Significance
The detection of rare tumor-specific somatic mutations in “liquid biopsies” is limited by the high error rate of DNA sequencing technologies. By sequencing peritoneal fluid from women with high-grade serous ovarian cancer, we demonstrate that duplex sequencing, currently the most accurate sequencing technology, is able to detect one cancer cell among tens of thousands of normal cells. This unprecedented sensitivity also revealed a striking prevalence of extremely low frequency TP53 mutations in normal tissue. Women with and without cancer harbored TP53 mutations of pathogenic consequences, both in peritoneal fluid and peripheral blood. These mutations likely represent a premalignant mutational background that accumulates in cancer and aging.
Keywords: TP53 mutations, ultra-deep sequencing, ovarian cancer, clonal hematopoiesis, premalignant mutations
Abstract
Current sequencing methods are error-prone, which precludes the identification of low frequency mutations for early cancer detection. Duplex sequencing is a sequencing technology that decreases errors by scoring mutations present only in both strands of DNA. Our aim was to determine whether duplex sequencing could detect extremely rare cancer cells present in peritoneal fluid from women with high-grade serous ovarian carcinomas (HGSOCs). These aggressive cancers are typically diagnosed at a late stage and are characterized by TP53 mutations and peritoneal dissemination. We used duplex sequencing to analyze TP53 mutations in 17 peritoneal fluid samples from women with HGSOC and 20 from women without cancer. The tumor TP53 mutation was detected in 94% (16/17) of peritoneal fluid samples from women with HGSOC (frequency as low as 1 mutant per 24,736 normal genomes). Additionally, we detected extremely low frequency TP53 mutations (median mutant fraction 1/13,139) in peritoneal fluid from nearly all patients with and without cancer (35/37). These mutations were mostly deleterious, clustered in hotspots, increased with age, and were more abundant in women with cancer than in controls. The total burden of TP53 mutations in peritoneal fluid distinguished cancers from controls with 82% sensitivity (14/17) and 90% specificity (18/20). Age-associated, low frequency TP53 mutations were also found in 100% of peripheral blood samples from 15 women with and without ovarian cancer (none with hematologic disorder). Our results demonstrate the ability of duplex sequencing to detect rare cancer cells and provide evidence of widespread, low frequency, age-associated somatic TP53 mutation in noncancerous tissue.
The detection of tumor-specific mutations in clinically accessible samples has enormous potential to transform cancer diagnostics, monitoring, and screening. However, a major limitation is insufficiently accurate sequencing methods. Conventional next-generation sequencing (NGS) technologies have a high false positive error rate, which precludes reliable detection of mutations at frequencies <1/100 (1). “Molecular tagging” of single-stranded DNA decreases the rate of false mutations to less than 1 per 10,000 sequenced nucleotides and has been successfully applied to the detection of mutant cancer DNA in a variety of clinical samples (2–6). However, this false positive error rate limits the specificity of this method in challenging situations in which ultra-deep sequencing is needed to detect extremely low frequency mutant molecules (e.g., <1/10,000), as is the case of ovarian cancer DNA in Pap smears (5). Because true mutations are indistinguishable from artifacts, compromised specificity leads to lower sensitivity and overall low diagnostic accuracy. Duplex sequencing is an NGS technology that employs molecular tagging of both strands of DNA independently. True mutations are defined as mutations that are present at the same position in both strands of DNA and that are complementary (7). This internal error correction effectively reduces false positive mutations because PCR and sequencing artifacts are very unlikely to occur at both strands of DNA at the same position and with complementary nucleotide changes (theoretical false positive rate is ∼4 × 10−10) (7). Previous studies have demonstrated that duplex sequencing is able to detect a single point mutation among >107 sequenced nucleotides (7, 8), an unprecedented accuracy that holds significant potential for early cancer detection.
High-grade serous ovarian carcinoma (HGSOC) is the most common and most aggressive type of ovarian cancer, with a dismal 5-y survival rate of 10–30% (9). A main cause of poor prognosis is the absence of effective screening tools to detect early-stage disease. HGSOC frequently metastasizes through the peritoneal cavity, the anatomic potential space between abdominal and pelvic organs and the abdominal walls. The putative premalignant lesion to HGSOC is intraepithelial neoplasia in the distal fallopian tube (also known as serous tubal intraepithelial carcinoma), which is in direct continuity with the peritoneal cavity (10–12). Even in the absence of gross metastasis to the peritoneum, ovarian cancer cells can frequently be found in peritoneal fluid upon cytopathological examination, and the presence of these cells has prognostic value in the current clinical staging system (13). Thus, peritoneal fluid is routinely collected during surgery for women with suspected ovarian cancer. We reasoned that peritoneal fluid is an optimal clinical biopsy for high-sensitivity early detection of ovarian cancer because HGSOC disseminates early and preferentially to the peritoneal cavity, suggesting that cancer cells may be more abundant in peritoneal fluid than in the relatively distant uterine cavity or cervix, as previously attempted by other studies (5, 14).
An important feature of HGSOC, which may facilitate early detection by high-sensitivity sequencing, is the high prevalence of tumor protein p53 gene (TP53) mutations (>96%) (15, 16), even in premalignant lesions (17). Moreover, >95% of TP53 mutations cluster in exons 4–10 (15), which provides a relatively small target to perform cost-efficient ultra-deep sequencing (8). Thus, we used duplex sequencing to sequence TP53 exons 4–10 in peritoneal fluid from patients with HGSOC with known TP53 mutations and control patients without ovarian cancer. Our goal was to provide proof of principle of the ability of duplex sequencing to detect very rare cancer cells, and thus the study was enriched for subjects with early stage disease or negative peritoneal fluid by traditional cytological evaluation.
