Significance
Myosins containing MyTH4-FERM (myosin tail homology 4-band 4.1, ezrin, radixin, moesin, or MF) domains in their tails are found in wide range of phylogenetically divergent organisms. Interestingly, evolutionarily distant MF myosins have similar roles in the extension of actin-filled membrane protrusions, such as filopodia, and microtubule binding, suggesting that their core functions have been highly conserved over evolution. A structural analysis of mammalian and Dd myosin MF domains in combination with comparison of diverse MF myosin sequences illustrate how tuning of existing features can give rise to new structures while preserving the general properties of myosin tails. Thus, tinkering with the MF domain enables it to serve as a multifunctional platform for cooperative recruitment of various partners, allowing common properties to arise through convergent evolution.
Keywords: protein evolution, molecular tinkering, microtubules, filopodia
Abstract
Myosins containing MyTH4-FERM (myosin tail homology 4-band 4.1, ezrin, radixin, moesin, or MF) domains in their tails are found in a wide range of phylogenetically divergent organisms, such as humans and the social amoeba Dictyostelium (Dd). Interestingly, evolutionarily distant MF myosins have similar roles in the extension of actin-filled membrane protrusions such as filopodia and bind to microtubules (MT), suggesting that the core functions of these MF myosins have been highly conserved over evolution. The structures of two DdMyo7 signature MF domains have been determined and comparison with mammalian MF structures reveals that characteristic features of MF domains are conserved. However, across millions of years of evolution conserved class-specific insertions are seen to alter the surfaces and the orientation of subdomains with respect to each other, likely resulting in new sites for binding partners. The MyTH4 domains of Myo10 and DdMyo7 bind to MT with micromolar affinity but, surprisingly, their MT binding sites are on opposite surfaces of the MyTH4 domain. The structural analysis in combination with comparison of diverse MF myosin sequences provides evidence that myosin tail domain features can be maintained without strict conservation of motifs. The results illustrate how tuning of existing features can give rise to new structures while preserving the general properties necessary for myosin tails. Thus, tinkering with the MF domain enables it to serve as a multifunctional platform for cooperative recruitment of various partners, allowing common properties such as autoinhibition of the motor and microtubule binding to arise through convergent evolution.
The evolution of new functions is often driven by the reuse of existing structures, a process François Jacob called “molecular tinkering” (1). Conservation of critical residues is often necessary for enzymatic activities, whereas structural motifs mostly involved in protein recognition present more opportunities for molecular tinkering. Molecular motors such as myosins are of particular interest for exploring protein evolution because they contain both a rather conserved motor domain and a more diverse C-terminal tail region. These multifunctional motors have central roles in a wide range of cellular activities, which require precise coupling of their motor function to specific partners. Myosins use a highly conserved mechanism of force production that involves rearrangement of the motor domain leading to lever arm swing (2), and members of the superfamily seem to have acquired new cellular functions by modification of key regions controlling recruitment of partners and regulating motor functions (3). This is in large part achieved by the gain of structural domains in the C-terminal cargo binding region and the evolution of their sequence by molecular tinkering (e.g., ref. 4). A particularly interesting subgroup of myosins includes those that have either one or two MyTH4-FERM domains (MF; myosin tail homology 4-band 4.1, ezrin, radixin, moesin) in their C-terminal tail region (Fig. 1). These MF myosins are widespread and likely quite ancient because they are found in many different branches of the phylogenetic tree (5, 6), including Opisthokonts (which includes Metazoa, unicellular Holozoa, and Fungi), Amoebozoa, and the SAR (Stramenopiles, Alveolates, and Rhizaria) (Fig. 1 A and B). Over the course of hundreds of millions years of parallel evolution the MF myosins have acquired or maintained roles in the formation of specialized actin-based structures such as filopodia (7, 8) and/or cross-linking microtubules (MT) to actin filaments (9–11).
Fig. 1.
Evolutionarily distant myosins with a shared conserved MF. (A) Schematic illustration of the MF myosin family showing the tail domain organization. (B) Distribution of MF myosins through phylogeny. A schematized phylogenetic tree (Left) illustrating the relative positions of major phyla and the MF myosins found in representative species (Right) (5). ●, myosin present; ○, myosin not found in this branch of the tree. (C) Ribbon representation illustrating the X-ray structures of HsMyo10MF (27), MmMyo7aMF1 (30), DdMF1, and DdMF2. The structures are all presented with their F1 lobe in a similar orientation. Note the variation in how the F2 and F3 lobes are positioned compared with the F1 lobe in each FERM domain. The F2-H1 helix (red star) orientation is, however, similar in all structures because it participates in strong interactions that maintain the cloverleaf configuration (see also Fig. 6).
The Metazoan Myo10 and Amoebozoan Dictyostelium discoideum Myo7 (DdMyo7) myosins are both essential for the extension of filopodia, plasma membrane protrusions filled with parallel bundles of F-actin (7, 8, 12), suggesting a high degree of functional conservation throughout evolution. Strikingly, both mammalian Myo10 and DdMyo7 are localized at the tips of filopodia (7, 8) and are thought to play roles in mediating extension of actin filaments against the membrane as well as transporting receptors and regulators along filopodia as they extend (13). Other mammalian MF myosins such as Myo15 and Myo7a and Myo7b have roles in the extension or organization of actin-filled protrusions such as stereocilia and microvilli (14–17). Mammalian Myo10 and DdMyo7 are also required for cell–substrate adhesion (8, 18), and mammalian Myo10 and Drosophila Myo15 have roles in mediating cadherin-dependent cell–cell adhesion in epithelial cells (19, 20). Thus, across a remarkable range of cell types and evolutionarily diverse organisms, the MF family of myosin motors has maintained a core set of shared functions.
The defining feature of the MF myosins is the bipartite structural domain consisting of an N-terminal MyTH4 followed by a FERM domain. The FERM domain serves as a protein interaction module, and in different myosins this domain has been shown to bind to adhesion and signaling receptors as well as actin binding proteins (18, 20–22). The FERM domain has also been implicated in autoinhibition of mammalian Myo7a and Myo10 and Drosophila Myo7a (23–26). Less is known about the partners that bind to the MyTH4 domain, and the main role identified so far is MT binding (10, 27, 28). Indeed, it has been shown that both the mammalian Myo10 and the DdMyo7 MyTH4 domains bind to MTs (9, 10, 27), which has important implications for the role of these motors in the generation of force between or cross-linking of the two main cellular cytoskeleton networks.
A combination of structural and functional studies of evolutionarily distant myosin MF domains can provide insight into how molecular tinkering has guided the appearance of conserved and potentially novel functions of this widespread group of motors. The structures of the MF domains from both mammalian Myo7a and 10 have been solved recently, revealing that the MyTH4 domain is a compact helical bundle closely apposed to the FERM domain. The large interaction surface between the MyTH4 and FERM domains results in a supramodular organization (29, 30), which restricts the relative orientation of these domains (Fig. 1C). However, it is not known whether the supramodular feature is a conserved property of MF domains across many phyla and whether it has mostly a structural or a functional role. Studies of MF domains from phylogenetically distant organisms are necessary to reveal how evolution of a shared domain can diversify myosin function or result in the emergence of conserved functions. A detailed analysis of the MF domains of amoeboid DdMyo7 and comparison with the MF domains of mammalian Myo7a and Myo10 offers a unique opportunity to address the question of structural and functional conservation of the MF domain across over 600 million years of independent evolution.
Results and Discussion
Overall Description of the MyTH4-FERM Structures.
Four high-resolution structures describing WT and MT binding loss of function mutant forms (discussed below) of the N-terminal MF (MF1) and C-terminal MF (MF2) domains of the amoeboid DdMyo7 have been solved (Fig. 1C, Materials and Methods, and Table S1). Each of these MF domains has been described from crystal structures that correspond to distinct crystal packing environments (Fig. S1 A–C). Interestingly, the rmsd between the mutant and WT structures for each of the MF domains are low. Comparison of the WT and mutant MF1 structures shows that the rmsd is 0.498 Å (for 392 atoms) and, similarly, comparison of the WT and mutant MF2 domains yields an rmsd of 0.829 Å (for 411 atoms), despite these structures being composed of four subdomains (one MyTH4 domain and three FERM lobes (F1, F2, and F3 lobes) and relatively low sequence identity (Fig. S1A and Table S2). The similarity in the two structures for each MF indicates that the relative orientation of the four subdomains does not exhibit large variations. In contrast, drastic differences in the relative orientation of the MyTH4 and FERM subdomains are observed when the DdMF1 and DdMF2 are compared, as indicated by the large rmsd between the whole MyTH4-FERM domains (5.199 Å, for 373 atoms). Similarly, the rmsd between the MF domains of DdMF1 and DdMF2 are also large when each is compared with the Myo10 MF (2.475 Å, for 320 common atoms and 5.021 Å, for 342 common atoms, respectively).
Table S1.
Data collection, phasing, and refinements statistics
| Data collection | DdMF1 | DdMF1 mutant* | DdMF2 native | DdMF2 SeMet | DdMF2 mutant† |
| Space group | P212121 | P212121 | P1 | P1 | P212121 |
| Cell dimensions | |||||
| a, b, c, Å | 60.2, 90.5, 148.7 | 53.3, 61.5, 172.6 | 40.0, 59.0, 64.4 | 39.9, 59.0, 64.4 | 40.17, 158.8, 194.7 |
| α, β, γ, ° | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.1, 96. 8, 108.5 | 89.9, 83.1, 71.7 | 90.0, 90.0, 90.0 |
| Molecules per asymmetric unit | 1 | 1 | 1 | 1 | 2 |
| Wavelength, Å | 0.97242 | 0.97934 | 0.98010 | 0.97918 | 0.97918 |
| Resolution, Å | 50.00–1.85 (1.96–1.85) | 45.36–2.19 (2.32–2.19) | 50.00–2.0 (2.10–2.0) | 50.00–2.30 (2.40–2.30) | 46.51–2.70 (2.87–2.70) |
| No. of reflections | |||||
| Total | 414,273 (69,693) | 263,767 (26,122) | 65,009 (7,261) | 339,869 (19,417) | 456,116 (45,812) |
| Unique | 56,017 (10,885) | 29,999 (2,944) | 36,454 (3,520) | 24,296 (2,391) | 35,131 (3,412) |
| Completeness, % | 99.3 (97.1) | 99.97 (99.97) | 97.51 (95.07) | 99.2 (98.0) | 99.7 (96.9) |
| Redundancy | 5.9 (5.14) | 8.8 (8.9) | 2.0 (2.1) | 14.0 (8.1) | 12.9 (13.4) |
| I/σ | 18.36 (2.27) | 18.15 (6.14) | 10.90 (2.14) | 14.74 (3.73) | 26.42 (3.95) |
| Rsym, % | 5.7 (63.0) | 9.6 (36.3) | 6.5 (44.1) | 14.3 (63.4) | 8.9 (67.1) |
| Refinement | DdMF1 | DdMF1 mutant* | DdMF2 | DdMF2 mutant† | |
| Resolution, Å | 43.31–1.90 | 19.01–2.19 | 37.54–2.0 | 38.91–2.70 | |
| Rcryst/Rfree, % | 19.4/23.0 (27.2/32.3) | 16.7/20.1 (20.2/22.2) | 18.2/22.1 (25.8/28.5) | 20.4/23.4 (28.9/33.8) | |
| Average B factor | 28.60 | 32.6 | 40.10 | 79.30 | |
| Rmsd bonds | |||||
| Distances, Å | 0.007 | 0.014 | 0.015 | 0.023 | |
| Angles, ° | 0.97 | 1.67 | 1.68 | 1.86 | |
| No. of atoms | |||||
| Nonhydrogen | 4,461 | 4,617 | 4,386 | 8,269 | |
| Protein | 3,923 | 4,105 | 4,087 | 7,927 | |
| Other | 35 | 0 | 28 | 0 | |
| Water | 503 | 512 | 271 | 342 | |
| Ramachandran plot | |||||
| Favored, % | 97.4 | 96.0 | 97.0 | 96.6 | |
| Allowed, % | 1.8 | 3.4 | 2.6 | 3.19 | |
| Outliers, % | 0.8 | 0.6 | 0.4 | 0.21 | |
Values in parentheses are for the highest-resolution shell.
