Significance
Almost all bacteria require Fe(III) for survival and growth. To compete successfully for this essential nutrient, bacteria developed very efficient Fe(III) uptake mechanisms based on high-affinity Fe(III) chelators, so-called siderophores. To gain a competitive advantage, many bacteria have evolved to scavenge and effectively poach siderophores from other species. Enterobactin, one of the strongest Fe(III) chelators known, is produced and secreted by many enteric bacteria. We show that a key protein involved in Fe(III) uptake in the foodborne pathogen Campylobacter jejuni is adapted to scavenge enterobactin hydrolysis products, a strategy that may enable the pathogen to more efficiently exploit siderophores produced by other bacteria and hence their resources.
Keywords: siderophore, Fe(III) uptake, periplasmic binding protein, enterobactin, Campylobacter jejuni
Abstract
To acquire essential Fe(III), bacteria produce and secrete siderophores with high affinity and selectivity for Fe(III) to mediate its uptake into the cell. Here, we show that the periplasmic binding protein CeuE of Campylobacter jejuni, which was previously thought to bind the Fe(III) complex of the hexadentate siderophore enterobactin (Kd ∼ 0.4 ± 0.1 µM), preferentially binds the Fe(III) complex of the tetradentate enterobactin hydrolysis product bis(2,3-dihydroxybenzoyl-l-Ser) (H5-bisDHBS) (Kd = 10.1 ± 3.8 nM). The protein selects Λ-configured [Fe(bisDHBS)]2− from a pool of diastereomeric Fe(III)-bisDHBS species that includes complexes with metal-to-ligand ratios of 1:1 and 2:3. Cocrystal structures show that, in addition to electrostatic interactions and hydrogen bonding, [Fe(bisDHBS)]2− binds through coordination of His227 and Tyr288 to the iron center. Similar binding is observed for the Fe(III) complex of the bidentate hydrolysis product 2,3-dihydroxybenzoyl-l-Ser, [Fe(monoDHBS)2]3−. The mutation of His227 and Tyr288 to noncoordinating residues (H227L/Y288F) resulted in a substantial loss of affinity for [Fe(bisDHBS)]2− (Kd ∼ 0.5 ± 0.2 µM). These results suggest a previously unidentified role for CeuE within the Fe(III) uptake system of C. jejuni, provide a molecular-level understanding of the underlying binding pocket adaptations, and rationalize reports on the use of enterobactin hydrolysis products by C. jejuni, Vibrio cholerae, and other bacteria with homologous periplasmic binding proteins.
With the rapid rise in bacterial resistance to antibiotics, a better understanding of cooperative behavior in microbial communities is urgently needed for the development of novel approaches to controlling infections caused by resistant bacteria (1, 2). As an essential nutrient, iron is often a growth-limiting factor for beneficial, commensal, and pathogenic bacteria alike, not only due to its low solubility in water under aerobic conditions at and around neutral pH, but also because the host organism and competing microbes actively limit its availability (3, 4). Microorganisms evolved efficient Fe(III) uptake mechanisms to overcome this challenge, a common strategy being the production of siderophores, small Fe(III)-chelating molecules with high affinity and selectivity for Fe(III), with over 500 examples known to date (5). The sharing of siderophores is a recognized example for positive cooperativity that has been linked to bacterial virulence (6). The best-characterized siderophores are hexadentate ligands that form coordinatively saturated, octahedral 1:1 complexes with Fe(III) (3, 5, 7), the most studied being the triscatecholate enterobactin (H6-ENT) produced by many enteric bacteria (8).
In Escherichia coli, enterobactin is synthesized within the cell and secreted through the cell membranes to capture Fe(III) from the environment. The resulting Fe(III)-enterobactin complex [Fe(ENT)]3− is recognized by the outer membrane receptor FepA and actively transported into the periplasm. In the periplasm, [Fe(ENT)]3− is sequestered by the periplasmic binding protein (PBP) FepB, which transfers it to an inner membrane transporter for further transport into the cytoplasm (9). Once there, [Fe(ENT)]3− is hydrolyzed by an intracellular esterase to release Fe(III) for use in the cell (8).
Along with the development of structurally diverse siderophores, microorganisms adapted their associated receptor and transport proteins for the uptake of the appropriate Fe(III) complexes (9). To gain a competitive advantage, many bacteria have evolved to poach siderophores produced by other bacteria. Campylobacter jejuni, for example, does not itself produce siderophores yet possesses an uptake system that is able to use siderophores from competing species (10). Initially, it was proposed that in C. jejuni [Fe(ENT)]3− is transported across the outer membrane by the receptors CfrA and CfrB (11). Once in the periplasm, [Fe(ENT)]3− was proposed to bind to the PBP CeuE, the resulting complex enabling the transport of the ferric siderophore into the cytoplasm (12, 13).
In addition, increasing numbers of lower-denticity siderophores are being isolated from bacterial cultures and found to coordinate Fe(III) and mediate its uptake (14–18). For example, the trilactone backbone of enterobactin makes it prone to hydrolysis, and although this lability is necessary to allow the intracellular release of Fe(III) from the siderophore, it in addition leads to its slow degradation in aqueous media (7, 19–21). Three hydrolysis products are formed: tris(2,3-dihydroxybenzoyl-l-Ser) (H7-trisDHBS), bis(2,3-dihydroxybenzoyl-l-Ser) (H5-bisDHBS), and 2,3-dihydroxybenzoyl-l-Ser (H3-monoDHBS), with all three found in the growth medium of E. coli (Fig. 1). Although enterobactin, once secreted, is also available to other cells (producers or nonproducers), it can only be used once because Fe(III) release requires its hydrolysis. The enterobactin hydrolysis products, however, could be used again as secondary, lower-denticity siderophores.
Fig. 1.
Molecular structure of enterobactin, its hydrolysis products, the siderophore mimic H6-MECAM, and a selection of tetradentate siderophores.
It has been demonstrated that the human pathogens C. jejuni (10, 22, 23) and Vibrio cholerae (24), the causes of food poisoning and cholera, respectively, can use enterobactin hydrolysis products for the uptake of Fe(III) from their environment. Both are known not to produce enterobactin.
An alternative Campylobacter Fe(III) acquisition model that relies on these linear hydrolysis products was recently proposed based on the finding that Cee, the sole trilactone esterase of C. jejuni and Campylobacter coli, is located in the periplasm, i.e., these bacteria are unable to degrade enterobactin within the cytoplasm (25). The model suggests that, once the Fe(III) complex of enterobactin enters the periplasm, its ester backbone is cleaved by Cee, which is highly efficient in hydrolyzing both the Fe(III) complex and apo-enterobactin. The resulting hydrolysis products, mainly the tetradentate ligand bisDHBS5− and the bidentate ligand monoDHBS3−, are then used to mediate the subsequent transport of Fe(III) into the cytoplasm.
The identification of the esterase Cee and in particular the observation that bisDHBS5− can be used independently of enterobactin (25) raise important questions about the siderophore preference of the PBP CeuE and its role in the iron uptake in C. jejuni. By using siderophore mimics, we previously demonstrated that CeuE can bind the Fe(III) complexes of both hexadentate and tetradentate catecholate ligands (26, 27). Our cocrystal structures revealed that CeuE interacts with the coordinatively saturated Fe(III) complex of the hexadentate mimic MECAM6− through electrostatic interactions and hydrogen bonding, whereas it binds the coordinatively unsaturated complex of the tetradentate mimic 4-LICAM4− by recruiting the side chains of two amino acid residues (His227 and Tyr288) to complete the coordination sphere of the Fe(III) center. We established that His227 and Tyr288 are conserved among a subset of related PBPs, including VctP from V. cholerae, and suggested that this subset of PBPs undergo similar structural changes to adapt to the binding of lower-denticity sidero-phores (27). The recent report that V. cholerae most efficiently uses trisDHBS7− and bisDHBS5− for the acquisition of Fe(III) provided a partial confirmation of this prediction (24).
Here, we report that CeuE binds [Fe(bisDHBS)]2− with much higher affinity than the Fe(III) complex of enterobactin, reveal the structural basis for this difference in binding strength, and examine key aspects of the relevant Fe(III) coordination chemistry in solution.
Results
Crystal Structures of CeuE-[Fe(bisDHBS)]2− and CeuE-[Fe(monoDHBS)2]3−.
A previously reported total synthesis of enterobactin was adapted to synthesize H5-bisDHBS, with modifications aimed at simplifying the procedures (28). The Fe(III) complex of bisDHBS5− was soaked into apo-CeuE crystals, grown as reported previously (27). Experimental details for the preparation of the Fe(III) complex of bisDHBS5−, crystal growth conditions, data collection, and refinement are provided in Supporting Information. Crystallographic statistics and a discussion of unit cell dimensions and average B values can be found in Supporting Information (Table S1).
Table S1.
