Significance
Contraction of heart muscle is triggered by calcium binding to the actin-containing thin filaments but modulated by structural changes in the myosin-containing thick filaments. We showed that phosphorylation of the myosin regulatory light chain generates a structural signal that is transmitted between myosin molecules in the thick filament and from the thick to the thin filaments, altering their calcium sensitivity. A closely related dual-filament signaling pathway underlies the enhanced contractility of heart muscle when it is stretched. These coordinated and cooperative changes in thick and thin filament structure are an essential component of contractile regulation in the healthy heart, and their impairment is likely to underlie the functional effects of mutations in thick filament proteins in heart disease.
Keywords: cardiac muscle regulation, myosin regulatory light chain, phosphorylation
Abstract
Contraction of heart muscle is triggered by calcium binding to the actin-containing thin filaments but modulated by structural changes in the myosin-containing thick filaments. We used phosphorylation of the myosin regulatory light chain (cRLC) by the cardiac isoform of its specific kinase to elucidate mechanisms of thick filament-mediated contractile regulation in demembranated trabeculae from the rat right ventricle. cRLC phosphorylation enhanced active force and its calcium sensitivity and altered thick filament structure as reported by bifunctional rhodamine probes on the cRLC: the myosin head domains became more perpendicular to the filament axis. The effects of cRLC phosphorylation on thick filament structure and its calcium sensitivity were mimicked by increasing sarcomere length or by deleting the N terminus of the cRLC. Changes in thick filament structure were highly cooperative with respect to either calcium concentration or extent of cRLC phosphorylation. Probes on unphosphorylated myosin heads reported similar structural changes when neighboring heads were phosphorylated, directly demonstrating signaling between myosin heads. Moreover probes on troponin showed that calcium sensitization by cRLC phosphorylation is mediated by the thin filament, revealing a signaling pathway between thick and thin filaments that is still present when active force is blocked by Blebbistatin. These results show that coordinated and cooperative structural changes in the thick and thin filaments are fundamental to the physiological regulation of contractility in the heart. This integrated dual-filament concept of contractile regulation may aid understanding of functional effects of mutations in the protein components of both filaments associated with heart disease.
Contraction of heart muscle is initiated by an intracellular Ca2+ transient followed by binding of Ca2+ to troponin in the thin filaments. The resulting change in thin filament structure allows myosin heads or motor domains from the thick filaments to bind to actin in the thin filaments, and a change in the conformation of the actin-attached head domain linked to ATP hydrolysis drives force generation and filament sliding (1–3). During this “working stroke,” small conformational changes in the catalytic domain of the myosin head associated with release of ATP hydrolysis products are amplified by its light chain domain, containing the essential and regulatory light chains (4), to produce a nanometer-scale displacement at the connection of the head to the thick filament backbone.
Contractility of heart muscle is also controlled by multiple posttranslational modifications of both thick and thin filament regulatory proteins, and these changes in filament proteins have been widely implicated in the modulation of cardiac output in health and disease (5–7). In the present study, we focused on phosphorylation of the cardiac isoform of the regulatory light chain (cRLC) as a well-characterized and experimentally accessible example of modification of a thick filament component. cRLC is at the thick filament end of the myosin head, where it joins the coiled–coil tail that forms the thick filament backbone. cRLC is partially phosphorylated in vivo under basal conditions (8–12), and changes in its phosphorylation level are linked to heart disease (8, 11, 13, 14). cRLC mutations associated with hypertrophic cardiomyopathy abolish cRLC phosphorylation in vitro (15), and mice expressing nonphosphorylatable cRLCs show severe cardiac dysfunction (10, 16). In the vertebrate heart, cRLCs are phosphorylated almost exclusively by the cardiac isoform of myosin light chain kinase (cMLCK) (17, 18), and cMLCK gene ablation leads to severe cardiac hypertrophy (19).
The mechanisms responsible for the regulation of cardiac function by cRLC phosphorylation are poorly understood at the molecular, cellular, and organ levels. Mechanistic hypotheses at the molecular level have been largely based on studies of smooth and skeletal muscle RLCs, which have similar molecular structures and contain phosphorylatable serines analogous to that (Ser15) in cRLC (4, 20–22). Phosphorylation of the analogous serine in smooth muscle RLC (smRLC) is the primary mechanism of contractile regulation in that tissue. Phosphorylation of skRLC plays a similar role in some invertebrate skeletal muscles (23, 24) and enhances contractility in mammalian skeletal muscle (25).
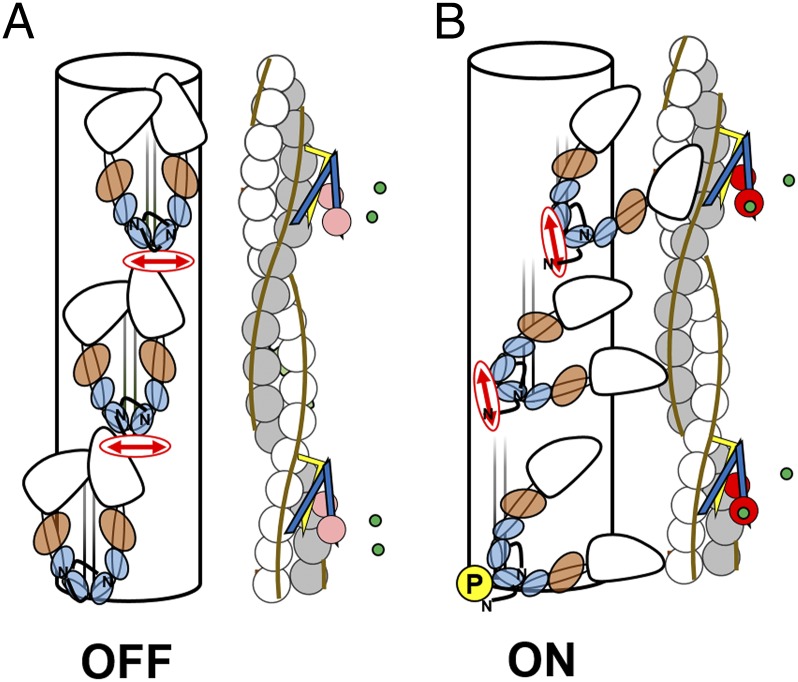
Electron microscopy studies of isolated myosins and thick filaments from all these sources (21, 26, 27) suggest a conserved molecular mechanism in which RLC phosphorylation activates or potentiates contractility by disrupting a compact OFF conformation of myosin in which the myosin heads are folded back on the myosin tail (20, 21, 28) (Fig. 1A). In striated muscles, this folded OFF conformation is linked to the formation of an ordered helical lattice of myosin head domains on the surface of the thick filaments, with the long axis of the head roughly parallel to the filament axis. This surface lattice of myosin heads is stabilized by intermolecular interactions between adjacent myosin molecules and possibly between myosin and two other thick filament components, titin, and myosin-binding protein C (MyBP-C). This characteristic folded OFF state of myosin is also present in the C-zone of cardiac thick filaments: the region that contains MyBP-C (26, 27). Phosphorylation of skRLC in isolated thick filaments of skeletal muscle disrupts the lattice, releasing the myosin heads from the filament surface (Fig. 1B). These structural studies on isolated proteins and filaments led to the hypothesis that RLC phosphorylation potentiates contractility in skeletal and cardiac muscle by increasing the number of myosin heads available for actin interaction.