DdMF1 mutant: K1157E, H1159E, K1161E, and K1174E.
DdMF2 mutant: K1181E, R1882E, K1909E, K1912E, and K1913E.
Fig. S1.
Structural comparison of DdMyo7 WT and mutant DdMF1 and DdMF2. (A, Left) Superimposition of the DdMF1 WT (orange) and mutant #2 K1157E, H1159E, K1161E, and K1174E (magenta) structures (rmsd = 0.485 Å for 381 atoms). (Right) Comparison between the DdMF2 WT (blue) and mutant #1 K1181E, R1882E, K1909E, K1912E, and K1913E structures (magenta, rmsd = 0.829 Å for 411 atoms). Note that a part of the N-terminal sequence of the mutant is not visible in electron density and not modeled (arrow). (B) Comparison of the crystal packing of DdMF1 WT (dark orange) and DdMF1 mutant #2 (light orange) structures. Symmetry molecules are in gray (DdMF1 WT) and pink (DdMF1 mutant #2). Note that the environment of the MF molecule differs in the two crystal forms. The position of the different domains (MyTH4 and the three FERM lobes F1, F2, and F3) found in the crystal is thus not greatly influenced by the interactions with other molecules in the crystal. (C) Comparison of the crystal packing of DdMF2 WT (dark blue) and mutant #1 structures (light blue, molecule A). Symmetry molecules of the DdMF2 WT molecule are in gray. Note that the asymmetric unit of the mutant includes two molecules; molecule B is shown in pink. The environment of the WT and the mutant molecules is clearly different. (D) The surface analysis shows a difference in the electrostatic potential between the WT and the mutant MFs that accounts for the difference in MT affinity.
Table S2.
Protein identity (blue) and similarity (yellow) for the MF proteins studied
 |
For detail on the sequences used, see Materials and Methods.
The MyTH4 and FERM domains of both DdMF1 and DdMF2 are tightly coupled together to form a supramodule (Fig. 1C), similar to the mammalian Myo7a and Myo10 MyTH4-FERM domains (29). The association is mediated by conserved residues at the end of all MyTH4 domains (the last helix of the domain, H7, and the H6.H7 linker that precedes it) and the F1 lobe of the FERM domain (Fig. 2 and Fig. S2 C and D). Electrostatic and hydrophobic interactions link both subdomains together, characterized by a conserved Pro Arg Glu motif (Pro1686 and the salt bridge forming residues Arg1682 and Glu1690 in the Myo10 sequence) that are strictly conserved in DdMyo7 MF1 and MF2 as well as in other distant MyTH4-FERM domains (Fig. 2B and Fig. S2C). The H6.H7 linker and the H7 helix adopt a conformation in the DdMF1 and DdMF2 structures similar to that found in Myo10 MF and Myo7a MF1. Unexpected differences at the end of the MyTH4 of DdMF2, however, result in a supramodule with significantly distinct relative positions between the MyTH4 and FERM (Fig. S2 D and E). There is a large (∼23°) difference in the orientation of the core MyTH4 bundle and the linker-region/F1 module when DdMF2 is compared with other MF structures (Fig. S2E). The H6 of the DdMF2 MyTH4 core is shorter by four residues and the linker region that follows contains two prolines that modify the orientation of the linker, whereas helix H7 is extended on its C terminus by three residues. This leads to different interactions of the linker/H7 helix with the surrounding elements in DdMF2 (Fig. S2 D and E) compared with Myo7a MF1, Myo10 MF, and DdMF1, which are quite similar to each other. Interestingly, strong but distinct hydrophobic interactions maintain the linker in tight contact with the MyTH4 core, establishing a new way to form the supramodule with drastically different relative positions for the MyTH4 and FERM domains. Thus, whereas the tight linkage between the two domains is maintained for evolutionarily distant MF domains, variations can exist in the overall shape of the supramodule.
Fig. 2.
Conservation of the MyTH4 domain and the MyTH4-FERM interface. (A) Ribbon representation of the MyTH4 domain from the crystallized MF domains. (Left) The core of the MyTH4 domain formed by a bundle of six helices (represented by the gray cylinders, from H1 to H6), which interacts with a conserved N-terminal linker (red) on one surface and with a C-terminal linker and helix H7 (cyan or blue) on the opposite surface. (Middle and Right) The nonconserved long inserts of different MyTH4 domains as well as the N-terminal regions (Nt) are represented using the indicated color code. See also Fig. S2A. The large insertions that are characteristic of the Myo10 and Myo7a classes, respectively, are displayed as M10-L0 (Myo10) and M7a-L1 (Myo7a) and cover the surface of the H1 and H2 helices. (B) Structure-based sequence alignment analysis of representative MyTH4 domains. Residues that are absolutely conserved are indicated in bold. Residues that are highly conserved are highlighted in yellow (hydrophobic residues), blue (positively charged), red (negatively charged), and green (other residues). The Cen2 binding pocket formed in part by the shorter H5 and H6 helices in mammalian Myo7a is indicated as Myo7 deletion (pink arrows). The last helix of the domain that serves as a linker and participates in the interface with the F1 lobe is indicated in black (H7). The conserved residues participating in this interface [Arg, Pro, and Glu (29)] are indicated with black circles. Note the longer linker before the H7 helix in DdMF2 (highlighted in violet) that modifies the orientation of this helix and the supramodule overall shape. Other myosin MyTH4 domains also have longer linkers that may affect the supramodule (Dataset S1). Residues shown to participate (solid stars) or are part of the MT binding surface (open stars) are indicated using the color code defined in A. The MF1, MF2, Hs, Aea, Gnp, Dd, and At abbreviations stand for N-terminal MyTH4-FERM domain, C-terminal MyTH4-FERM domain, Homo sapiens, Aedes aegypti, Gonapodya prolifera, Dictyostelium discoideum, and Arabidopsis thaliana, respectively.
Fig. S2.
Variable features of the MyTH4 domain. (A) The N-terminal variable region of Myo7a (pink), Myo10 (green), DdMF1 (orange), and DdMF2 (blue) are shown. This region is near the conserved N-ter linker and modifies the surface of the H3 and H5 helices. In DdMF1 and Myo7a MF1, it adopts a similar conformation and partially covers the H6 surface (black arrow). The N-terminal residue is indicated with a circle using the same color code. (B) Binding pocket in Myo7a for the CEN2 peptide as described (30) (Left). Some residues of the pocket are labeled. (Right) The same region in Myo10 (green) is shown for comparison. Note that the longer H5 and H6 helices in Myo10 and their slightly different orientation compared with Myo7a as well as the conformation of the H3.H4 linker close the cleft and thus prevent peptide binding to this surface in Myo10. (C–E) The supramodule MyTH4-FERM interface differs in DdMF2 compared with DdMF1. (C) The C-ter linker that connects the H6 and H7 helices (cyan) strongly interacts with the MyTH4 and F1 domains (shown here for DdMF1). The strictly conserved arginine, proline, and glutamate (27) residues of the linker that stabilize the supramodule interface between the MyTH4 and FERM domains are also shown using sticks representation (R, E, and P, respectively). In D, the DdMF2 structure (blue) is superimposed on the DdMF1 structure (orange) using the F1 lobe as a reference. Note the difference in orientation of the H6, H4, and H2 helices that reflect the difference in orientation of the whole MyTH4 domain, relative to the F1 lobe. The H7 helix (black arrow) superimposes in both structures and interacts similarly with the F1 lobe. In E, the DdMF2 structure is compared with the DdMF1 structure using the core of the MyTH4 domain as a reference. Note the large differences in the C-ter linker position (blue and cyan) that result in a reorientation of the H7 helix together with the F1-lobe by ∼23°, represented here using the F1-H2 as a reference.
Differences in the MyTH4 and FERM domain coupling due to sequence insertions or variation have the potential to lead to significant functional consequences. A particular orientation of these two domains is likely to be optimized for binding to a specific partner, especially if the recognition involves more than one subdomain. For example, a significant change in the overall disposition of myosin subdomains can also be seen for the vertebrate and yeast Myo5 cargo binding domains (4). The drastic difference seen in the disposition of the myosin tail MyTH4 and FERM domains suggests new possibilities for negative or positive cooperativity in partner binding, and the likely emergence of new functions for the myosin from one class to another.
Conservation and Divergence in the MyTH4 Domain of Evolutionarily Distant MF Myosins.
Myosins with common functions might be expected to have conserved features necessary for their regulation or their specific cellular roles whereas class-specific variations could be the signature of acquisition of new functions. Comparison of the MyTH4 domains for which a structure is available and sequence analysis of phylogenetically diverse MF proteins revealed the defining, conserved features of the MyTH4 domain. The core of the MyTH4 domain is formed by a conserved six-helix bundle, in which all of the helices are nearly parallel or antiparallel and with a conserved Nter linker preceding the first helix (Fig. 2A and Fig. S2A). The connections between the second, third, fourth, and fifth helices are short and conserved among the different MyTH4 domains. Despite this overall conservation, structural comparison highlights how class-specific inserted variable sequences (analysis summarized in Table S3 and Dataset S1) drastically change the interfaces available for binding to the MyTH4 domain and have the potential to provide binding partner specificity to each myosin class.
Table S3.
MyTH4 domain inserts and variable loops
 |
First, major variability is observed among MyTH4 domains in the N-terminal variable region before the conserved Nter linker (Fig. 2A; see alignment in Fig. 2B), making it difficult to define the start of the MyTH4 domain. Distinct differences are seen in how this Nter sequence covers the surface of helices H3, H5, and H6 (Fig. 2A, Middle and Right). The structure of the Nter variable region is, however, strikingly similar in the MF1 domains from two distant Myo7 myosins (mammalian and Dd) (Fig. S2A). Interestingly, in DdMyo7 MF2, different basic residues of this Nter variable region cluster are part of the MT binding site (discussed below).
Second, the connection between the first and second helices is highly variable among MyTH4 domains and is particularly long in mammalian Myo7a (M7a–L1, residues 1055–1147) and Myo7b (residues 1027–1086) (Fig. 2A, Middle and Right and 2B). This insertion in Myo7a covers the surface of the H1 and H2 helices that are otherwise exposed in the other MyTH4 domains. This surface is also covered in Myo10, in part, by a long inserted sequence (M10–L0) that forms a loop between the conserved Nter linker and the first helix, H1 (Fig. 2A).
A third variable characteristic of Myo7 MF1s is found at the end of the H5 helix and its connection with the H6 helix (residues 1219–1222 of Myo7a). The shorter H5 and H6 helices in the Myo7a MF1 domain allow the formation of a pocket (not found in other MyTH4 domains) that is exploited for recognizing the Cen2 motif of its cellular partner SANS (30) (Fig. S2B).
The comparison of four different MyTH4 domains reveals that molecular tinkering by insertion and deletion of sequences at specific locations results in drastic changes of the surface of the MyTH4 domain while conserving the overall domain integrity and supramodule organization provided by the six core helices (Table S3). Interestingly, these modifications are largely conserved in sequence within each distinct myosin class. Overall, these inserted sequences have the potential to play an important role in defining new functions of the MyTH4 domain for different myosin classes during evolution either by hiding previously existing binding sites or by creating new opportunities for the recruitment of partners.
Is the MT Binding Site Conserved Among MF Myosins?
