Significance
The Hedgehog protein signal (Hh), covalently modified by cholesterol, functions to coordinate embryonic tissue patterning and postembryonic tissue maintenance. Cholesterol and several of its side-chain oxidized derivatives also function in Hh response by augmenting the activity of Smoothened, an essential seven-transmembrane protein. Here, we show that a distinct class of sterols, oxidized in the B-ring, also affect Hh response, but act by a distinct mechanism to inhibit Smoothened activity. These sterols and their precursor, 7-dehydrocholesterol, accumulate in the human genetic disease Smith–Lemli–Opitz syndrome, providing a rationale for diminished Hedgehog pathway activity in Smith–Lemli–Opitz syndrome and suggesting new candidates as potential modulators of Smoothened activity in normal cells.
Keywords: Hedgehog signaling, SLOS, Smoothened, DHCEO, cyclodextrin
Abstract
Cellular lipids are speculated to act as key intermediates in Hedgehog signal transduction, but their precise identity and function remain enigmatic. In an effort to identify such lipids, we pursued a Hedgehog pathway inhibitory activity that is particularly abundant in flagellar lipids of Chlamydomonas reinhardtii, resulting in the purification and identification of ergosterol endoperoxide, a B-ring oxysterol. A mammalian analog of ergosterol, 7-dehydrocholesterol (7-DHC), accumulates in Smith–Lemli–Opitz syndrome, a human genetic disease that phenocopies deficient Hedgehog signaling and is caused by genetic loss of 7-DHC reductase. We found that depleting endogenous 7-DHC with methyl-β-cyclodextrin treatment enhances Hedgehog activation by a pathway agonist. Conversely, exogenous addition of 3β,5α-dihydroxycholest-7-en-6-one, a naturally occurring B-ring oxysterol derived from 7-DHC that also accumulates in Smith–Lemli–Opitz syndrome, blocked Hedgehog signaling by inhibiting activation of the essential transduction component Smoothened, through a mechanism distinct from Smoothened modulation by other lipids.
The Hedgehog signaling pathway plays essential roles in embryonic patterning, as indicated by congenital malformations that are associated with mutations in pathway components (1–4). Hedgehog signal transduction is initiated by binding of the Hedgehog signaling protein to its receptor, Patched, which relieves suppression and allows ciliary accumulation of Smoothened, a seven-transmembrane (7TM) protein structurally related to G protein-coupled receptors (GPCRs). Patched is hypothesized to suppress Smoothened activity by controlling the availability of an unknown lipidic Smoothened ligand in the primary cilium (5, 6).
Some of the developmental malformations observed in mice and humans with mutations in Sonic hedgehog (Shh), especially holoprosencephaly (HPE), are phenocopied by chemical or mutational inactivation of the cholesterol biosynthetic enzyme, 7-dehydrocholesterol reductase (DHCR7), which converts 7-dehydrocholesterol (7-DHC) to cholesterol (7, 8). As a result, cholesterol is depleted, whereas 7-DHC accumulates and is converted into abnormal derivatives. One of the underlying reasons for the malformations in Smith–Lemli–Opitz syndrome (SLOS) (DHCR7 deficiency) is thought to be cholesterol deficiency, and pharmacologic depletion of cellular sterols by methyl-β-cyclodextrin (MCD) inhibits Smoothened activity in wild-type cells (9, 10). It is not clear whether the sterols depleted by MCD directly activate Smoothened or act as permissive factors for Smoothened activation, as noted for sterol regulation of many other GPCR superfamily members (11). As an alternative to a stimulatory/permissive role for sterols that are depleted in SLOS, some evidence suggests a contribution to SLOS pathology by sterols that accumulate to abnormal levels (12–14); how such sterols might affect Smoothened activity is unknown.
Various small-molecule agonists and antagonists that bind to the Smoothened transmembrane domain have been discovered, including the steroidal plant compounds cyclopamine and jervine and synthetic Smoothened antagonists and agonists (SAGs) (15, 16). Several small molecules including cyclopamine derivatives and other synthetic compounds that have been characterized as inhibiting Smoothened by binding to the “cyclopamine pocket” are in clinical trials or have been approved for use as anticancer drugs (17). Recent structural studies define the cyclopamine pocket in atomic detail, but mutations affecting this pocket generally fail to disrupt regulation by Patched (15), suggesting a distinct site of action for an endogenous mediator of Patched regulation.
