/JCI119232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurose T, et al. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. Am J Physiol. 1990;258(1 Pt 1):E220–E227. doi: 10.1152/ajpendo.1990.258.1.E220. [DOI] [PubMed] [Google Scholar]
- 42.Taborsky GJ, Jr, Mundinger TO. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology. 2012;153(3):1055–1062. doi: 10.1210/en.2011-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab. 2009;297(1):E231–E235. doi: 10.1152/ajpendo.00222.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 45.Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977;196(4297):1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 46.Hücking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111(2):257–264. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1763–R1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- 48.Orban BO, Routh VH, Levin BE, Berlin JR. Direct effects of recurrent hypoglycaemia on adrenal catecholamine release. Diab Vasc Dis Res. 2015;12(1):2–12. doi: 10.1177/1479164114549755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381(6581):415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 50.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 51.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]