A shared property of MyTH4 domains is their ability to interact with MTs, suggesting that they bind via a highly conserved MT binding site. DdMyo7 has two MF domains and the N-terminal MF domain (DdMF1) has been shown to bind MTs (9). Sequence alignments show that the DdMF1 MyTH4 harbors a positively charged sequence similar to that of the MT binding site of Myo10 (27), suggesting that this might be a signature motif for MF domain binding to MTs (Fig. 3A). However, this motif is absent in the DdMF2 MyTH4 sequence, suggesting that this domain would not bind to MTs. The MT binding of DdMF1, DdMF2, and mammalian Myo10 MF domains purified from bacteria was compared directly to test this possibility. Equilibrium MT cosedimentation binding assays were performed for each MF domain (see Table 1 for summary). Similar to what had been found for DdMF1 purified from Dictyostelium cells (9), the bacterially expressed domain also bound MTs with a moderate apparent Kd, (1.7 ± 0.48 µM, n = 3) (Fig. 3B) that is weaker than that of the mammalian Myo10 MF domain and exhibits a lower fractional binding (0.24 vs. 0.91; Table 1). Unexpectedly, DdMF2 also bound MTs with a higher affinity (apparent Kd = 0.47 ± 0.09 µM, n = 4, fractional binding 0.9; Table 1) than DdMF1 (Fig. 3C). The apparent affinity of DdMF2 is only slightly weaker than that of the mammalian Myo10 MF domain (apparent Kd = 0.14 ± 0.08 µM, n = 4) (Fig. 3D), and MT binding by DdMyo7 is significant overall when one considers that the DdMyo7 tail has two tandem MF domains that can each bind to MTs and this myosin is likely able to dimerize, as are mammalian Myo10 and Myo7a (23, 25). These results are consistent with MT binding being a core, conserved property of all MyTH4 domains but reveals that this interaction can be mediated by distinct binding sites.
Fig. 3.
MF domain binding to MTs. (A) Conservation of the Myo10 MT binding motif. The residues previously implicated in MT binding in the Myo10 MyTH4 domain (27) are shown in light blue. The additionally identified residues participating in the interaction of the Myo10 MyTH4 domain are shown in dark blue (positively charged), yellow (tyrosine), and red (prolines). The organisms for this alignment are Homo sapiens (human), Bos taurus (bovine), Xenopus tropicalis (frog), Danio rerio (zebrafish), Ciona intestinalis (ciona), Monosiga brevicollis (monosiga), Capsaspora owczarzaki (capsaspora), and Dictyostelium discoideum (DdMF1 and DdMF2). (B and C) Equilibrium MT binding assays to measure the apparent binding affinity of the MF domains of DdMyo7 MF1 and MF2. (D–F) Equilibrium MT binding assays to measure the apparent binding affinity of the MF domains of HsMyo10 [whole MF domain, MyTH4 alone, Myo10 binding to subtilisin-treated MTs (S-MTs)]. (G) Fractional binding of Myo10 MF with different MyTH4 mutants (see Table 1) at 2 µM tubulin polymer.
Table 1.
MT binding affinities for Myo10 MF, DdMF1, and DdMF2
| MF protein | Apparent Kd, µM | SD | Frac bind* | n |
| HsMyo10 | ||||
| MF | 0.14 | 0.08 | 0.91 | 4 |
| MyTH4 | 0.24 | 0.09 | 0.68 | 3 |
| MF† | 4.98 | 1.13 | 0.51 | 2 |
| MF mut#1 R1643A, K1647A, K1650E | 6.35 | 3.53 | 0.24 | 3 |
| MF mut#5 P1546E, P1548E, R1600E | 0.37 | 0.16 | 0.77 | 3 |
| MF K1647D | 0.66 | 0.16 | 0.71 | 2 |
| MF mut#2 R1643A, K1647A, K1650E, K1654E, R1657E | 16% of WT | 4 | ||
| MF mut#3 R1643A, K1647A, K1650E, P1546E, P1548E, R1600E | 10% of WT | 3 | ||
| MF mut#4 R1643A, K1647A, K1650E, K1654E, R1657E, Y1673A | 13% of WT | 3 | ||
| DdMyo7 | ||||
| DdMF1‡ | 1.70 | 0.05 | 0.25 | 1 |
| DdMF1 | 1.76 | 0.48 | 0.24 | 3 |
| DdMF1 mut#1 K1257E–K1261E | 1.96 | 0.31 | 0.19 | 3 |
| DdMF1 mut#2 K1157E, H1159E, K1161E, K1174E | 15% of WT | 2 | ||
| DdMF2 | 0.47 | 0.09 | 0.9 | 4 |
| DdMF2† | 0.83 | 0.39 | 0.64 | 5 |
| DdMF2 mut#1 K1896E, K1900E, K1909E, K1921E | 4% of WT | 2 | ||
| DdMF2 mut#2 K1881E, R1882E, K1909E, K1912E, K1913E | No binding detected | 3 | ||
Frac bind is fractional binding at saturating concentrations of tubulin polymer; percent of binding is fraction bound at saturating tubulin (2 µM for Myo10 MF and DdMF2 and 7 µM for DdMF1) compared with the WT MF.
Subtilisin-treated MT.
Purified from Dictyostelium cells (9).
Characterization of the Myo10 MT Binding Site.
The mammalian Myo10 MF domain binds to MTs via charged interactions with the C-terminal tails of tubulin (27). The tubulin C-terminal tail is an unstructured region of ∼20 aa residues that points away from the MT lattice and is the most variable among tubulin isoforms due to both sequence variation and posttranslational modifications (31). Additionally, binding of the FERM domain of Myo10 to the cytoplasmic tail of the netrin receptor DCC was found to inhibit MT binding (27), suggesting that both the MyTH4 and FERM domain contribute to MT binding. However, the affinity of the MyTH4 domain alone for MTs is quite similar to that of the full MF domain (apparent Kd = 0.24 ± 0.09 µM, n = 3) (Fig. 3E), indicating that MT binding is almost exclusively occurring via the MyTH4 domain in mammalian Myo10. To assess whether the main interaction of the Myo10 MF with MT requires the C-terminal tail of MTs, as previously proposed (27), cosedimentation experiments with subtilisin-treated MTs that lack the acidic C-terminal tubulin tails were carried out. Interestingly, although removal of the C-terminal tubulin tails did result in a reduction in MF binding (apparent Kd = 4.98 ± 1.13 µM, n = 2) (Fig. 3F), their loss did not abolish MF binding completely, establishing that MT binding involves more than an interaction with the C-terminal tubulin tails. Similarly, mutation of two positive residues previously implicated in MT binding [K1647 and K1650 (27)] as well as the nearby R1643 substantially reduced but did not eliminate MT binding (mut#1; apparent Kd = 6.35 ± 3.53 µM, n = 3) (Fig. 3G and Fig. S3A). Thus, additional residues of the Myo10 MyTH4 domain contribute to the interaction with MTs, binding to both the MT lattice and the exposed Cter tails.
Fig. S3.
Identification of the MyTH4 MT binding sites. (A) Equilibrium MT binding assays to measure the binding affinity of the MF domains of HsMyo10 mutant #1. (B–D) Identification of additional Myo10 MF residues required for MT binding. MT binding assays to identify the full MT binding site of Myo10 MF domain. Shown are examples of the binding analysis using SDS/PAGE (top) and quantification of the binding for two different concentrations of tubulin polymer at approximately half maximal or saturation binding (0. 5 µM and 2 µM, respectively). (B) Mutant #2 R1643A, K1647A, K1650E, K1654E, and R1657E. (C) Mutant #3 R1643A, K1647A, K1650E, R1657E, and R1600E. (D) Mutant #4 R1643A, K1647A, K1650E, P1546E, P1548E, and R1600E. (E) Mutant #5 P1546E, P1548E, and R1600E. (F–H) MT binding assays to identify the MT binding sites of the DdMF1 and DdMF2 MF domains. Shown are representative examples of binding analysis using SDS/PAGE (top) and quantification of the binding for two different concentrations of tubulin polymer at approximately half maximal or saturation binding (1 and 7 µM for DdMF1; 0.5 and 2 µM, respectively, for DdMF2). (F) DdMF1 mutant #2 K1157E, H1159E, K1161E, and K1174E. (G) DdMF2 mutant #1 K1896E, K1900E, K1909E, and K1912E. (H) DdMF2 mutant #2 K1881E, R1882, K1909E, K1912E, and K1913E. (I) The DdMF1 and DdMF2 MT binding surfaces are different. Ribbon representation of the MT binding surface of DdMF1 (Left) and DdMF2 (Middle). The two surfaces are directly compared (Right) in the same orientation as in Fig. 4 B and C. Residues participating in MT binding are highlighted. Residues identified by mutagenesis are shown in dark colors (orange, DdMF1; blue, DdMF2) and other residues in this surface that could participate to the MT binding are in lighter colors.
Careful study of the surface of the Myo10 MyTH4 domain revealed additional positively charged and hydrophobic residues that could contribute to MT binding. A set of six mutants were generated and tested for MT binding (Materials and Methods), establishing that, besides the previously implicated residues (R1643, K1647, and K1650), three additional charged residues contribute to MT binding: K1654, R1657, and R1600 (Table 1 and Fig. S3 B–E). The binding surface (MTB) is found to be extended and includes two regions with basic and hydrophobic residues (Fig. 4A): The first part corresponds to the originally described site (27) that includes several basic residues and a nonstrictly conserved tyrosine (Y1673) and the second part of the site involves R1600 and two nearby Pro residues. In fact, MT binding activity is drastically reduced only when both sites on this surface are mutated (see M10-mut #3 and #4; Fig. 3G and Fig. S3 C and D). Interestingly, conservation of these surface residues among different Myo10 sequences, from the evolutionarily distant Choanoflagallate Monosiga brevicollis to human (Fig. 3A), suggests that the interaction with MTs [which is crucial in vertebrates for orienting the mitotic spindle by anchoring it to the cortex (11, 32)] is likely to be an ancient property of Myo10 myosins.
Fig. 4.
The MT binding surfaces of distant MF myosins are distinct. (A) Surface representation of the MyTH4-FERM domain from human Myo10. The MT binding residues in the MyTH4 domains are highlighted using the same color code as in Fig. 3A. The central and right figures show the MyTH4 domain only. (B and C) Surface of the MyTH4 domain from DdMF1 (B) and DdMF2 (C). The sequence-predicted MT binding site (equivalent to that of Myo10) is shown in light blue with K1261. The MT binding residues identified by mutagenesis are shown in dark blue and the residues on this surface that likely participate in MT binding are in green (charged residues) and yellow (hydrophobic residues noted as P1 for P1898; I for I1915, Y for 1928, and P2 for P1929). Note that the MT binding site of the DdMF1 and DdMF2 are on the opposite surface compared with that of Myo10MF (light blue predicted residue).
Characterization of the DdMyo7 MT Binding Sites.
Candidate MT binding sites for both DdMF1 and DdMF2 were identified using a similar approach. First, the equivalent residues implicated in MT binding by Myo10 that are conserved in DdMF1, R1257, and K1261 (Fig. 3A) were mutated and assayed. Charge reversal of both residues did not affect binding of DdMF1 to MTs (mut#1; apparent Kd = 1.96 ± 0.31 µM, n = 3) (Table 1), revealing that despite sequence conservation these basic residues of DdMF1 are not part of the MT binding site. In fact, these residues are not free to participate in MT binding in DdMF1, because they are involved in stabilization of the N-terminal extension, which differs greatly between the Myo10 and DdMF1 MyTH4 domains (Fig. S2A). The overall surface of the MT is negatively charged (Fig. S4A), although recognition via hydrophobic residues is also likely necessary for specificity. Analysis of the surface of the MyTH4 domain on the opposite side from the Myo10 MTB (Fig. 4A) revealed the presence of a cluster of exposed, positively charged, and hydrophobic residues that seemed to be good candidates to serve as an MT binding site. Mutation of four charged residues on this surface, K1157E, H1159E, K1161E, and K1174E, abolished almost all MT binding (Fig. 4B and Fig. S3F). The structure of this MF1 mutant #2 shows that the mutations do not affect the protein structure and only influence the MT binding surface (Fig. S1C), which might in fact be extended by three other basic residues found nearby K1145, K1147, and K1149 (Fig. 4B). These results reveal that the MyTH4 MT binding site is not generally conserved but that positively charged motifs with surface-exposed hydrophobic side chains found elsewhere in the myosin MyTH4 domain can serve as an MT binding site.