Results
Patients and Sequencing Information.
Sixteen patients with HGSOC (“ovarian cancers”) (SI Appendix, Table S1) and 20 patients without detected gynecologic malignancy (“controls”) (SI Appendix, Table S2) were included in this study. Seven ovarian cancer patients and 10 control patients had germline BRCA1 or BRCA2 breast cancer genes mutations. Half of the cancers were early stage (0–II). TP53 mutations were determined by NGS in all primary tumors except three occult microscopic cancers, for which Sanger sequencing was used due to the limited amount of DNA (SI Appendix, SI Materials and Methods) (18–20). A single clonal TP53 mutation was found in all tumors (SI Appendix, Table S3). No additional clonal or subclonal TP53 mutations were found in any of the primary tumors (NGS average depth was ∼300×). Peritoneal fluid was centrifuged, and DNA was extracted from the cell pellet, obtaining an average of 11.9 μg of DNA (range 1.1–112 μg). Duplex sequencing for TP53 exons 4–10, which cover >95% of mutations in HGSOC (15), was performed. Molecular tagging of both strands of DNA allowed us to group raw reads sharing the same molecular tag into a single-strand consensus sequence (SSCS) and to collapse the two SSCSs with complementary tags into a single, highly accurate duplex consensus sequence (DCS) (SI Appendix, Fig. S1). The median DCS depth was calculated as the median DCS coverage at each genomic position in the capture target, and this value essentially corresponds to the total number of unique haploid genomes sequenced. The median DCS depth for the 37 peritoneal fluid samples ranged from 1,689 to 36,133. Sequencing information is presented in Dataset S1.
Duplex Sequencing Detected Tumor-Specific TP53 Mutations in Almost All Ovarian Cancer Peritoneal Fluid Samples.
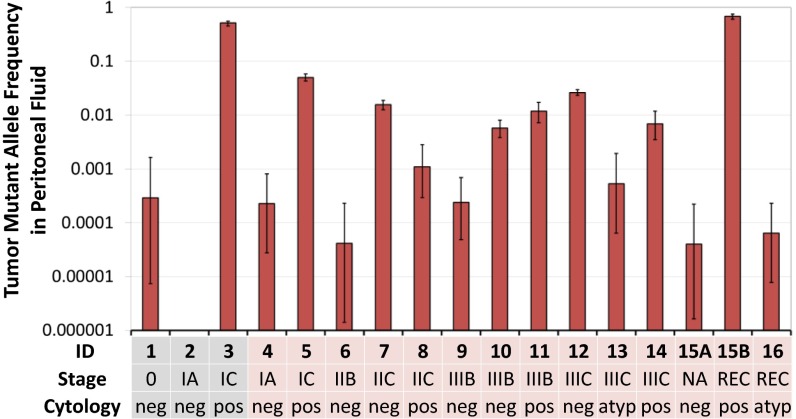
The TP53 mutation identified in the primary tumor was detected in 94% (16/17) of HGSOC peritoneal fluid samples (Fig. 1), including 9 peritoneal fluids without malignant cytopathology. In one of the cases (case 16), the mutation was not detected in DCS reads but was present in two SSCS reads well above background. The only cancer missed was an occult stage IA carcinoma with negative cytopathology. Importantly, half of the tumor mutations (8/16) were found at a frequency at or below 0.001 (3/16 at a frequency at or below 0.0001) and would not be reliably discernible from technical errors with less accurate sequencing techniques.
Fig. 1.
TP53 mutations detected in ovarian tumors are also present in peritoneal fluid. Sample ID, Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) stage, and cytopathological results are indicated in the x axis. Samples 1–3 correspond to cancers incidentally discovered at prophylactic surgery in women with hereditary BRCA mutations. Samples 15A and 15B correspond to primary and recurrence surgeries from the same patient. Sample 15A was unstaged because this patient was undergoing chemotherapy for a previously diagnosed breast malignancy. Tumor mutant allele frequency was calculated as the number of Duplex Consensus Sequence (DCS) reads with the given mutation divided by the total DCS nucleotides sequenced at the mutation coordinate. Tumor 16 was the only one with an indel, and matching peritoneal fluid showed the indel mutations in single-strand consensus sequences, but not DCS. Errors bars represent the exact/Clopper–Pearson 95% confidence interval. atyp, atypical; NA, not available; neg, negative; pos, positive; REC, recurrence.
In one patient, peritoneal fluid samples were available from two different surgeries: primary surgery and subsequent surgery for recurrent disease 2 y later. The exact same tumor-specific TP53 mutation was found in both. The tumor mutant allele frequency (TMAF), which should approximate the fraction of tumor cells in the sample, increased dramatically from 0.000039 to 0.685 from the initial peritoneal wash to the recurrent ascites.