Fig. 1.
OFF and ON conformations of the thick and thin filaments in heart muscle. (A) OFF conformations at low [Ca2+] in the absence of phosphorylation of the cRLC (blue). (B) ON conformations with calcium (green) bound to troponin C (pink/red) and cRLC phosphorylated (yellow circle, P). Troponin I, troponin T, and tropomyosin are yellow, blue, and sepia, respectively. Essential light chain is brown. BC-cRLC probe dipole is indicated by red double arrows.
The aim of the present work was to understand the role of interactions between thick and thin filament-based regulatory mechanisms in a heart muscle cell, in which the normal structure, organization, and interrelationship of the filaments is preserved. We measured the structural changes in the thick filaments of ventricular trabeculae by polarized fluorescence from bifunctional probes attached to the cRLC, exploiting the fact that the native cRLCs in demembranated trabeculae can be efficiently replaced by labeled cRLCs with negligible effect on trabecular function (29, 30). We used in situ phosphorylation of cRLC by an expressed cMLCK to control the regulatory state of the thick filament and further exploited the cRLC exchange protocol to introduce cRLC mutants for mechanistic studies. We studied the relationship between thick and thin filament regulation using calcium titrations with probes on both the cRLC in the thick filaments and troponin in the thin filaments. The results presented below show that integration of thick and thin filament-based signaling pathways is essential for the normal regulation of contractility in the heart.
Results and Discussion
cMLCK Phosphorylates cRLC Specifically in Rat Heart Muscle.
We showed previously that recombinant human cMLCK phosphorylates serine 15 of isolated human or rat cRLC in a Ca2+ and calmodulin (CaM)-dependent manner (30). Here we tested the specificity of cMLCK in rat cardiac myofibrils by incubation in 1 μmol/L cMLCK in the presence of Ca2+ and CaM (1 μmol/L), using 2,3-butanedione-monoxime (BDM; 30 mmol/L) to prevent contraction. cRLC was specifically phosphorylated by cMLCK in situ to about 0.5 mol/mol in a 60-min incubation as indicated by Pro-Q Diamond phospho-protein stain and Western blot (SI Appendix, Figs. S1 and S2). Similar results were obtained in isolated demembranated trabeculae from rat ventricle (SI Appendix, Fig. S3). cRLC phosphorylation was undetectable before cMLCK treatment (<0.05 mol/mol), and the extent of phosphorylation after treatment depended on the preparation and protocol as reported in detail below. The phosphorylation profiles of myosin binding protein-C (cMyBP-C) and troponin I (cTnI) were not altered by incubation in cMLCK/Ca2+/CaM in these conditions (SI Appendix, Figs. S1 and S3–S5). In vitro kinase assays using cMyBP-C isolated from rat ventricle confirmed that cMLCK does not phosphorylate this protein (SI Appendix, Fig. S4). These results are consistent with previous reports that cTnI and cMyBP-C phosphorylation levels in intact rodent cardiomyocytes are unaffected by conditional cMLCK gene ablation (12).
cRLC Phosphorylation Increases Ca2+ Sensitivity of Isometric Force in Ventricular Trabeculae.
cRLC phosphorylation was undetectable (<0.05 mol/mol) in freshly demembranated trabeculae from rat ventricle (30). After a 1-h incubation in cMLCK/CaM/Ca2+, the cRLC phosphorylation level was 0.49 ± 0.10 mol/mol (mean ± SEM, n = 8; SI Appendix, Fig. S6). Isometric force during maximal Ca2+ activation from a relaxed sarcomere length of 1.9 μm was slightly increased by cRLC phosphorylation (Fig. 2A, Inset), and normalized force at submaximal [Ca2+] was clearly increased (Fig. 2A and SI Appendix, Table S1). Hill equation fits to the force-pCa data indicated that cRLC phosphorylation increased pCa for half-maximal force (pCa50) by 0.07, consistent with previous reports (25, 31–33), with no significant change in the Hill coefficient nH (SI Appendix, Table S1). Treatment of rat trabeculae with in situ phosphorylation buffer lacking cMLCK did not change Ca2+ sensitivity, cooperativity, or maximum Ca2+-activated force (SI Appendix, Fig. S7), and the cRLC phosphorylation level in these trabeculae was 0.02 ± 0.01 mol/mol (mean ± SEM, n = 4).
Fig. 2.
cRLC phosphorylation controls myosin head conformation. Calcium-dependence of force (A) and BSR-cRLC-BC probe orientation measured as the <P2> order parameter (B) before (●) and after (○) incubation with cMLCK at 1.9-μm sarcomere length. Maximal isometric force before (black bars) and after cMLCK treatment (open bars) are shown in the Inset in A. Means ± SEM (n = 5). The pictograms on the right indicate the orientation of BSR labeled (red) and phosphorylated (yellow circle; P) cRLCs before (Lower) and after cMLCK treatment (Upper) with respect to the filament axis (vertical arrow). The angular changes are exaggerated for clarity.
cRLC Phosphorylation Alters the Orientation of the Myosin Light-Chain Domain.
A bifunctional sulforhodamine (BSR) probe cross-linking helices B and C in the N-terminal lobe of the cRLC (BSR-cRLC-BC), roughly perpendicular to the long axis of the myosin head (Fig. 1), provides a sensitive signal for the change in orientation of the RLC region of the myosin heads in cardiac thick filaments induced by cRLC phosphorylation (29, 34). In the phosphorylated state of the thick filament, the light chain domains of the myosin heads are more perpendicular to the filament axis, and the dipole of the BC probe (red arrow) becomes more parallel to the filament axis (Fig. 1B) (29, 34). In the polarized fluorescence technique used here, this is reported as a more positive value of the order parameter <P2>, which measures the time-averaged orientation of the BC probe dipole with respect to the filament axis (35). <P2> can take values between +1 for a dipole parallel to the filament axis and −0.5 for a perpendicular dipole.