Crystallographic statistics
| Structure | ||
| Crystallographic parameters | {(CeuE)3-[Fe(bisDHBS)]2−2[Fe]} Crystal II | {(CeuE)3[Fe(bisDHBS)]2−[Fe(DHBS)2]3−2} Crystal III |
| Data collection | ||
| Beamline | Diamond I04-1 | Diamond I02 |
| Wavelength, Å | 0.920 | 0.979 |
| Space group | P1 | P1 |
| Cell parameters, Å | a = 58.46, b = 63.02, c = 67.01 | a = 58.07, b = 63.09, c = 67.16 |
| Cell parameters, ° | α = 83.36, β = 76.57, γ = 78.24 | α = 83.09, β = 76.90, γ = 79.21 |
| Resolution range, Å | 65.01–1.90 | 65.19–2.10 |
| Number of reflections | 308,233 | 107,607 |
| Unique reflections | 70,279 | 51,094 |
| Monomers in AU | 3 | 3 |
| Completeness, %* | 98.1 (96.1) | 96.3 (95.4) |
| I/I(σ) | 17.5 (2.2) | 7.7 (1.7) |
| CC(1/2) | 0.996 (0.683) | 0.957 (0.541) |
| Average multiplicity | 4.4 (4.2) | 2.1 (2.1) |
| Rmerge, %† | 9.5 (89.5) | 9.1 (80.7) |
| Refinement statistics | ||
| %age Rfree reflections | 5 | 5 |
| (%)Rcryst = Σ∣∣Fo∣−∣Fc∣ ∣/Σ∣Fo∣, % | 22.7 | 21.8 |
| Free R factor, % | 26.4 | 25.9 |
| Bond distances,‡ Å | 0.015 (0.019) | 0.013 (0.019) |
| Bond angles, ° | 1.775 (1.995) | 1.653 (2.001) |
| Chiral centers, Å3 | 0.104 (0.200) | 0.096 (0.200) |
| Planar groups, Å | 0.010 (0.021) | 0.009 (0.021) |
| Average B values | ||
| Main chain, Å2 | 41.0 | 29.7 |
| Side chain B, Å2 | 45.1 | 33.7 |
| Ramachandran plot | ||
| Preferred regions, % | 95.3 | 95.4 |
| Allowed regions, % | 3.9 | 4.0 |
| Outliers, % | 0.8 | 0.6 |
| PDB ID code | 5ADW | 5ADV |
Values in parentheses correspond to the highest resolution shell.
Rmerge is defined as 100 × Σ∣I − <I>∣/ Σ I, where I is the intensity of the reflection.
rms deviations from ideal geometry (target values are given in parentheses).
Three crystals were selected; crystal I was soaked for 90 min, crystal II for 24 h (PDB ID code 5ADW), and crystal III for 11 d (PDB ID code 5ADV). As expected, all three crystals were in space group P1 with three protein monomers per asymmetric unit, consistent with the apo-CeuE structure (PDB ID code 3ZKW) (27). Crystal I showed no additional electron density in the CeuE binding pockets, indicating that the 90-min soaking time was too short.
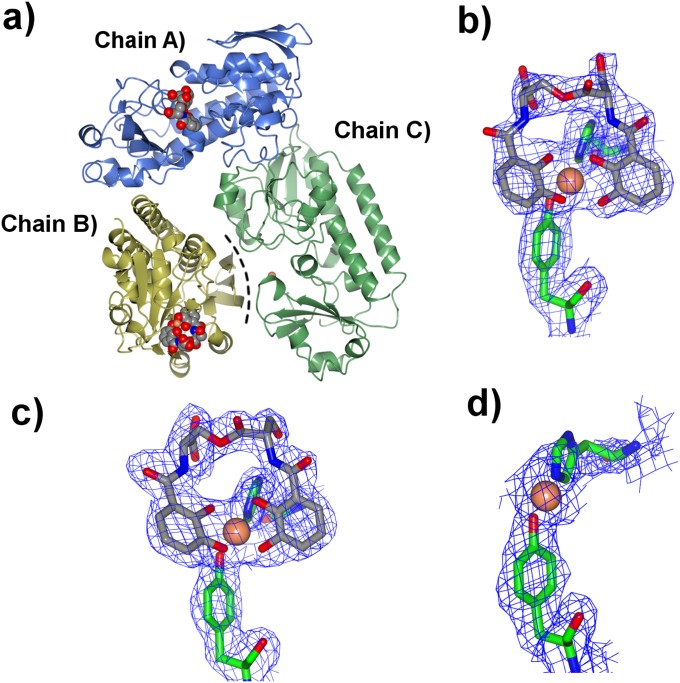
Crystal II has additional electron density in the binding pockets of each of the three independent CeuE monomers, which was modeled as [Fe(bisDHBS)]2− in chains A and B (Fig. 2 and Fig. S1). CeuE binds a single [Fe(bisDHBS)]2− species per monomer in an Fe(III):siderophore:protein ratio of 1:1:1. BisDHBS5− chelates the Fe(III) center in a tetradentate fashion with the four catecholate oxygen donors coordinated. The remaining two coordination sites of the distorted octahedral Fe(III) center are occupied by donor atoms provided by the side chains of His227 and Tyr288. In addition, electrostatic interactions and hydrogen bonds involving residues Arg118, Arg205, Arg249, and Lys121 contribute to the binding (Fig. 3). The diserine linker in bisDHBS5− forces the oxygen donors in the ortho and meta position to the amide groups on the catechol units to occupy adjacent coordination sites on the Fe(III) center. Consequently, the remaining two coordination sites are not equivalent. The site opposite the weaker catecholate donor (ortho position) is occupied by the oxygen donor of Tyr288, whereas that opposite the stronger oxygen donor (meta position) is occupied by the nitrogen donor of His227, consistent with their respective trans-influence (29). Although the observed Fe(III) binding mode is similar to that which we reported previously for the C2-symmetric siderophore mimic 4-LICAM4− (PDB ID code 5A1J) (27), the diserine backbone of bisDHBS5− plays a key role in determining the orientation of the unsymmetrical natural siderophore. In both chains A and B, the carboxylate group in the backbone of bisDHBS5− provides an additional negative charge and accepts a hydrogen bond from Arg249 (Fig. 3). This high degree of complementarity indicates that the binding pocket of CeuE is perfectly suited to the binding of the tetradentate ligand.
Fig. 2.
(A) Ribbon representation of the three independent CeuE chains in the unit cell of crystal II (PDB ID code 5ADW) with a black dashed line representing steric clash of chain B N terminus with the binding pocket of chain C. (B–D) Cylinder representation of the different ligand binding orientations found in crystals II and III with electron density shown as 2Fobs-Fcalc, contoured at 1σ: (B) crystal II chain B; (C) crystal III chain A; and (D) crystal III chain C. Ligands are shown as cylinders: gray, carbon; blue, nitrogen; red, oxygen; and iron, coral; and Arg, Tyr, and His side chains are shown as cylinders with carbon atoms colored as their ribbons.
Fig. S1.
(A) Ribbon representation of the three independent CeuE chains in the unit cell of {(CeuE)3-[Fe(bisDHBS)]2−2[Fe]} (crystal II; PDB ID code 5ADW) with black dashed line representing steric clash of chain B N terminus with the binding pocket of chain C. (B–D) Cylinder representation of the ligands bound in each of the three binding pockets with electron density map shown as 2Fobs-Fcalc, contoured at 1σ: (B) chain A; (C) chain B; and (D) chain C. Ligands are shown as cylinders: gray, carbon; blue, nitrogen; red, oxygen; and iron, coral.
Fig. 3.
(A) Overlay of the three independent [Fe(bisDHBS)]2− units and key residues in the respective substrate binding pocket of CeuE shown as cylinders with the carbon atoms colored according to chain: crystal II chains A (blue) and B (gold), crystal III chain B (green), dashed H bonds exemplified for crystal II chain B. (B) Schematic diagram of key hydrogen-bonding interactions (charges omitted for clarity).
In the binding pocket of chain C, there was much less density and it was modeled as a half-occupied Fe(III) center with no coordinating ligand. The anomalous difference Fourier maps confirm a peak of about 50% of that seen for the Fe(III) in chains A and B. There is some residual density around the Fe(III), but it was not possible to satisfactorily model this as bisDHBS5− or monoDHBS3−. Although the binding pockets of chains A and B are both open to solvent, access to the pocket of chain C is restricted by the N terminus of chain B. The resulting steric constraints explain the only partial occupation of this pocket (Fig. 2A).
Crystal III has similar cell dimensions and packing to crystal II (Table S2). There is electron density in each of the three binding pockets (Figs. S1 and S2), the density is weak in the region corresponding to the backbone of bisDHBS5− (below the 1σ level), suggesting that the di-serine ester-linkage of bisDHBS5− was highly mobile or had hydrolyzed or a combination of both. The best fit to the density was attained when chain B was modeled with [Fe(bisDHBS)]2− bound while chains A and C were modeled with two monoDHBS3− ligands coordinating the Fe(III) center (Fig. 2). Remarkably, the orientation of the monoDHBS3− ligands in chain C differed from that in chain A. In A, the oxygen donors in both the ortho and meta position of the catecholamide units occupy adjacent coordination sites on the Fe(III) center, as seen in [Fe(bisDHBS)]2−. In contrast, in chain C, one of the monoDHBS3− ligands was in a flipped position, with the attached serine of the backbone pointing into the binding pocket. Here, the donors in the ortho position of the two catecholamide units coordinate trans to one another, whereas the donors in the meta position remain coordinated cis to one another. In this isomer, His227 and Tyr288 occupy coordination sites trans to the catecholate oxygen donor atoms in the meta position (Fig. 2). This change in orientation of the ligands is likely to be due to steric constraints imposed by the N terminus of chain B (Fig. S3). Crystal III thus reveals two alternative binding modes for the Fe(III) complex of the monomeric enterobactin hydrolysis product monoDHBS3−.
Table S2.
Comparison of crystallographic statistics of apo-CeuE, Crystal II, and Crystal III
| Structure | |||
| Crystallographic parameters | apo-CeuE | {(CeuE)3-[Fe(bisDHBS)]2−2[Fe]} Crystal II | {(CeuE)3[Fe(bisDHBS)]2−[Fe(DHBS)2]3−2} Crystal III |
| Average B value for whole chain | |||
| Chain A, Å2 | 29.3 | 39.1 | 30.4 |
| Chain B, Å2 | 28.8 | 49.1 | 33.5 |
| Chain C, Å2 | 30.2 | 41.0 | 30.5 |
| Cell parameters | |||
| Cell parameters, Å | a = 56.95, b = 62.74, c = 67.98 | a = 58.46, b = 63.02, c = 67.01 | a = 58.07, b = 63.09, c = 67.16 |
| Cell parameters, ° | α = 82.19, β = 76.74, γ = 75.96 | α = 83.36, β = 76.57, γ = 78.24 | α = 83.09, β = 76.90, γ = 79.21 |
| Cell volume, Å3 | 228,507 | 234,627 | 234,495 |
| PDB ID code | 3ZKW | 5ADW | 5ADV |
Fig. S2.