In univariate analyses of dependent variables vs. TMAF in peritoneal fluid, TMAF was not significantly associated with preoperative CA-125 (Spearman test), germline BRCA status, clinical stage, or future recurrence (Mann–Whitney test). Higher TMAF was, however, significantly associated with positive peritoneal fluid cytology (mean ± SD, 0.212 ± 0.30 in positive cytology vs. 0.004 ± 0.009 in negative or atypical cytology, Mann–Whitney P = 0.009) and ascites as opposed to peritoneal washes (mean ± SD, 0.132 ± 0.27 in ascites vs. 0.048 ± 0.15 in peritoneal washes, Mann–Whitney P = 0.009). After adjusting for possible confounding of one variable by another in multivariate models, higher TMAF remained associated with ascites but not with positive cytology. In addition to ascites, older age also associated with higher TMAF in all models (SI Appendix, Table S4).
Low Frequency TP53 Mutations Were Found in Peritoneal Fluid from Nearly All Patients.
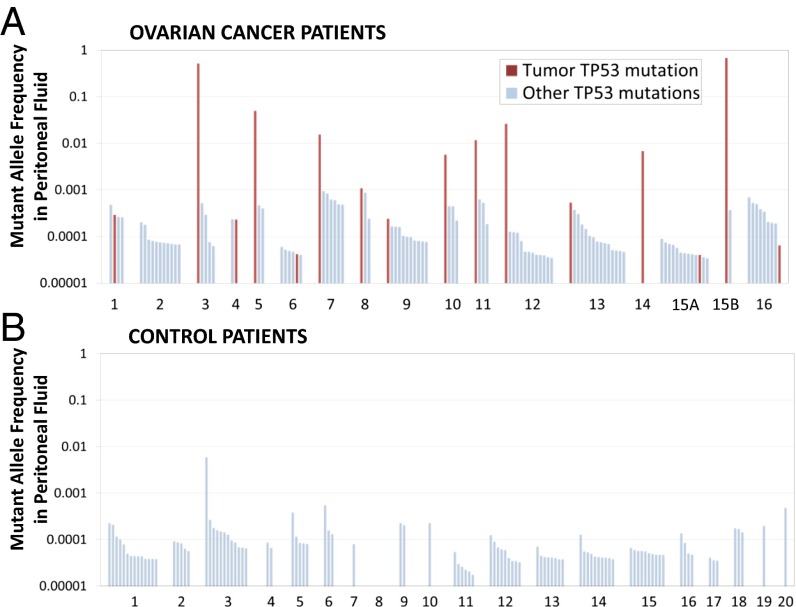
We detected low frequency (<0.001) TP53 mutations in nearly all of the peritoneal fluid samples from women with and without cancer (Fig. 2) (16/17 cancers and 19/20 controls). A total of 197 mutations were found (Dataset S2): 97 mutations in ovarian cancer patients and 100 mutations in controls. It was previously demonstrated that duplex sequencing is able to uniquely detect a single mutation among >107 sequenced nucleotides, which places the false positive rate of this technology below 10−7 (7). Using this figure as a conservative estimate of the false positive error rate and given the fact that a total of ∼3.8 × 108 DCS nucleotides were sequenced in this study, we calculated that potentially ∼38 mutations could be artifacts. This figure represents only 20% of all of the mutations found and leaves >150 mutations unlikely to be explained as technological error. To distinguish them from the tumor mutations, we termed these highly prevalent low frequency TP53 mutations “biological background.”
Fig. 2.
Mutant allele frequency of TP53 mutations (exons 4–10) in peritoneal fluid from patients with ovarian cancer (A) and controls (B). Each bar represents a unique mutation observed at least once. Mutations are ordered by descending mutant allele frequency within each patient. Magenta bars indicate tumor mutations.
The mean number of biological background mutations per sample was 5.3 (range, 0–14). The number of mutations was directly proportional to the DCS depth for each sample (i.e., the deeper a sample was sequenced, the more mutations were found) (SI Appendix, Fig. S2). For cancer patients, the tumor mutation was more abundant than biological background mutations in the majority of peritoneal fluid samples (11/16) and was at least 10-fold more abundant than biological background mutations in 50% (8/16) of samples (Fig. 2A).
Low Frequency TP53 Mutations in Peritoneal Fluid Are Similar to Cancer-Specific TP53 Mutations.
