Significance
Complex, nongradual responses to environmental conditions are commonplace in nature and perhaps most extreme in polyphenic insects where continuous variation in nutrition experienced in early development gives rise to discrete alternative castes or morphs. This research shows that the hedgehog (Hh) pathway has acquired a novel and highly unusual role in the nutrition-dependent regulation of polyphenic development of a beetle. Experimental repression of Hh signaling returns a highly discontinuous response to nutrition to its presumed ancestral, gradual state. Our results suggest that recruitment of the Hh signaling pathway may have been a key step in the evolution of trait thresholds and the corresponding origin of alternative phenotypes and complex allometries.
Keywords: co-option, modularity, developmental plasticity, allometry, threshold trait
Abstract
The recruitment of modular developmental genetic components into new developmental contexts has been proposed as a central mechanism enabling the origin of novel traits and trait functions without necessitating the origin of novel pathways. Here, we investigate the function of the hedgehog (Hh) signaling pathway, a highly conserved pathway best understood for its role in patterning anterior/posterior (A/P) polarity of diverse traits, in the developmental evolution of beetle horns, an evolutionary novelty, and horn polyphenisms, a highly derived form of environment-responsive trait induction. We show that interactions among pathway members are conserved during development of Onthophagus horned beetles and have retained the ability to regulate A/P polarity in traditional appendages, such as legs. At the same time, the Hh signaling pathway has acquired a novel and highly unusual role in the nutrition-dependent regulation of horn polyphenisms by actively suppressing horn formation in low-nutrition males. Down-regulation of Hh signaling lifts this inhibition and returns a highly derived sigmoid horn body size allometry to its presumed ancestral, linear state. Our results suggest that recruitment of the Hh signaling pathway may have been a key step in the evolution of trait thresholds, such as those involved in horn polyphenisms and the corresponding origin of alternative phenotypes and complex allometries.
Understanding the genetic and developmental mechanisms underlying the origin and diversification of novel, complex traits is a fundamental objective of evolutionary biology. The recruitment of modular developmental genetic components into new developmental contexts has been proposed as a central mechanism enabling the origin of novel traits without necessitating the origin of novel genes or developmental pathways (1, 2). At the same time, redeployment of “old” genes and pathways into novel developmental contexts has the potential to create critical new opportunities for morphological diversification: for example, the recruitment of appendage patterning genes has played a key role in the origin of butterfly eye spots, a remarkable evolutionary novelty (3). Moreover, by being placed in the context of wing patterning, the further evolution of eye spot formation could exploit preexisting patterning mechanisms, ultimately enabling eye spot formation to diversify depending on which wing, wing surface, or position it has occurred (4). Therefore, newly deployed gene regulatory networks can contribute to both macroevolution (e.g., butterfly eyespot generation) and microevolution (e.g., diversification of the eyespot pattern).
One particularly significant axis of diversification receiving growing attention from evolutionary and developmental biologists concerns environment-responsive trait formation (5, 6). Environment-responsive development or developmental plasticity is ubiquitous across trait types and taxa and creates especially significant evolutionary degrees of freedom for novel, complex traits (5, 7). Here, polyphenic development—the most extreme case of developmental plasticity, whereby environmental factors cue the development of two or more discrete morphs or castes—has become increasingly recognized for its importance as a potential facilitator of adaptive radiations (8). For instance, in many butterfly species, seasonal conditions critically alter selective environments, and seasonal sensitivity in eye spot formation is able to adjust wing phenotypes, thereby maintaining high fitness across fluctuating environments (9). Similarly, environment-dependent induction of carnivory in spadefoot toad tadpoles (10, 11) or tooth formation and bacterial predation in nematodes (12), two striking and complex evolutionary novelties, greatly affect the adaptive significance of each innovation, thereby facilitating their adaptive radiations (13). Here, we investigate the functional significance of the Hedgehog (Hh) signaling pathway, a deeply conserved cellular transduction pathway, in the development of beetles horns, a striking evolutionary novelty (14), and specifically, the origins of environment-responsive horn formation, alternative male phenotypes, and body size thresholds, emergent phenotypes that have greatly impacted patterns of morphological radiation among horned beetles (15–17).
Several thousands of species of horned beetles exist worldwide, with the greatest diversity found in the rhinoceros (Dynastinae) and scarab (Scarabaeinae) beetles, two subfamilies that independently evolved horns. Beetle horns are primarily used as weapons in male competition (18), restricted to or greatly exaggerated in males (19), and in most species studied thus far, greatly affected in their development by the nutritional conditions experienced during the larval stage (20). Here, the evolution of nutritional responsiveness in the context of horn formation is thought to have played an especially critical role in horned beetle diversification by (i) enabling the evolution of some of the most extreme examples of condition-dependent exaggerated male secondary sexual traits (21); (ii) facilitating the origin of horn polyphenisms and discrete hornless sneaker and horned fighter morphs adapted for alternative reproductive niches (18); (iii) establishing sharp threshold body sizes that decouple alternative developmental trajectories and enable the integration of two separate, complex, disparate male phenotypes from the same genome (16, 22); and (iv) contributing to the enormous radiation of horned beetles through the evolution of morph-specific morphologies, behaviors, and physiologies and the diversification of size thresholds, perhaps best illustrated in the >2,000 species-rich genus Onthophagus (16, 17, 19, 23).
Previous studies have shown that horn development is instructed by many of the same genes that regulate the formation of regular appendages, such as leg gap genes (14), that nutrition responsiveness of horn development may be coupled to insulin signaling (24, 25), and that sex- and morph-specific horn exaggeration may be facilitated by doublesex (dsx), a master regulator of somatic sex determination (23, 26, 27). However, how beetle horns achieve their strikingly dimorphic expression in many species and the developmental mechanisms that enable the definition of a sharp body size threshold separating alternate morphs from what is presumed to be a continuous range of nutritional conditions are entirely unknown. Here, we investigate the function of the Hh signaling pathway, a highly conserved pathway best understood for its role in patterning anterior/posterior (A/P) polarity in body segments and appendages (28, 29), in the development of horns, horn polyphenisms, and threshold body sizes. Specifically, we show that, although interactions among pathway members remain conserved and involved in regulating A/P polarity in traditional appendages, such as legs, the Hh signaling pathway has acquired a novel and highly unusual role in the nutrition-dependent regulation of horn polyphenisms by actively suppressing horn formation in low-nutrition males. Our results suggest that recruitment of the Hh signaling pathway may have been a key step in the evolution of trait thresholds, such as those involved in horn polyphenisms and the corresponding origin of alternative phenotypes and complex allometries.