(A) Ribbon representation of the three independent CeuE chains in the unit cell of {(CeuE)3[Fe(bisDHBS)]2−[Fe(monoDHBS)2]3−2} (crystal III; PDB ID code 5ADV). (B–D) Cylinder representation of the three binding pockets with electron density map shown as 2Fobs-Fcalc, contoured at 1σ: (B) chain A; (C) chain B; and (D) chain C. Ligands are shown as cylinders: gray, carbon; blue, nitrogen; red, oxygen; and iron, coral.
Fig. S3.
Worm representation, scaled by B value, of the three independent CeuE chains in the unit cell of {(CeuE)3[Fe(bisDHBS)]2−[Fe(monoDHBS)2]3−2} (crystal III; PDB ID code 5ADV).
In crystals II and III, the Fe(III) complexes are bound with the metal center in the Λ-configuration, as previously observed in the cocrystal structures of (CeuE)2-[{Fe(MECAM)}2]6− (26), CeuE-[Fe(4-LICAM)]− (27), FeuA-[Fe(bacillibactin)]3− (30), FeuA-[Fe(MECAM)]3−, and FeuA-[Fe(ENT)]3− (31).
Relevant Fe(III) Coordination Chemistry in Solution.
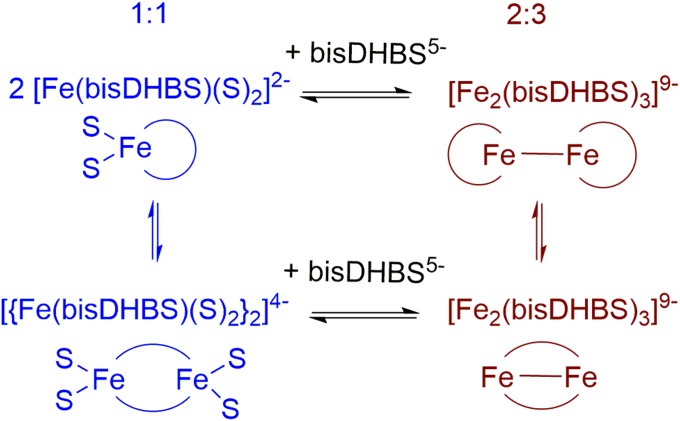
The identification of the Λ-configured 1:1 complex [Fe(bisDHBS)]2− in the cocrystal structures prompted us to investigate whether this complex is also the predominant species formed in solution. Due to the mismatch between the four donor atoms provided by the two chelating catechol units of bisDHBS5− and the preferred sixfold coordination environment of Fe(III), the composition and structure of the complex(es) formed have long been a matter of debate. Based on a UV-visible absorbance spectroscopic study carried out in aqueous solution at pH 9, it was suggested that bisDHBS5− forms a 1:1 complex with Fe(III) under these conditions and that the remaining two coordination sites are occupied by solvent molecules (15). In contrast, NMR spectroscopic investigations carried out in d6-DMSO with Ga(III) as a diamagnetic substitute for Fe(III) indicated a metal-to-ligand ratio of 2:3. Consequently, the formation of a dinuclear complex of composition {Fe2(bisDHBS)3} was proposed (32).
Stoichiometric control allowed the isolation of two different Fe(III) complexes of a tetradentate siderophore mimic that consists of two ethyl-bridged catecholamides. A metal-to-ligand ratio of 2:3 resulted in the crystallization of a dinuclear triple-stranded helicate of composition [Fe2(L)3]6−, whereas a basic solution with metal-to-ligand ratio of 1:1 produced crystals of hydroxo-bridged dinuclear complexes of composition [{Fe(L)(OH)}2]4− (29).
We carried out experiments to rationalize these observations and related the results to biologically relevant conditions.
To probe the metal-to-ligand ratio, we used Job’s method of continuous variation (33, 34), monitoring complex formation by both electronic absorbance and CD spectroscopies. The spectroscopic measurements were carried out in aqueous solution [5% (vol/vol) DMSO] at pH 7.5 using Fe(III) nitrate as the Fe(III) source. Nitrilotriacetic acid (NTA) was added to prevent the precipitation of Fe(III) hydroxides. Control experiments ascertained that the presence of NTA does not interfere with the reaction of Fe(III) with bisDHBS5− under these conditions.
Electronic absorbance spectra were recorded at selected metal-to-ligand ratios in the region of the ligand-to-metal charge transfer (LMCT) band of [Fe(bisDHBS)]2− (Fig. 4A). The maximum of the LMCT band shifts significantly upon variation of the metal-to-ligand ratio. A red complex forms under conditions of excess ligand. The λmax at ∼510 nm is consistent with the formation of a tris(catecholate) species (35, 36). When the metal-to-ligand ratio approaches 1:1, the color of the solution changes from red to purple. The observed λmax of around 565 nm is consistent with the formation of a bis(catecholate) species (36, 37). The Job plot confirms that a λmax around 510 nm is consistent with a metal-to-ligand ratio of 2:3, whereas a λmax around 565 nm is consistent with a metal-to-ligand ratio of 1:1 (Fig. 4B).
Fig. 4.
(A) Selected electronic absorbance spectra [colors from black to light blue ordered by metal-to-ligand ratio as indicated; [M] + [L] = 0.4 mM; 0.1 M Tris⋅HCl with 5% (vol/vol) DMSO; pH 7.5]. (B) Job plot for the reaction of H5-bisDHBS with Fe(III), obtained by following the absorbance at 512 nm (red triangles) and 563 nm (blue circles). The absorbance values are averages of two experiments (error bars indicate the difference between the runs).
In summary, the electronic absorbance spectra demonstrate that in aqueous solution near neutral pH, both 1:1 and 2:3 complexes are formed upon reaction of Fe(III) with H5-bisDHBS (Fig. S4), with the species distribution being controlled by stoichiometry.
Fig. S4.
Conceivable species formed upon reaction of Fe(III) with H5-bisDBHS in aqueous solution at pH 7.5 (charges assigned assuming deprotonation of the two catechol units and the carboxylic acid group of H5-DHBS; S, solvent).
Similarly, the visible region CD spectra vary with the metal-to-ligand ratio (Fig. 5A). At metal-to-ligand ratios of 3:2 and 1:1, the CD spectra show an intense positive band around 320 nm, a weaker positive band around 410 nm, and a weak bisignate band centered at 595 nm, which is near the LMCT absorption maximum of the 1:1 species. Scarrow et al. (15) reported a similar CD spectrum for [Fe(bisDHBS)]2− isolated from growth cultures and assigned the intense near-UV band to ligand-based transitions and the weaker visible region features to LMCT transitions. Because the latter are indicative of the chirality at the Fe(III) center, the low intensity of the signal indicates that the chiral induction by the l-serine residues in the backbone is only weak.
Fig. 5.
(A) CD spectra of solutions of H5-bisDHBS or Fe(III) plus H5-bisDHBS at key metal-to-ligand ratios [0.1 M Tris⋅HCl buffer with 5% (vol/vol) DMSO, pH 7.5, 150 mM NaCl, [M] + [L] = 0.4 mM]. (B and C) CD spectra of solutions of Fe(III) plus H5-bisDHBS (B) or Fe(III) plus enterobactin (C) recorded after addition of increasing increments of CeuE as indicated (0.1 M Tris⋅HCl buffer, pH 7.5, 150 mM NaCl).
Upon changing the metal-to-ligand ratio to 2:3, the maximum of the near-UV band shifts from 320 to 340 nm and the visible region now shows a bisignate band with a positive signal at 415 nm and a negative signal at 550 nm, consistent with a negative Cotton effect. The position of the zero CD crossover at 503 nm is close to the LMCT absorption maximum (∼510 nm) of the Fe(III)-bisDHBS5− 2:3 species. The resulting CD spectrum is characteristic of a solution of Fe(III) tris(catecholamide) complexes in which the Δ-configuration dominates (38). This characteristic change in the CD profile coincides with the color change from purple to red reflected in the electronic absorbance spectra (Fig. 4A). The concomitant increase in amplitude is consistent with the increase in the number of coordinated catechol units, not only because each is expected to contribute to the chiral induction effect, but also due to the pairwise additivity of exciton-coupled chromophores (39). The effect is also consistent with the diastereoselective formation of a dinuclear triple helicate, in which the enantiomerically pure ligand predetermines the helical direction and hence the chirality of both metal centers, as seen in similar supramolecular assemblies (40, 41).
Similar observations were reported for the amonabactins, a family of bis(catecholate) siderophores in which the catecholate units are attached to the Nε atom of lysine residues present in their peptide-based backbones (16, 42, 43). However, the increase in Cotton effect upon decrease of the metal-to-ligand ratio from 1:1 to 2:3 is more pronounced with H5-bisDHBS than for the amonabactins. This is probably due to a stronger chiral induction from the Cα of the serine backbone in the linear dimer, which is closer to the Fe(III) center than the Cα of the lysine residues in the backbone of the amonabactins (Fig. 1).
Interactions Between CeuE and the Fe(III) Complexes of Siderophores in Solution.
CD.
To probe the stereochemistry of the Fe(III) center in the presence of CeuE in solution, CD spectra were recorded in the wavelength range of the LMCT band. Series of spectra were obtained during a titration of a solution of CeuE into solutions of Fe(III) plus H5-bisDHBS or Fe(III) plus enterobactin in buffer (pH 7.5). The titrations allowed the changes in spectral features upon binding of the respective Fe(III) siderophore complex to CeuE to be observed (Fig. 5 B and C).
The first CD spectrum was obtained after addition of Fe(III) and H5-bisDHBS to the buffer, followed by equilibration. It confirms that, in the absence of CeuE, the l-serine backbone in bisDHBS5− has only a weak influence on the stereochemistry at the Fe(III) center, with Δ-configured complexes in slight excess.