In the present experiments, about 40% of the native cRLCs in demembranated trabeculae were replaced by BSR-cRLC-BC (SI Appendix, SI Methods). Incubation of the trabeculae in cMLCK led to in situ phosphorylation of these BSR-cRLC-BCs to 0.50 ± 0.05 mol/mol (mean ± SEM, n = 10; SI Appendix, Fig. S6), the same as the fraction observed for native cRLC in the same protocol. The effect of cRLC phosphorylation on the Ca2+ sensitivity and cooperativity of isometric force in trabeculae containing BSR-cRLC-BC was also similar to that observed for native cRLC (SI Appendix, Table S1).
cRLC phosphorylation increased <P2> for the BSR-cRLC-BC probe at all [Ca2+] (Fig. 2B). The higher value of <P2> indicates that the probe dipole has become more parallel to the filament axis and therefore that the light chain domain of the myosin heads is more perpendicular (Fig. 1B). Calcium activation of the thin filament produced a further increase in <P2>, but the effect of RLC phosphorylation on probe orientation was greater at lower [Ca2+]. The Ca2+ dependence of probe orientation between pCa 6.5 and pCa 4.5 was well fitted by the Hill equation, both before and after cMLCK incubation. In the unphosphorylated state (●), pCa50 for probe orientation was 5.61 ± 0.01 and nH was 8.1 ± 0.8 (means ± SEM, n = 5; SI Appendix, Table S1; sarcomere length 1.9 μm). Both these parameters are larger than the corresponding values for force (SI Appendix, Table S1), as reported previously (29). cRLC phosphorylation induced a reproducible increase in pCa50 for probe orientation by 0.10 pCa units with no significant change in nH (SI Appendix, Table S1).
cRLC Phosphorylation Induces a Structural Change in Unphosphorylated Myosins by Signaling Between Myosin Head Domains.
In the protocol used for Fig. 2, myosin heads carrying the BSR-cRLC-BC probe and unlabeled myosin heads were phosphorylated equally. To investigate whether phosphorylated myosin heads can influence the conformation of neighboring unphosphorylated heads in the trabeculae, we attached the probe to myosin heads containing a cRLC that could not be phosphorylated because its serine 15 had been replaced by alanine. We confirmed that this labeled cRLC, referred to as S15A-BSR-cRLC-BC, was not phosphorylated by cMLCK in vitro (SI Appendix, Fig. S8). Moreover these S15A-BSR-cRLC-BCs, identified in Phostag gels by their BSR fluorescence, were not phosphorylated by cMLCK treatment in trabeculae (SI Appendix, Fig. S8). cRLC phosphorylation of the endogenous unlabeled heads in this protocol corresponded to 0.23 ± 0.03 mol/mol of total trabecular cRLC content (SI Appendix, Fig. S8).
This extent of in situ phosphorylation of the unlabeled cRLCs produced similar effects on Ca2+-activated force as those described in the previous section for labeled cRLCs with a phosphorylatable Ser-15. In particular, pCa50 for force increased by 0.10 pCa units with no significant change in nH (Fig. 3A and SI Appendix, Table S2). Moreover, despite the fact that the myosin heads carrying the cRLC probe were not phosphorylated in this experiment, the effect of cMLCK incubation on probe orientation (Fig. 3B) was similar to that observed for labeled cRLCs with a phosphorylatable Ser-15 (Fig. 2B). cRLC phosphorylation increased <P2> at all [Ca2+], corresponding to a more perpendicular orientation of the myosin heads, although the effect of phosphorylation was more prominent at low [Ca2+]. Moreover pCa50 for the orientation change of the labeled unphosphorylated myosin heads was still increased by 0.12 pCa units by cMLCK treatment (SI Appendix, Table S2). These results show that cRLC phosphorylation by cMLCK induces a cooperative change in the structure of the thick filament that allows a relatively low level of cRLC phosphorylation (about 20%) to alter the orientation of unphosphorylated myosin heads.
Fig. 3.
cRLC phosphorylation controls the conformation of unphosphorylated myosin heads via interhead signaling. (A and B) Calcium dependence of force (A) and <P2> (B) in trabeculae exchanged with nonphosphorylatable S15A-BSR-cRLC-BC before (●) and after (○) cMLCK treatment. (C and D) Calcium dependence of force (C) and <P2> (D) in trabeculae coexchanged with BSR-cRLC-BC and either unphosphorylated (●; cRLCwt) or phosphorylated WT cRLC (○; cRLCwt-S15-Pi). Sarcomere length, 1.9 μm. Means ± SEM (n = 5).
To further test this conclusion and to eliminate any residual uncertainty about the specificity of in situ phosphorylation by cMLCK, we introduced a mixture of labeled unphosphorylated and unlabeled phosphorylated cRLCs by an independent protocol. Unphosphorylated BSR-cRLC-BC was mixed with either purified in vitro-phosphorylated unlabeled cRLC (cRLCwt-S15-Pi) or unphosphorylated unlabeled cRLC (cRLCwt) as a control and coexchanged into trabeculae. This protocol produced roughly the same distribution of labeled/phosphorylated cRLC species in the trabeculae as the S15A method described above. None of the labeled cRLCs were phosphorylated (SI Appendix, Fig. S8), and 0.20 ± 0.05 mol/mol of total cRLC in the exchanged trabeculae was phosphorylated in this protocol (SI Appendix, Fig. S8). Ca2+ sensitivity of both isometric force (Fig. 3C and SI Appendix, Table S2) and probe orientation (Fig. 3D and SI Appendix, Table S2) were reproducibly increased by about 0.04 pCa units by the inclusion of cRLCwt-S15-Pi, and <P2> was higher at all [Ca2+] (Fig. 3D), corresponding to a more perpendicular orientation of the myosin heads. These results strongly support the conclusion of the experiments described in the previous paragraph that a relatively low molar ratio of cRLC phosphorylation (about 0.2 mol/mol) influences the orientation of unphosphorylated myosin heads through cooperative interactions between myosin heads.
The Parallel Orientation of the Myosin Heads Requires the N-Terminal Extension of the cRLC.