Results and Discussion
To investigate the role of the Hh pathway in Onthophagus development, we first cloned fragments of Onthophagus taurus hh (the morphogen in the absence of which Hh signaling is deactivated), patched (ptc; the Hh receptor that inhibits Hh signaling unless bound to Hh protein), and smoothened (smo; a membrane protein that, in the absence of Hh protein, is constitutively inhibited by ptc but disinhibited in the presence of Hh, thereby activating the pathway) using sequence data from an earlier study (30). Below, we describe the effects of dsRNA injections beginning with their role in the regulation of general postembryonic development and followed by their function in the context of horn morphogenesis, focusing on nutrition-sensitive horn formation. We discuss the implications of our results for our understanding of the origin of environment-sensitive trait formation and the evolution of trait thresholds and nonlinear allometries.
Functional Significance of the Hh Pathway in Late Onthophagus Development.
RNAi-mediated knockdown of hh and smo resulted in overall similar phenotypes, consistent with their presumed position within the Hh pathway and suggesting that the functions of both genes are at least partly overlapping (Fig. 1). After smoRNAi, animals exhibited a survival rate of 32% (98 of 305). We observed developmental defects in legs and wings (56 of 64 males and 28 of 34 females showed defects in either one or both appendage types) (Fig. 1 and Fig. S1). For instance, compared with control-injected animals, the tibia and femur of smoRNAi animals were more slender, the ventral bristle patterning of the femur was disrupted and reduced, and the coxa was disproportionately shorter (Fig. 1 A and B and Fig. S1). Moreover, legs in animals with severe knockdown phenotypes became fully vestigial. These results suggest that smo plays an important role in appendage formation beyond embryogenesis and well into prepupal and pupal development (Table S1 shows a summary of RNAi phenotypes).
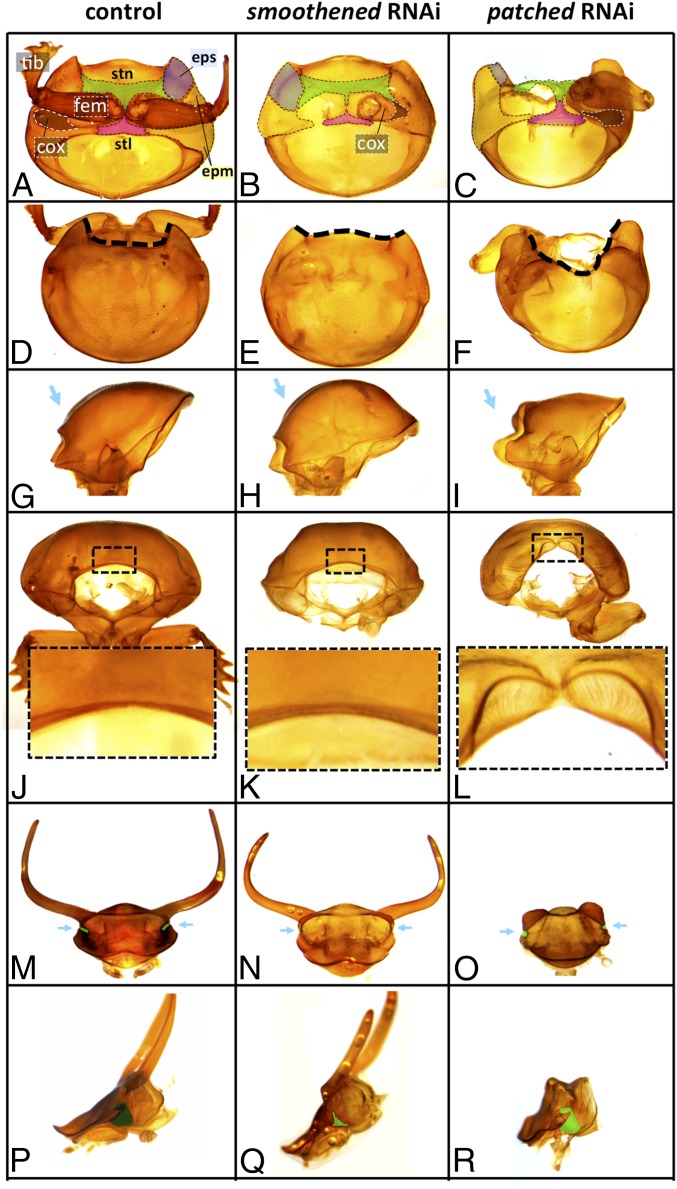
Fig. 1.
Effect of hh, smo, and ptc dsRNA injection on Onthophagus development. Prothorax and head of (Left) control-injected, (Center) smoRNAi, and (Right) ptcRNAi animals are shown. (A–C) Comparison of the prothorax from the ventral side. The differences of smo and ptc function in prothoracic development are most obvious in the sternum (stn; green), episternum (eps; blue), and epimeron (epm; yellow). Note that in situ size of the coxa (area shaded black) is reduced in smoRNAi individuals relative to control-injected and ptcRNAi animals. (D–F) Development of the notum is also affected by RNAi. (E) Note that the anterior edge of the pronotum (thick dashed lines in D–F) extends anteriorly in smoRNAi animals, whereas (F) ptcRNAi results in significant reduction of the anterior pronotal edge. (G–I) Lateral view of the same prothoraces; smoRNAi animals exhibit prominent bulging of the pronotum, whereas ptcRNAi animals lack the anterior region (blue arrows). (J–L) Frontal view of the same prothoraces. (K) Effect of smoRNAi was minimal on the anterior edge, whereas (L) ptcRNAi resulted in development of ectopic bristles. (M–O) Frontal view of the head. Compound eyes are labeled in green and highlighted by blue arrows. Head shape was only lightly affected by RNAi. (N) All but one smoRNAi animal developed full-sized horns, whereas (O) horns in all ptcRNAi animals were vestigial. (P–R) Lateral view of the head. (Q) Compound eyes are significantly reduced in smoRNAi animals but (R) not reduced in ptcRNAi animals. Images of thoraces as well as heads are taken under the same magnification. cox, coxa; fem, femur; stl, sternellum; tib, tibia.
Fig. S1.
Effect of smoRNAi and ptcRNAi in leg development. (Left) Ventral views of head and prothorax of control, smoRNAi, and ptcRNAi males. Right femur is labeled in blue. Animals’ anterior is to the top. (Right) Details of foreleg phenotypes, with coxa (cox), femur (fem), and tibia (tib) indicated. The femur is outlined to contrast differences in morphology between treatments. Note changes in bristle pattern on the femur, which was affected by RNAi, suggesting disruption of axis formation during late leg development. Arrows indicate anterior (A), posterior (Po), distal (D), and proximal (Pr) axes.
Table S1.