The spectra recorded upon addition of CeuE indicate inversion of configuration at the metal center with isodichroic points at 305 and 544 nm. The positive band at 320 nm changes into a negative band at 330 nm. The weak positive signal across the LMCT range develops into a broad negative band with a minimum at 395 nm and a positive band with a maximum at 600 nm, consistent with a Λ-configured Fe(III) center (38). The series of spectra confirms that CeuE selects for Λ–configured [Fe(bisDHBS)]2− and shows that the diastereoisomers that remain in solution are able to reequilibrate quickly.
For comparison, an analogous titration was carried out with a solution containing Fe(III) and enterobactin (Fig. 5C). As expected from previous reports, the initial CD spectrum of [Fe(ENT)]3− is characteristic of Fe(III) tris(catecholamide) complexes with Δ-configuration (38). Upon addition of CeuE, the bands decrease in intensity, indicating a shift in the equilibrium between Δ- and Λ-configured complexes is caused by the Fe(III) complex of enterobactin binding to CeuE. However, even after addition of 1.6 equivalents of CeuE, Δ-configured [Fe(ENT)]3− still predominates.
The observed selection of Λ-configured complexes by CeuE indicates that the chiral l-serine backbone, which has a Δ-configuration–inducing effect, slightly hinders rather than facilitates protein binding. The resulting moderation of binding affinity is consistent with the intermediary role of the periplasmic binding protein within the iron transport pathway. A precise tuning of affinity is essential to allow the transfer of the cargo to the inner membrane transporter in the final step of the Fe(III) transport chain.
Fluorescence spectroscopy.
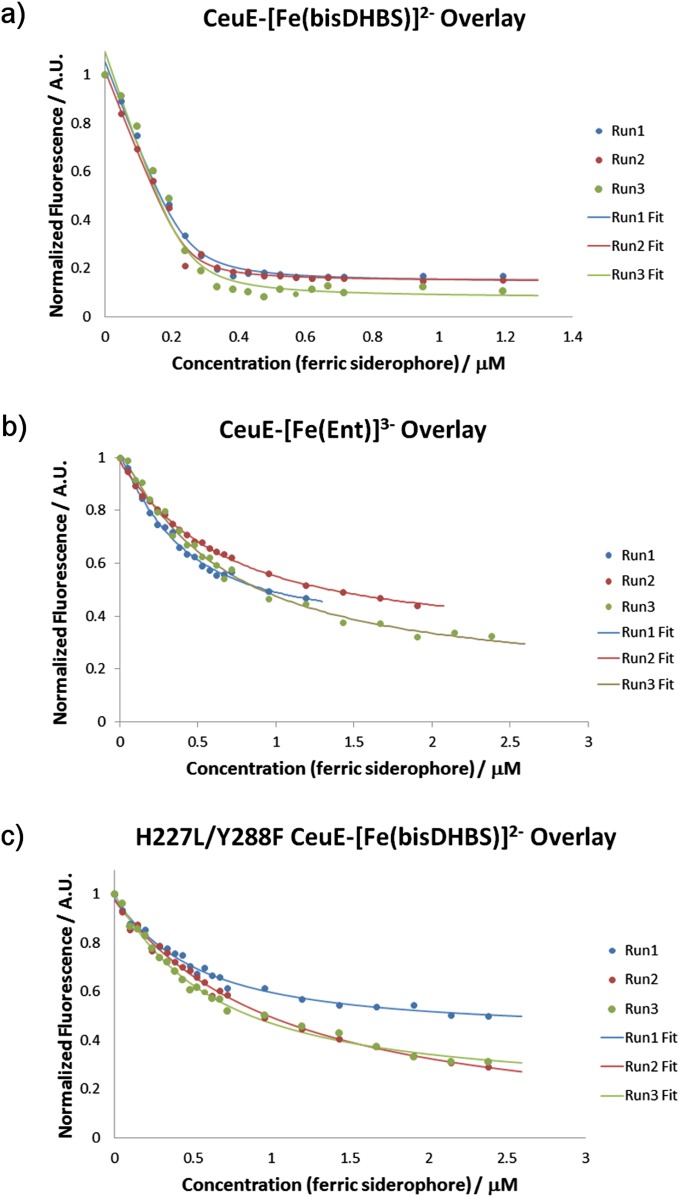
To allow a quantitative comparison of the binding strength, the affinity of CeuE for [Fe(bisDHBS)]2− and [Fe(ENT)]3− was determined using intrinsic fluorescence quenching experiments. Aliquots of the respective Fe(III)-siderophore solution (1:1 ratio, 12 μM in aqueous 40 mM Tris⋅HCl, 150 mM NaCl) were added successively to a solution of CeuE (240 nM in aqueous 40 mM Tris⋅HCl, 150 mM NaCl, pH 7.5) and the fluorescence intensity was measured after equilibration (λexc = 280, λem = 310–410 nm) (Fig. S5). The dissociation constants (Kd) were obtained from three independent measurements, as described in Supporting Information.
Fig. S5.
(A) Fluorescence quenching analyses of CeuE with [Fe(bisDHBS)]2−. Run 1, Kd = 12.6 ± 3.4 nM; run 2, Kd = 7.7 ± 2.9 nM; run 3, Kd = 12.3 ± 5.6 nM. (B) Fluorescence quenching analyses of CeuE with [Fe(ENT)]3−. Run 1, Kd ∼ 254 ± 35 nM; run 2, Kd ∼ 512 ± 31 nM; run 3, Kd ∼ 529 ± 54 nM. (C) Fluorescence quenching analyses of H227L/Y288F CeuE with [Fe(bisDHBS)]2−. Run 1, Kd ∼ 379 ± 49 nM; run 2, Kd ∼ 882 ± 90 nM; run 3 Kd ∼ 482 ± 47 nM. Titrations recorded with 240 nM CeuE in 40 mM Tris⋅HCl, pH 7.5, 150 mM NaCl; data shown as circles; lines give the nonlinear least-squares calculated fits.
Surprisingly, CeuE was found to bind [Fe(bisDHBS)]2− with much higher affinity (Kd = 10.1 ± 3.8 nM) than [Fe(ENT)]3− (Kd ∼ 0.4 ± 0.1 µM). This observation shows that the direct coordination of the His227 and Tyr288 side chains to the Fe(III) center more than compensates for the loss of electrostatic attraction caused by the decrease in negative charge upon moving from a hexadentate to a tetradentate catecholate siderophore.
To confirm the importance of His227 and Tyr288 for the binding affinity of CeuE to [Fe(bisDHBS)]2−, site-directed mutagenesis was used to replace the Fe(III)-coordinating amino acids with noncoordinating, yet sterically similar residues. As anticipated, mutation of His227 to leucine (H227L) and Tyr288 to phenylalanine (Y288F) resulted in a clear drop in binding affinity. The dissociation constant obtained with the double mutant (Kd ∼ 0.5 ± 0.2 µM) indicates weak binding, comparable to that observed for CeuE-[Fe(ENT)]3− (Kd ∼ 0.4 ± 0.1 µM). Both dissociation constants are about two orders of magnitude higher than that for CeuE-[Fe(bisDHBS)]2− (Kd = 10.1 ± 3.8 nM). In contrast, the dissociation constant obtained for CeuE-[Fe(bisDHBS)]2− is similar to those previously published for the binding of hexadentate tris(catecholate) siderophore complexes to PBPs, such as FeuA and FepB (10–50 nM) (30, 31, 44–46).
Discussion
Enterobactin is one of the most efficient mediators of bacterial Fe(III) uptake, and increasing evidence indicates that its hydrolysis products, in particular bisDHBS5−, play an important role in the competition for essential Fe(III) because they can be exploited by siderophore poachers, such as C. jejuni.
Our studies demonstrate that CeuE, the periplasmic binding protein of C. jejuni, selects Λ-[Fe(bisDHBS)]2− from a mixture of species in solution. In the absence of the protein, bisDHBS5− was found to coordinate to Fe(III) in metal-to-ligand ratios of both 1:1 and 2:3, depending on the relative concentrations of Fe(III) and siderophore present in solution. In both complexes, chiral induction from the Cα of the l-serine backbone causes the Fe(III) center(s) to adopt preferentially the Δ-configuration in aqueous buffer at pH 7.4, with weak (1:1 complex) to moderate (2:3 complex) diastereoselectivity. The ability of CeuE to select a defined Fe(III) complex from a mixture is consistent with its role in the Fe(III) transport process, where it functions as a periplasmic gatekeeper and is required to deliver the correct Fe(III) complex to the inner membrane transporter. CD spectra recorded during the titration of CeuE into a solution of [Fe(bisDBHS)]2− indicated an inversion of configuration from Δ to Λ upon addition of the protein. The effect is caused by CeuE driving the equilibrium by selecting for Λ-configured complexes; the two diastereomers that remain in solution reequilibrate quickly.
Of particular significance is the revelation that CeuE binds [Fe(bisDBHS)]2− ∼100 times more tightly than [Fe(ENT)]3−, with Kd = 10.1 ± 3.8 nM and Kd ∼ 0.4 ± 0.1 µM, respectively. This marked difference strongly suggests that [Fe(bisDHBS)]2− is the preferred ligand for the protein rather than [Fe(ENT)]3− as previously thought.
Our cocrystal structures provide molecular-level insights into the binding pocket adaptations that give rise to this pronounced difference in affinity and are the first (to our knowledge) to reveal the interactions of a natural tetradentate siderophore with its cognate PBP. In addition to electrostatic and hydrogen-bonding interactions between the binding pocket and [Fe(bisDHBS)]2−, the crystal structure showed the direct coordination of two amino acid side chains (His227 and Tyr288) to the Fe(III) center. In contrast, the binding of the Fe(III) complex of MECAM, the hexadentate enterobactin mimic, was found earlier to rely solely on electrostatic interactions and hydrogen bonding (26). The oxygen donor of Tyr288, which is directly coordinated in CeuE-[Fe(bisDHBS)]2−, is positioned 3.5 Å away from the metal center in (CeuE)2-[{Fe(MECAM)}2]6−, where it participates in hydrogen bonding. His227 is disordered forming part of a flexible loop in both the apo-CeuE and (CeuE)2-[{Fe(MECAM)}2]6− structures. Hence, we propose that His227 and Tyr288 enable CeuE to preferentially bind the tetradentate enterobactin hydrolysis product rather than intact hexadentate enterobactin. This proposal is supported by the observation that the replacement of His227 and Tyr288 with noncoordinating amino acids leads to a drastic drop in affinity for [Fe(bisDHBS)]2−.