A distinct class of Smoothened ligand consisting largely of oxygenated cholesterol derivatives and exemplified by 20(S)-hydroxycholesterol [20(S)-OHC] can activate Smoothened by binding to the cysteine-rich domain (CRD) that is N-terminal to the 7TM helical region (18–20). Although 20(S)-OHC is not known to be made naturally, we described two other enzymatically derived sterols that can similarly activate Smoothened via its CRD (18). Mutations in the relevant enzymes fail to exhibit phenotypes related to Hedgehog pathway dysregulation (21), however, and the physiologic relevance of these sterols therefore remains unclear. Furthermore, we demonstrated that Patched regulation of a Smoothened construct lacking the CRD is intact (18), indicating that any endogenous Smoothened-modulating ligand controlled by Patched must bind elsewhere. The role of endogenous CRD or cyclopamine pocket ligands thus remains unclear, and the putative mediator controlled by Patched, whether an agonist or an antagonist, is unknown.
To identify novel lipids that can activate or inhibit Smoothened activity, we undertook an unbiased, activity-guided fractionation of a Chlamydomonas lipid extract that showed inhibitory activity in a mammalian Hedgehog pathway reporter assay. We purified and identified ergosterol endoperoxide (5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol) as the inhibitory species, which led us to test the involvement of its mammalian analogs derived from 7-DHC in Hedgehog pathway regulation. Using the ability of the synthetic Smoothened agonist SAG to circumvent the requirement for stimulatory/permissive sterols, we show that MCD actually potentiates SAG stimulation of Hedgehog pathway activity and that this effect of MCD correlates with its extraction of 7-DHC. Finally, we show that 7-DHC derivatives such as 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), the most abundant oxysterol in the brain of a SLOS animal model (22), can inhibit Hedgehog signal response.
Results
Ergosterol Endoperoxide Isolated from Chlamydomonas reinhardtii Inhibits Hedgehog Signaling.
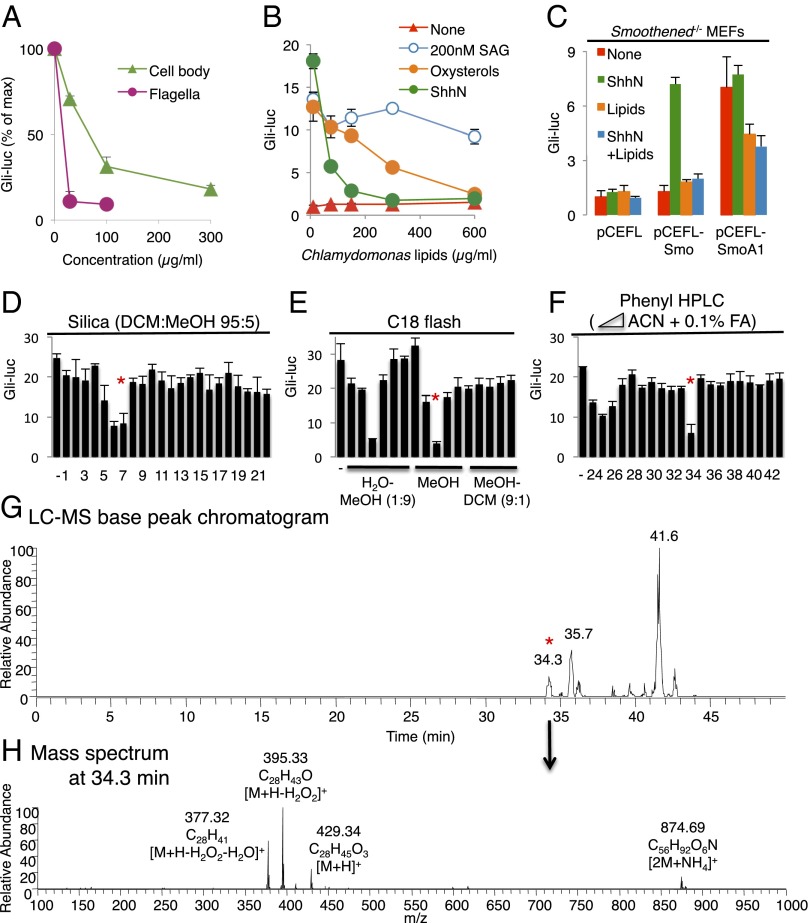
To find novel Smoothened agonists and antagonists, we tested the effect of a total lipid extract prepared from C. reinhardtii flagella, which are structurally and functionally related to mammalian primary cilia and amenable to biochemical isolation (23, 24). We also prepared lipid extracts from various other sources, including Hedgehog-responsive NIH 3T3 cells, various mouse tissues, lipoproteins from mammals and Drosophila larvae, as well as whole Drosophila larval homogenates. Whereas none of these extracts stimulated Hedgehog pathway activity in cultured mammalian cells, in experiments in which the pathway was activated by incubation of cells with the N-terminal signaling domain of murine Sonic hedgehog (ShhN), inhibition was observed, in the following sequence of decreasing relative potency: Chlamydomonas flagella > whole Chlamydomonas cells ≥ Drosophila lipophorin > whole Drosophila larvae (Fig. 1A). Chlamydomonas whole-cell lipid extract antagonized activation by ShhN or oxysterols, but only weakly antagonized activation by SAG (Fig. 1B). Furthermore, the constitutively active, Patched-resistant SmoothenedA1 mutant conferred significant resistance to the inhibitory activity of Chlamydomonas lipids, indicating that these lipids block Hedgehog signaling at the level or upstream of Smoothened (25) (Fig. 1C).