Summary of RNAi phenotypes, survival, and penetrance as a function of RNAi target and sex
| RNAi target | Survival (%) | Representative phenotype (sex: penetrance %) | ||
| Appendages (legs and wings) | Body segments | Eye/head development | ||
| hh | 48 | Narrowed femur and tibia (male: 71.1; female: 78.3) | Slight anterior bulge in pronotum (male: 26.3; female: 21.7) | Not measured |
| smo | 32 | Severely narrowed femur and tibia (male: 87.5; female: 82.4) | Prominent anterior bulging in pronotum (male: 84.3; female: 82.4) | Reduced eyes associated with overproliferation of lateral/posterior head (male: 85.7; female: 69.2) |
| ptc | 57 | Thickened, widened femur and tibia (male: 100; female: 92.3) | Partial anterior depletion in pronotum (male: 85.7; female: 69.2) | Malformation of head, especially anterior to eyes (male: 78.6; female: 53.8) |
The development of the first thoracic sternellum (nomenclature follows refs. 31–35) was also affected by smoRNAi in a direction similar to but more severe than in hhRNAi animals (Fig. 1). In some animals, the sternal region was clearly separated into two parts, with additional defects in the ventral posterior regions (epimeron) (Fig. 1B). Specifically, 54 of 64 males and 28 of 34 females showed defects in this region. In contrast, the dorsal prothorax exhibited prominent bulging of the anterior pronotum (52 males and 24 females) (Fig. 1 H and K) and an anterior shift of the pronotal edge (dashed lines in Fig. 1 D–F).
In Drosophila, inactivation of hh during larval development causes abdominal bristles to exhibit a mirror image double-anterior pattern, suggesting that the Hh signaling pathway is required to establish and maintain the A/P axis within body segments (36). However, we failed to observe any obvious anteriorization in the posterior regions of any thoracic segments in smoRNAi animals. Instead, the posterior portion of the prothorax (epimeron) showed obvious malformation (Fig. 1B). This result suggests that smo’s function in late Onthophagus development is to redefine the borders between subsegmental regions within the prothorax. Furthermore, smoRNAi resulted in the development of reduced compound eyes in 43 of 64 males and 22 of 34 females, a phenotype that parallels hh inactivation in Drosophila (compare Fig. 1P with Fig. 1Q) (refs. 28 and 29 discuss corresponding Drosophila phenotypes).
After hhRNAi, 48% (61 of 126) of injected larvae survived and emerged to adults. Of those 61, 45 (27 males and 18 females) exhibited similar but generally weaker developmental defects in appendages (legs and/or wings) compared with smoRNAi animals. Collectively, these results indicate that hh and smo are involved in largely overlapping processes critical for proper pupal and adult appendage and segment differentiation in Onthophagus.
ptc dsRNA injection also resulted in strong but largely opposite phenotypic consequences compared with hhRNAi and smoRNAi (Figs. 1 and 2). Here, 57% (27 of 47) of animals survived, and almost all (26 of 27 animals; 14 males and 12 females) showed significant developmental defects in appendages and/or the prothorax. In contrast to the more slender tibiae and femurs observed in smoRNAi individuals, ptcRNAi animals developed much broader, wider legs along the A/P axis, with the most extreme phenotypes being observable in the proximal femur. Here, we also observed extended transverse rows of bristles on anterior prothoracic femurs (S1). These results suggest that ptc plays an important role in facilitating proper axis formation during leg development but that this role is different from and largely opposite to those of smo and hh.
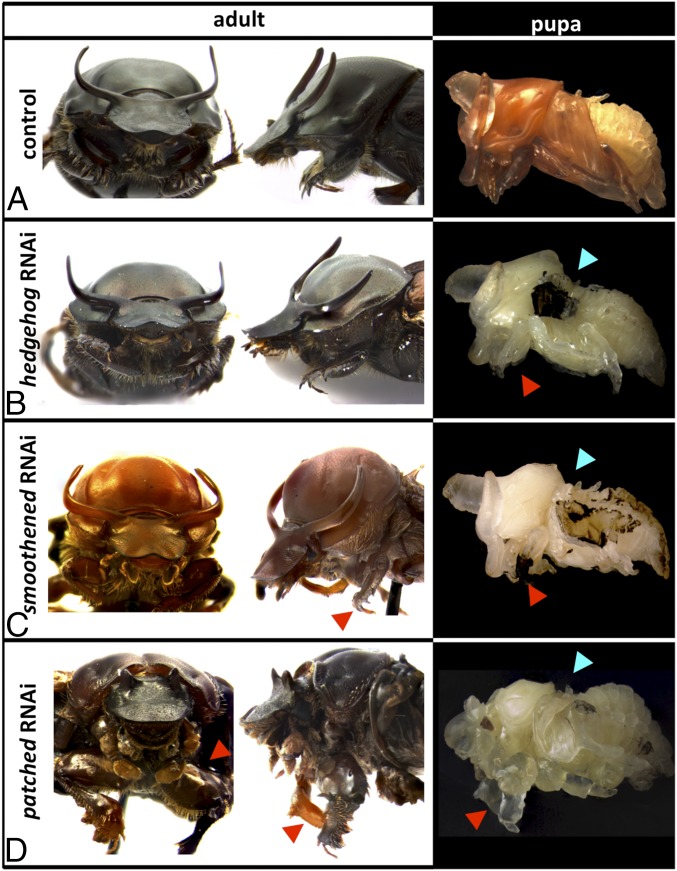
Fig. 2.
Global effects of dsRNA injection. Representative phenotypes of (Left) adults (frontal view is on the left, and lateral view is on the right) and (Right) pupae (lateral view) injected with (A) a control construct and dsRNA targeting (B) hh, (C) smo, and (D) ptc, respectively. Arrowheads indicate legs on the first thoracic segment (red) and wings on pupae (light blue).
Prothoracic development was also affected by ptcRNAi in ways largely opposite to what we had observed for hhRNAi and smoRNAi. Specifically, the prothoracic sternum (Fig. 1 A and C) was severely malformed and reduced, resulting in the detachment of the sternum from the pleural region. The anterior region of the prothoracic episternum was also reduced in ptcRNAi animals (Fig. 1 A and C). Most animals showed one or both of these phenotypes. Furthermore, animals exhibited a reduction or complete loss of the prothoracic horn in pupae (Fig. 2D). Along with this developmental defect in the prothoracic horn, adults exhibited severe malformation of the anterior pronotum (12 males and 9 females) (Figs. 1 F, I, and L and 2D). In addition, we observed ectopic bristles on the anterior edge of the pronotum (compare with Fig. 1 J–L). Lastly, unlike smoRNAi, ptcRNAi did not seem to affect eye formation; however, a particular part of the head immediately adjacent to the eyes (arrows in Fig. 1 M–O) failed to develop its proper shape in 11 males and 7 females, suggesting that ptc may be involved in the head subsegmental area specification. Taken together, our results suggest that the Hh pathway plays fundamental roles during late postembryonic development in Onthophagus, particularly with respect to pupal/adult appendage formation and the establishment and redefinition of pupal/adult subsegmental regions within the prothorax and head.