There is an equivalent direct coordination of His227 and Tyr288 in the crystal structure of CeuE-[Fe(monoDHBS)2]3−, in which two monoDHBS3− ligands, the hydrolysis products of bisDHBS5−, coordinate to the Fe(III) center. In both crystals, the Fe(III) complexes are bound with the metal center in Λ-configuration.
A sequence homology search of reported 3D structures (Fig. S6) suggests that a subset of related Fe(III)-siderophore binding proteins shares a preference for the hydrolysis products of enterobactin over intact enterobactin. The PBP of the Vct uptake system in V. cholerae has a high similarity to CeuE and contains the coordinating histidine and tyrosine residues (27). Hence, our results rationalize the recent discovery that V. cholerae is able to use the linear derivatives of enterobactin, but not enterobactin itself (24).
Fig. S6.
Plot of Z scores in descending order for structures identified by the Dali server using chain A of the apo-CeuE structure (PDB ID code 3ZKW) as search model. Only a single chain was selected for each entry, and the apo plus one ligand complex where available for each protein. Duplicates are not shown.
We therefore propose that CeuE preferentially selects for [Fe(bisDHBS)]2− in the periplasm and mediates its uptake into the cell. As a result, the Fe(III) complex of enterobactin is retarded in the periplasm where it is exposed to esterase-catalyzed hydrolysis to its linear components. Because it has been reported that C. jejuni can use bisDHBS5− as a siderophore independently of enterobactin (10) and the discovery of the trilactone esterase Cee in the periplasm of C. jejuni (25), it appears that CeuE, previously considered to be a [Fe(ENT)]3− binding protein, is instead adapted to preferentially bind [Fe(bisDHBS)]2−. This ability to use the enterobactin hydrolysis product for Fe(III) uptake and hence to avoid the metabolic costs of siderophore production provides C. jejuni with a competitive advantage when it encounters enterobactin-secreting bacteria either within the host or contaminated water.
In contrast, in E. coli, the PBP FepB does not possess equivalent tyrosine and hisitidine residues and the esterase that cleaves the Fe(III) complex of enterobactin is located in the cytoplasm. Consequently, the Fe(III)-siderophore uptake systems of the siderophore scavenger C. jejuni and the siderophore producer E. coli show significant differences.
Taken together, these insights strongly suggest an important in vivo role for the tetradentate siderophore and its cognate PBP in the uptake of Fe(III) by Campylobacter and a number of other pathogenic bacteria.
Methods
Experimental details including general methods, compound synthesis (Fig. S7) and characterization, site-directed mutagenesis, protein crystallization, X-ray data collection, structure solution and refinement (Tables S1 and S2), and fluorescence quenching data (Figs. S5 and S8) can be found in Supporting Information.
Fig. S7.
Synthesis of H5-bisDHBS. Reagents and conditions: (a) Boc2O, NEt3, MeCN, reflux, 94%; (b) Boc-O-benzyl-l-serine, EDC, HOBt, MeCN, 0 °C to room temperature (RT), 60%; (c) TFA, CH2Cl2, RT, 89%; (d) 2,3-bis(benzyol)benzoyl chloride 5, DIPEA, CH2Cl2, RT, 44%; (e) H2, Pd-C 10%, EtOH, RT, 91%.
Fig. S8.
Weighted average and error calculation used for Kd determination.
SI Methods
Data Collection.
1H and 13C NMR spectra were recorded on a Jeol ECS 400 (400 MHz for 1H, 100 MHz for 13C) at ambient temperature. Chemical shifts are reported relative to residual solvent peaks [CDCl3 δH 7.26 δC 77.2, CD3OD δH 3.31 δC 49.0, (CD3)2SO δH 2.50 δC 39.5 ppm] and coupling constants (J) are given in hertz. Data are reported as follows: chemical shift (multiplicity, coupling constants, number of protons). Multiplicity abbreviations are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. High-resolution electrospray ionization mass spectra were recorded on a Bruker microTOF electrospray mass spectrometer. Infrared (IR) spectra were recorded on a Thermo Nicolet Avatar 370 FT-IR spectrophotometer using KBr pellets covering a wavenumber range of 4,000–1,000 cm−1 or on a PerkinElmer Spectrum Two (ATIR), covering a wavenumber range of 4,000–650 cm−1. Elemental analyses were carried out on an Exeter CE-440 elemental analyzer, and the results obtained are within ±0.4% of calculated values. The CD spectroscopic measurements were performed on a Jasco J810 CD spectropolarimeter at 20 °C under constant nitrogen flush, and the specific rotation was recorded on a Jasco DIP-370 digital polarimeter. Fluorescence spectra were recorded on a Hitachi F-4500 fluorescence spectrophotometer.
Chromatography.
Analytical TLC was performed using Merck silica gel 60 F253 aluminum-backed plates using specified solvent systems and visualized using a Chromato-vue Model CC-10 UV lamp. Flash column chromatography was carried out using Fluka Silica (pore size, 60 Å; 220–440 mesh; 35–75 μm).
Solvents and Reagents.
All solvents and reagents were used as supplied, unless otherwise stated.
SI Experimental Procedures
For a reaction scheme that illustrates the synthesis of H5-bisDHBS, please see Fig. S7.
l-Serine-N-[(1,1-dimethylethoxy)carbonyl]-O-(phenylmethyl)-ester (2).
To a stirred suspension of l-serine benzyl ester hydrochloride (1.15 g, 5 mmol) in acetonitrile (20 mL), triethylamine (0.60 mL, 6.0 mmol) was added and the resulting solution was allowed to stir for 10 min. Di-tert-butyl dicarbonate (1.15 g, 5 mmol) was then added and the mixture left to stir for 3 h at reflux. Once the reaction was completed, the solvent was removed in vacuo and the resulting oil dissolved in dichloromethane (50 mL). The solution was washed with formic acid (15 mL, 0.1 M) and water until the aqueous layer was at neutral pH (3 × 25 mL minimum). The organic layer was dried over MgSO4, filtered, and the solvent removed in vacuo, yielding a white solid (1.38 g, 94%). mp: 66.8–68.2 °C; TLC (CH2Cl2:MeOH, 9:1 vol/vol): Rf = 0.63; *1H NMR (400 MHz, CDCl3): δ 7.39–7.34 (m, 5H), 5.49 (br s, 1H), 5.23 (d, J = 12.5 Hz, 1H), 5.20 (d, J = 12.5 Hz, 1H), 4.42 (br s, 1H), 3.99 (dd, J = 11.0, 4.0 Hz, 1H), 3.91 (dd, J = 11.0, 4.0 Hz, 1H) 2.14 (br s, 1H), 1.44 (s, 9H); *13C NMR (100 MHz, CDCl3): δ 170.7, 155.7, 135.2, 128.6, 128.4, 128.1, 80.3, 67.3, 63.5, 55.8, 28.2; IR (KBr): 3,420, 3,365, 2,981, 2,936, 1,760, 1,700, 1,526, 1,368, 1,350, 1,203, 1,160, 1,087, 1,069 cm−1; *High-resolution mass spectrometry (HRMS) (m/z): [M+Na]+ calcd. for C15H21NO5Na, 318.1312; found, 318.1305; analysis (calcd., found for C15H21NO5): C(61.00, 61.14), H(7.17, 7.12), N(4.74, 4.63).
l-Serine-N-[(1,1-dimethylethoxy)carbonyl]-O-(phenylmethyl)-ester-O-l-serine, N-[(1,1-dimethylethoxy)carbonyl]-O-(phenylmethyl)-ether (3).
Boc-O-benzyl-l-serine (1.89 g, 6.4 mmol), compound 2 (1.72 g, 5.8 mmol), and HOBt (1.23 g, 9.1 mmol) were suspended in 10 mL of dry acetonitrile, and the mixture was stirred on ice. Once the mixture was cooled, EDC (1.41 g, 7.4 mmol) was added to the suspension, followed by the addition of 15 mL of acetonitrile. The mixture was stirred for 2 h on ice, followed by 48 h at room temperature. The volatiles were removed in vacuo and the remaining oil dissolved in dichloromethane (100 mL). The solution was washed with brine (50 mL) and saturated NaHCO3 solution (10 mL), followed by brine (2 × 50 mL). The organic layer was dried over MgSO4, filtered, and the solvent was removed in vacuo. The crude product was purified via column chromatography, 9:1 chloroform:acetonitrile, to yield a pure white solid (2.03 g, 60%). mp: 80.1–82.2 °C; TLC (CH3Cl:MeCN, 4:1 vol/vol): Rf = 0.47; †1H NMR (400 MHz, CDCl3): δ 7.37–7.27 (m, 10H), 5.50 (br d, J = 8.8 Hz, 1H), 5.30 (br d, J = 8.8 Hz, 1H), 5.21 (d, J = 12.4 Hz, 1H), 5.17 (d, J = 12.0 Hz, 1H), 4.68–4.56 (m, 3H), 4.49–4.43 (m, 2H), 4.35–4.31 (m, 1H), 3.72 (dd, J = 9.6, 3.6 Hz, 1H), 3.58 (dd, J = 9.6, 3.2 Hz, 1H) 1.43 (s, 9H), 1.40 (s, 9H); †13C NMR (100 MHz, CDCl3): δ 170.6, 170.0, 155.7, 155.7, 137.5, 135.4, 128.8, 128.8, 128.7, 128.6, 128.2, 128.0, 127.8, 80.2, 80.1, 73.2, 69.6, 67.5, 65.0, 53.8, 53.0, 28.0, 27.9; IR (KBr): 3,459, 2,979, 2,934, 2,876, 1,744, 1,714, 1,505, 1,457, 1,367, 1,337, 1,252, 1,158, 1,115, 1,067, 1,025 cm−1; †HRMS (m/z): [M+Na]+ calcd. for C30H40N2O9Na, 595.2626; found, 595.2602; analysis (calcd., found for C30H40N2O9): C(62.92, 63.37), H(7.04, 7.04), N(4.89, 4.86).
l-Serine-O-(phenylmethyl)-ester-O-l-serine-O-(phenylmethyl)-ether trifluoroacetic acid salt (4).