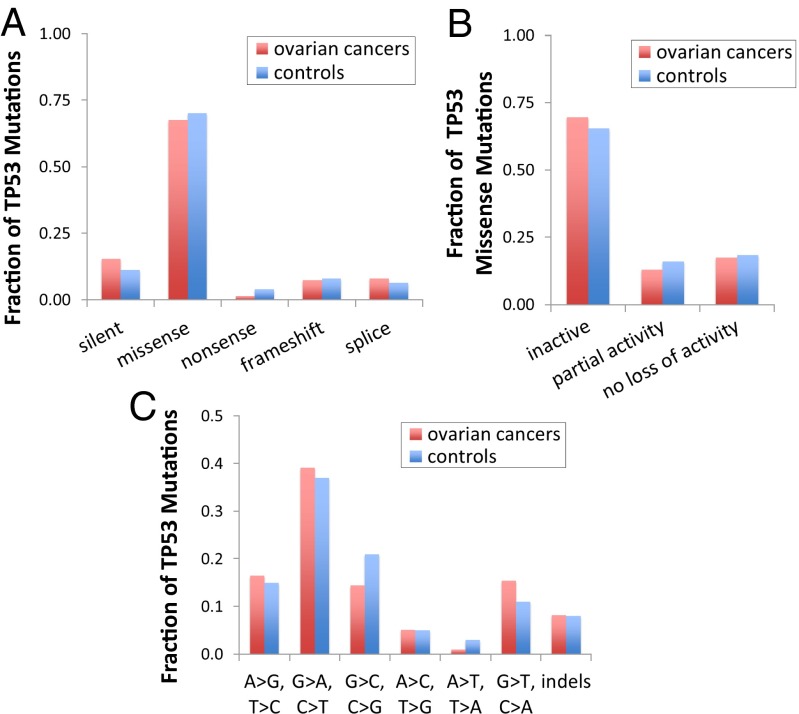
To explore whether these low frequency TP53 mutations were biologically relevant, we first analyzed their type. Most of the background mutations were missense, both in women with (67/97) and without cancer (72/100) (Fig. 3A). The prevalence of missense mutations is consistent with clonal TP53 mutations most commonly seen in human cancers in general and in ovarian cancers in particular, according to the International Agency for Research on Cancer (IARC) ovarian cancer database (21, 22) (SI Appendix, Fig. S3). Specifically, within exons 5–8, which encode the protein’s DNA-binding domain and harbor the majority of TP53 mutations found in human cancers, there are 1,567 possible single nucleotide substitutions, which are expected to produce 73.4% missense, 3.7% nonsense, and 22.9% silent mutations (23). However, in our study, silent mutations were less than half the expected frequency, in both cases and controls (SI Appendix, Fig. S4). Nonsynonymous mutations, which include missense and nonsense, represented 89.8% (53/59) and 90.0% (63/70) of all single nucleotide substitutions in these exons in cases and controls, respectively, significantly above the 77.1% expected in the absence of selection (P = 0.027 in cancers; P = 0.013 in controls, by Fisher’s exact test). Approximately 80% of these missense mutations were projected to generate an inactive or partially inactive p53 protein (Fig. 3B) as determined by TP53-MUT 2.00, an online tool that predicts the biological activity of known TP53 mutations (24). In addition, missense mutations were enriched in the 9 most commonly mutated “hotspot” TP53 codons (175, 179, 195, 220, 237, 245, 248, 273, and 282) in ovarian carcinomas according to the IARC database (21, 22). These codons represent only 2.7% of the codons in our capture set; however, 10/67 missense mutations in cancers (14.9%) and 13/72 missense mutations in controls (18.1%) clustered in those codons (P = 2 × 10−4 and P < 1 × 10−5, respectively, by Fisher’s exact test). Importantly, when cancers and controls were subdivided by BRCA germline status, the distribution of mutation type and the functional impact of missense mutations remained very similar for each of the groups (SI Appendix, Fig. S5). To demonstrate that the findings were not driven by outlier individuals, we also plotted the distribution of mutation type (SI Appendix, Fig. S6) and predicted functional impact of missense mutations (SI Appendix, Fig. S7) for each patient in the study. The overwhelming majority of patients (including 19/20 controls) harbored at least one deleterious mutation. Next, we analyzed the mutational spectra of TP53 mutations found in ovarian cancers and controls (Fig. 3C). The spectrum was similar in both groups and showed an abundance of C:G→T:A transitions. Furthermore, 38.6% of C:G→T:A mutations occurred at CpG sites, which are known to be highly mutable and prone to deamination. These data are consistent with known mutational signatures in cancer associated with age, specifically signatures 1A and 1B reported in Alexandrov et al. (25, 26).
Fig. 3.
Characterization of “biological background” TP53 mutations found in peritoneal fluid of patients with ovarian cancer (97 mutations) and controls (100 mutations). The fraction of mutations is indicated for categories of mutation type (A), pathogenicity (B), and spectrum (C). TP53 activity of missense mutations for B was determined via “MUT-TP53 2.00” (24).
Low Frequency TP53 Mutations Increase with Cancer and with Age.
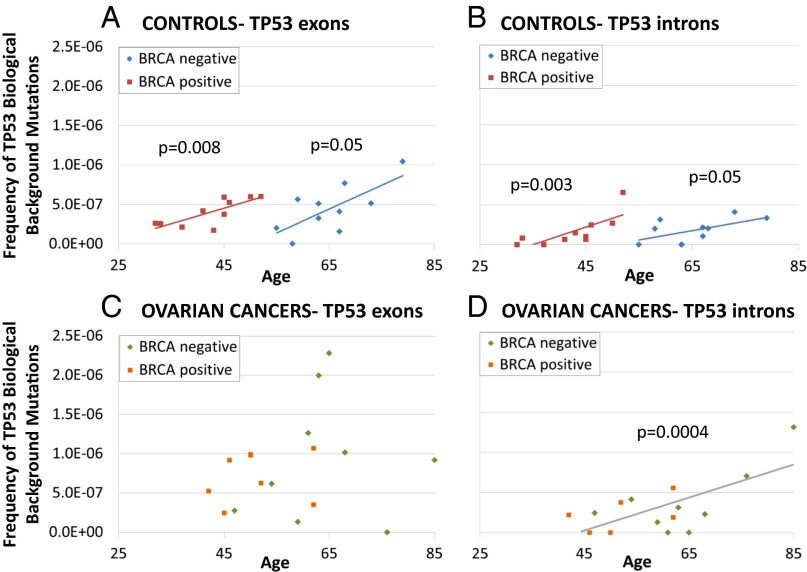
Next, we quantified the frequency of biological background TP53 mutations for each individual as the number of mutations divided by the total number of DCS nucleotides. Interestingly, women with ovarian cancer had a significantly higher frequency of TP53 mutations in exons 4–10 than cancer-free women (8.4 × 10−7 in cancers vs. 4.23 × 10−7 in controls, Mann–Whitney P = 0.02) (SI Appendix, Fig. S8), and this significant difference remained after adjusting for age and BRCA status (P = 0.004). Although our study was focused on TP53 coding mutations, the sequencing protocol resulted in the incidental capture of intronic regions contiguous with TP53 exons 4–10, which also contain low-frequency biological background mutations. The frequency of mutations in introns was higher in cancers than in controls, but this comparison was not statistically significant (SI Appendix, Fig. S8). Notably, in control women, we found that the frequency of TP53 biological background mutations increased with age for mutations found in exons (Fig. 4A) as well as in introns (Fig. 4B). The frequency of mutations increased by 2.2 × 10−7 for every 10-y increase in age for exons (P = 0.001 after adjusting for BRCA status) and by 1.1 × 10−7 for every 10-y increase in age for introns (P = 0.01 after adjusting for BRCA status). These regressions also were done separately for women with and without germline BRCA mutations because of the near complete age separation between those two groups and were statistically significant for mutations in exons and introns regardless of germline mutational status (Fig. 4 A and B) with near parallel slopes, as assumed in the common regression model. In women with ovarian cancer, there was no association of exonic TP53 mutations with age, but the intronic mutation frequency showed a significant increase of 3.0 × 10−7 with every 10-y increase in age after adjusting for BRCA status (P = 0.0004) (Fig. 4 C and D).