Genetic Relationships Between hh, smo, and ptc.
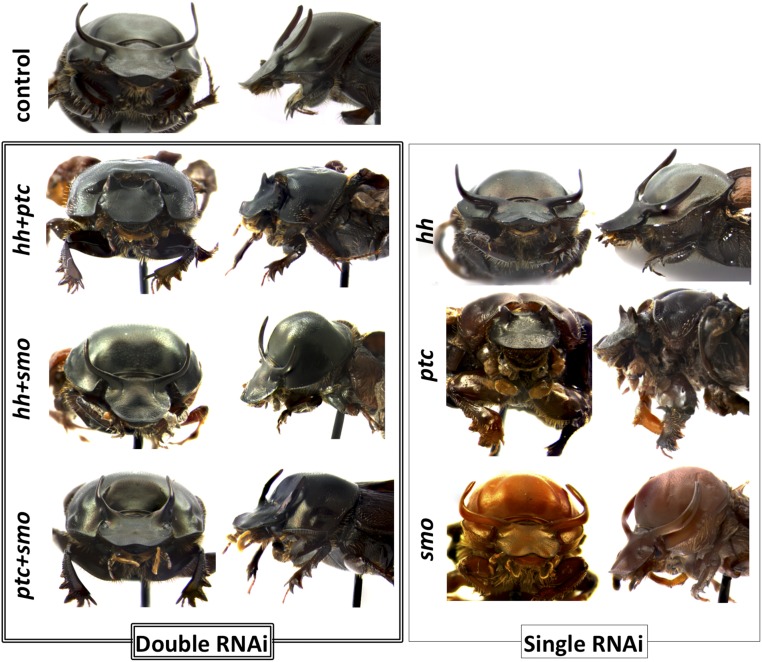
RNAi phenotypes obtained in this study suggest that hh and smo possess similar function during Onthophagus development. In contrast, ptcRNAi generated very different and in part, opposite phenotypes. For example, smoRNAi mostly affected the posterior region of the prothorax and resulted in reduced legs, whereas ptcRNAi animals lacked the anterior prothoracic region and developed thicker femurs and tibiae (S1). These results match expectations derived from the functional relationships between hh, smo, and ptc as documented in other taxa (37) [i.e., the hh gene product inhibits the function of ptc (the receptor for Hh), which in the absence of Hh, inhibits Smo (membrane protein)]. To further examine whether the genetic relationships among these three genes is as conserved in Onthophagus as it is in other taxa, we performed double knockdowns. Specifically, we predicted that, if the genetic relationship among hh, ptc, and smo is conserved, then double-RNAi phenotypes should correspond to the most downstream pathway component. We observed that hh/smoRNAi resulted in the same phenotypes seen in smoRNAi individuals, whereas hh/ptcRNAi resulted in phenotypes closer to those seen in ptcRNAi individuals (Fig. S2). Both results are consistent with a conservation of the genetic relationship among the three pathway members, supporting the notion that the genetic relationships within the Hh signaling pathway in Onthophagus are likely conserved compared with those in other taxa, including Drosophila.
Fig. S2.
Representative phenotypes obtained from double knockdowns. (Upper) Control-injected large male. (Lower Left) hh/ptcRNAi, hh/smoRNAi, and ptc/smoRNAi from top to bottom, respectively. (Lower Right) Single-gene RNAi phenotypes for comparison (images are the same as in Fig. 2): hhRNAi, ptcRNAi, and smoRNAi from top to bottom, respectively.
Functional Significance of Hh Signaling in the Development of Horns and Horn Polyphenisms.
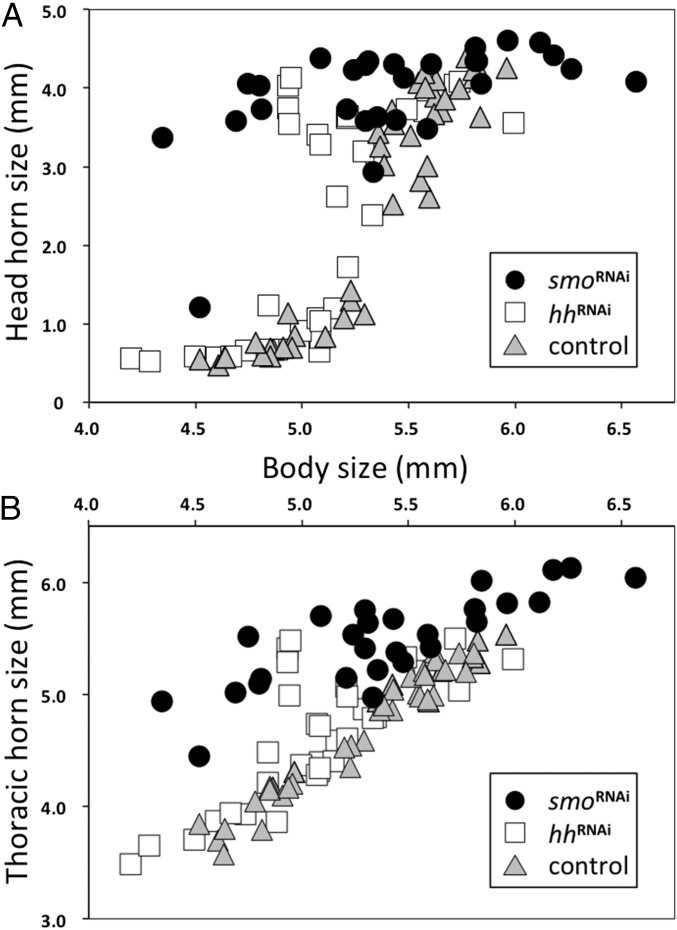
Paralleling the phenotypic effects described above, hhRNAi and smoRNAi resulted in similar horn phenotypes. However, neither horn location nor shape was affected in these animals; rather, hhRNAi and smoRNAi affected horn size. Furthermore, this effect was limited to males of small body size that had experienced suboptimal feeding conditions. Control-injected males, like WT males, only develop a full set of head horns when nutritional conditions allow them to reach or exceed a critical body size threshold (in this research, around 5.3 mm in pupal thorax width) (Fig. 3A). Male larvae that fail to reach this threshold size metamorphose into smaller, largely hornless morphs. However, almost all smoRNAi males developed large head horns, including small individuals normally fated to remain hornless (t31 = −5.80; P < 0.0001). We observed a similar, albeit less extreme, effect in hhRNAi males (Fig. 3A) (t58 = −3.81; P = 0.0031). The apparent linearization of the allometry among smoRNAi animals precluded additional comparison with control-injected males. However, pairwise comparison of individual model parameters was possible between control-injected and hhRNAi animals and found a highly significant difference in the allometric slope at the inflection point of the sigmoidal scaling relationship (t73 = 2.75; P < 0.0001) but not at any other model parameter (Fig. 3A).