Compound 3 (0.1078 g, 0.18 mmol) was dissolved in dichloromethane (4 mL), and the flask was purged with nitrogen. Trifluoroacetic acid (1 mL) was then added to the stirred solution, and the reaction was allowed to proceed at room temperature, monitored by TLC (∼2 h). The volatiles were removed in vacuo to yield a yellow oil, which was taken up in toluene (5 mL), which was subsequently removed in vacuo. This process was repeated twice to remove traces of trifluoroacetic acid, yielding a white solid (0.0908 g, 89%). mp: 64.3–67.7 °C; TLC (CH3Cl:MeCN, 4:1 vol/vol): Rf = 0; 1H NMR (400 MHz, CD3OD): δ 7.45–7.30 (m, 10H), 5.32 (d, J = 11.5 Hz, 1H), 5.27 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 12.5 Hz, 1H), 4.54 (d, J = 12.5 Hz, 1H), 4.28 (dd, J = 4.0, 3.2 Hz, 1H), 4.17 (dd, J = 4.0, 3.2 Hz, 1H), 4.02 (dd, J = 12.0, 4.4 Hz, 1H), 3.95 (dd, J = 12.0, 4.4 Hz, 1H), 3.95 (dd, J = 12.0, 4.4 Hz, 1H), 3.83 (dd, J = 12.0, 4.4 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ 169.8, 169.7, 139.0, 137.1, 130.2, 130.1, 130.0, 129.7, 128.7, 74.7, 69.4, 68.1, 60.9, 56.3, 54.6; IR (ATIR): 3,006, 1,764, 1,746, 1,674, 1,599, 1,539, 1,412, 1,185, 1,139, 956, 836, 802, 739, 721, 698 cm−1; HRMS (m/z): [M+H-2TFA]+ calcd. for C20H25N2O5, 373.1758; found, 373.1758; analysis (calcd., found for C24H26F6N2O9): C(48.01, 48.08), H(4.36, 4.28), N(4.67, 4.61).
l-Serine-N-[2,3-bis(phenylmethoxy)benzoyl]-O-(phenylmethyl)-ester-O-l-serine, N-[2,3-bis(phenylmethoxy)benzoyl]-O-(phenylmethyl)-ether (6).
Compound 4 (0.5779 g, 1.0 mmol) was dissolved in anhydrous dichloromethane (5 mL). Alternating amounts of N,N-diisopropylethylamine (DIPEA) (15 mmol, 2.6 mL) and crude compound 5 (49), (3.0 mmol) dissolved in dichloromethane (10 mL) were added. The resulting mixture was stirred for 10 min. The mixture was subsequently diluted with dichloromethane (30 mL), followed by the addition of formic acid (20 mL, 0.1 M, aqueous). The organic layer was separated and washed with water (2 × 20 mL), before drying over MgSO4. The organic layer was then filtered and dried in vacuo. The crude product was purified via column chromatography using 9:1 dichloromethane:acetonitrile as the solvent system to yield an off-white solid material (0.458 g, 44%). mp: 45.2–46.9 °C; TLC (CH2Cl2:MeCN, 9:1 vol/vol): Rf = 0.48; 1H NMR (400 MHz, CDCl3): δ 8.82 (d, J = 8.4 Hz, 1H), 8.80 (d, J = 7.6 Hz, 1H), 7.73–7.71 (m, 2H), 7.45–6.91 (m, 34H), 5.24 (d, J = 12.0 Hz, 1H), 5.22 (d, J = 12.0 Hz, 1H), 5.15–5.08 (m, 7H), 5.02 (dd, J = 10.4, 3.6 Hz, 2H), 4.56–4.52 (m, 2H), 4.35 (dd, J = 11.6, 3.6 Hz, 1H), 4.08 (s, 2H), 3.28 (dd, J = 9.6, 3.2 Hz, 1H), 3.18 (dd, J = 9.6, 3.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 170.2, 169.6, 165.6, 165.4, 152.3, 152.3, 147.4, 147.3, 137.7, 136.6, 136.5, 136.5, 135.5, 129.6, 129.5, 128.9, 128.9, 128.9, 128.8, 128.7, 128.6, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 127.7, 127.6, 127.1, 126.9, 124.7, 124.5, 123.4, 123.4, 117.4, 117.3, 76.1, 76.0, 72.6, 71.2, 71.2, 68.6, 67.6, 64.9, 52.8, 51.4; IR (ATIR): 3,363, 3,064, 3,032, 2,876, 1,747, 1,658, 1,576, 1,515, 1,454, 1,262, 1,201, 1,028, 956, 913, 852, 734, 695 cm−1; HRMS (m/z): [M+H]+ calcd. for C62H57N2O11, 1,005.3957; found, 1,005.3930; analysis (calcd., found for C62H56N2O110.0.25H2O): C(73.76, 73.46), H(5.64, 5.51), N(2.77, 2.79).
Bis(2,3-dihydroxybenzoyl-l-Ser) (H5-bisDHBS).
Compound 6, 0.359 g (0.35 mmol), was dissolved in dry toluene (8 mL) before dry ethanol (42 mL) was added. To the stirred solution, one small spatula tip of Pd-C (10%) was added. The mixture was purged with hydrogen and allowed to react for 18 h under a positive pressure of hydrogen. After the reaction was completed, the catalyst was filtered off and the solvent removed in vacuo to yield a white solid (0.165 g, 91%). mp: 197.4–200.1 °C; TLC (CH2Cl2:MeCN, 9:1 vol/vol): Rf = 0; ‡[α]20D = +15.8 (c 0.1, MeOH); ‡1H NMR (400 MHz, (CD3)2SO): δ 1H NMR ((CD3)2SO, 400 MHz), δ 9.00–8.96 (m, 2H), 7.39 (dd, J = 8.4, 1.6 Hz, 1H), 7.36 (dd, J = 8.0, 1.2 Hz, 1H), 6.95 (dd, J = 4.4, 1.2 Hz, 1H), 6.93 (dd, J = 4.8, 1.2 Hz, 1H), 6.74–6.68 (m, 2H), 4.81–4.76 (m, 1H), 4.61 (dd, J = 11.2, 4.4 Hz, 1H), 4.58–4.55 (m, 1H), 4.41 (dd, J = 11.2, 6.4 Hz, 1H), 3.82 (dd, J = 11.6, 6.4 Hz, 1H), 3.78(dd, J = 11.2, 4.0 Hz, 1H), 3.44 (q, J = 7.2 Hz, 2.79H, EtOH)§, 1.05 (t, J = 7.2 Hz, 2.75H, EtOH); ‡13C NMR (100 MHz, (CD3)2SO): δ 171.1, 170.7, 169.5, 169.3, 149.3, 148.9, 146.7, 146.7, 119.4, 119.3, 119.1, 118.7, 118.7, 118.6, 116.4, 116.0, 63.9, 60.9, 56.1 (EtOH), 55.4, 51.8, 18.4 (EtOH); IR (ATIR): 3,344, 1,738, 1,639, 1,584, 1,532, 1,457, 1,342, 1,231, 1,172, 1,129, 1,072, 843, 738 cm−1; ‡HRMS (m/z): [M-H]− calcd. for C20H19N2O11, 463.0994; found, 463.0994; analysis (calcd., found for C20H20N2O110.0.9 EtOH.1.1H2O): C(49.81, 49.52), H(5.29, 4.94), N(5.33, 5.27).
SI Continuous Variation Method: Fe-NTA and H5-bisDHBS
Electronic Absorbance.
A series of aqueous solutions containing the ligand of interest and Fe-NTA were prepared such that the sum of the concentrations of both remained constant (0.4 mM) in 0.1 M Tris⋅HCl, pH 7.5, 5% (vol/vol) DMSO. The ratio of ligand and metal was varied from 100% ligand to 100% metal. The absorbance at a wavelength characteristic for the system of interest was plotted against the ligand-to-metal ratio.
CD.
The instrument was operated with the following parameters: wavelength range, 300–700 nm; data pitch, 0.5 nm; scanning mode, continuous; scanning speed, 100 nm/min; response, 2 s; bandwidth, 2 nm; accumulation, 5; pathlength, 1 cm. The samples consisted of a total concentration of [L] + [M] of 0.4 mM, recorded in 0.1 M Tris⋅HCl, pH 7.5, 150 mM NaCl, 5% (vol/vol) DMSO, with ligand-to-metal ratios recorded at 1:0, 3:2, 1:1, 2:3, 0:1. The blank spectrum was subtracted from all spectra.
Ligand Solution.
A stock solution of H5-bisDHBS was prepared in DMSO (10 mM).
Fe-NTA Solution.
Nitrilotriacetic acid trisodium salt (0.1 mmol) was dissolved in a standardized Fe(NO3)3 solution (0.0179 M, 5.587 mL). This solution was then made up to 10 mL with distilled water to obtain a solution containing Fe(NO3)3 (0.01 M) and NTA (0.01 M).
Cloning, Expression, and Purification of CeuE and CeuE Mutants.
CeuE was obtained as previously described (27). PCR-based site-directed mutagenesis was used to create CeuE mutants. The plasmid DNA for the N-terminal truncated form of CeuE (24-330) in the YSBLic3C vector was used as a template for PCR, and the appropriate base mutations were incorporated into amplification primers.