Fig. 1.
Identification of ergosterol endoperoxide as a Chlamydomonas lipid that inhibits mammalian Hedgehog signaling. (A) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with ShhN-conditioned medium in combination with the indicated concentrations of organic extracts from Chlamydomonas cell bodies or flagella. (B) Dose–response analysis of Chlamydomonas whole-cell lipid extract in Shh-LIGHT2 cells with no stimulation (red), stimulation with ShhN-conditioned medium (green), 200 nM SAG (blue) or 5 μM 20(S)-OHC and 5 μM 22(S)-OHC (oxysterols, orange). (C) Gli-luciferase activity in Smoothened−/− MEFs transfected with the indicated Smoothened (Smo) constructs and stimulated with ShhN-conditioned medium and Chlamydomonas lipids as indicated. (D–F) Activity profiles following fractionation of Chlamydomonas lipids using the indicated columns and solvents. (G and H) In-line electrospray ionization–Fourier transform MS analysis, showing masses and formulas of in-source fragmentation products. Red asterisks in D and E indicate fractions that were used in subsequent chromatography steps. The asterisks in F and G indicate co-incidence of activity with a chromatographic peak.
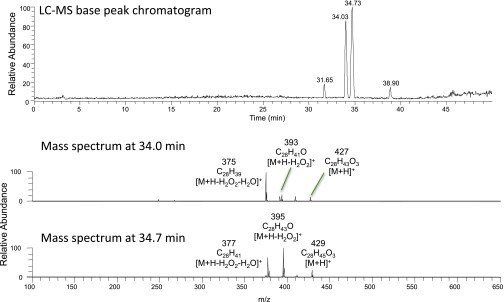
We undertook an activity-guided purification of the inhibitory lipid from whole Chlamydomonas cells using two steps of flash chromatography (Fig. 1 D and E) followed by reversed-phase HPLC (Fig. 1F) coupled with in-line Orbitrap mass spectrometry (positive-mode electrospray ionization) and parallel automated fraction collection. The activity eluting at 34 min corresponded to a chromatographic peak (Fig. 1G) and a collection of related ions that derive from spontaneous in-source fragmentation of a protonated species at m/z 429.34 (Fig. 1H). High mass accuracy permitted elucidation of a neutral molecular formula of C28H44O3. Sequential neutral losses of H2O2 (Δm 34) and H2O (Δm 18) indicated the presence of hydroxyl and peroxide (hydroperoxide or endoperoxide) functional groups (Fig. 1H). Interestingly, a partially purified inhibitory fraction obtained from Drosophila larval extract contained a species with identical retention time, accurate mass and in-source fragmentation spectrum, as well as a closely related species (C28H42O3) with one more double bond, based on similar retention time, a 2-Da smaller mass, and in-source neutral losses of H2O and H2O2 (Fig. S1).
Fig. S1.
Column fractionation of Drosophila larval extract yielded a partially purified inhibitory fraction containing two related species with the indicated masses of in-source fragmentation products.