As a step toward dissecting the molecular mechanism of the control of the orientation of the myosin heads in cardiac thick filaments by cRLC phosphorylation, we followed an approach previously applied to smooth muscle, which is regulated by phosphorylation of an analogous serine near the N terminus of its RLC (smRLC) (36). The folded OFF conformation of smooth muscle myosin heads in the unphosphorylated state is stabilized by this N-terminal region of the smRLC, and deletion of this region mimics the structural and regulatory effects of phosphorylation. We therefore prepared a truncated version of cRLC by deleting its first 12 amino acids (SI Appendix, Fig. S9) and labeled the BC helix of this truncated cRLC with BSR. This labeled truncated cRLC is subsequently referred to as Ndel-BSR-cRLC-BC. Although Ndel-BSR-cRLC-BC retains a serine in the position analogous to the native Ser-15 phosphorylation site, it is a poor substrate for cMLCK in vitro (SI Appendix, Fig. S9).
Replacement of 30–40% of the native cRLC of ventricular trabeculae by Ndel-BSR-cRLC-BC increased the calcium sensitivity of isometric force production at sarcomere length 1.9 μm by 0.10 pCa units compared with the BSR-cRLC-BC control (Fig. 4A, dashed and dotted lines show effect of phosphorylation of full-length cRLC from Fig. 2, and SI Appendix, Tables S1 and S3). Thus, deletion of the N terminus of cRLC sensitizes force production by about the same amount as phosphorylation of WT cRLC, consistent with the hypothesis that the N terminus of cRLC is required for stabilization of the heads-parallel state of the thick filament. Moreover there was no further significant change in pCa50 for force on in situ cRLC phosphorylation of trabeculae containing Ndel-BSR-cRLC-BC (Fig. 4A and SI Appendix, Table S3), despite a total cRLC phosphorylation level of 0.79 ± 0.02 mol/mol in these experiments (SI Appendix, Fig. S9). The Hill coefficient (nH) for the Ca2+ dependence of isometric force was reduced by in situ cRLC phosphorylation in the presence of Ndel-BSR-cRLC-BC (Fig. 4A and SI Appendix, Table S3), suggesting that the N-terminal extension of the cRLC is required for full cooperativity of the structural change in the thick filament in response to Ca2+ activation.
Fig. 4.
The cRLC N-terminal extension is required for the OFF conformation of the myosin head. Calcium dependence of force (A) and <P2> (B) in trabeculae exchanged with N-terminal deleted Ndel-BSR-cRLC-BC before (●) and after (○) cMLCK treatment. The Hill equation fits from Fig. 2 for BSR-cRLC-BC exchanged trabeculae before and after incubation with cMLCK are shown as dashed and dotted lines, respectively. Sarcomere length, 1.9 μm. Means ± SEM (n = 5).
<P2> for the BC probe was increased by deletion of the N-terminal extension of cRLC (Fig. 4B), indicating that the myosin heads in trabeculae containing Ndel-BSR-cRLC-BC become more perpendicular to the thick filament axis at all [Ca2+]. Addition of [Ca2+] in the absence of cRLC phosphorylation (Fig. 4B) induced a further increase in <P2>, and pCa50 for this orientation change was 5.76 ± 0.03 (SI Appendix, Table S3), much higher than that observed for control BSR-cRLC-BC. In situ phosphorylation of trabeculae containing Ndel-BSR-cRLC-BC produced an even more parallel orientation of the BC probes, which also became almost insensitive to [Ca2+], suggesting that, when about 80% of the cRLCs are phosphorylated in the presence of about 40% Ndel-BSR-cRLC-BC, thick filament structure as reported by the BC probe becomes almost insensitive to [Ca2+]. Full Ca2+ control of isometric force is retained in these conditions, albeit with lower cooperativity (Fig. 4A and SI Appendix, Table S3).
These results show that the N-terminal extension of the cRLC is required for stabilization of the OFF state of the unphosphorylated thick filament and for physiological cooperativity of calcium activation in heart muscle.
Calcium Sensitization by cRLC Phosphorylation Is Mediated by Structural Changes in the Thin Filament.
The results described above demonstrated a functional coupling between the structure of the thick filament and the sensitivity of that structural change and of isometric force to [Ca2+]. Because the calcium sensitivity of isometric force is primarily determined by the regulatory proteins of the thin filament, we used a bifunctional rhodamine (BR) probe on troponin to investigate the role of the thin filaments in calcium sensitization by cRLC phosphorylation. This probe (BR-cTnC-E) is parallel to the E helix in the C-terminal lobe of troponin C (TnC) and signals the regulatory state of the thin filament (29, 37). In this set of experiments, in situ cMLCK treatment led to cRLC phosphorylation of about 0.8 mol/mol (SI Appendix, Fig. S10) and enhancement of isometric force at pCa 4.5 of about 25% (Fig. 5A and SI Appendix, Table S4). The pCa50 for isometric force increased by 0.10 pCa units on cRLC phosphorylation at 1.9-μm sarcomere length with no change in nH (SI Appendix, Table S4), reproducing the effect observed in experiments with probes on the cRLC (Fig. 2 and SI Appendix, Table S1).
Fig. 5.
Phosphorylation of cRLC increases the calcium sensitivity of the thin filament. (A) Calcium-dependence of force before (●) and after (○) cMLCK treatment of BR-cTnC-E exchanged trabeculae. (B) Calcium dependence of <P2> for the BR-cTnC-E probe before (●) and after (○) cMLCK treatment and after inhibition of active contraction by Blebbistatin (gray triangles). (C and D) Calcium dependence of force (C) and <P2> (D) in the absence of Blebbistatin (●), in the presence of Blebbistatin (○), and after cMLCK treatment in the presence of Blebbistatin (gray triangles). Fitted pCa50 parameters for B and D are shown in the Insets. Sarcomere length, 1.9 μm. Means ± SEM (n = 6–7). Paired two-tailed Student t test: **P < 0.01; ***P < 0.001.
In control trabeculae, with very low levels of cRLC phosphorylation, the orientation of the BR-TnC-E probe has a biphasic dependence on [Ca2+] (36), although the data for pCa < 6 were reasonably well fitted by the Hill equation (Fig. 5B, ●). In situ cRLC phosphorylation by cMLCK increased <P2> for BR-cTnC-E slightly at both high and low [Ca2+], with no effect on the overall Ca2+-dependent change in probe orientation (Fig. 5B, ○). pCa50 for probe orientation increased by 0.08 pCa units, with no significant change in nH (SI Appendix, Table S4), similar to the effect on isometric force. These results show that phosphorylation of cRLC in the thick filament changes the Ca2+ sensitivity of force by altering the Ca2+ sensitivity of structural changes in the thin filament rather than by simply increasing the availability of myosin heads for thin-filament binding.