Fig. S4.
Model of Myo10 MF binding to MT illustrating steric hindrance by DCC binding. (A) Electrostatic surface potential of a mammalian protofilament [Left (83)] and a D. discoideum homology-modeled protofilament [Right, Swiss model (46)]. Note the abundance of negative charges (red) on both surfaces. The C-terminal region of both α-tubulin and β-tubulin are indicated with gray and black arrows, respectively. (B and C) Model of the interaction between HsMyo10 MF (green, B), DdMF2 (light blue, C) and the MT (red/blue) using the MT binding surface identified in Fig. 4. Note that the binding would be impaired if the FERM domain was engaged in binding partners via the F3 lobe, such as DCC (pink) (mode ①). In DdMF2, different restrictions for cooperative binding of partners and MT sites would apply because the MT binding site is located on the opposite surface of the MyTH4 domain for this myosin MF domain, compared with that of Myo10.
The DdMF2 MT binding interaction was characterized by first assessing its binding to subtilisin-treated MTs. In contrast to the substantial drop in binding that was observed with the Myo10 MF, DdMF2 binding to subtilisin-MTs was only modestly decreased (apparent Kd = 0.84 ± 0.39 µM, n = 5) (Table 1). Similar to DdMF1, the structure of DdMF2 allowed for the identification of surface-exposed charged residues that could serve as the MT binding site (Fig. 4C). A positively charged surface was found on the same face of the MyTH4 domain compared with the identified MT binding site of DdMF1 (Fig. S3I), opposite to that of Myo10. Two DdMF2 charge reversal mutants were tested for MT binding (mutant #1: K1896E, K1900E, K1909E, and K1912E; mutant #2: K1881E, R1882E, K1909E, K1912E, and K1913E). Both of these exhibited little or no binding to MTs (Fig. S3 G and H). The structure of the MF2 mutant #2 shows that the mutations do not affect the protein structure and only influence the MT binding surface (Fig. S1D and Table S1). The central basic residues of these mutants, K1896, K1900, and K1912 (which are equivalent to the K1157, K1161, and K1174 residues important for MT binding in MF1) are likely important for mediating the MT binding of DdMF2. Note, however, that significant differences exist on this surface between DdMF1 and DdMF2 due to the variation in the Nter sequence as well as in the loops prior and after helix 1 (Fig. S3I). Because the contribution from the C-terminal tail of tubulin is not a major part of the interaction with DdMF2, it is likely that the interaction surface with the MT is extended and involves other residues of this MyTH4 surface composed of basic residues and few hydrophobic residues such as P1898, I1915, Y1928, and P1929 (Fig. 4C).
Conservation of the MT Binding Motifs Among Evolutionarily Distant MF Myosins.
The MyTH4 domains thus bind to MTs using largely electrostatic interactions, although the exact interaction site differs greatly in the surface involved and its topography and composition. It is of interest to note that whereas the surface of the MT is found to contribute to the binding for both Myo10 and DdMF2, interaction with the tubulin C-tail contribution is mostly important for Myo10 binding. This recognition could differentially target Myo10 to specific populations of MTs depending on the posttranslational modifications on their C-tail. The differences in the MT binding sequence between the three MyTH4 domains raises the question of how conserved these sequences are within a MF myosin class and when they emerged during evolution. Alignment of the MyTH4 domains of various myosins reveals that the Myo10 MT binding surface is well conserved in this myosin class from Capsaspora, a distant unicellular relative of Metazoa, to humans (Fig. 3A). Note that although the Ciona Myo10 MyTH4 has a Gln in place of the second Lys in the MT binding region this residue is not critical for MT binding based on the finding that mutation of this residue in Myo10 (K1647D) does not significantly affect MT binding (Table 1). Examination of the same region of other metazoan and fungal myosin MyTH4 domains reveals poorer conservation, consistent with the identified MT-binding site being specific for Myo10. Similarly, the MT-binding sequences for both DdMF1 and DdMF2 are highly conserved among the different social amoeba groups that are believed to have been evolving independently of Metazoa for over 600 million years (33). Thus, the MT binding properties of MyTH4 domains are a good example of convergent evolution.
Variability and Characteristic Features of the FERM Domains from Evolutionarily Distant Myosins.
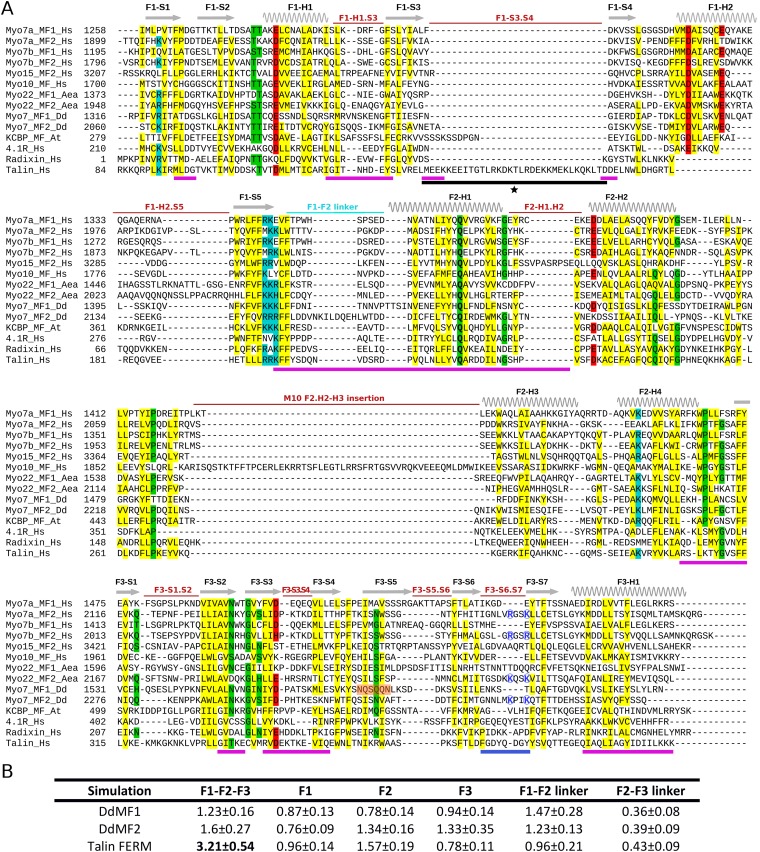
The FERM domains of MF myosin tails are a tripartite protein interaction module composed of the F1, F2, and F3 lobes, the same as the ERM and talin proteins. They mediate binding to a number of different partner proteins, including membrane receptors, and they are implicated in autoinihibition of motor activity (18, 20, 26, 34). Little is known about the features of the FERM domain that determine myosin autoinhibition, specificity of the interaction with several partners, and ability for these partners to bind cooperatively or to exclude each other on a particular myosin. A structure-based comparison of FERM sequences was carried out to reveal the characteristic properties of this domain that contribute to its function in myosin motors. Comparison of the structures of the four MF myosin FERM domains reveals conservation in the fold of the individual lobes of the myosin FERM domains as well as with other FERM domains (Fig. 1C). However, there is notable variability in the loops that connect the structural elements of each lobe (Fig. 5A and Fig. S5A). In addition, the lobes are arranged together to form the canonical cloverleaf configuration, in contrast to the elongated conformation found in talin (35) (Fig. 6). This analysis shows that several of the structural features that determine the formation of the cloverleaf are variable. Insertions are found on two different surfaces: On one side, important variations in the loops of all three lobes are found on the same surface as that where large insertions exist for the MyTH4 domains of Myo7a MF1 and Myo10 (Tables S3 and S4 and Fig. 5). Insertions are also found on the opposite side of the FERM domain, arising from variations in loops of the F1 and F2 subdomains as well as the F1-F2 linker. Structural differences are likely to correlate with diversification and specificity for partner binding and thus the special cellular functions of these myosins. We have thus analyzed whether functional roles, such as autoregulation or binding to specific partners, can be associated with these differences.
Fig. 5.
Variability of the FERM domain. (A) Structural representation of the Myo10 MF domain with the conserved core of the MyTH4 and FERM lobes (gray) and the variable regions among FERM domains (in red, yellow, and cyan). Shown also is the Myo10 insert in the MyTH4 domain (green cylinder). The orientation (indicated schematically with cartoons on the right) was chosen to visualize the two opposite surfaces that show variability (red features on the same side as that of the M10-L0 MyTH4 insert, and yellow and blue on the opposite surface). A 120° view of this surface is shown in B (lower panel) to better visualize the opposite surface. (B) Representation of the MF domain (gray) in which the position of partners that have been cocrystallized with a FERM domain are shown (see Table S5). The four modes of binding to the FERM domain previously identified are indicated by ① through ④. Several partners have been shown to bind the C-terminal F3 lobe, most of them by extending the beta sheet near the S5 strand (mode ①). The interface between the three lobes (binding mode ④) is exploited by some partners, such as Cen1 and Heg1. The dashed line represents the rest of the CEN structure that is not modeled in the X-ray structure. (C) Note that the DdMF1 F3 structure is not compatible with the binding in mode ① due to a deformation of the S5 strand. (D) The extended cleft buried in between the three FERM lobes is drastically shortened at the interface between the F2-F3 lobes in Myo10 due to the specific F2-H2.H3 insertion (red). Note that steric hindrance would occur between this insertion and a peptide bound in the cleft, thus peptide binding in the cleft would require following a different path (black arrow).
Fig. S5.
Structure-based alignment of representative FERM domains and molecular dynamics. (A) Residues that are absolutely conserved are indicated in bold. Residues that are highly conserved are highlighted in yellow (hydrophobic residues), blue (positively-charged), red (negatively-charged), and green (other residues). The variable regions between the conserved elements of each lobe are indicated in red, on top of the sequences. Residues present on the surfaces that participate in the maintenance of the cloverleaf structure (Fig. 6) are indicated with a magenta line below the sequences. Highlighted in orange is the F3-S5 strand that adopts a different conformation in DdMF1 as shown in Fig. 5C (note the absence of the conserved hydrophobic residues). The conserved insertion found in the F1 lobe of talin is indicated with a black line and a star below its sequence. The blue line below the sequence highlights the Myo7 regulation loop between the S6 and S7 strands of the MF2 F3 lobe. The conserved lysines in this loop are highlighted with blue circles. Interestingly, basic residues for this loop are not conserved for other myosins such as Myo10 MF (see also Fig. S6) and Myo15 MF2. (B) Rmsd of the backbone atoms of the secondary structure elements after least-square fit on the respective crystal structures. The average rmsd ± SD (angstroms) of the F1-F2-F3 assembly in molecular dynamics simulations is significantly higher in the elongated talin FERM than in myosin MF domains, which feature the cloverleaf organization. The high value observed for the F1-F2 linker in DdMF1 and DdMF2 is due to punctual rearrangements rather than higher flexibility, as demonstrated by the similar values in the rmsd standard deviations. The F2-F3 linker and the lobes themselves are conformationally stable.