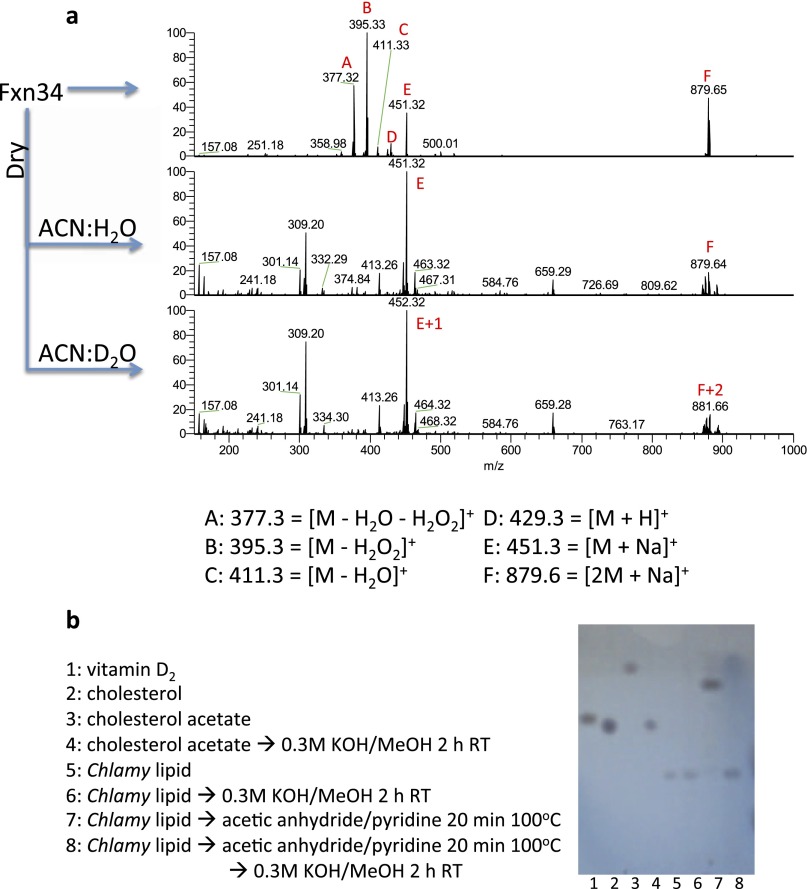
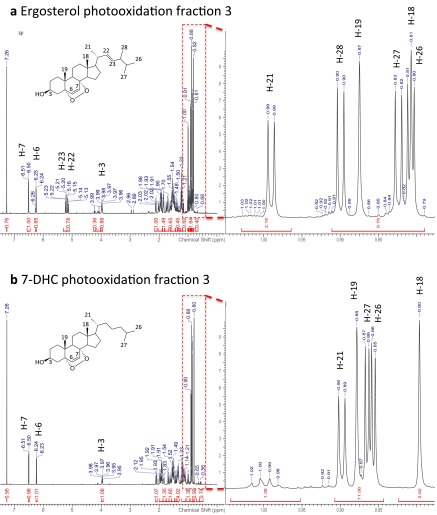
A search of metabolite and lipid databases with the accurate mass obtained from mass spectrometry revealed 35 sterols or vitamin D derivatives. Chemical analyses indicated a sterol with a single exchangeable hydrogen corresponding most likely to the 3β-OH group of sterols and ruling out the presence of an additional exchangeable hydroperoxide functionality (Fig. S2A). Furthermore, the presence of an ester bond was ruled out by resistance of the molecule to base hydrolysis at room temperature (Fig. S2B). A scaled-up version of the final HPLC step yielded ∼0.5 mg of purified compound and allowed definitive structure elucidation by NMR. Proton (1H) and 13C chemical shifts matched those previously reported for ergosterol endoperoxide, a natural compound isolated from various fungi (26) (Table S1), with mass and other features that are consistent with the compounds isolated from Chlamydomonas.
Fig. S2.
Chemical characterization of the purified Chlamydomonas lipid. (A) Chlamydomonas lipid has a single exchangeable proton. HPLC-purified Chlamydomonas lipid was infused directly into the mass spectrometer (Top), dried and redissolved in 1:1 acetonitrile:water (ACN:H2O) (Middle), or acetonitrile:deuterium oxide (ACN:D2O) (Bottom). Note that the formic acid from the HPLC eluent that promotes formation of the protonated species evaporates after drying, which leaves the sodium adduct as the only means of ionization. Fragmentation of m/z 452 species first caused a neutral loss of 19, confirming that a hydroxyl proton had exchanged with a deuterium (not shown). (B) Chlamydomonas lipid is not an ester. Even though conventional saponification at 80 °C destroyed the purified species and Hedgehog pathway-inhibitory activity (not shown), room temperature saponification under the indicated conditions did not (lanes 5 and 6). As a control, cholesterol acetate (lanes 3 and 4) and preacetylated Chlamydomonas lipid (lanes 7 and 8) were saponified and analyzed by TLC.
Table S1.