We first produced two single CeuE mutants: H227L (FWD_H227_L ataaaagtaggcacactcggaaaaagtatcaat; Rev_H227_L attgatactttttccgagtgtgcctacttttat) and Y288F (FWD_Y288_F gatccagaatactggtttttagcaagtggaaat; REV_Y288_F atttccacttgctaaaaaccagtattctggatc).
The plasmid DNAs of the CeuE single mutants were used for the incorporation of a second mutation to get the double-mutant H227L/Y288F. The E. coli BL21 (DE3) expression strain was used for the transformation of all mutants with transformed cells being grown at 37 °C in lysogenic broth supplemented with antibiotic (kan).
The mutant proteins were purified according to standard procedures including a nickel affinity chromatography column, His-tag cleavage by 3C protease, a second nickel affinity chromatography column, and finally gel filtration. Eluted fractions containing pure mutants were concentrated to ∼20–40 mg/mL protein and stored at −80 °C.
Correct folding was confirmed by CD spectroscopy. Experiments were carried out at 20 °C using a 0.1-cm pathlength quartz cell and 5 mM Tris⋅HCl buffer at pH 8.5. Random error and noise were reduced for each spectrum by averaging of five scans in the wavelength range of 260–190 nm. The signal acquired for the buffer used for dilution of the proteins was subtracted from the spectra acquired for the proteins.
SI Crystallography
Crystallization.
A CeuE stock (5 mg/mL; 50 mM Hepes, pH 7.5, 150 mM NaCl) was used for the crystal screening. Initial screening was carried out using a Mosquito Crystal Robot (TTP LabTech) with 150 nL of protein solution plus 150 nL of reservoir solution in 96-well format plates (MRC two-well crystallization microplate; Swissci) equilibrated against 54 µL of reservoir solution. Experiments were carried out at 20 °C with the PACT (Molecular Dimensions) commercial crystal screen.
Ligand Soaking.
Ligand soaking was performed in the 96-well plate in which the apo-crystals were grown. The addition of 3 μL of the 10 mM Fe-NTA stock solution, followed by 3 μL of the 10 mM H5-bisDHBS DMSO stock solution to the crystallization reservoir allowed the equilibrium of H5-bisDHBS and Fe-NTA to be established before contact with apo-CeuE crystals. The addition of 1 μL of the reservoir solution to the sitting drop, followed by resealing the well allowed time for the Fe(III)-bisDHBS complex to diffuse through the crystal. At time intervals, crystals were harvested from the drop and flash frozen in liquid nitrogen for examination.
Data Collection, Structure Solution, and Refinement.
All computations were carried out using programs from the CCP4 suite (51), unless otherwise stated. Crystals II and III were vitrified at 110 K with no cryoprotection. For both complexes, data were recorded at the Diamond Light Source on beamlines I04-1 and I02, respectively. For crystal II, advantage was taken of the availability of a minikappa device on the beamline and two sweeps of 220° of data were collected, one at kappa = 0° and one at kappa = 40°. In space group P1, this improves both the completeness and the scaling of the data. For crystal III, a single sweep of 220° was collected. All images were processed using XDS and pointless/aimless as implemented in XIA2 (52–54). Both structures are isomorphous with the apo-CeuE structure (PDB ID code 3ZKW) (27), which was used as the starting model. The structures were refined using REFMAC (55) iterated with manual model building/correction in COOT (56). Processing and refinement statistics are given in Table S1.
SI Biophysical Measurements
CD Spectra of CeuE-[Fe(bisDHBS)]2−.
Spectra were recorded using the parameters stated above. The samples consisted of 50 μM Fe(III)-bisDHBS in 0.1 M Tris⋅HCl, pH 7.5, 150 mM NaCl (1,000 μL) to which 20-μL aliquots of a CeuE stock solution (17.5 mg/mL in 20 mM Tris⋅HCl, pH 8.0, 10 mM NaCl) were added. The blank spectrum was subtracted from all spectra.
Fluorescence Quenching Analysis.
Fluorescence spectroscopic measurements were carried out at room temperature (excitation slit width, 10 nm; emission slit width, 20 nm; scan speed, 60 nm/min with automatic response). The detector voltage was set at 950 V. CeuE was excited at 280 nm, and the emission spectrum was recorded from 285 to 415 nm. For each measurement, a 240 nM CeuE solution (2,000 μL), 40 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, was placed in a 1-cm quartz cuvette and titrated stepwise with concentrated Fe(III)-siderophore stock solution. After each addition, the solution was thoroughly mixed and allowed to equilibrate for 1 min. The normalized integrated emission band (corrected for photomultiplier tube response and buffer subtracted) from 310 to 410 nm was used for plotting and binding constant calculation, using the fitting program DynaFit (57). A more accurate determination of the micromolar Kd values by using higher protein and ligand concentrations was attempted, but compromised by increased absorbance of the ligand at the excitation wavelength.
Fe-Siderophore Solution.
Enterobactin was purchased from EMC Microcollections and used as supplied after compound purity was confirmed by HPLC and extinction coefficient determination. A 12 μM stock solution was prepared by addition of 2 μL of a 10 mM H5-bisDHBS or enterobactin DMSO stock solution and 2 μL of a 10 mM Fe(III)-NTA stock solution into 1,696 μL of 40 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, followed by thorough mixing.
SI Notes
Discussion of Unit Cell Dimensions and B Values of Crystals II and III.
In the apo-CeuE structure, all three independent chains were well ordered overall, with average B values of 29.3, 28.8, and 30.2 Å2 for chains A, B, and C, respectively. There was a single break in chain A at residue 256. The loop around residues 96–100 had lower density with some disorder in chains A and B, and the run of four charged residues 102–106 have poorly ordered side chains. The loop 222–227 showed considerable flexibility in all three chains: this loop included His227 involved in binding to the Fe(III) center in the complexes.
Crystal II (a = 58.46 Å, b = 63.02 Å, c = 67.01 Å, α = 83.36°, β = 76.57°, γ = 78.24°) shows a small change in unit cell dimensions compared with apo-CeuE (a = 56.95 Å, b = 62.74 Å, c = 67.98 Å, α = 82.19°, β = 76.74°, γ = 75.96°), the most significant being a ∼2.5° change in γ, with the cell volume increasing by 2.5%. These changes are coupled with a small but significant shift in the orientation of the three molecules relative to one another. A superposition based on all three chains gives an rms deviation in Cα positions of just over 1 Å, while superposing individual equivalent chains reduces this to 0.6 Å.
The B factors in crystal II (24 h) are 39.1, 49.1, and 41.0 Å2 for chains A, B and C, respectively, higher than in apo-CeuE. Chain A is well ordered with only the loops 222–227 and 252–258 showing low density, similar to what was observed in the apo-crystal. In contrast, chain B is more poorly ordered. There is very weak electron density for the N-terminal residues 24–48, and their conformation is largely modeled on that seen in apo-CeuE. The B values for this region are on average about 78 Å2, much higher than for the rest of the molecule (Fig. S3). In addition, several other loops have much greater flexibility than in the apo B chain. Chain C has a similar degree of order to A, with a few extra loops showing increased flexibility compared with apo-CeuE. However, the loop containing residues 222–227 has very poor density, and this region has been modeled based on the conformation observed in the other two chains. In contrast, the three chains in crystal III are all well ordered similar to the apo form.
The binding pockets of chains A and B are both open to solvent; access to the pocket of chain C is restricted by the N terminus of chain B (Figs. S1 and S2). The resulting steric constraints explain the only partial occupation of the pocket in crystal II and the change in orientation of the ligands in crystal III. In crystal II, the soaking of the [Fe(bisDHBS)]2− can be assumed to explain the disruption of the conformation of the N terminus of chain B.
Fluorescence Quenching Statistical Data Analysis.
The average dissociation constants were calculated using a weighted average and associated errors estimated using the method outlined in Fig. S8. For CeuE-[Fe(ENT)]3− and CeuE(H227L/Y288F)-[Fe(bisDHBS)]2−, only weak binding was observed. The endpoint of the titrations could not be reached because the high ligand concentrations required gave rise to interfering ligand absorbance at the excitation wavelength. Hence, only approximate values for these dissociation constants are given.
Acknowledgments
We thank Mr. S. Hart for assistance with data collection, Dr. A. Leech for help with CD spectroscopy, and the Diamond Light Source for access to beamlines I02 and I04 (proposal; mx-7864). We thank the UK Biotechnology and Biological Sciences Research Council and the Engineering and Physical Sciences Research Council (Grant EP/L024829/1) for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5ADW and 5ADV). Experimental datasets associated with this paper have been deposited with the University of York library (www.york.ac.uk/library/info-for/researchers/datasets/).
*Characterization data are consistent with literature data (47).
†Characterization data are consistent with literature data (48).
‡Characterization data are consistent with literature data (50).
§Obscured by water signal.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520829113/-/DCSupplemental.