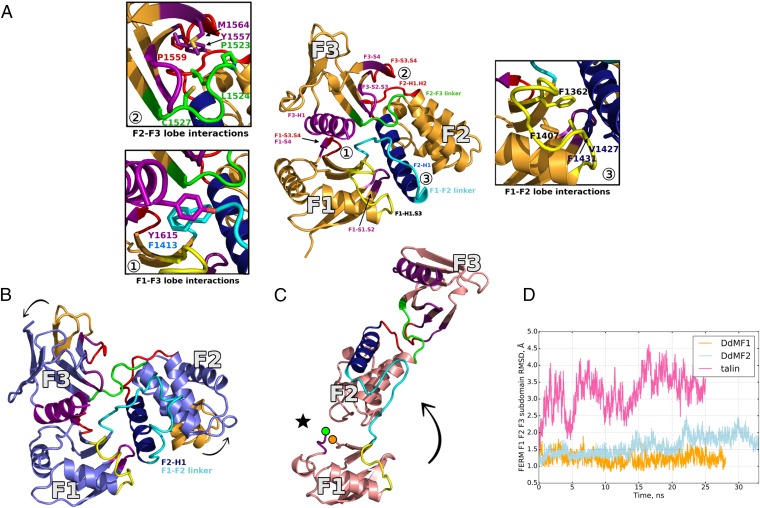
Fig. 6.
Conservation of the FERM cloverleaf structure. (A) Major contacts between the lobes of the FERM domain. The cloverleaf configuration is stabilized by interlobe interactions as illustrated here on the DdMF1 structure (orange). The variable regions involved in these interactions are shown in red and yellow, except for the interlobe linkers shown in cyan (F1-F2) and green (F2-F3). Other less variable regions at the interface of the three lobes are shown in purple and blue (F2-H1 helix). The three major surfaces participating in the maintenance of the trilobe structure are shown in detail: The F1-F3 surface (①) is mainly maintained by hydrophobic interactions between the F1-S3.S4 loop, the F1-S4 strand and hydrophobic residues present in the F3-H1 surface; the F2-F3 surface (②) is formed by the F2-H1.H2 loop, the F2-F3 linker, and the F3-S2.S3 and F3-S3-S4 loops; and the F1-F2 interface (③) includes the F1-S1.S2 and F1-H1.S3 loops of the F1 lobe and the highly variable F1.F2 linker between these two lobes as well as the first helix (F2-H1) of the F2 lobe. (B) The DdMF2 structure is shown in purple, with the elements participating on the maintenance of the FERM structure highlighted using the same color code as in A. The F1 lobe is kept in the same position as in A, but note that the F2 and F3 lobes are displaced (black arrows, part of the DdMF1 lobes are shown in orange as a reference). The F1-F2 linker (cyan) is bigger in DdMF2, forcing the F2 lobe to rotate by ∼30° (black arrows), and this also changes the position of the F3 lobe. (C) Structure of the Talin FERM domain (35). Note how talin adopts an extended conformation, rather than the canonical trilobe FERM structure. The F1 lobe is represented in the same orientation as in A and B, but the F2 lobe position differ (black arrow). Note the difference in the F1.F2 linker (blue) that interacts with the F2 lobe. The position of a large (31 residues) and flexible F1-S3.S4 linker (see Fig. S5) not modeled in the crystal structure is indicated with a black star (the beginning and the end of the linker is indicated with a green and an orange dot, respectively). (D) Conformational dynamics of the FERM domain from molecular dynamics simulations. The plot shows the rmsd from the crystal structure of the backbone atoms of FERM lobes F1, F2, and F3 for DdMF1 (orange), DdMF2 (light blue), and talin (pink). The rmsd time series show that the cloverleaf organization of the myosin FERM is stable, whereas the talin FERM domain undergoes significantly higher fluctuations.
Table S4.
FERM domain inserts and variable loops
 |
Hinges of the FERM in red: controls the overall shape of the FERM domain. See Fig. 6.
Surface binding sites in blue: binding modes①, ②, and ③; see Fig. 5.
Inner cleft binding mode ④ in green; see Fig. 5.
Sequences from the following organisms were aligned: Metazoa: Ce (Caenorhabditis elegans), Ci (Ciona intestinalis), Hs (Homo sapiens), Hb (Heterorhabditis bacteriophora); Placozoa: Tia (Trichoplax adhaerens); Ctenophora: Mnl (Mnemiopsis leidyi); Filasterea: Co (Capsaspora owczarzaki); Ichthyosporea: Aop (Amoebidium parasiticum); Nucleariidae: Foa (Fonticula alba); Fungi: Gnp (Gonapodya prolifera), Prs (Piromyces); Amoebozoa: Ays (Acytostelium subglobosum), Ppp (Polysphondylium pallidum) (Dataset S1).
Myosin activity must be tightly regulated in cells so that the motor is only active at the right place and at the right time. This control is especially crucial because a number of myosins are multifunctional and can bind to a variety of cargos (3). A prevalent mode of myosin regulation is via head–tail autoinhibition, although the exact molecular mechanism for this control is not well understood for many myosins. Because this is a commonly used mode of regulation it might be anticipated that the MF myosin FERM domains would have a conserved structural feature that plays a role in this regulation. Residues in the F3 S6.S7 loop of the fly Myo7a C-terminal FERM domain have been implicated in mediating head–tail autoinhibition of this myosin (26). Interestingly, sequence alignments reveal that these basic residues are in a conserved loop in several C-terminal MF2s (e.g., mammalian Myo7a and Myo7b, Drosophila Myo7a, as well as representatives of both amoeboid Myo7 and Myo22 classes). Notably, this loop is longer in the mammalian Myo7 and DdMyo7 MF2s than in MF1s such as Myo7a MF1 or DdMF1 (Figs. S5A and S6 and Dataset S1). However, this region is not highly conserved in all C-terminal MF domains—the loop is missing in Myo10 MF and the Myo15 MF2 sequence is quite different and lacks the critical charged amino acids. The high degree of sequence conservation in this regulatory loop raises the question of whether this is an ancient feature of MF myosins. Alternatively, convergent evolution may have guided the ability to conserve motor/cargo interactions in these distant MF classes for a similar inhibitory autoregulation mechanism in the Myo7/Myo22 class, whereas different mechanisms of motor autoregulation might have developed for other classes in which inserts or deletions would not favor intramolecular interactions to occur via the F3-S6.S7 loop, as seems to be the case for Myo10 (Fig. S6). Further combined structural and functional studies of the MF myosin FERM domain and its role in regulation are needed to gain a full understanding of the underlying molecular mechanism of the intramolecular control of myosin function.
Fig. S6.
The conserved MF2 autoinhibition loop. Comparison of the regulatory F3-S6.S7 loop in the F3 subdomain. Mutations in two conserved basic residues of this loop (blue spheres) in the Drosophila Myo7a MF2 domain cause the myosin to be constitutively active as they impede the head–tail interaction (26). These two lysines are conserved in the MF2 domains of Myo7s and Myo22, but not in their MF1 domains (Fig. S5). For comparison, the F3 lobe of the Myo10 is shown in magenta using the ribbon representation. Note that the F3-S6.S7 loop is smaller than in DdMF2. (Inset) A detail of the F3 lobe.
FERM domains bind to different partners and there are several distinct modes of FERM binding by partners mediated by the three-lobed cloverleaf structure (Fig. 5 B and C and Table S5). Comparison of the binding between several different FERM domains and their partners, for which an atomic structure has been solved, reveals four major sites of interaction (Fig. 5). A well-characterized binding site is located in the groove between the S5 strand and H1 helix of the F3 lobe that is tuned for binding to partners such as the cytoplasmic tails of β-integrin and DCC (18, 34) (Fig. 5B, mode ①). The cytoplasmic tails of β-integrin also bind to the same site on the F3 lobe of talin (36). Interestingly, an insertion in the DdMF1 sequence changes the surface of F3, and recognition via this F3 lobe cannot occur as found for other MF lobes. This illustrates again how insertions in the MF sequence can lead to a loss of function. Another main binding site of interest is the central cavity between the three lobes of the FERM where Cen1 interacts with Myo7a MF1 and Heg1 with the KRIT FERM domain (Fig. 5B, mode ④). This binding site is more buried and its accessibility in each FERM is modulated by class-specific variations in all three lobes. Note in particular how differences in Myo10 (in the F3-H1 helix, the variable loops F1-S3.S4 and F1-H2.S5) as well as the presence of inserts in the F2-H2.H3 loop remodel the central peptide binding site (Fig. 5D).
Table S5.
Interactions observed for the MyTH4 and the FERM domains
| Binding protein | MyTH4/FERM | Binding mode | RCSB PDB ID code | Ref. |
| MyTH4 binding protein | ||||
| SANS Cen2 | Myo7a MF1 | 3PVL | 30 | |
| FERM binding protein | ||||
| CD43 | Radixin FERM | ① | 2EMS | 73 |
| CD44 | Radixin FERM | ① | 2ZPY | 74 |
| DCAF1 | Merlin FERM | ① | 3WA0 | 75 |
| 4P7I | 76 | |||
| DCC | Myo10 MF | ① | 3AU4 | 27 |
| 3PZD | 29 | |||
| ICAM-2 | Radixin FERM | ① | 1J19 | 77 |
| Integrin | Talin FERM | ① | 1MK7 | 36 |
| KRIT1 | SNX17 FERM | ① | 4TKN | 78 |
| NEP | Radixin FERM | ① | 2YVC | 79 |
| RIAM | Talin FERM | ① | 2MWN | 80 |
| Selectin | SNX17 FERM | ① | 4GXB | 81 |
| Moesin C-ter | Moesin FERM | ② | 1EF1 | 38 |
| Rap1 | KRIT1 FERM | ③ | 4DXA | 37 |
| SANS Cen1 | Myo7a MF1 | ④ | 3PVL | 30 |
| Heg1 | KRIT1 FERM | ④ | 3U7D | 82 |
For the proteins binding to the FERM domain, the four modes of binding previously identified are indicated by ① through ④; see Fig. 5. The coordinates for the complexes as well as the reference that describes the interactions are given.
Analysis and comparison of FERM domains highlights how the inserts and variable regions in the MF domains are crucial to the interaction with different partners. In addition to fine-tuning of the sequence in conserved binding sites for specific partner recognition, inserts introduced during evolution likely contribute to emergence of function by restricting or modulating the accessibility of certain binding modes. It remains to be seen whether such inserted sequences also represent new binding modes highly specific to a particular myosin class; their sequence conservation within a particular class would favor such a hypothesis (Tables S3 and S4).
Maintenance of the Cloverleaf Configuration over a Billion Years of Evolution.
The myosin FERM domains have a cloverleaf organization typical of ERM proteins and several of the binding modes involve more than one lobe (Figs. 5 and 6). Maintenance of the overall orientation and organization of the three lobes into the cloverleaf is thus critical for these interactions. Comparison of the structures and sequences of different myosin FERM domains shows that the relative orientation of the lobes for each FERM domain is distinct, although general features are conserved between the different myosins and the ERM family member FERM domains (Fig. 1C and Fig. S5A). The amino acids that participate in maintaining the cloverleaf are not highly conserved, although they are similar in nature. In addition, connecting loops fine-tune the position and, likely, the dynamics of each subdomain. Controlled flexibility within the FERM lobes is critical to favor selective binding in the central region (Fig. 5B, mode ④) or to binding sites that extend over two lobes such as that of Rap1 and the Cter of Moesin (37, 38) (Fig. 5B, modes ② and ③).
The cloverleaf configuration of the myosin FERM domains and ERM proteins contrasts with the distinct open conformation of the talin FERM domain (35) (Fig. 6). A change in the linker between the F1 and F2 lobes has a dramatic effect on the orientation of the two domains. In the case of talin, the linker adopts a different, hairpin-like, conformation and strongly interacts with the F2 subdomain, but it does not interact with the F1 lobe (compare Fig. 6 B and C). Molecular dynamics simulations indicate that the talin FERM domain exhibits an increased flexibility, which results from enhanced interlobe rigid-body movements (Fig. 6D and Fig. S5B). In sharp contrast, the cloverleaf configuration of the myosin FERM domains (DdMF1 and DdMF2) seems to be stable on the simulation timescale. The reduced flexibility of the myosin FERM cloverleaf would favor the emergence of cooperative binding sites, promoting efficient recognition by binding partners. Thus, it seems that the lobes of myosin FERM domains have evolved to maintain their cloverleaf interactions, remarkably, even though rather large sequence variation is observed for the residues that mediate these interlobe interactions (Fig. S5A).