Fig. 3.
Effect of hhRNAi and smoRNAi on horn development and relative horn sizes in male O. taurus pupae. (A) Control-injected animals (gray triangles) exhibited the species-typical sigmoidal relationship between body size (x axis) and head horn size (y axis); smoRNAi (●), in contrast, resulted in nearly all males developing full-sized horns regardless of body size and a complete linearization of the body size horn length allometry. Lastly, hhRNAi (□) also resulted in relatively longer horns, but this effect was limited to a subset of males of intermediate body size. (B) hhRNAi (□) modestly increased thoracic horn length in some animals, whereas smoRNAi (●) increased horn length in all individuals, especially small, low-nutrition individuals.
Lastly, a partly corresponding effect was observed in pupal thoracic horns. Like all other species studied so far, O. taurus pupa develop a horn on the pronotum, which aids in the shedding of the larval head capsule during the larval to pupal molt (38). However, in a subset of species, including O. taurus, the pupal thoracic horn is fully resorbed before the adult molt (39). In WT as well as control-injected animals, this pupal thoracic horn scales linearly with body size, whereas smoRNAi animals developed significantly longer horns relative to their body size compared with control-injected individuals (Fig. 3B) (ANCOVA on log-transformed data: Ftreatment = 115.88; P < 0.0001; Ftreatment×size = 98.97; P < 0.0001). Similarly, although hhRNAi males exhibited thoracic horn scaling relationships matching those of control-injected individuals for most of the body size range, males of intermediate body sizes around the body size threshold for head horns (∼5.3 mm) had a tendency to also develop greatly exaggerated thoracic horns, resulting in significant differences in horn length residuals between control-injected and hhRNAi males (Fig. 3B) (residual analysis: t37 = −3.16; P = 0.0031).
In contrast, ptcRNAi resulted in a horn phenotype largely opposite to those generated by hhRNAi and smoRNAi (Fig. 1 N and O). Note that, because ptcRNAi also affected the prothorax to a degree that rendered it no longer useable for precise body size estimation, we were unable to quantify the exact relationship between horn length and body size. However, in all ptcRNAi males (n = 14), not a single individual exhibited fully developed head horns regardless of overall body size, including in individuals of approximately similar or larger body size than their fully horned control-injected counterparts. Similarly, pupal prothoracic horns were reduced in size or in some cases, missing completely in both males and females (Fig. 2B). Taken together, our RNAi results, therefore, suggest that hh and smo inhibit horn development in small, low-nutrition males, whereas ptc promotes horn formation, at least in high-nutrition individuals.
Developmental Evolution and Integration of Nutrition Sensitivity, Allometry, and Threshold Sizes.
Signal transduction pathways have been proposed to constitute developmental/genetic modules that, on one side, maintain a high level of conservation within, whereas on the other side, are easily dissociated from their original biological process and co-opted into novel developmental contexts, where they can then facilitate the evolution of novel traits and trait characteristics (40). Previous work suggests that polyphenic, sigmoidal relationship between body size and horn size evolved from linear and isometric scaling relationships (16). Recent work has implicated the sex determination gene dsx in promoting horn formation in males subject to optimal nutritional condition in both the rhinoceros beetle Trypoxylus dichotomus (which exhibits a linear, highly positive allometry) and O. taurus (which exhibits a more derived sigmoidal allometry, including a sharp body size threshold separating alternative male morphs). In both taxa, dsx seems to play a critical role in the nutrition-dependent exaggeration of horn growth, and several studies suggest further that insulin signaling, juvenile hormone signaling, or a combination of the two may interact with dsx and sensitize dsx action to nutritional conditions (24, 25, 27). This research highlights a third highly conserved but previously overlooked pathway, the Hh signaling pathway, which—like dsx signaling—has been co-opted into the regulation of horn development and acquired nutrition sensitivity in the process but exerts its regulatory function in, to the best of our knowledge, a thus far unprecedented manner, namely by actively inhibiting horn growth in low-nutrition individuals only (Fig. 4A).
Fig. 4.
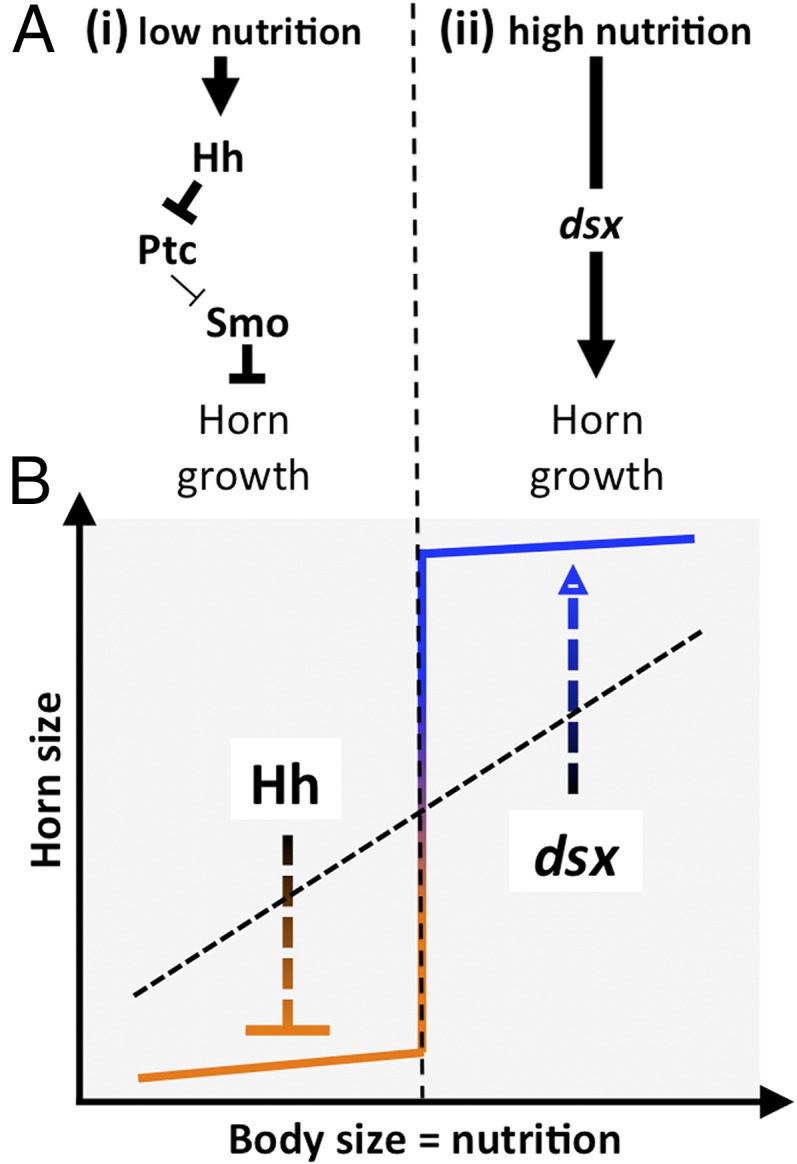
Proposed models for the development of nutrition-dependent expression of alternative horned and hornless male morphs separated by a sharp body size threshold. (A) Results presented here suggest that (i) the Hh signal pathway negatively regulates horn growth in low-nutrition male beetles, whereas (ii) previous work implicated the somatic sex determination gene dsx in mediating nutrition-dependent exaggeration of horns under high nutrition. (B) Combined, dsx-mediated promotion of horns in high-nutrition individuals and Hh-mediated inhibition of horns in low-nutrition individuals have the potential to transform ancestral, linear into strongly sigmoidal scaling relationships characterized by a bimodal distribution of male phenotypes and the establishment of a sharp allometric size threshold.