To investigate the mechanism of this effect, we inhibited active force generation using 25 μmol/L Blebbistatin (38). Addition of Blebbistatin after cRLC phosphorylation reduced the overall amplitude of the change in BR-cTnC-E orientation and reversed the increase in pCa50 induced by cRLC phosphorylation (Fig. 5B, gray triangles and inset histogram, and SI Appendix, Table S4). We confirmed the antagonistic effects of cRLC phosphorylation and Blebbistatin on the Ca2+ sensitivity of the thin filament in a separate set of experiments in which Blebbistatin was added to trabeculae containing unphosphorylated cRLC, causing a decrease in pCa50 by 0.09 units (Fig. 5D, ○, inset histogram, and SI Appendix, Table S3). Subsequent in situ phosphorylation of cRLC to 0.86 mol/mol (SI Appendix, Fig. S10) by cMLCK in the presence of Blebbistatin caused a significant but smaller increase in pCa50 for the BR-cTnC-E orientation change by 0.05 pCa units (Fig. 5D, inverted triangles, inset histogram, and SI Appendix, Table S4).
These results show that phosphorylation of cRLC in the thick filaments alters the Ca2+-sensitivity of the thin filament. The effect is still present when active force generation and strong binding of myosin heads to the thin filaments are abolished by Blebbistatin.
Length-Dependent Activation and cRLC Phosphorylation May Use the Same Dual-Filament Signaling Pathway.
Length-dependent activation, the sarcomere-level phenomenon that is thought to underlie the Frank–Starling relationship between systolic contractility and end diastolic volume in the heart (39), is characterized by increases in both maximum Ca2+-activated force and Ca2+ sensitivity over the physiological range of sarcomere length, effects that are also produced by cRLC phosphorylation (Fig. 2), suggesting that the two effects might share part of a signaling pathway. To test this idea, we measured the sarcomere length dependence of the effects of cRLC phosphorylation. In situ phosphorylation of cRLC by cMLCK to about 0.5 mol/mol (SI Appendix, Table S1) increased maximum Ca2+-activated force at sarcomere length 2.3 μm by about 12% and pCa50 by 0.09 (Fig. 6A and SI Appendix, Table S1). Comparison with the results obtained at sarcomere length 1.9 μm (Fig. 2 fits reproduced as the dashed and dotted lines in Fig. 6A) shows that the effects of cRLC phosphorylation and increased sarcomere length are additive. However, the Hill coefficient nH was significantly reduced by cRLC phosphorylation at the longer sarcomere length (Fig. 6A and SI Appendix, Table S1), in agreement with some previous reports (32, 33).
Fig. 6.
cRLC phosphorylation and increased sarcomere length have synergistic effects on myosin head conformation. Calcium dependence of force (A) and BSR-cRLC-BC probe orientation (B) before (●) and after (○) incubation with cMLCK at 2.3-μm sarcomere length. Maximal isometric force before (black bars) and after (open bars) cMLCK treatment shown in the Inset in A. Dashed and dotted lines represent the Hill equation fits before and after cMLCK incubation, respectively, at sarcomere length 1.9 μm; data from Fig. 2. Means ± SEM (n = 5).
Increasing sarcomere length from 1.9 to 2.3 μm significantly increased <P2> for the BSR-cRLC-BC probe at all activating [Ca2+] in the cRLC-unphosphorylated state (Fig. 6B, compare filled circles with dashed line) and at all [Ca2+] in the phosphorylated state (open circles and dotted line). Thus, increased sarcomere length, like cRLC phosphorylation, is associated with a more perpendicular orientation of the light chain domains of the myosin heads (Fig. 6B). Moreover both interventions lead to an increase in calcium sensitivity, and these effects are also additive; increasing sarcomere length from 1.9 to 2.3 μm increased pCa50 from 5.56 to 5.63 in one set of trabeculae; cRLC phosphorylation at the longer sarcomere length in another set of trabeculae increased it further from 5.65 to 5.74 (SI Appendix, Table S1). However, at the longer sarcomere length, the phosphorylation-dependent increase in Ca2+ sensitivity was accompanied by a decrease in nH (Fig. 6B), as also observed for the isometric force (SI Appendix, Table S1).
The similar changes in thick filament structure and calcium sensitivity induced by cRLC phosphorylation and length-dependent activation suggest the involvement of a common mechanistic pathway.
Two Regulatory States of the Thick Filament in Heart Muscle.
Many aspects of the above results can be integrated and explained in terms of the concept of a regulatory transition in the conformation of the myosin heads in the thick filaments of heart muscle analogous to that in smooth muscle myosin (20, 28) and the thick filaments of skeletal muscle (21, 40). By analogy with those other muscle types and with direct support from EM reconstructions of isolated thick filaments from cardiac muscle (26, 27), we may consider an OFF state of the cardiac thick filament (Fig. 1A) in which the long axes of the myosin heads, and those of their light chain domains, are roughly parallel to the filament axis. The myosin heads are folded back onto the subfragment-2 region of the myosin tail, and this OFF structure is likely to be stabilized by intramolecular head–tail and head–head interactions, intermolecular interactions between myosins in adjacent layers along the filaments, and probably additional interactions with other thick filament components, myosin binding protein C (MyBP-C), and titin. Although many structural details of these interactions remain unclear, their effect is to stabilize a population of myosin heads, probably the majority of heads in the region of the filament containing MyBP-C, in a roughly helical lattice on the surface of the thick filaments (Fig. 1A), in which they are unavailable for binding to actin in the thin filaments or hydrolyzing ATP (41, 42), features that define this as a functional OFF state. These interactions between filament components are lost in the ON state of the thick filament (Fig. 1B), in which the myosin heads are released from the filament surface, and their light chain domains become more perpendicular to the filament axis. The ON heads are available for actin binding provided that the thin filament has been switched on by calcium binding to troponin.