MF Domain Organization Is Critical for Selective and Simultaneous Binding of Partners for Each Cellular Role of a Myosin.
Dynamics of the FERM lobes is important for binding of peptides in the central cleft but the mobility must be restricted to favor efficient recognition of the binding sites by the partners, in particular when binding requires more than one lobe/domain. Although the MF myosins are multifunctional motors, restriction in the MF supramodule mobility could also favor selectivity in the assembly of several partners to the tail of these myosins. It can favor simultaneous binding of cargos for cotransport in cells while preventing other associations, such as binding to MTs, from occurring if partners are bound elsewhere. This concept of negative or positive cooperativity is illustrated in Fig. S4 B and C. Although simultaneous MT and DCC binding is not possible for Myo10 (27), it is interesting to note that the DdMF2 MT binding site could be compatible with partner binding to the F3 lobe (mode ①). Note also that the orientation of the FERM domain upon recruitment by membrane-bound partners could restrict the availability of the surface that binds to MTs, and that these restrictions would be quite different in DdMyo7 compared with Myo10, which have their MT binding sites defined on opposite surfaces. Without strict orientation of the domain by the supramodule or restriction in lobe dynamics via the cloverleaf, such cooperation between binding sites would be lost. Interestingly, MF myosin tail evolution has kept these general features among classes without strict conservation of the sequences involved, allowing not only the conservation of the general dynamic properties of the MF supramodule but also its adaptation to increase emergence of new functions. This indeed illustrates how, upon gene duplication and evolution, distinct MF classes can acquire new functionalities. The restriction in the dynamics of the supramodule along with the variability on the surface of the MF domain can lead to drastic change in the location of the surface involved in a functionality, and this can lead to new combinations/restrictions for simultaneous partner binding and thus new cellular roles for these myosins.
Conclusion
The myosin MF domain has maintained a high degree of structural similarity over the 600 million years of independent evolution between Metazoa and Amoebozoa. Structural analysis reveals that for both human and Dicty myosin MF domains the MyTH4 and FERM domains form a supramodule and their FERM subdomains are organized in a cloverleaf configuration. Class-specific sequence variations allow for both changes in the accessible surface and modification of the dynamics between different subdomains that, in concert, determine the specificity for binding partners. Contrary to what one might have expected, the results presented here highlight how conservation of function may not require a strict conservation of a sequence motif involved in protein recognition. Interactions of similar nature between the myosin MyTH4 domains and the MT can be established with different surfaces of the same domain in evolutionarily distant MF myosins. In the case of MyTH4 binding to MTs, keeping a generally basic surface with some hydrophobic residues that can interact with the acidic C-terminal tail as well as with the surface of the MT is an important general feature. This corresponds to a mechanism of gain/loss of function via sequence motifs on different surfaces that have conserved features/properties. Furthermore, conserved residues that are part of a motif that can be identified in a sequence alignment may, in fact, not be able to play their role in the context of different MyTH4 domains due to inserted sequences that result in major surface remodeling (Fig. 2 and Fig. S2A). The high degree of conservation of this activity throughout evolution suggests that it is an important feature of MyTH4 proteins in general and, in particular, MF myosin functions. Much remains to be discovered about the different modes of interaction between MyTH4-MTs and the functional importance of this interaction that potentially cross-links and allows force production between the two main cytoskeleton tracks in eukaryotic cells.
One of the most intriguing aspects of MF domains is the high degree of conservation of the supramodule and the cloverleaf configuration that controls the rotational freedom of the MyTH4 and the three lobes of the FERM domain. The MyTH4 and FERM domains can exist stably apart from each other, suggesting that the supramodule does not simply have a structural role but rather a functional role in restraining the movement between the subdomains. The control and tuning of the dynamics of the MF supramodule ensures efficient cooperative or exclusive binding of partners on different sites of this supramodule. This likely determines the assembly of an MF–partner complex that together defines a particular role for this group of multifunctional motors. Structural studies of myosin MF domains in combination with functional data are needed to uncover the principles encoded in a sequence that allow a particular myosin to play similar roles in different organisms.
The multifunctional myosin tails are particularly interesting because they interact with different partners as well as having critical roles in regulating myosin activity. MF domains bind to the cytoplasmic tails of signaling receptors and adhesion receptors and interact with adapter proteins (18, 20, 21, 34) and contribute to autoinhibition (26). Maintaining the motor in an off-state is crucial for all myosins, preventing the needless hydrolysis of ATP and keeping a pool of the motor protein readily available for cargo binding. Activation occurs by binding of specific partners that may be localized or made available in response to cellular signals. The MF myosin tails exemplify these features of myosin function. Interactions with new partners that could change myosin function undoubtedly arose through the evolution of the MF domain. In contrast, other features have been maintained because they provide a core function for an entire group of myosins, as is exemplified by the maintenance of the highly conserved autoinhibition loop in the F3 lobe of the FERM domain seen in the Myo7, Myo22, and amoeboid Myo7 MF2 domains. In the case of the MF myosins, one particular mechanism emerged early on and has been maintained throughout evolution.
Materials and Methods
Construct Design.
Bacterial expression clones for WT and mutant C-terminally His-tagged DdMF1 (residues 1119–1620), DdMF2 (residues 1835–2357), the MyTH4-FERM cassette (residues 1501–2058), or MyTH4 domain alone (of human Myo10 gene, residues 1871–1906) were generated as described in SI Materials and Methods. The previously described pDTi225 plasmid (9) was used to express DdMF1 in Dictyostelium.
Protein Expression, Purification, and Crystallization.
His-tagged proteins were purified using His-trap columns followed by gel filtration on a Sephadex 200-16/60 column. Crystals of the DdMF1 construct (9.3 mg/mL) were obtained by hanging drop vapor diffusion at 290 K in 18% (wt/vol) PEG 8000 and 0.1 M MES, pH 6.0. Crystals of the bacterially expressed DdMF1 mutant #2 were obtained in 10% (wt/vol) PEG 20K, 20% (wt/vol) PEG 550 MME, 0.1M Mops, pH 7.5, 0.1 M Hepes, pH 7.5, 20 mM dl-alanine, 20 mM dl-glutamic acid, 20 mM glycine, 20 mM dl-lysine HCl, and 20 mM dl-serine using a protein solution at 11 mg/mL. DdMF2 native crystals appeared using a 13 mg/mL protein mixed with 18% (wt/vol) PEG 1500 and 0.1 M MMT (dl-malic Acid, MES, and Tris, pH 8.0). The selenomethionine derivative crystals were obtained in 13% (wt/vol) PEG 1500 and 0.1 M MMT, pH 8.0, using a protein solution at the same concentration as for the native crystals. Finally, the DdMF2 mutant #1 crystallized by mixing a 13 mg/mL protein solution with 11.9% (wt/vol) PEG 3350 and 0.1M Bis-Tris propane pH 7.5). More detail in the protein separation, crystallization and structural determination can be found in SI Materials and Methods.
Equilibrium MT Cosedimentation Assays.
Cosedimentation assays were performed as described (9). Reaction mixes included either 2 µM MF protein or 2.5 µM of DdMyo7 MF1 domain with increasing concentrations of MTs in 20 mM Pipes, pH 6.9, 1 mM MgSO4, 1 mM EGTA, 40 µM taxol, and 1 mM GTP. Equal volumes of supernatant and pellet samples were subjected to SDS/PAGE on either a 10% SDS/PAGE gel or an 8% Bis-Tris gel (Invitrogen) followed by staining with Coomassie Brilliant Blue G-250. The resulting gel was scanned and then quantified using the Analyze Gel module in Fiji (39). The data were plotted as the fraction of MF or DdMyo7 MF1 domain that partitioned to the pellet as a function of increasing MT concentration using KaleidaGraph (Synergy). The following quadratic equation was fit to the data:
where MF⋅MT/MF0 is the fraction of total micromolar MF that partitions to the MT pellet, MF0 is the total micromolar MF, MT0 is the total tubulin polymer in the assay, and Kd is the apparent dissociation constant. The normalization factor a takes into account the fraction of MF0 that partitions to the MT at saturation binding and therefore represents the fraction of the population that is competent to bind MTs. The low fractional binding for DdMF1 (Fig. 3B) is hypothesized to be due to a slow equilibrium of DdMF1 in solution that generates a population that is competent to bind MTs relatively tightly and a population whose conformation cannot. The observation that there is not a significant amount of DdMF1 that partitions to the pellet in the absence of MTs, as would be expected for degraded protein, supports this interpretation.
Structure and Sequence Analysis.
The sequence alignment of the MyTH4 and FERM domains were carried out using the MUSCLE multiple sequence alignment program (40) or online using PROMALS 3D (41) with the human Myo10 MF (PDB ID code 3AU5) and Myo7A MF-SH3 (PDB ID code 3PVL) domains as input structures. All MF myosin sequences used in the alignment are available from CyMoBase (www.cymobase.org/cymobase) (5). Representative sequences from the Amoebozoa, SAR, and Opisthokont (from Fungi to Metazoa) branches of the evolutionary tree were used for the alignments. All structural figures were prepared by PyMOL (www.pymol.org/). Methods for the molecular dynamics simulations can be found in SI Materials and Methods.
SI Materials and Methods
Construct Design.
The regions of the Dictyostelium Myo7 gene (GenBank accession no. L35321.2) encoding either the DdMF1 (1119–1620) or DdMF2 (1835–2357) domains were PCR cloned (Strataclone; Agilent) using a full-length genomic clone as a template (42). The regions were then cloned into a pET-23 plasmid (Novagen/EMD Millipore) for expression in Escherichia coli. The resulting plasmids encode the MF domain followed by a 6xHis tag at the C terminus. DNA fragments encoding the MyTH4-FERM cassette (residues 1501–2058) of human Myo10 gene (GenBank accession no. NP_036466) were amplified by PCR and cloned into a modified pET-14 plasmid (Novagen/EMD Millipore) that lacks the thrombin cleavage site. As previously described (29), 36 residues were deleted from the FERM domain to avoid protein degradation (Myo10MF: residues 1871–1906). The truncated Myo10-MyTH4 construct (Myo10MyTH4: residues 1501–1699) was generated by inverse PCR. The resulting plasmids encode an N-terminal 6xHis tag followed by an SSG linker then the protein of interest. Mutations eliminating positively charged or nearby surface residues, mutated by themselves or in combination with charge reversal mutations of the MT binding residues, were introduced either using the Quickchange (Agilent) or Q5 Mutagenesis (New England Biolabs) systems per manufacturer’s instructions. The sequences of all PCR generated or mutated clones were verified.
Protein Expression and Purification.
The previously described DdMyo7 MF1 domain [amino acids 1085–1620 (9]) was either purified from E. coli (discussed below) or from D. discoideum cells using a modified protocol. The Dictyostelium expressed S1-MF1 fusion was released from a cytoskeletal fraction with 10 mM MgATP and then digested with tobacco etch virus protease. MF1 was purified from the total digest by FPLC (GE Healthcare) by ion exchange chromatography on a CM-650 column and the MF1 eluted using a linear 20–200 mM NaCl gradient. MF1-containing peak fractions were identified by SDS/PAGE, pooled, dialyzed into 50 mM NaCl, 25 mM Hepes, pH 7.5, and 0.1 mM DTT, and concentrated by ultrafiltration using a 30,000 molecular weight cutoff membrane (Amicon), and then small aliquots were snap-frozen in liquid N2 and stored at −80 °C.