References
- 1.Jiricny N, et al. Fitness correlates with the extent of cheating in a bacterium. J Evol Biol. 2010;23(4):738–747. doi: 10.1111/j.1420-9101.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 2.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430(7003):1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 3.Schalk IJ, Guillon L. Fate of ferrisiderophores after import across bacterial outer membranes: Different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids. 2013;44(5):1267–1277. doi: 10.1007/s00726-013-1468-2. [DOI] [PubMed] [Google Scholar]
- 4.Raymond KN, Allred BE, Sia AK. Coordination chemistry of microbial iron transport. Acc Chem Res. 2015;48(9):2496–2505. doi: 10.1021/acs.accounts.5b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27(5):637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 6.West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc Biol Sci. 2003;270(1510):37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71(3):413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond KN, Dertz EA, Kim SS. Enterobactin: An archetype for microbial iron transport. Proc Natl Acad Sci USA. 2003;100(7):3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 2008;1778(9):1781–1804. doi: 10.1016/j.bbamem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Naikare H, et al. Campylobacter jejuni ferric-enterobactin receptor CfrA is TonB3 dependent and mediates iron acquisition from structurally different catechol siderophores. Metallomics. 2013;5(8):988–996. doi: 10.1039/c3mt20254b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu F, Zeng X, Haigh RD, Ketley JM, Lin J. Identification and characterization of a new ferric enterobactin receptor, CfrB, in Campylobacter. J Bacteriol. 2010;192(17):4425–4435. doi: 10.1128/JB.00478-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CE, Williams PH, Ketley JM. Pumping iron: Mechanisms for iron uptake by Campylobacter. Microbiology. 2009;155(Pt 10):3157–3165. doi: 10.1099/mic.0.032425-0. [DOI] [PubMed] [Google Scholar]
- 13.Stahl M, Butcher J, Stintzi A. Nutrient acquisition and metabolism by Campylobacter jejuni. Front Cell Infect Microbiol. 2012;2:5. doi: 10.3389/fcimb.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knosp O, von Tigerstrom M, Page WJ. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984;159(1):341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarrow RC, Ecker DJ, Ng C, Liu S, Raymond KN. Iron(III) coordination chemistry of linear dihydroxyserine compounds derived from enterobactin. Inorg Chem. 1991;30(5):900–906. [Google Scholar]
- 16.Telford JR, Raymond KN. Amonabactin: A family of novel siderophores from a pathogenic bacterium. J Biol Inorg Chem. 1997;2(6):750–761. [Google Scholar]
- 17.Brandel J, et al. Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 2012;41(9):2820–2834. doi: 10.1039/c1dt11804h. [DOI] [PubMed] [Google Scholar]
- 18.Miethke M. Molecular strategies of microbial iron assimilation: From high-affinity complexes to cofactor assembly systems. Metallomics. 2013;5(1):15–28. doi: 10.1039/c2mt20193c. [DOI] [PubMed] [Google Scholar]
- 19.Brickman TJ, McIntosh MA. Overexpression and purification of ferric enterobactin esterase from Escherichia coli. Demonstration of enzymatic hydrolysis of enterobactin and its iron complex. J Biol Chem. 1992;267(17):12350–12355. [PubMed] [Google Scholar]
- 20.Cohen SM, Meyer M, Raymond KN. Enterobactin protonation and iron release: Hexadentate tris-salicylate ligands as models for triprotonated ferric enterobactin. J Am Chem Soc. 1998;120(25):6277–6286. [Google Scholar]
- 21.Lin H, Fischbach MA, Liu DR, Walsh CT. In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J Am Chem Soc. 2005;127(31):11075–11084. doi: 10.1021/ja0522027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock RE, Hantke K, Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- 23.Hantke K. Dihydroxybenzoylserine—a siderophore for E. coli. FEMS Microbiol Lett. 1990;55(1-2):5–8. doi: 10.1016/0378-1097(90)90158-m. [DOI] [PubMed] [Google Scholar]
- 24.Wyckoff EE, Allred BE, Raymond KN, Payne SM. Catechol siderophore transport by Vibrio cholerae. J Bacteriol. 2015;197(17):2840–2849. doi: 10.1128/JB.00417-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng X, Mo Y, Xu F, Lin J. Identification and characterization of a periplasmic trilactone esterase, Cee, revealed unique features of ferric enterobactin acquisition in Campylobacter. Mol Microbiol. 2013;87(3):594–608. doi: 10.1111/mmi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller A, Wilkinson AJ, Wilson KS, Duhme-Klair A-K. An [Fe(mecam)2]6− bridge in the crystal structure of a ferric enterobactin binding protein. Angew Chem Int Ed Engl. 2006;45(31):5132–5136. doi: 10.1002/anie.200601198. [DOI] [PubMed] [Google Scholar]
- 27.Raines DJ, Moroz OV, Wilson KS, Duhme-Klair A-K. Interactions of a periplasmic binding protein with a tetradentate siderophore mimic. Angew Chem Int Ed Engl. 2013;52(17):4595–4598. doi: 10.1002/anie.201300751. [DOI] [PubMed] [Google Scholar]
- 28.Rastetter WH, Erickson TJ, Venuti MC. Synthesis of iron chelators. Enterobactin, enantioenterobactin, and a chiral analog. J Org Chem. 1981;46(18):3579–3590. [Google Scholar]
- 29.Enemark EJ, Stack TDP. Spectral and structural characterization of two ferric coordination modes of a simple bis(catecholamide) ligand: Metal-assisted self-assembly in a siderophore analog. Inorg Chem. 1996;35(10):2719–2720. [Google Scholar]
- 30.Peuckert F, Miethke M, Albrecht AG, Essen LO, Marahiel MA. Structural basis and stereochemistry of triscatecholate siderophore binding by FeuA. Angew Chem Int Ed Engl. 2009;48(42):7924–7927. doi: 10.1002/anie.200902495. [DOI] [PubMed] [Google Scholar]
- 31.Peuckert F, et al. The siderophore binding protein FeuA shows limited promiscuity toward exogenous triscatecholates. Chem Biol. 2011;18(7):907–919. doi: 10.1016/j.chembiol.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Bergstrom CP, Lu MC, Bell CL. NMR studies of dimeric 2,3-dihydroxy-N-benzoyl serine. J Nat Prod. 1991;54(4):1003–1008. [Google Scholar]
- 33.Job P. Formation and stability of inorganic complexes in solution. Ann Chim. 1928;9:113–203. [Google Scholar]
- 34.Renny JS, Tomasevich LL, Tallmadge EH, Collum DB. Method of continuous variations: Applications of job plots to the study of molecular associations in organometallic chemistry. Angew Chem Int Ed Engl. 2013;52(46):11998–12013. doi: 10.1002/anie.201304157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris WR, et al. Coordination chemistry of microbial iron transport compounds. 19. Stability constants and electrochemical behavior of ferric enterobactin and model complexes. J Am Chem Soc. 1979;101(20):6097–6104. [Google Scholar]
- 36.Harris WR, Raymond KN, Weitl FL. Ferric ion sequestering agents. 6. The spectrophotometric and potentiometric evaluation of sulfonated tricatecholate ligands. J Am Chem Soc. 1981;103(10):2667–2675. [Google Scholar]
- 37.Murakami Y, Nakamura K. The chelating behavior of catechol-4-sulfonate with iron (III) ion. Bull Chem Soc Jpn. 1963;36(11):1408–1411. [Google Scholar]
- 38.Karpishin TB, Stack TDP, Raymond KN. Stereoselectivity in chiral iron(III) and gallium(III) tris(catecholate) complexes effected by nonbonded weakly polar interactions. J Am Chem Soc. 1993;115(14):6115–6125. [Google Scholar]
- 39.Liu M, Zhang L, Wang T. Supramolecular chirality in self-assembled systems. Chem Rev. 2015;115(15):7304–7397. doi: 10.1021/cr500671p. [DOI] [PubMed] [Google Scholar]
- 40.Enemark EJ, Stack TDP. Synthesis and structural characterization of a stereospecific dinuclear gallium triple helix: Use of the trans-influence in metal-assisted self-assembly. Angew Chem Int Ed Engl. 1995;34(9):996–998. [Google Scholar]
- 41.Albrecht M, et al. “Induced fit” in chiral recognition: Epimerization upon dimerization in the hierarchical self-assembly of helicate-type titanium(IV) complexes. Angew Chem Int Ed Engl. 2011;50(12):2850–2853. doi: 10.1002/anie.201006448. [DOI] [PubMed] [Google Scholar]
- 42.Telford JR, Leary JA, Tunstad LMG, Byers BR, Raymond KN. Amonabactin: Characterization of a series of siderophores from Aeromonas hydrophila. J Am Chem Soc. 1994;116(10):4499–4500. [Google Scholar]
- 43.Telford JR, Raymond KN. Coordination chemistry of the amonabactins, bis(catecholate) siderophores from Aeromonas hydrophila. Inorg Chem. 1998;37(18):4578–4583. doi: 10.1021/ic980090x. [DOI] [PubMed] [Google Scholar]
- 44.Abergel RJ, Zawadzka AM, Hoette TM, Raymond KN. Enzymatic hydrolysis of trilactone siderophores: Where chiral recognition occurs in enterobactin and bacillibactin iron transport. J Am Chem Soc. 2009;131(35):12682–12692. doi: 10.1021/ja903051q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprencel C, et al. Binding of ferric enterobactin by the Escherichia coli periplasmic protein FepB. J Bacteriol. 2000;182(19):5359–5364. doi: 10.1128/jb.182.19.5359-5364.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miethke M, Skerra A. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with l-norepinephrine-mediated bacterial iron acquisition. Antimicrob Agents Chemother. 2010;54(4):1580–1589. doi: 10.1128/AAC.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sowinski JA, Toogood PL. Synthesis of an enantiomerically pure serine-derived thiazole. J Org Chem. 1996;61(22):7671–7676. doi: 10.1021/jo961408a. [DOI] [PubMed] [Google Scholar]
- 48.Yu X, Dai Y, Yang T, Gagné MR, Gong H. Facile synthesis of salmochelin S1, S2, MGE, DGE, and TGE. Tetrahedron. 2011;67(1):144–151. [Google Scholar]
- 49.Schobert R, Stangl A, Hannemann K. Conjugates of methyl 6-aminopenicillanate with biscatechol-hydroxamate chelators: Synthesis and siderophoric activity. Tetrahedron. 2006;62(33):7799–7808. [Google Scholar]
- 50.Igarashi Y, et al. Catechoserine, a new catecholate-type inhibitor of tumor cell invasion from Streptomyces sp. J Antibiot (Tokyo) 2012;65(4):207–209. doi: 10.1038/ja.2011.137. [DOI] [PubMed] [Google Scholar]
- 51.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter G. Xia2: An expert system for macromolecular crystallography data reduction. J Appl Cryst. 2010;43(1):186–190. [Google Scholar]
- 53.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans P. Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 1):72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- 55.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuzmič P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal Biochem. 1996;237(2):260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]