The BC-cRLC probe provides a sensitive and reproducible measure of the transition between these OFF and ON states of the thick filaments in ventricular trabeculae. Because the BC probe dipole is parallel to the short hook helix of the myosin heavy chain in the N-terminal lobe of the cRLC, which itself is perpendicular to the long axis of the light chain domain (4), the order parameter <P2> for BC-cRLC probe orientation, calculated from the polarization of its fluorescence, is higher for the ON (heads-perpendicular) than for the OFF (heads-parallel) state. In general, a probe in the light chain or lever-arm domain of the myosin head might also be expected to be sensitive to changes in the orientation of the light chain domain associated with the force-generating working stroke in the myosin head (43, 44). However, the BC-cRLC probe is relatively insensitive to active force generation per se, as shown by the very small orientation changes reported by this probe on maximal calcium activation of myosin heads with phosphorylated and N-terminal deleted cRLCs (Fig. 4B, ○). This insensitivity of BC-cRLC orientation to active force generation is likely to be related to the filament-docking function of the N-terminal lobe of the cRLC and the relatively low fraction of myosin heads bound to actin during active contraction in heart muscle, as discussed previously (34).
The present results show that the regulatory transition in the N-lobe of cRLC reported by the BC-cRLC probe is controlled by cRLC phosphorylation, by calcium activation, and by sarcomere length. All three interventions promote a more ON state of the cRLC N-lobe and by implication of the cardiac thick filaments, but their effects are additive; no single intervention can produce the fully ON state. These results provide strong support for the proposal that a pool of noncycling or “superrelaxed” myosin cross-bridges is retained during physiological activation of cardiac muscle (41).
The Regulatory Transition in the Thick Filament Is Highly Cooperative.
Several independent lines of evidence indicate that the regulatory transition in the thick filament is highly cooperative. First, calcium activation from the most OFF state of the filament we studied, that with dephosphorylated cRLC at sarcomere length 1.9 μm (Fig. 2A, ●), is associated with a structural change in the thick filament with a Hill coefficient (nH) of about 8, much higher than that for active isometric force or for the structural change in the thin filament under the same conditions (SI Appendix, Tables S1 and S4). This high value of nH suggests that the regulatory structural transition in the thick filament is even more cooperative than that in the thin filament. Neither cRLC phosphorylation nor increasing sarcomere length alone alters the cooperativity reported by nH for the structural change in the thick filament (SI Appendix, Table S1), but a combination of cRLC phosphorylation and deletion of the cRLC N terminus or increased sarcomere length reduces it (SI Appendix, Tables S1 and S2). Thus, calcium-dependent cooperativity is attenuated as the low-calcium structure of the thick filament is displaced toward the ON state.
Second, the series of experiments in which the orientation of the cRLC regions of unphosphorylated heads was measured in response to the phosphorylation of neighboring heads (Fig. 3) showed directly that the regulatory transition in the thick filament reported by the BC-cRLC probe is transmitted between myosin heads, as expected for a cooperative transition in the filament mediated by head–head interactions.
Finally, both the regulatory structural transition in the thick filament (Fig. 7A) and the change in calcium sensitivity of isometric force (Fig. 7B) have a highly nonlinear dependence on the extent of cRLC phosphorylation. Although these data show considerable scatter associated with the fact that each point comes from a different trabecula, it is clear that the full effect of cRLC phosphorylation on both the structural change in the thick filaments (Fig. 7A) and the change in calcium sensitivity (Fig. 7B) is attained with cRLC phosphorylation of less than 0.2 mol/mol. This marked nonlinearity may be responsible for the observation that both nH and calcium sensitivity (pCa50) are larger for the change in thick filament structure than the corresponding parameters for force or thin filament structure (SI Appendix, Tables S1 and S4). A low level of partial activation of the thin filament, allowing binding of a very small fraction of the myosin heads, leads to a larger fractional activation of the thick filament. Cooperative activation of the thick filament is likely to have more general significance in the regulation of contraction in heart muscle, for example, in transmitting the effects of cMyBP-C in the C zone to the remainder of the thick filament (29).
Fig. 7.
Dependence of myosin head conformation, calcium sensitivity, and force on extent of cRLC phosphorylation (RLC-P/RLCtotal). (A) Increase in <P2> for BSR-cRLC-BC at ∼pCa 6.2. (B) Increase in calcium sensitivity (ΔpCa50 for isometric force). (C) Increase in maximal calcium activated isometric force. Dotted lines show linear regressions, excluding the zero phosphorylation points in A and B.
The highly nonlinear response to cRLC phosphorylation of thick filament structure and calcium sensitivity is in marked contrast with the roughly linear dependence of the maximum isometric force enhancement on extent of cRLC phosphorylation in the same experiments (Fig. 7C). The difference suggests that cRLC phosphorylation has two distinct functional consequences: a highly cooperative effect on thick filament structure linked to the change in calcium sensitivity and a linear increase in isometric force that corresponds to 35 ± 11% (95% CI) for full (1 mol/mol) cRLC phosphorylation as indicated by linear regression of the data in Fig. 7C. This value is in the middle of the wide range reported by previous studies on heart muscle preparations that used noncardiac isoforms of MLCK (25, 31–33) and similar to the value (30–40%) reported for β-cardiac myosin in vitro (45). Enhancement of maximum isometric force by cRLC phosphorylation is also observed for skeletal muscle myosin (25), and the effects of RLC phosphorylation on the motor function of a range of muscle myosin isoforms have been attributed to an increase in the myosin lever arm stiffness (46, 47) or step size (48).
Signaling Between Thick and Thin Filaments in the Heart.
The regulatory structural change in the thick filament described above is closely coupled to the change in the calcium sensitivity (pCa50) for active force; pCa50 for the structural change in the thick filament is higher in the ON state. pCa50 and the orientation of the BC-cRLC probe have the same nonlinear dependence on the extent of cRLC phosphorylation (Fig. 7 A and B). Finally, the other two interventions that were found to stabilize the ON state of the thick filament, deletion of the N terminus of the cRLC and increased sarcomere length, also increased pCa50 (SI Appendix, Tables S1 and S2). However, probes on troponin in the thin filament showed essentially the same change in pCa50 on cRLC phosphorylation as that reported for the thick filament by the BC-cRLC probe (Fig. 5 and SI Appendix, Table S4). Thus, phosphorylation of cRLC in the thick filament alters the calcium sensitivity of troponin in the thin filament.