All other human and Dictyostelium MF domains were expressed in E. coli. Expression of the fusion proteins was carried out in E. coli BL21-AI (Invitrogen) or E. coli BL21 (DE3) Gold (Agilent) in 2× YT medium at 20 °C and induced by the addition of 0.2% l-arabinose or 0.2 mM isopropyl β-d-1-thiogalactopyranoside, respectively. Selenomethionine derivatives were prepared using E. coli 834 (DE3) and SelenoMet medium from Molecular Dimensions. The cells were collected by centrifugation, and the pellets were frozen in liquid N2 and stored at −80 °C. On the day of purification, the frozen pellets were thawed rapidly and then lysed using a Broyeur TS cell disruptor (Celld). Following centrifugation the supernatant was applied to a Histrap column (GE Healthcare) and the His-fused proteins eluted with 300 mM imidazole. The resulting peak of MF protein was pooled, dialyzed into 80 mM Pipes, pH 6.8, 40 mM NaCl, 1 mM MgSO4, and 1 mM EGTA then concentrated and further purified by gel filtration on a Sephadex 200-16/60 column in the same buffer. The final pool was clarified by centrifugation, concentrated, and stored in small aliquots at −80 °C.
Taxol-stabilized MTs were polymerized from purified bovine brain tubulin (43). Tubulin was cold-depolymerized, clarified, and polymerized with 1 mM MgGTP at 37 °C, followed by MT stabilization with 40 µM taxol.
Crystallization, Data Collection, and Structure Determination.
The DdMF1 construct was crystallized using the hanging drop vapor diffusion method at 290 K by mixing 1 µL of 9.3 mg/mL protein solution with 1 µL of reservoir solution [18% (wt/vol) PEG 8000 and 0.1M MES, pH 6.0]. The crystals appeared overnight and were subsequently optimized using the microseeding technique and cryocooled in liquid nitrogen in a final solution containing 18% (wt/vol) PEG 8000, 0.1 M MES, pH 6.0, and 25% (wt/vol) PEG 400. Crystals of the bacterially expressed K1157E, H1159E, K1161E, and K1174E DdMF1 mutant were grown by mixing 1 µL of 11 mg/mL protein solution with 1 µL of reservoir solution [10% (wt/vol) PEG 20,000, 20% (wt/vol) PEG 550 MME, 0.1 M Mops, pH 7.5, 0.1 M Hepes, pH 7.5, 20 mM dl-alanine, 20 mM dl-glutamic acid, 20 mM glycine, 20 mM dl-lysine HCl, and 20 mM dl-serine]. Crystals were flash-frozen in the crystallization condition. Crystals of DdMF2 were obtained using the same technique. For the native form of the protein, the crystals appeared using a 13 mg/mL protein solution mixed with a reservoir solution containing 18% (wt/vol) PEG 1,500 and 0.1 M MMT (dl-malic Acid, MES, and Tris, pH 8.0). Crystals grew spontaneously as plates and were cryocooled in liquid nitrogen in 22% (wt/vol) PEG 1,500, 0.1 M MMT, pH 8.0, and 20% ethylene glycol. The selenomethionine derivative protein was crystallized in 13% (wt/vol) PEG 1,500 and 0.1 M MMT, pH 8.0, using a protein solution at the same concentration as for the native crystals. Finally, crystals of the DdMF2 K1881E, R1882E, K1909E, K1912E, and K1913E mutant were obtained by mixing a 13 mg/mL protein solution with a reservoir solution containing 11.9% (wt/vol) PEG 3,350 and 0.1 M Bis-Tris propane, pH 7.5. The crystals appeared overnight and were subsequently optimized using the microseeding technique and cryocooled in liquid nitrogen in a final solution containing 12% (wt/vol) PEG 8,000, 0.1 M MES, pH 6.0, and 25% (wt/vol) PEG 400.
X-ray datasets were collected at the Proxima 1 and Proxima 2 beamlines (SOLEIL Synchrotron). The diffraction datasets were indexed and scaled with XDS (44). The DdMF1 model was solved by molecular replacement with Phaser (45) using a homology model based on mouse Myo7 MF1 (3PVL) built using SwissModel (46). Model editing and refinement was carried out with ARP/wARP (47), Coot (48), and phenix.refine (49). The model of the DdMF1 K1157E, H1159E, K1161E, and K1174E mutant was solved using the structure of DdMF1 WT as a search model for molecular replacement. No molecular replacement solutions were found for the DdMF2 dataset using the MyTH4-FERM published structures, neither using the full structure nor the different domains separately. Phases were calculated by the single-wavelength anomalous dispersion method using the selenomethionine derivative crystals and collecting the data at the peak wavelength of selenium. Seventeen Se sites were found by the PHENIX AutoSol wizard (50). The model generated was refined with autoBUSTER (51) and used as a search model for molecular replacement with the native data. The model was subsequently edited and refined with Coot and autoBUSTER, respectively. Loop building was carried out using buccaneer (52) and ARP/wARP. The model of the DdMF2 K1181E, R1882E, K1909E, K1912E, and K1913E mutant was solved using the structure of DdMF2 WT as a search model for molecular replacement. Statistics on the data collection for the all of the structures and crystallographic statistics of the final models are summarized in Table S1.
Molecular Dynamics Simulations.
Simulations were carried out for DdMF1 (28 ns), DdMF2 (33 ns), and talin F0-F1-F2-F3 module (3IVF, 25 ns). Missing loops in the crystal structures were built using the MODELER program (53). All models were submitted to the Molprobity server to optimize the sidechain flips (54). Hydrogens were added using the hbuild tool of the CHARMM program [version 38b1 (55)]. The most likely protonation states at pH 7 for histidine residues were determined using a multisite titration approach (56). The solvent and protein interior were modeled as continuums with respective dielectric constants of 4.0 and 80.0. A Boltzmann-distributed charge density, corresponding to 150 mM of NaCl at 300 K, was added to model ions in solution. Numerical resolution of the Poisson–Boltzmann equation was done using the Adaptive Poisson-Boltzmann Solver (APBS) (57), through tAPBS (agknapp.chemie.fu-berlin.de/karlsberg). Finally, the protonation probabilities were obtained by Monte Carlo optimization via the Karlsberg2 program (58, 59). All of the other titratable residues were assumed to be in their standard state. Molecular dynamics simulations were run using the CHARMM36 force field (60) with the highly scalable package NAMD 2.10 (61). Short-range nonbonded interactions were truncated using a cutoff of 12 Å and a force-switching function starting at 10 Å. Periodic boundary conditions were applied and the long-range electrostatics was treated by the particle mesh Ewald method with a grid spacing of 1 Å and a sixth-order B-spline charge interpolation scheme. Each structure was first solvated in an orthorhombic box of TIP3P water molecules (62). Na+ and Cl− ions [with the Roux and coworkers parameters (63)] were added so as to ensure the electroneutrality of the system and reach a physiological concentration of 150 mM of salt.
After 5,000 steps of energy minimization, each system was progressively heated by random velocity reassignment from 0 to 300 K during 600 ps while imposing harmonic restraints on the backbone atoms with a force constant of with 10 kcal⋅mol−1⋅Å−2. Restraints were not applied on the reconstructed loops to permit full structural relaxation. Heating was followed by a 2-ns equilibration run at constant temperature (300 K) and pressure (1 atm) in which the restraints were gently decreased to zero. Production runs of ∼30 ns at the same constant temperature and volume were then carried out.
All molecular dynamics runs use the r-RESPA multiple timestep algorithm with a 2-fs timestep for bonded and short-range nonbonded forces and a 4-fs timestep for long-range electrostatics (64). The SHAKE algorithm was used to constrain the length of covalent bonds involving hydrogen atoms (65).
Temperature control at 300 K for equilibration and production was ensured using a Langevin thermostat (friction constant 1/ps for equilibration and 0.1/ps for production). Pressure control at 1 atm was achieved using a Berendsen barostat (66), in which the system is weakly coupled to a bath with a 400-fs time constant and the compressibility of liquid water 4.57 × 10−5/bar. During production dynamics, the orientation of the protein was restrained using an external harmonic potential on the orientation quaternion with a force constant of 1,000 kcal/mol (67). This allowed the use of an anisotropic box by cancelling the overall rotation of the protein, which effectively reduced the total number of water molecules in the system increasing the computational efficiency. Trajectory analysis was done with VMD (68) and Wordom (69, 70), along with ipython (71) and matplotlib (72).
SI Alignment
A structure-based alignment of MF domains from a range of phylogenetically diverse organisms illustrating class-specific insertions in the MyTH4 and FERM domains (see Tables S3–S5). Alignments were made using PROMALS 3D (41) with the human Myo10 and mouse Myo7a MF1 structures (PDB ID codes 3AU5 and 3PVL, respectively) as references. Full-length MF myosin sequences from the following organisms are available at www.cymobase.org/cymobase (5):
Metazoa: Ecdysozoa - (Cag) Crassostrea gigas (oyster), (Lg) Lottia gigantean (owl limpet), (Dap) Daphnia pulex (water flea), (Hb) Heterohabditis bacteriophora (nematode), (Dm) Drosophila melanogaster (fruit fly), (Aea) Aedes aegypti (mosquito); Chordata (Hs) Homo sapiens, (Mm) Mus musculus (mouse), (Xt) Xenopus tropicalis (frog), (Tag) Taeniopygia guttata (finch); Urochordata - (Ci) Ciona intestinalis (sea squirt); Cephalochordata - (Bf) Branchiostoma floridae (lancelet); Echinodermata - (Spt) Strongylocentrotus purpuratus (sea urchin); Hemichordata - (Sck) Saccoglossus kowalevskii (acorn worm)
Cnidaria: (Nv) Nematostella vectensis (sea anemone), (Hm) Hydra magnipapillata
Placozoa: (Tia) Trichoplax adherens
Porifera: (Amq) Amphimedon queenslandica (sponge)
Ctenophora: (Mnl) Mnemiopsis leidyi (water comb jelly)
Choanoflagellatea: (Mb) Monosiga brevicollis, (Pro) Proterospongia
Filasteria: (Co) Capsaspora owczarzaki
Ichtyosporea: (Aop) Amoebedium parasiticum
Fungi: (Gnp) Gonapodya prolifera, (Prs) Piromyces sp. (Chytrid fungus)
Stramenopiles, Alveolata, Rhizaria (SAR): (Alk) Aplanochytrium kerguelense PBS07 (marine protist), (Pyc) Phytophthora capsici (oomycete)
Amoebozoa: (Dd) Dictyostelium discoideum (social amoeba), (Dif) Dictyostelium fasciculatum (social amoeba), (Ac) Acanthemoba castellanii, (Bama) Balamuthia mandrillaris (pathogenic amoeba)
Supplementary Material
Acknowledgments
We thank Pierre Legrand and Andrew Thompson and beamline scientists of PROXIMA1 and PROXIMA2 (SOLEIL synchrotron) for excellent support during data collection; Dr. Holly Goodson (University of Notre Dame), Dr. Daniel Picot, Dr. Isabelle Callebaut, and Karl Petersen for comments on the manuscript; and Karl Petersen for assistance with the alignments. This work was supported by NIH Grant R37 GM054141 (to S.P.G.) and the Fondation pour la Recherche Médicale (FRM) (M.C.). This project was granted access to the high-performance computing resources of Centre Informatique National de l’Enseignement Supérieur under Allocation 2015076644 made by Grand Équipement National de Calcul Intensif. A.H. was supported by grants from the Centre National de la Recherche Scientifique, ANR-13-BSV8-0019-01, FRM, Ligue Nationale Contre le Cancer, and Association pour la Recherche sur le Cancer Subvention Fixe. The A.H. team is part of Labex CelTisPhyBio 11-LBX-0038, which is part of the Initiative d’Excellence at PSL Research University (ANR-10-IDEX-0001-02 PSL). M.A.T. was supported by National Science Foundation Grants MCB-0923743 and MCB-1244235.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.rcsb.org/pdb/home/home.do [PDB ID codes 5EJY (DdMF1), 5EJR (DdMF2), 5EJQ (DdMF1 mutant 2, K1157E, H1159E, K1161E, and K1174E), and 5EJS (DdMF2 mutant 2, K1881E, R1882E, K1909E, K1912E, and K1913E)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600736113/-/DCSupplemental.