Intriguingly, these findings raise the possibility that a combination of dsx-mediated promotion of horn growth under high nutrition and Hh signal-mediated inhibition of horn growth under low nutrition may have played a critical role in the evolution of sigmoid allometries and the origin of critical size thresholds, thereby enabling the evolution of discrete, alternative, nutritionally cued morphs common in the genus Onthophagus (Fig. 4B). Exactly how these pathways interact with each other as well as nutrition-sensing and location-determining pathways represents an exciting area for future research. For example, recent work on beetles and social insects implicate the insulin pathway, juvenile hormone, and methylation-mediated signaling through the EGF receptor pathway as putative candidates by which growth could become coupled to nutrition (24, 25, 41–43). More generally, our results suggest that, during the evolution of novel traits, gene co-option and mode of functional modification of co-opted gene networks can be remarkably dynamic, allowing highly conserved modules to acquire new roles in the development of novel traits and trait characteristics.
Materials and Methods
Beetle Care.
O. taurus was collected near Chapel Hill, North Carolina, and Bloomington, Indiana to establish a laboratory colony. About 300 adults per colony were kept at 25 °C in a sand/soil mixture at a 16-h:8-h light:dark cycle. Animals were fed homogenized cow manure twice a week. To obtain larvae, a set of five females and three males were selected from the laboratory colony, kept in a small plastic container with packed moist sand/soil mixture with cow manure, and allowed to produce brood balls as described previously (44). Brood balls were collected after 8 d, and larvae were transferred to 12-well plates after 7 additional days as described in the work by Shafiei et al. (45). Larvae and pupae were kept in an incubator at 24 °C and a 16-:8-h light:dark cycle.
Cloning and Sequencing of Onthophagus Genes.
Sequence information of hh, ptc, and smo was first obtained from a previously published study (28). Briefly, RT-PCR was used to amplify cDNA fragments from O. taurus pupal male RNA samples (Table S2 shows primer sequences). Amplified fragments were subsequently cloned into pSC-A vector (StrataClone PCR Cloning Kit; Agilent). Sequencing reactions from cloned fragments were carried out using BigDye Cycle Sequencing Chemistry (Life Technologies). We were able to obtain 282-bp partial sequence fragments for hh and ptc, respectively. We used these fragments for dsRNA synthesis (see below). A 242-bp partial smo fragment for dsRNA synthesis was cloned from a putatively 2,769-bp full-length sequence.
Table S2.
Primer sequences used in this study
| Primer name | Sequence | Use |
| OthhF | GAAGGTACAGCTGCTGATAGA | Gene cloning |
| OthhR | TCACTGATACATCCTATAGGGAAT | |
| OthhRQF | TTATGGCACCGATTAGAATGG | Cloning of the region used for RNAi; qPCR |
| OthhRQR | TCACTGATACATCCTATAGGGAAT | |
| OtsmoF | ATGATTTTTGATGTAGTTGTGGT | Gene cloning |
| OtsmoR | TCATTTTAGCCTGTTTCGTTTT | |
| OtsmoRQF | TGATAATAATCGGCGATCAGAA | Cloning of the region used for RNAi; qPCR |
| OtsmoRQR | TCATTTTAGCCTGTTTCGTTTTATG | |
| OtptcF | ATTTAAAAGATTTGCGCACAACTC | Gene cloning; cloning of the region used for RNAi; qPCR |
| OtptcR | TTCCAGTGGATTAATATGCAACAAA |
qPCR, quantitative PCR.
dsRNA Generation, Injection, and Control Treatments.
To knockdown hh, ptc, and smo, dsRNA was generated as described in ref. 23. The region of interest was amplified and cloned using the StrataClone PCR Cloning Kit followed by BigDye Sequencing (Table S2 shows primer sequences). We used 282-bp partial sequence fragments for hh and ptc. A 242-bp partial smo fragment was cloned from a putatively 2,769-bp full-length sequence. The vector containing the fragment was purified using the QIAprep Spin Miniprep Kit (Qiagen). The purified plasmid vector was used as a template for PCR with M13 primers (IDT), and the PCR product was used for in vitro transcription. Forward and reverse RNA strands were produced using the MEGAscript T7 and T3 Kit (Life Technologies) followed by DNase I treatment to remove DNA template. The synthesized RNA was precipitated by lithium, dissolved in injection buffer, denatured at 75 °C for 10 min, chilled on ice, and mixed at a 1:1 ratio by weight. This mixture was incubated in a water bath at 80 °C until room temperature was reached. The concentration of the annealed RNA was measured and confirmed by gel electrophoresis, and it was stored at −80 °C until injection. We used a Hamilton syringe and a 32-gauge needle for injection. Amounts of injection for smo, ptc, and hh dsRNA were 0.5, 3, and 6 µg, respectively. Most larvae were injected once during the first 5 d of the final third instar, and a few larvae were injected late in the second instar. Control injections were carried out as described in the work by Moczek and Rose (14). Briefly, we reared all of the animals under the same conditions as dsRNA-injected animals but injected them with dsRNA from a 220-bp PCR product derived from a pSC-A vector; 1 µg dsRNA was injected into larvae during the first 5 d of the final third instar.
Quantitative RT-PCR.