1H and 13C NMR data of Chlamydomonas lipid (in CDCl3)
| 1H NMR | 13C NMR | ||
| C-1 | 34.90 | ||
| C-2 | 30.34 | ||
| H-3 | 3.97 (m) | C-3 | 66.69 |
| C-4 | 37.14 | ||
| C-5 | 82.36 | ||
| H-6 | 6.24 (d, 8.6) | C-6 | 135.61 |
| H-7 | 6.50 (d, 8.6) | C-7 | 130.96 |
| C-8 | 79.63 | ||
| C-9 | 51.29 | ||
| C-10 | 37.17 | ||
| C-11 | 23.62 | ||
| C-12 | 39.55 | ||
| C-13 | 44.77 | ||
| C-14 | 51.89 | ||
| C-15 | 20.85 | ||
| C-16 | 28.87 | ||
| C-17 | 56.40 | ||
| H-18 | 0.82 (s) | C-18 | 13.09 |
| H-19 | 0.88 (s) | C-19 | 18.40 |
| C-20 | 39.96 | ||
| H-21 | 1.00 (d, 6.6) | C-21 | 21.09 |
| H-22 | 5.15 (dd, 15.4, 8.6) | C-22 | 135.40 |
| H-23 | 5.22 (dd, 15,2, 7.8) | C-23 | 132.50 |
| C-24 | 42.98 | ||
| C-25 | 33.28 | ||
| H-26 | 0.82 (d, 6.8) | C-26 | 19.86 |
| H-27 | 0.84 (d, 6.8) | C-27 | 20.17 |
| H-28 | 0.91 (d, 6.8) | C-28 | 17.78 |
Values in parentheses are coupling constants in hertz. Assignments were verified by 1H-1H correlation spectroscopy, heteronuclear single quantum coherence, and heteronuclear multiple-bond correlation spectroscopy, and matched those provided for ergosterol peroxide by ref. 26 and references therein.
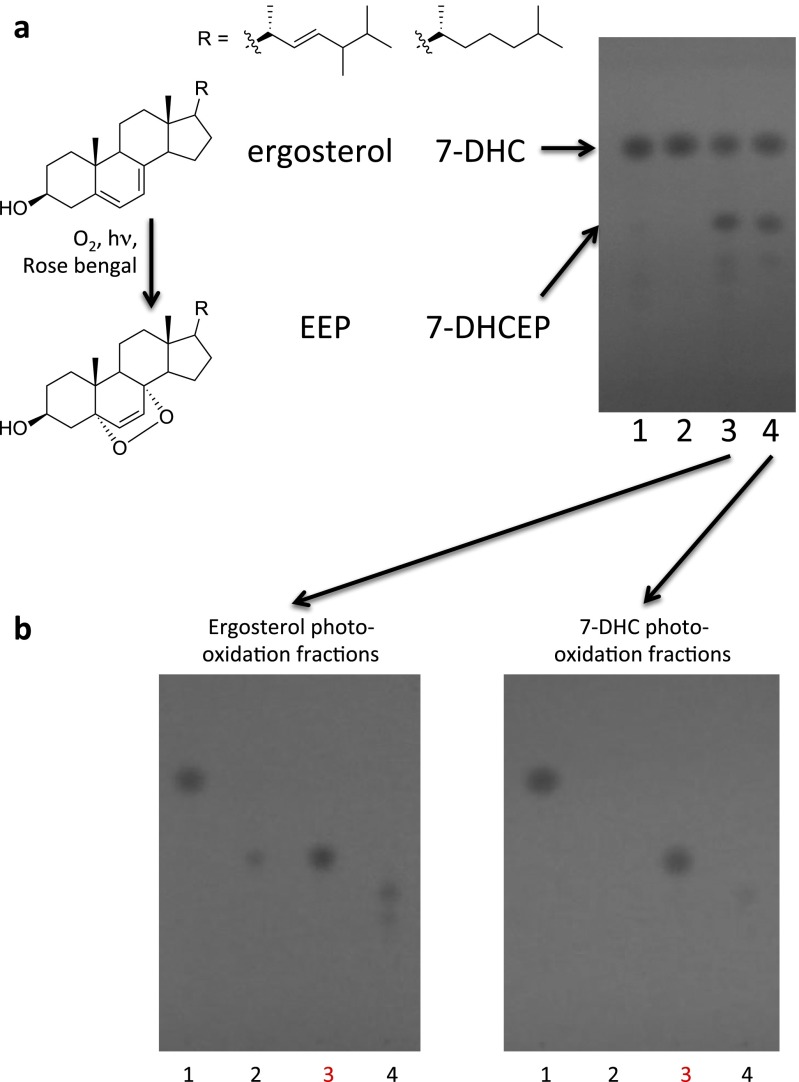
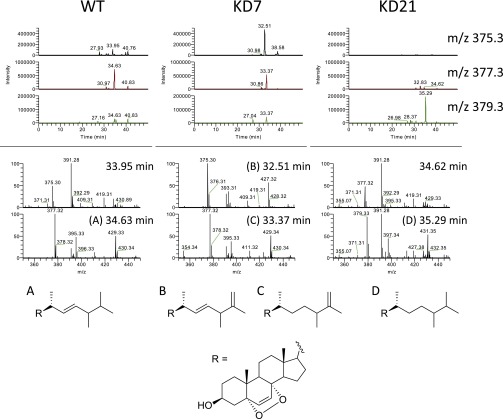
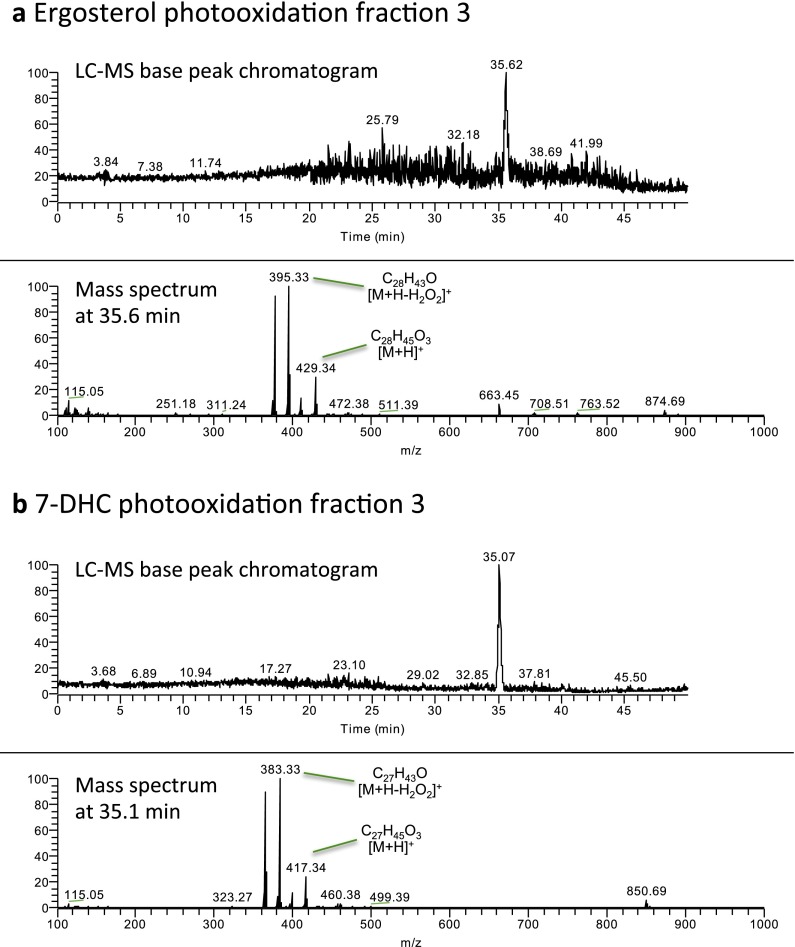