Fig. 4.
Frequency of biological background mutations (number of mutations divided by total number of DCS nucleotides) detected in TP53 exons 4–10 and corresponding introns in peritoneal fluid of women with and without ovarian cancer. Patients’ age is indicated in the x axis, and germline BRCA status is color-coded for ovarian cancers and controls. Women without ovarian cancer showed significant associations between the frequency of biological background TP53 mutations and age, both for mutations found in TP53 exons (A) and introns (B). Because control women were segregated into young age and old age depending on germline BRCA status, the correlations with age (regression lines and Spearman’s test P values) are presented separately for women with and without BRCA germline mutations. Women with cancer did not show a significant association between the frequency of biological background TP53 mutations in exons and age (C). For introns, the association was significant after adjusting for BRCA status (D).
TP53 Mutation Burden in Peritoneal Fluid Distinguishes Individuals With and Without Ovarian Cancer.
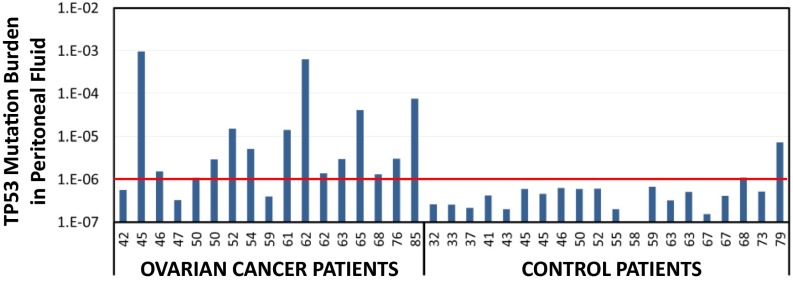
We sought to assess whether the TP53 mutations detected in peritoneal fluid samples could distinguish individuals with ovarian cancer from controls without knowledge of the primary tumor TP53 mutation. As previously shown, almost all cancer samples harbor the tumor mutation (some at relatively high allele frequency) (Fig. 1), and most cancer samples carry more TP53 biological background mutations than controls (Fig. 4C vs. Fig. 4A). Thus, we reasoned that the total burden of mutated TP53 molecules found in a sample could be a useful biomarker to distinguish individuals with and without cancer. For each sample, the mutation burden was calculated as the total number of mutant TP53 molecules in exons 4–10 divided by the total number of DCS nucleotides sequenced. Fourteen out of 17 peritoneal fluid samples from cancer patients had a TP53 mutation burden >10−6 (Fig. 5). In contrast, only 2 out of 20 controls reached that threshold, and, interestingly, they were among the oldest controls in the study (Fig. 5). These frequencies correspond to 82% sensitivity and 90% specificity for distinguishing cancer patients from controls.
Fig. 5.
TP53 mutation burden in peritoneal fluid distinguishes women with ovarian cancer from controls. Within each group, patients are sorted by ascending age, indicated in the x axis. For each sample, the mutation burden was calculated as the total number of mutant TP53 molecules (exons 4–10) divided by total DCS nucleotides sequenced. A threshold of 10−6 (red line, corresponding to one mutation for one million nucleotides) distinguishes cancers and controls with 82% (14/17) sensitivity and 90% specificity (18/20).
Low Frequency TP53 Mutations Are also Found in Peripheral Blood.
The observation that TP53 biological background mutations are present in the peritoneal fluid of essentially all patients (with and without ovarian cancer) provides evidence for the emerging concept that somatic mutation in classically cancer-associated “driver” genes may occur in “normal” tissues (27). However, because peritoneal fluid consists of a heterogeneous mixture of cell types, we sought to assess whether rare TP53-mutated subclones were present in a different sample source. We chose peripheral blood because multiple studies have demonstrated that clonal hematopoiesis with mutations in driver genes occurs in a subset of normal individuals (28–33).