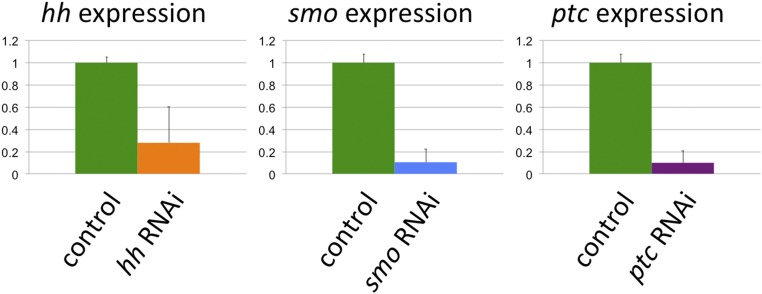
Real-time quantitative RT-PCR was conducted to validate the effect of larval RNAi (Fig. S3). Larvae were injected with hh, smo, or ptc dsRNA and reared as described above. Nine hh-, smo-, and ptc-RNAi males as well as nine control-injected individuals were collected within 24 h after pupation. Total RNA was extracted using TRIzol (Sigma-Aldrich) and stored in −80 °C until further use. We performed two-step quantitative RT-PCR using the QuantiTect Reverse Transcription Kit and the QuantiTect SYBR Green PCR Kit (Qiagen). Quantitative PCR was conducted with Applied Biosystems 7500 Real-Time PCR Systems (Life Technologies); 1 µg total RNA was used for the reverse transcription followed by quantitative PCR using an equivalent of 100 ng reverse-transcribed RNA for each sample (including two technical replicates). Reaction condition was as follows: 95 °C for 15 min followed by 40 cycles of 95 °C for 15 s, 52 °C for 30 s, and 72 °C for 30 s. ΔΔCt method was used to obtain relative expression levels of hh, smo, and ptc normalized against actin as an internal control (46).
Fig. S3.
Validation of RNAi by quantitative RT-PCR. Animals injected with hh, smo, or ptc dsRNA were harvested as early pupae and subjected to two-step quantitative RT-PCR. The effect of RNAi was assessed relative to each gene’s expression levels in control animals.
Allometric Measurements.
RNAi and control pupae as well as adults were measured using a 2D image analysis setup consisting of a dissecting microscope (Leica) mounted with a digital camera (Scion) and using ImageJ software (47). Thorax width was used as the measure of pupal and adult body size. Head and thoracic horn length were measured as described in the work by Moczek (48). Measurements were recorded to the nearest 0.01 mm.
Imaging.
Beetle images in this manuscript were collected using the same setup as used for allometric measurements. For the images of animals, five to ten images with different focal planes were aligned and merged using Adobe Photoshop Creative Suite 5.
Statistical Analysis.
Allometric scaling relationships of head and pronotal horns were analyzed as in previous studies (14, 23, 49). In brief, because male head horns exhibit a strongly sigmoidal allometry, we first contrasted horn length residuals across treatment groups. Residuals were calculated as the difference between horn lengths observed in a given individual and those expected for a given individual’s body size based on the scaling relationship seen in control-injected individuals modeled by a modified four-parameter sigmoid Hill equation. Two-tailed t tests were used for pairwise comparisons. Because hhRNAi but not smoRNAi individuals retained a sigmoidal scaling relationship similar in shape to that observed in control-injected individuals, we were able to further compare hhRNAi males with control-injected males by contrasting individual model parameter estimates for the y intercept, horn amplitude, threshold body size (point of inflection of the sigmoid), and slope at the point of inflection using Welch’s t tests.
In contrast, because pupal thoracic (pronotal) horns exhibit linear scaling relationships, we first executed an ANCOVA with horn length as the dependent variable, treatment as a fixed factor, thorax width (body size) as a covariate, and treatment x thorax width as an interaction term. We first executed this analysis by including all three treatment groups and repeated it for the raw as well as log-transformed data, but we discovered significant differences in error variance among samples with both approaches. We then executed the same analysis in pairwise comparisons. Here, analysis of log-transformed data permitted pairwise comparisons between control-injected and smoRNAi as well as between hhRNAi and smoRNAi males, respectively, but not between control-injected and hhRNAi males. Lastly, we also replicated the residual-based analysis executed for head horns for thoracic horns. We used a simple linear regression to model the allometry of control-injected males and calculated residuals as the difference between horn lengths observed in a given individual and those expected for a given individual’s body size based on this regression. Two-tailed t tests were used for pairwise comparisons. For simplicity, figures show raw data only. Results from t tests are presented as tdf = test statistic.
Acknowledgments
We thank Justin Kumar, Carolyn Spratford, and Bonnie Weasner for useful discussions on Hh and related genes. Justin Kumar also provided insightful suggestions to earlier versions of the manuscript. We thank Anna Macagno for assistance with the statistical analysis; Adam Moore, Alexander Neufeld, Justin Song, and Melanie Stamper for help with beetle care; and Vagner Benedito and Lina Yang for help with the quantitative PCR analysis. Alberto Ballerio provided useful information on beetle thorax anatomy. Funding for this study was provided by National Science Foundation Grants IOS 1256689 (to A.P.M.) and IOS 1120209 (to A.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601505113/-/DCSupplemental.