The interfilament signaling pathway responsible for this effect does not seem to involve binding of force-generating myosin heads to the thin filaments, because cRLC phosphorylation can still increase pCa50 when active force is completely abolished by Blebbistatin (Fig. 5D). The change in pCa50 observed in the presence of Blebbistatin is smaller than that in its absence, but this difference is probably due to stabilization of the OFF structure of the thick filament by Blebbistatin, which abolishes the effect of calcium on the orientation of the BC-cRLC probe (29). Moreover the effect of Blebbistatin on pCa50 for the thin filament (37) (SI Appendix, Table S4) may be explained by communication of this more OFF thick filament structure to the thin filament by the same interfilament signaling pathway operating in the opposite direction from the ON/calcium sensitization effect induced by cRLC phosphorylation, in contrast with mechanisms based on removal of the force generating myosin heads that have been considered previously.
Thus, it seems unlikely that signaling between thick and thin filaments is wholly mediated by the interaction between myosin heads and actin, suggesting the existence of an additional signaling pathway. Although other pathways cannot be excluded, an attractive candidate mechanism, suggested by the observation that N-terminal fragments of MyBP-C can alter the structure of the thin filaments and stimulate active force generation in the absence of calcium (29, 49), is that the N terminus of MyBP-C is bound to the thick filament surface in its OFF state, but binds to the thin filament and sensitizes it to calcium when the thick filament is ON. Because the interaction of the N terminus of MyBP-C with actin is inhibited by MyBP-C phosphorylation (29, 50), such a mechanism would also provide a potential pathway for contractile modulation by kinases that act through MyBP-C (5, 51).
The direct action of MyBP-C as an interfilament signaling protein would be confined to the C-zone, the central one-third of each half filament to which MyBP-C is confined, but cooperative intermolecular interactions in both the thin and thick filaments are expected to spread the signal from the C zone toward the rest of the sarcomere (29). Functional differences between the C-zone and non–C-zone of the sarcomere may remain, however, and this may explain the observation that, despite the highly cooperative effect of cRLC phosphorylation on thick filament structure, calcium activation of the thin filament, which mobilizes myosin heads throughout the sarcomere, has an additional effect (Fig. 2).
Length-Dependent Activation.
The mechanism by which increased sarcomere length enhances force generation of heart muscle—length-dependent activation—has been the subject of many previous studies, although the molecular and cellular basis of the effect has been unclear (39). The present results suggest that the regulatory state of the thick filament is an intermediate step in the mechanism of length-dependent activation, a step that would then be followed by transmission of the signal to the thin filament as discussed above.
Moreover recent X-ray studies on skeletal muscle (52) have demonstrated a mechano-sensing signaling mechanism that couples increased thick filament stress to the transition to its ON state, which is also associated with a slightly longer periodicity of packing of the myosin tails in the filament backbone. Because the thick filaments of skeletal and heart muscle contain similar isoforms of the same proteins with a similar structural organization, this mechano-sensing mechanism may also be present in heart muscle. Moreover, heart muscle has a much higher resting stiffness than skeletal muscle, associated with its shorter titin isoform, so an increase in the sarcomere length of ventricular trabeculae from 1.9 to 2.3 μm, as in the present experiments, is associated with an increase in resting force (53), which is transmitted to the thick filaments by the titin links between the tips of the thick filaments and the end of the sarcomere. X-ray measurements on resting skeletal (54) and cardiac muscle (55) provide further support for the hypothesis of a titin- and resting stress-mediated change in the structure of the thick filament.
The combination of the present results with those of the X-ray studies mentioned above suggests the following hypothesis for the signaling pathway underlying length-dependent activation. Increasing sarcomere length stretches the stiff titin links at the tips of the thick filaments, resulting in increased thick filament strain and the transition to the longer thick filament periodicity. The latter weakens the intermolecular interactions between myosin molecules along the thick filament, promoting the transition to its ON state. Finally, interfilament signaling mechanisms, possibly involving MyBP-C, transmit the ON signal to the thin filament, increasing its calcium sensitivity as described in the previous section. Other mechanisms, for example, involving posttranslational modifications of thin filament regulatory proteins (56), may modify or extend this hypothetical signaling pathway.
Implications for the Physiology and Pathology of Contractile Regulation in the Heart.
In the conventional paradigm for contractile regulation in striated muscles, including the heart, the thick filaments are not usually considered to have a primary role, although it is well established that posttranslational modification of or mutations in thick filament components including cRLC can modulate contractility. The results presented above reveal some of the mechanisms by which contractility is controlled by cRLC phosphorylation, including the strong cooperativity of the effect, the role of the N terminus of cRLC in stabilizing the OFF state of the thick filament, and the role of signaling between thick and thin filaments in mediating calcium sensitization. However, they also show that these features are not specific to cRLC phosphorylation; similar effects seem to be involved in length-dependent activation. The cooperative structural change in the thick filament induced by either cRLC phosphorylation or increased sarcomere length is therefore likely to be an intrinsic component of the normal physiological control of cardiac contractility. Regulation of contractility in the heart involves the coordinated activation of both thick and thin filaments, although many of the underlying molecular mechanisms are still poorly understood. This concept of dual filament regulation may be useful in understanding the origins of impaired contractility associated with pathological changes in both thick and thin filament proteins.
Methods
All experiments involving animals were performed under the Schedule 1 procedure in accordance with UK Animal Scientific Procedure Act, 1986 (please see SI Appendix, SI Methods), and therefore this work has no licensing or approval requirements. Protein production, preparation of cardiac trabeculae, protein exchange protocols, and fluorescence polarization experiments were performed according to routine protocols published elsewhere. Details of materials and methods are provided in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank Mathias Gautel, Birgit Brandmeier, Alexander Alexandrovich, David Trentham, and Mark Pfuhl for help and advice. This work was supported by the British Heart Foundation (Grants PG/12/52/29713 and FS/09/001/26329).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602776113/-/DCSupplemental.