As this compound was not commercially available, we synthesized it by photooxidation of ergosterol, purified it, and demonstrated its identity with the lipid isolated from Chlamydomonas and Drosophila by retention time, MS, and NMR analyses (Figs. S3–S5). We confirmed that this synthetic preparation of ergosterol endoperoxide inhibited ShhN signaling with similar potency to that of the compound purified from Chlamydomonas (Fig. 2A). Pure ergosterol endoperoxide also inhibited the constitutive pathway activity in Patched1-deficient mouse embryonic fibroblasts (MEFs) and blocked Smoothened ciliary accumulation, consistent with action on Smoothened in a Patched-independent manner (Fig. 2 B–D). Lipid extracts from two Chlamydomonas mutants with defects at late stages of ergosterol biosynthesis showed similar activity due to formation of sterol endoperoxides with side chains that are distinct from that of ergosterol endoperoxide found in wild-type Chlamydomonas (27) (Fig. S6). A mutant defective in formation of the 5,7-diene structure required for endoperoxide formation has not been described, precluding a test of whether this intermediate is critical for the inhibitory activity of the Chlamydomonas extract.
Fig. S3.
Synthesis and purification of EEP and 7-DHCEP. (A) TLC analysis of ergosterol (lane 1), 7-DHC (lane 2), photooxidation products of ergosterol (lane 3), and 7-DHC (lane 4). (B) Photooxidation products of ergosterol and 7-DHC were fractionated using flash chromatography and analyzed by TLC. Major reaction products were found in pure forms in fractions 3 (shown in red) and were analyzed further (Figs. S4 and S5).
Fig. S5.
1H NMR spectra of purified EEP (A) and 7-DHCEP (B).
Fig. 2.