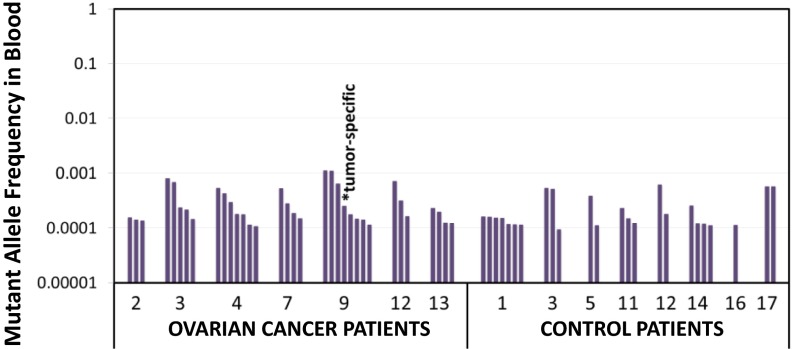
We applied duplex sequencing to whole blood samples from 15 women in our study (7 with ovarian cancer and 8 without cancer). No patients had known history of hematologic disease, and all but one were chemotherapy-naive at sample collection. We identified at least one low frequency TP53 mutation in all patients (15/15), with a range of 1–8 mutations per sample (Fig. 6). A comprehensive list of these mutations can be found in Dataset S2. Similar to the findings in peritoneal fluid, these mutant clones were exclusively present at extremely low mutant allele frequency (≤0.001), and, thus, they are undetectable by less accurate sequencing methods. Importantly, 22% (13/58) of the biological background mutations in peripheral blood were also detected in the matched peritoneal fluid sample from the same patient, further proving the validity of these mutations. Leukocytes are common in peritoneal fluid (34), and, thus, overlap of mutations between matched blood and peritoneal fluid is expected. In one patient with ovarian cancer, the primary tumor TP53 mutation was identified in blood DNA, consistent with the presence of circulating tumor cells or cell free tumor DNA in whole blood. In the other six ovarian cancer patients, the tumor-specific TP53 mutation was not found in blood.
Fig. 6.
TP53 mutations in peripheral blood. Patient ID is indicated in the x axis. Each bar represents a unique mutation observed in at least one DCS. Mutations are ordered by descending mutant allele frequency within each sample. The tumor TP53 mutation was detected in peripheral blood of case 9 but was not found in any other cases.
The type, pathogenicity, and spectrum of the TP53 mutations found in blood were very similar to mutations found in peritoneal fluid (SI Appendix, Fig. S9). In addition, similar to peritoneal fluid, 21.6% (8/37) of the nontumor missense mutations were present at one of the nine previously mentioned TP53 hotspot codons although these codons represent only 2.7% of the total codons in TP53 exons 4–10 (P = 6 × 10−5). Furthermore, consistent with our findings in peritoneal fluid and reported age-related clonal hematopoiesis (28–30, 32, 33), the frequency of TP53 biological background mutations in peripheral blood increased significantly with patient age (P = 0.011).
Discussion
This study demonstrates that duplex sequencing—the most accurate NGS technique currently available—is able to identify tumor DNA at mutant allele frequencies as low as ∼1/25,000, which is beyond the capabilities of other sequencing technologies. Ultra-deep sequencing of TP53 by duplex sequencing enabled the detection of tumor DNA in peritoneal fluid from women with ovarian cancer with 94% sensitivity, despite enriching cases for early stage and negative peritoneal cytology. Tumor DNA was present in some cases that would otherwise challenge clinical detection, in particular two cancers that were microscopic and identified only at risk-reducing surgery. These results provide evidence that ovarian cancer cells are present in the peritoneum even at the earliest detectable stages of disease and before clinically apparent metastasis.
The high resolution afforded by duplex sequencing uncovered the presence of extremely low frequency, cancer-like TP53 mutations in peritoneal fluid and peripheral blood from nearly all patients with and without ovarian cancer. We termed these mutations “biological background” to distinguish them from tumor-specific TP53 mutations. Previous studies have reported somatic mutation of cancer-associated genes in normal tissue (blood and skin), but generally in relatively small subsets of older individuals (27–33, 35). Here, we show that low frequency, cancer-like TP53 mutations in noncancerous tissue are very common, but almost exclusively occur at allele frequencies <0.001, which is below the limit of detection of conventional sequencing methods. Remarkably, the only biological background mutation at frequency >0.001 was found in the oldest woman in the control group. Thus, our results demonstrate widespread, low frequency TP53 mutagenesis, in nearly all individuals, that increases with aging and cancer. These mutations are likely present in both the mesothelial lining of the peritoneal cavity (the dominant cell type in peritoneal fluid samples) as well as in leukocytes. We speculate that biological background mutation may be a phenomenon common to all healthy replicative tissues, but larger studies including multiple tissue samples from healthy individuals across a wide range of ages will be necessary to fully explore this concept.
Several lines of evidence confirm that the majority of these biological background mutations are functional and not technical artifacts. First, they closely resemble TP53 mutations in cancers: nearly all of them are predicted to partially or completely inactivate TP53, they are predominantly C:G→T:A transitions, and they cluster in TP53 hotspot codons. Second, they are more abundant in women with cancer than in women cancer-free, and, in the latter, they increase with age. The age effect was observed for mutations detected in both exons and introns, and independently in the analysis of peritoneal fluid and peripheral blood. This age dependency is in concordance with prior studies of somatic mutations in noncancerous tissues (28–30, 32, 33) and supports the notion that “clock-like mutations” commonly found in cancers accumulate in normal cells with aging before the development of cancer (25). Third, a proportion (13/58) of the low frequency mutations found in blood were also detected in peritoneal fluid from the same patient. Because peritoneal fluid is known to contain a variable number of leukocytes (34), this overlapping is expected and demonstrates the reproducibility of our approach.