References
- 1.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457(7231):818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro A, Podlaha O. Wings, horns, and butterfly eyespots: How do complex traits evolve? PLoS Biol. 2009;7(2):e37. doi: 10.1371/journal.pbio.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro A. Origin, development, and evolution of butterfly eyespots. Annu Rev Entomol. 2015;60:253–271. doi: 10.1146/annurev-ento-010814-020942. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti CR, et al. The generation and diversification of butterfly eyespot color patterns. Curr Biol. 2001;11(20):1578–1585. doi: 10.1016/s0960-9822(01)00502-4. [DOI] [PubMed] [Google Scholar]
- 5.Pfennig DW, et al. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol. 2010;25(8):459–467. doi: 10.1016/j.tree.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Moczek AP, et al. The role of developmental plasticity in evolutionary innovation. Proc Biol Sci. 2011;278(1719):2705–2713. doi: 10.1098/rspb.2011.0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moczek AP, et al. The significance and scope of evolutionary developmental biology: A vision for the 21st century. Evol Dev. 2015;17(3):198–219. doi: 10.1111/ede.12125. [DOI] [PubMed] [Google Scholar]
- 8.Pitnick S, Pfennig DW. Evolutionary biology: Brotherly love benefits females. Nature. 2014;505(7485):626–627. doi: 10.1038/nature12853. [DOI] [PubMed] [Google Scholar]
- 9.Prudic KL, Stoehr AM, Wasik BR, Monteiro A. Eyespots deflect predator attack increasing fitness and promoting the evolution of phenotypic plasticity. Proc Biol Sci. 2015;282(1798):20141531. doi: 10.1098/rspb.2014.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom S, et al. Developmental origins of a novel gut morphology in frogs. Evol Dev. 2013;15(3):213–223. doi: 10.1111/ede.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledon-Rettig CC, Pfennig DW, Nascone-Yoder N. Ancestral variation and the potential for genetic accommodation in larval amphibians: Implications for the evolution of novel feeding strategies. Evol Dev. 2008;10(3):316–325. doi: 10.1111/j.1525-142X.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 12.Ragsdale EJ, Müller MR, Rödelsperger C, Sommer RJ. A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell. 2013;155(4):922–933. doi: 10.1016/j.cell.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 13.Susoy V, Ragsdale EJ, Kanzaki N, Sommer RJ. Rapid diversification associated with a macroevolutionary pulse of developmental plasticity. eLife. 2015;4:4. doi: 10.7554/eLife.05463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moczek AP, Rose DJ. Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc Natl Acad Sci USA. 2009;106(22):8992–8997. doi: 10.1073/pnas.0809668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moczek AP, Nijhout HF. Rapid evolution of a polyphenic threshold. Evol Dev. 2003;5(3):259–268. doi: 10.1046/j.1525-142x.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 16.Emlen DJ, Hunt J, Simmons LW. Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: Phylogenetic evidence for modularity, evolutionary lability, and constraint. Am Nat. 2005;166(Suppl 4):S42–S68. doi: 10.1086/444599. [DOI] [PubMed] [Google Scholar]
- 17.Emlen DJ, Marangelo J, Ball B, Cunningham CW. Diversity in the weapons of sexual selection: Horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae) Evolution. 2005;59(5):1060–1084. [PubMed] [Google Scholar]
- 18.Snell-Rood EC, Moczek AP. Horns and the role of development in the evolution of beetle contests. In: Hardy ICW, Briffa M, editors. Animal Contests. Cambridge Univ Press; Cambridge, United Kingdom: 2010. pp. 178–198. [Google Scholar]
- 19.Moczek AP. The evolution and development of novel traits, or how beetles got their horns. Bioscience. 2005;55(11):937–951. [Google Scholar]
- 20.Kijimoto T, Pespeni M, Beckers O, Moczek AP. Beetle horns and horned beetles: Emerging models in developmental evolution and ecology. Wiley Interdiscip Rev Dev Biol. 2013;2(3):405–418. doi: 10.1002/wdev.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emlen DJ. The evolution of animal weapons. Annu Rev Ecol Evol Syst. 2008;39(1):387–413. [Google Scholar]
- 22.Snell-Rood EC, et al. Developmental decoupling of alternative phenotypes: Insights from the transcriptomes of horn-polyphenic beetles. Evolution. 2011;65(1):231–245. doi: 10.1111/j.1558-5646.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kijimoto T, Moczek AP, Andrews J. Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns. Proc Natl Acad Sci USA. 2012;109(50):20526–20531. doi: 10.1073/pnas.1118589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science. 2012;337(6096):860–864. doi: 10.1126/science.1224286. [DOI] [PubMed] [Google Scholar]
- 25.Snell-Rood EC, Moczek AP. Insulin signaling as a mechanism underlying developmental plasticity: The role of FOXO in a nutritional polyphenism. PLoS One. 2012;7(4):e34857. doi: 10.1371/journal.pone.0034857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito Y, et al. The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep. 2013;14(6):561–567. doi: 10.1038/embor.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotoh H, et al. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via juvenile hormone signaling in stag beetles. PLoS Genet. 2014;10(1):e1004098. doi: 10.1371/journal.pgen.1004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 29.Mohler J. Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics. 1988;120(4):1061–1072. doi: 10.1093/genetics/120.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi JH, et al. Gene discovery in the horned beetle Onthophagus taurus. BMC Genomics. 2010;11:703. doi: 10.1186/1471-2164-11-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritcher P. Morphology of the posterior procoxal bridges in Scarabaeoidea (Coleoptera) Coleopt Bull. 1969;23(4):89–92. [Google Scholar]
- 32.Snodgrass RE. Principles of Insect Morphology. McGraw-Hill; New York: 1935. [Google Scholar]
- 33.Matsuda R. Morphology and evolution of the insect thorax. Mem Entomol Soc Can. 1970;76(S76):5–429. [Google Scholar]
- 34.Hlavac TF. The prothorax of Coleoptera: Origin, major features of variation. Psyche (Stuttg) 1972;79(3):123–149. [Google Scholar]
- 35.Hlavac TF. The prothorax of Coleoptera (except Bostrichiformia - Cucujiformia) Bull Mus Comp Zool. 1975;147(4):137–183. [Google Scholar]
- 36.Kopp A, Muskavitch MA, Duncan I. The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development. 1997;124(19):3703–3714. doi: 10.1242/dev.124.19.3703. [DOI] [PubMed] [Google Scholar]
- 37.Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Biol. 2005;6(4):306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 38.Moczek AP, Cruickshank TE, Shelby A. When ontogeny reveals what phylogeny hides: Gain and loss of horns during development and evolution of horned beetles. Evolution. 2006;60(11):2329–2341. [PubMed] [Google Scholar]
- 39.Kijimoto T, Andrews J, Moczek AP. Programed cell death shapes the expression of horns within and between species of horned beetles. Evol Dev. 2010;12(5):449–458. doi: 10.1111/j.1525-142X.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 40.Gerhart J, Kirschner M. The theory of facilitated variation. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8582–8589. doi: 10.1073/pnas.0701035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotoh H, et al. Juvenile hormone regulates extreme mandible growth in male stag beetles. PLoS One. 2011;6(6):e21139. doi: 10.1371/journal.pone.0021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319(5871):1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 43.Alvarado S, Rajakumar R, Abouheif E, Szyf M. Epigenetic variation in the Egfr gene generates quantitative variation in a complex trait in ants. Nat Commun. 2015;6:6513. doi: 10.1038/ncomms7513. [DOI] [PubMed] [Google Scholar]
- 44.Moczek AP, Nagy LM. Diverse developmental mechanisms contribute to different levels of diversity in horned beetles. Evol Dev. 2005;7(3):175–185. doi: 10.1111/j.1525-142X.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 45.Shafiei M, Moczek AP, Nijhout HF. Food availability controls the onset of metamorphosis in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae) Physiol Entomol. 2001;26(2):173–180. [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 48.Moczek AP. Pupal remodeling and the development and evolution of sexual dimorphism in horned beetles. Am Nat. 2006;168(6):711–729. doi: 10.1086/509051. [DOI] [PubMed] [Google Scholar]
- 49.Emlen DJ. Artificial selection on horn length-body size allometry in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae) Evolution. 1996;50(3):1219–1230. doi: 10.1111/j.1558-5646.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]