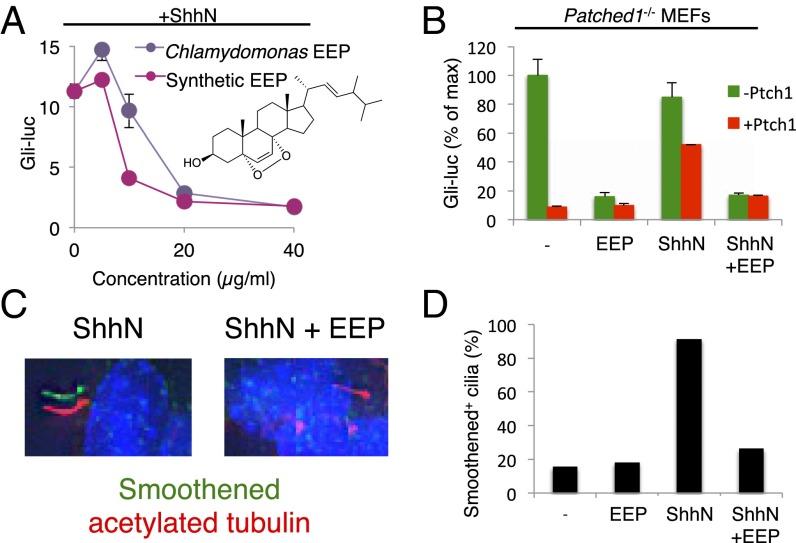
Ergosterol endoperoxide (EEP) inhibits Hedgehog pathway downstream of Patched1 and at the level of Smoothened. (A) Dose–response analysis of EEP purified from Chlamydomonas (purple) or synthesized by photooxidation (red). (B) Gli-luciferase activity in Patched1−/− MEFs transfected with or without Patched1 and treated with ShhN-conditioned medium and 25 μM EEP as indicated. (C and D) Ciliary accumulation of endogenous Smoothened in NIH 3T3 cells treated with ShhN-conditioned medium and 25 μM EEP as indicated. Fixed cells were analyzed by immunofluorescence using antibodies against Smoothened (green) and acetylated tubulin (red) with DAPI counterstain (blue). Representative images for selected conditions are displayed as shifted overlays of Smoothened and acetylated tubulin stains. (Magnification: 63×.)
Fig. S6.
Chlamydomonas mutants have sterol endoperoxides with side chains distinct from wild-type. Extracted ion chromatograms of partially purified lipid extracts for the indicated m/z values (Top), mass spectra at the indicated retention times (Middle), and predicted structures of the active species (Bottom). A–D indicate spectra corresponding to each of the proposed side chain structures.
Fig. S4.
LC-MS analyses of purified EEP (A) and 7-DHCEP (B).
An Endogenous Sterol Inhibits Mammalian Hedgehog Signaling.
Identification of ergosterol endoperoxide from Chlamydomonas and Drosophila as a Smoothened antagonist raised the possibility that an endogenous sterol may act in a similar fashion in mammalian cells. One approach to demonstrating the existence of such a negatively acting sterol would be to remove it from cells by preferential extraction with MCD (Fig. 3A). We know, however, that MCD treatment impairs response to ShhN stimulation, presumably by removing from cells the essential permissive/activating function of cholesterol (9). We therefore tested whether Smoothened agonists might function in a cholesterol-independent manner, as such activation might then be enhanced by MCD extraction of endogenous negatively acting sterols.
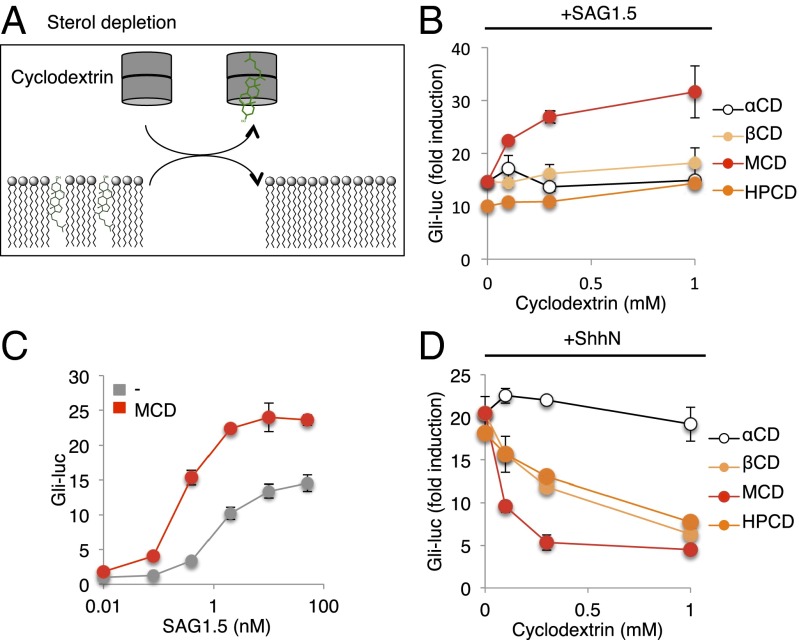
Fig. 3.