Our results indicate that noncancerous tissue carries clones with positively selected driver TP53 mutations. This finding is in agreement with a recent report of somatic cancer mutations in normal human skin (36). Multiple cancer-associated driver genes, including TP53, seemed to be mutated and clonally expanded in large patches of morphologically normal skin cells. Although the high prevalence of these clones was surprising, it is consistent with the concept that cancer is the result of clonal evolution over the lifespan of an individual. It is conceivable for multiple competing clones with cancer driver mutations to coexist and remain untransformed within normal tissue, and it is expected that the number and size of these clones would increase with age. It is also expected that the individuals that harbor more of these clones have higher probability of developing cancer. Indeed, individuals in previous studies carrying detectable somatic mutations in blood were at increased risk of developing hematopoietic malignancies (28–31, 33). Elevated mutagenesis is thought to be a characteristic feature of premalignant and malignant tissues (37), and our finding that women with HGSOC have a significantly higher burden of TP53 mutations in peritoneal fluid is consistent with this concept. Although the allele frequency of tumor-specific mutations was not always distinguishable from the level of biological background in cases, the total burden of mutated TP53 molecules in peritoneal fluid (including tumor and biological background mutations) discriminated women with and without ovarian cancer with 82% sensitivity and 90% specificity. Importantly, these estimates are based on a small number of patients and should be validated in larger studies.
In summary, this proof-of-principle study demonstrates that duplex sequencing shows promise for the detection of rare tumor alleles in “liquid biopsies.” Peritoneal lavage is an invasive procedure that is unsuitable for screening, but duplex sequencing could be applied to a wide range of clinical samples. Whereas previous studies using other sequencing approaches to identify tumor-specific mutations in Pap smear (5) or uterine lavage DNA (14) had suboptimal sensitivities, the coupling of duplex sequencing with these minimally invasive biopsies may generate more useful tools for early ovarian cancer screening. The exquisite sensitivity of duplex sequencing demonstrated here could also be applicable to the identification of cancer cells from other cancer types in liquid biopsies, a promising approach to improve early cancer detection, monitoring, and prognosis. Finally, we have also demonstrated that cancer mutations are found in noncancerous tissue in association with aging and cancer. This phenomenon may challenge diagnostic strategies that hinge on the identification of tumor-specific TP53 alleles above the biological background in a given tissue. However, the finding that individuals with cancer had higher burdens of TP53 mutation in peritoneal fluid samples suggests that mutational burden should be considered in future cancer biomarker studies.
Materials and Methods
Patients and Samples.
Blood, tumor, and peritoneal fluid were obtained from the University of Washington Gynecologic Oncology Tissue Bank, which collected specimens and clinical information after informed consent under protocol number 27077 approved by the University of Washington Human Subjects Division institutional review board. The study included 16 patients with HGSOC (“cancers”) (SI Appendix, Table S1) and 20 patients who had gynecologic surgery but were found to have benign pathology (“controls”) (SI Appendix, Table S2). All cancer patients had surgery for suspected ovarian malignancy, except 3 BRCA1 mutation carriers who had occult disease discovered during risk-reducing salpingo-oophorectomy. The 20 control patients included 10 BRCA1 or BRCA2 mutation carriers who underwent risk-reducing salpingo-oophorectomy and had no gross or microscopic pathology detected. The other 10 control subjects did not have inherited BRCA mutations and had benign pathology at gynecological surgery. All patients were chemotherapy-naive except 3 ovarian cancer patients and 2 controls, all of whom had a history of breast cancer (SI Appendix, Tables S1 and S2). All patients donated one peritoneal fluid sample collected at surgery, except 1 cancer patient who donated two (from primary and recurrence surgeries). Peritoneal fluid could be of two types: ascites (undiluted fluid present in the peritoneal cavity at the time of surgery) and peritoneal wash (in the absence of ascites, a saline lavage of the peritoneum performed after surgical entry to the peritoneal cavity).
DNA Extraction.
DNA was extracted from peripheral whole blood and peritoneal fluid sample cell pellets using the QIAamp DNA Mini kit (Qiagen) and was quantified with a QuBit HS dsDNA kit (ThermoFisher Scientific).
Duplex Sequencing.
DNA was prepared into libraries for duplex sequencing according to published protocols (7, 38). For peritoneal fluid libraries, up to 10 μg of genomic DNA (mean 5.4 μg) was used. For peripheral blood libraries, 3.5 μg of starting DNA was used. DNA was sonicated, end-repaired, a-tailed, and ligated with duplex sequencing adaptors (SI Appendix, SI Materials and Methods and Fig. S1). After PCR, capture of TP53 exons 4–10 was performed by two consecutive rounds of hybridization with xGen Lockdown probes. Indexed libraries were pooled and sequenced on an Illumina HiSeq2500. Data processing was performed as previously described (38). Raw reads sharing a common molecular tag were grouped to form SSCS. Next, complementary SSCS (corresponding to the two original strands of DNA) were collapsed into DCS. Only variants present in complementary SSCS were considered true mutations.
MUT-TP53 2.00.