References
- 1.Jacob F. Evolution and tinkering. Science. 1977;196(4295):1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney HL, Houdusse A. Structural and functional insights into the Myosin motor mechanism. Annu Rev Biophys. 2010;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 3.Hartman MA, Finan D, Sivaramakrishnan S, Spudich JA. Principles of unconventional myosin function and targeting. Annu Rev Cell Dev Biol. 2011;27:133–155. doi: 10.1146/annurev-cellbio-100809-151502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pylypenko O, et al. Structural basis of myosin V Rab GTPase-dependent cargo recognition. Proc Natl Acad Sci USA. 2013;110(51):20443–20448. doi: 10.1073/pnas.1314329110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odronitz F, Kollmar M. Drawing the tree of eukaryotic life based on the analysis of 2,269 manually annotated myosins from 328 species. Genome Biol. 2007;8(9):R196. doi: 10.1186/gb-2007-8-9-r196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebé-Pedrós A, Grau-Bové X, Richards TA, Ruiz-Trillo I. Evolution and classification of myosins, a paneukaryotic whole-genome approach. Genome Biol Evol. 2014;6(2):290–305. doi: 10.1093/gbe/evu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4(3):246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 8.Tuxworth RI, et al. A role for myosin VII in dynamic cell adhesion. Curr Biol. 2001;11(5):318–329. doi: 10.1016/s0960-9822(01)00097-5. [DOI] [PubMed] [Google Scholar]
- 9.Moen RJ, Johnsrud DO, Thomas DD, Titus MA. Characterization of a myosin VII MyTH/FERM domain. J Mol Biol. 2011;413(1):17–23. doi: 10.1016/j.jmb.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431(7006):325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 11.Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol. 2008;182(1):77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci USA. 2006;103(33):12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerber ML, Cheney RE. Myosin-X: A MyTH-FERM myosin at the tips of filopodia. J Cell Sci. 2011;124(Pt 22):3733–3741. doi: 10.1242/jcs.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belyantseva IA, et al. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7(2):148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 15.Crawley SW, et al. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell. 2014;157(2):433–446. doi: 10.1016/j.cell.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Amraoui A, Petit C. Usher I syndrome: Unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118(Pt 20):4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- 17.Probst FJ, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280(5368):1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, et al. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6(6):523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 19.Liu KC, Jacobs DT, Dunn BD, Fanning AS, Cheney RE. Myosin-X functions in polarized epithelial cells. Mol Biol Cell. 2012;23(9):1675–1687. doi: 10.1091/mbc.E11-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, et al. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development. 2008;135(1):53–63. doi: 10.1242/dev.011437. [DOI] [PubMed] [Google Scholar]
- 21.Boëda B, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21(24):6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi X, et al. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179(7):1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai T, et al. Structure and regulation of the movement of human myosin VIIA. J Biol Chem. 2015;290(28):17587–17598. doi: 10.1074/jbc.M114.599365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umeki N, et al. The tail binds to the head-neck domain, inhibiting ATPase activity of myosin VIIA. Proc Natl Acad Sci USA. 2009;106(21):8483–8488. doi: 10.1073/pnas.0812930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umeki N, et al. Phospholipid-dependent regulation of the motor activity of myosin X. Nat Struct Mol Biol. 2011;18(7):783–788. doi: 10.1038/nsmb.2065. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, et al. A FERM domain autoregulates Drosophila myosin 7a activity. Proc Natl Acad Sci USA. 2009;106(11):4189–4194. doi: 10.1073/pnas.0808682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano Y, et al. Structural basis of cargo recognition by the myosin-X MyTH4-FERM domain. EMBO J. 2011;30(13):2734–2747. doi: 10.1038/emboj.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narasimhulu SB, Reddy AS. Characterization of microtubule binding domains in the Arabidopsis kinesin-like calmodulin binding protein. Plant Cell. 1998;10(6):957–965. doi: 10.1105/tpc.10.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Z, Yan J, Lu Q, Pan L, Zhang M. Cargo recognition mechanism of myosin X revealed by the structure of its tail MyTH4-FERM tandem in complex with the DCC P3 domain. Proc Natl Acad Sci USA. 2011;108(9):3572–3577. doi: 10.1073/pnas.1016567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Pan L, Wei Z, Zhang M. Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo. Science. 2011;331(6018):757–760. doi: 10.1126/science.1198848. [DOI] [PubMed] [Google Scholar]
- 31.Janke C. The tubulin code: Molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206(4):461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon M, Bagonis M, Danuser G, Pellman D. Direct microtubule-binding by Myosin-10 orients centrosomes toward retraction fibers and subcortical actin clouds. Dev Cell. 2015;34(3):323–337. doi: 10.1016/j.devcel.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidel AJ, et al. Phylogeny-wide analysis of social amoeba genomes highlights ancient origins for complex intercellular communication. Genome Res. 2011;21(11):1882–1891. doi: 10.1101/gr.121137.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu XJ, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9(2):184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
- 35.Elliott PR, et al. The Structure of the talin head reveals a novel extended conformation of the FERM domain. Structure. 2010;18(10):1289–1299. doi: 10.1016/j.str.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Alvarez B, et al. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11(1):49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 37.Li X, et al. Structural basis for small G protein effector interaction of Ras-related protein 1 (Rap1) and adaptor protein Krev interaction trapped 1 (KRIT1) J Biol Chem. 2012;287(26):22317–22327. doi: 10.1074/jbc.M112.361295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101(3):259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 39.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RC. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei J, Kim BH, Grishin NV. PROMALS3D: A tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008;36(7):2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titus MA. A class VII unconventional myosin is required for phagocytosis. Curr Biol. 1999;9(22):1297–1303. doi: 10.1016/s0960-9822(00)80051-2. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert SP, Johnson KA. Expression, purification, and characterization of the Drosophila kinesin motor domain produced in Escherichia coli. Biochemistry. 1993;32(17):4677–4684. doi: 10.1021/bi00068a028. [DOI] [PubMed] [Google Scholar]
- 44.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langer G, Cohen SX, Lamzin VS, Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc. 2008;3(7):1171–1179. doi: 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 4):352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terwilliger TC, et al. Decision-making in structure solution using Bayesian estimates of map quality: The PHENIX AutoSol wizard. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 6):582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bricogne G, et al. 2011. BUSTER version 2.10.1 (Global Phasing Ltd, Cambridge, UK)
- 52.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 9):1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 53.Fiser A, Šali A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- 54.Davis IW, et al. MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35(Web Server issue):W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacKerell AD, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102(18):3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 56.Bashford D, Karplus M. Multiple-site titration curves of proteins: An analysis of exact and approximate methods for their calculation. J Phys Chem. 1991;95:9556–9561. [Google Scholar]
- 57.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kieseritzky G, Knapp EW. Optimizing pKA computation in proteins with pH adapted conformations. Proteins. 2008;71(3):1335–1348. doi: 10.1002/prot.21820. [DOI] [PubMed] [Google Scholar]
- 59.Rabenstein B, Knapp EW. Calculated pH-dependent population and protonation of carbon-monoxy-myoglobin conformers. Biophys J. 2001;80(3):1141–1150. doi: 10.1016/S0006-3495(01)76091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25(11):1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 61.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durell SR, Brooks BR, Ben-Naim A. Solvent-induced forces between two hydrophilic groups. J Chem Phys. 1994;98(8):2198–2202. [Google Scholar]
- 63.Luo Y, Roux B. Simulation of osmotic pressure in concentrated aqueous salt solutions. J Phys Chem Lett. 2010;1(1):183–189. [Google Scholar]
- 64.Tuckerman M, Berne BJ, Martyna GJ. Reversible multiple time scale molecular dynamics. J Chem Phys. 1992;97:1990. [Google Scholar]
- 65.Ryckaert JP, Ciccotti G, Berendsen HJ. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J Comput Phys. 1977;23(3):327–341. [Google Scholar]
- 66.Berendsen HJ, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 67.Fiorin G, Klein ML. Using collective variables to drive molecular dynamics simulations. Mol Phys. 2013;111(22–23):3345–3362. [Google Scholar]
- 68.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14(1):33–38, 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 69.Seeber M, Cecchini M, Rao F, Settanni G, Caflisch A. Wordom: A program for efficient analysis of molecular dynamics simulations. Bioinformatics. 2007;23(19):2625–2627. doi: 10.1093/bioinformatics/btm378. [DOI] [PubMed] [Google Scholar]
- 70.Seeber M, et al. Wordom: A user-friendly program for the analysis of molecular structures, trajectories, and free energy surfaces. J Comput Chem. 2011;32(6):1183–1194. doi: 10.1002/jcc.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perez F, Granger BE. IPython: A system for interactive scientific computing. Comput Sci Eng. 2007;9:21–29. [Google Scholar]
- 72.Hunter JD. Matplotlib: A 2D graphics environment. Comput Sci Eng. 2007;9:90–95. [Google Scholar]
- 73.Takai Y, Kitano K, Terawaki S, Maesaki R, Hakoshima T. Structural basis of the cytoplasmic tail of adhesion molecule CD43 and its binding to ERM proteins. J Mol Biol. 2008;381(3):634–644. doi: 10.1016/j.jmb.2008.05.085. [DOI] [PubMed] [Google Scholar]
- 74.Mori T, et al. Structural basis for CD44 recognition by ERM proteins. J Biol Chem. 2008;283(43):29602–29612. doi: 10.1074/jbc.M803606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mori T, Gotoh S, Shirakawa M, Hakoshima T. Structural basis of DDB1-and-Cullin 4-associated Factor 1 (DCAF1) recognition by merlin/NF2 and its implication in tumorigenesis by CD44-mediated inhibition of merlin suppression of DCAF1 function. Genes Cells. 2014;19(8):603–619. doi: 10.1111/gtc.12161. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Wei Z, Zhang J, Yang Z, Zhang M. Structural basis of the binding of Merlin FERM domain to the E3 ubiquitin ligase substrate adaptor DCAF1. J Biol Chem. 2014;289(21):14674–14681. doi: 10.1074/jbc.M114.551184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamada K, et al. Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. EMBO J. 2003;22(3):502–514. doi: 10.1093/emboj/cdg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stiegler AL, Zhang R, Liu W, Boggon TJ. Structural determinants for binding of sorting nexin 17 (SNX17) to the cytoplasmic adaptor protein Krev interaction trapped 1 (KRIT1) J Biol Chem. 2014;289(36):25362–25373. doi: 10.1074/jbc.M114.584011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terawaki S, Kitano K, Hakoshima T. Structural basis for type II membrane protein binding by ERM proteins revealed by the radixin-neutral endopeptidase 24.11 (NEP) complex. J Biol Chem. 2007;282(27):19854–19862. doi: 10.1074/jbc.M609232200. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, et al. Conformational activation of talin by RIAM triggers integrin-mediated cell adhesion. Nat Commun. 2014;5:5880. doi: 10.1038/ncomms6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghai R, et al. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci USA. 2013;110(8):E643–E652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gingras AR, Liu JJ, Ginsberg MH. Structural basis of the junctional anchorage of the cerebral cavernous malformations complex. J Cell Biol. 2012;199(1):39–48. doi: 10.1083/jcb.201205109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alushin GM, et al. High-resolution microtubule structures reveal the structural transitions in αβ-tubulin upon GTP hydrolysis. Cell. 2014;157(5):1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.