References
- 1.Huxley HE. The mechanism of muscular contraction. Science. 1969;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 2.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 3.Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 4.Rayment I, et al. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science. 1993;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 5.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283(40):26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng Y, et al. Top-down proteomics reveals concerted reductions in myofilament and Z-disc protein phosphorylation after acute myocardial infarction. Mol Cell Proteomics. 2014;13(10):2752–2764. doi: 10.1074/mcp.M114.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta MK, Robbins J. Post-translational control of cardiac hemodynamics through myosin binding protein C. Pflugers Arch. 2014;466(2):231–236. doi: 10.1007/s00424-013-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys. 2011;510(2):129–134. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeacocke SA, England PJ. Phosphorylation of myosin light chains in perfused rat heart. Effect of adrenaline and increased cytoplasmic calcium ions. Biochem J. 1980;188(3):763–768. doi: 10.1042/bj1880763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanbe A, et al. Abnormal cardiac structure and function in mice expressing nonphosphorylatable cardiac regulatory myosin light chain 2. J Biol Chem. 1999;274(30):21085–21094. doi: 10.1074/jbc.274.30.21085. [DOI] [PubMed] [Google Scholar]
- 11.Gregorich ZR, et al. Comprehensive assessment of chamber-specific and transmural heterogeneity in myofilament protein phosphorylation by top-down mass spectrometry. J Mol Cell Cardiol. 2015;87:102–112. doi: 10.1016/j.yjmcc.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AN, et al. Constitutive phosphorylation of cardiac myosin regulatory light chain in vivo. J Biol Chem. 2015;290(17):10703–10716. doi: 10.1074/jbc.M115.642165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem. 2011;286(12):9941–9947. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias FA, et al. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41(2):330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Szczesna D, et al. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J Biol Chem. 2001;276(10):7086–7092. doi: 10.1074/jbc.M009823200. [DOI] [PubMed] [Google Scholar]
- 16.Scruggs SB, et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem. 2009;284(8):5097–5106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JY, et al. Identification of cardiac-specific myosin light chain kinase. Circ Res. 2008;102(5):571–580. doi: 10.1161/CIRCRESAHA.107.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seguchi O, et al. A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. J Clin Invest. 2007;117(10):2812–2824. doi: 10.1172/JCI30804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding P, et al. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem. 2010;285(52):40819–40829. doi: 10.1074/jbc.M110.160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendt T, Taylor D, Trybus KM, Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci USA. 2001;98(8):4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodhead JL, et al. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436(7054):1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 22.Jung HS, et al. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc Natl Acad Sci USA. 2008;105(16):6022–6026. doi: 10.1073/pnas.0707846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somlyo AV, et al. Smooth muscle myosin: Regulation and properties. Philos Trans R Soc Lond B Biol Sci. 2004;359(1452):1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brito R, et al. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J Mol Biol. 2011;414(1):44–61. doi: 10.1016/j.jmb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney HL, Stull JT. Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol. 1986;250(4 Pt 1):C657–C660. doi: 10.1152/ajpcell.1986.250.4.C657. [DOI] [PubMed] [Google Scholar]
- 26.Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci USA. 2008;105(7):2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA. 2013;110(1):318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung HS, Komatsu S, Ikebe M, Craig R. Head-head and head-tail interaction: A general mechanism for switching off myosin II activity in cells. Mol Biol Cell. 2008;19(8):3234–3242. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampourakis T, Yan Z, Gautel M, Sun YB, Irving M. Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells. Proc Natl Acad Sci USA. 2014;111(52):18763–18768. doi: 10.1073/pnas.1413922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampourakis T, Irving M. Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle. J Mol Cell Cardiol. 2015;85:199–206. doi: 10.1016/j.yjmcc.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287(6):H2712–H2718. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- 32.Colson BA, et al. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588(Pt 6):981–993. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol. 2006;128(3):261–272. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kampourakis T, Sun YB, Irving M. Orientation of the N- and C-terminal lobes of the myosin regulatory light chain in cardiac muscle. Biophys J. 2015;108(2):304–314. doi: 10.1016/j.bpj.2014.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dale RE, et al. Model-independent analysis of the orientation of fluorescent probes with restricted mobility in muscle fibers. Biophys J. 1999;76(3):1606–1618. doi: 10.1016/S0006-3495(99)77320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikebe M, et al. Function of the NH2-terminal domain of the regulatory light chain on the regulation of smooth muscle myosin. J Biol Chem. 1994;269(45):28173–28180. [PubMed] [Google Scholar]
- 37.Sun YB, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587(1):155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279(34):35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 39.de Tombe PP, et al. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alamo L, et al. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384(4):780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: A potential cardioprotective mechanism in the heart. Biophys J. 2011;100(8):1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107(1):430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrie JE, et al. Dynamic measurement of myosin light-chain-domain tilt and twist in muscle contraction. Nature. 1999;400(6743):425–430. doi: 10.1038/22704. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins SC, et al. Orientation changes of the myosin light chain domain during filament sliding in active and rigor muscle. J Mol Biol. 2002;318(5):1275–1291. doi: 10.1016/s0022-2836(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 45.Karabina A, Kazmierczak K, Szczesna-Cordary D, Moore JR. Myosin regulatory light chain phosphorylation enhances cardiac β-myosin in vitro motility under load. Arch Biochem Biophys. 2015;580:14–21. doi: 10.1016/j.abb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khromov AS, Somlyo AV, Somlyo AP. Thiophosphorylation of myosin light chain increases rigor stiffness of rabbit smooth muscle. J Physiol. 1998;512(Pt 2):345–350. doi: 10.1111/j.1469-7793.1998.345be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenberg MJ, et al. The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R265–R274. doi: 10.1152/ajpregu.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Ajtai K, Burghardt TP. Ventricular myosin modifies in vitro step-size when phosphorylated. J Mol Cell Cardiol. 2014;72:231–237. doi: 10.1016/j.yjmcc.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herron TJ, et al. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ Res. 2006;98(10):1290–1298. doi: 10.1161/01.RES.0000222059.54917.ef. [DOI] [PubMed] [Google Scholar]
- 50.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009;284(18):12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardswell SC, Cuello F, Kentish JC, Avkiran M. cMyBP-C as a promiscuous substrate: Phosphorylation by non-PKA kinases and its potential significance. J Muscle Res Cell Motil. 2012;33(1):53–60. doi: 10.1007/s10974-011-9276-3. [DOI] [PubMed] [Google Scholar]
- 52.Linari M, et al. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528(7581):276–279. doi: 10.1038/nature15727. [DOI] [PubMed] [Google Scholar]
- 53.Patel JR, Pleitner JM, Moss RL, Greaser ML. Magnitude of length-dependent changes in contractile properties varies with titin isoform in rat ventricles. Am J Physiol Heart Circ Physiol. 2012;302(3):H697–H708. doi: 10.1152/ajpheart.00800.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irving T, et al. Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys J. 2011;100(6):1499–1508. doi: 10.1016/j.bpj.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farman GP, et al. Myosin head orientation: A structural determinant for the Frank-Starling relationship. Am J Physiol Heart Circ Physiol. 2011;300(6):H2155–H2160. doi: 10.1152/ajpheart.01221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanft LM, Biesiadecki BJ, McDonald KS. Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24. J Physiol. 2013;591(18):4535–4547. doi: 10.1113/jphysiol.2013.258400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.