All missense biological background mutations were evaluated by MUT-TP53 2.00 (24). Using functional and statistical information derived from the UMD (Universal Mutation Database) p53 database, this matrix assesses the likely biological activity of each mutation and categorizes them as one of the following: “inactive,” “partially active,” or “no significant loss of activity.”
IARC Database.
TP53 mutations found in peritoneal fluid and blood were compared with mutations present in the IARC database of clonal TP53 mutations in serous ovarian carcinoma (21, 22). Somatic TP53 mutations with topography listed as “ovary” and morphology listed as “serous cystadenocarcinoma” were downloaded and filtered for exons 4–10 and associated splice regions (22). Silent mutations, indels >1 bp, and tandem or complex mutations were discarded, yielding a total of 930 mutations.
Statistical Analysis.
The exact/Clopper–Pearson method was used to calculate the 95% confidence interval of TMAF. Two-sample tests for differences in distribution were performed with the Mann–Whitney test, and correlation was tested with Spearman's rank test, due to high variability in the outcomes (nonnormality). For categorical variables, the Pearson χ2 or Fisher’s exact test was used as appropriate. Multivariate regression was performed after log-transformation of mutation frequencies and CA-125 and exclusion of one far outlying value of TMAF. All of the tests were two-sided at an alpha level (type 1 error rate) of 0.05. Statistical analyses were performed with SPSS and R.
Supplementary Material
Acknowledgments
We thank our patients who generously donated these samples. For technical assistance and critical reading, we thank Hye Son Yi, Marc R. Radke, Kate S. Reid-Bayliss, Edward J. Fox, and Jesse J. Salk. This work was supported by Mary Kay Foundation Grant 045-15 (to R.A.R.), the Wendy Feuer Research Fund for the Prevention and Treatment of Ovarian Cancer and the Cori and Tony Bates Novel Technologies for Cancer Prevention Fund (E.M.S.), and National Institutes of Health Grant CA1817712 (to L.A.L.).
Footnotes
Conflict of interest statement: M.W.S. and L.A.L. declare leadership, consulting role, and stock ownership at TwinStrand Biosciences, Inc.
This article is a PNAS Direct Submission.
Data deposition: Sequencing data from this paper have been deposited in the Sequence Read Archive [accession no. SRP072370 (BioProject ID: PRJNA316476)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601311113/-/DCSupplemental.
References
- 1.Fox EJ, Reid-Bayliss KS, Emond MJ, Loeb LA. Accuracy of next generation sequencing platforms. Next Gener Seq Appl. 2014;1:106. doi: 10.4172/jngsa.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettegowda C, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7(293):293ra104. doi: 10.1126/scitranslmed.aaa8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA. 2015;112(31):9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinde I, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5(167):167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108(23):9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt MW, et al. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci USA. 2012;109(36):14508–14513. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt MW, et al. Sequencing small genomic targets with high efficiency and extreme accuracy. Nat Methods. 2015;12(5):423–425. doi: 10.1038/nmeth.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badgwell D, Bast RC., Jr Early detection of ovarian cancer. Dis Markers. 2007;23(5-6):397–410. doi: 10.1155/2007/309382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindelberger DW, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przybycin CG, Kurman RJ, Ronnett BM, Shih IeM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34(10):1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 13.Prat J. FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Maritschnegg E, et al. Lavage of the uterine cavity for molecular detection of Müllerian duct carcinomas: A proof-of-concept study. J Clin Oncol. 2015;33(36):4293–4300. doi: 10.1200/JCO.2015.61.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vang R, et al. Molecular alterations of TP53 are a defining feature of ovarian high-grade serous carcinoma: A rereview of cases lacking TP53 mutations in the Cancer Genome Atlas ovarian study. Int J Gynecol Pathol. 2016;35(1):48–55. doi: 10.1097/PGP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn E, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma: Evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226(3):421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norquist BM, et al. The molecular pathogenesis of hereditary ovarian carcinoma: Alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116(22):5261–5271. doi: 10.1002/cncr.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swisher EM, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol. 2005;193(3 Pt 1):662–667. doi: 10.1016/j.ajog.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 20.Walsh T, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108(44):18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hjortsberg L, et al. (2008) The p53 Mutation Handbook, Version 2.0. Available at p53.free.fr. Accessed November 2015.
- 22.Petitjean A, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 23.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26(15):2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 24.Soussi T, et al. MUT-TP53 2.0: A novel versatile matrix for statistical analysis of TP53 mutations in human cancer. Hum Mutat. 2010;31(9):1020–1025. doi: 10.1002/humu.21313. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov LB, et al. Clock-like mutational processes in human somatic cells. Nat Genet. 2015;47(12):1402–1407. doi: 10.1038/ng.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandrov LB, et al. Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349(6255):1483–1489. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- 28.Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs KB, et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet. 2012;44(6):651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurie CC, et al. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet. 2012;44(6):642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong TN, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552–555. doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie M, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–282. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Score J, et al. Detection of leukemia-associated mutations in peripheral blood DNA of hematologically normal elderly individuals. Leukemia. 2015;29(7):1600–1602. doi: 10.1038/leu.2015.13. [DOI] [PubMed] [Google Scholar]
- 36.Martincorena I, et al. Tumor evolution: High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeb LA. Human cancers express mutator phenotypes: Origin, consequences and targeting. Nat Rev Cancer. 2011;11(6):450–457. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy SR, et al. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat Protoc. 2014;9(11):2586–2606. doi: 10.1038/nprot.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.