MCD potentiates SAG. (A) Scheme describing use of cyclodextrins to remove cellular sterols. Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with (B) 10 nM SAG1.5 or (D) ShhN-conditioned medium and increasing concentrations of α-cyclodextrin (αCD), β-cyclodextrin (βCD), MCD, or hydroxypropyl-β-cyclodextrin (HPCD). (C) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with increasing concentrations of SAG1.5 alone (gray) or together with 1 mM MCD (red).
We indeed found that pathway activation by Smoothened agonist SAG1.5 (a more potent SAG analog) (28) could be enhanced by treatment with MCD (Fig. 3B), and this enhancement was also manifested by an MCD-induced shift toward greater sensitivity in the dose–response curve of SAG1.5 (Fig. 3C). This enhancement was not observed with any other cyclodextrins, suggesting preferential specificity of MCD for the inhibitory sterol. Activation by ShhN, in contrast, was inhibited by all cyclodextrins except α-cyclodextrin (Fig. 3D), which preferentially depletes phospholipids (29). All of the ShhN-inhibitory cyclodextrins we tested are known to extract cholesterol (30), and this result is consistent with the previously described critical requirement for cholesterol in ShhN-mediated pathway activation (9).
Endogenous 7-DHC Derivatives Inhibit Hedgehog Response.
Ergosterol endoperoxide, the negatively acting sterol identified in Chlamydomonas and Drosophila (see above) is generated from ergosterol, which is not produced in mammalian cells, but 7-DHC, an important intermediate in cholesterol biosynthesis, has the same ring structure as ergosterol and accumulates in SLOS patients. In fact, a derivative of 7-DHC with a ring structure identical to that of ergosterol endoperoxide, 5α,8α-epidioxycholest-6-en-3β-ol (hereafter referred to as 7-DHC endoperoxide), has been reported in the plasma of SLOS patients and in various tissues of animal models of this disease (31–33). However, although 7-DHC endoperoxide indeed antagonized ShhN-mediated activation with a potency similar to ergosterol endoperoxide (Fig. 4A; synthesis and purification described in Figs. S3–S5), most if not all of this compound forms ex vivo by oxidation of 7-DHC (33), thus indicating that this is not the endogenous inhibitory sterol.
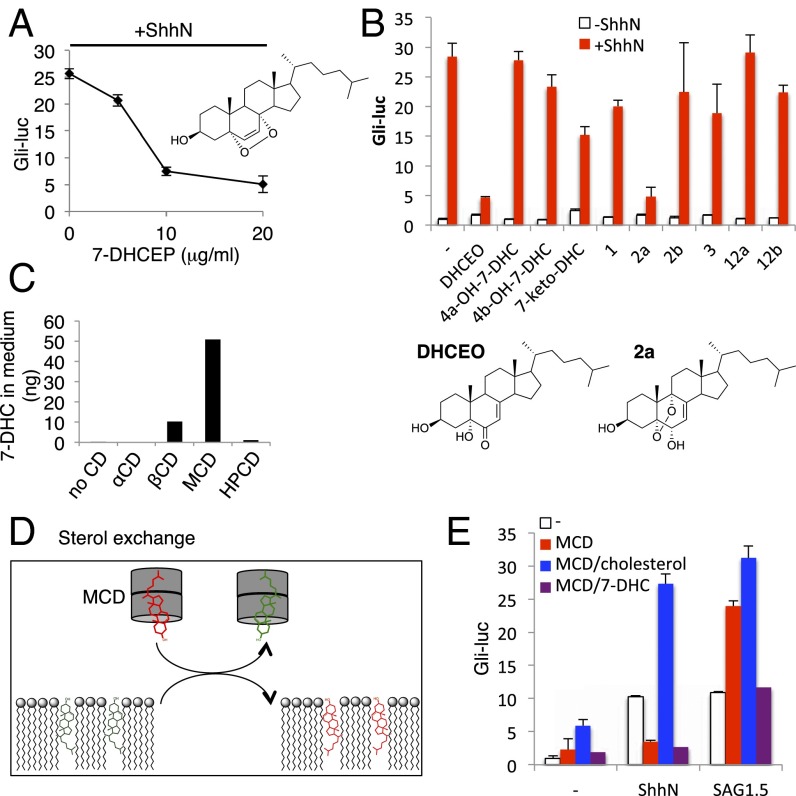
Fig. 4.
Endogenous 7-DHC derivatives inhibit Hedgehog signaling. (A) Dose–response analysis of 7-DHC endoperoxide (7-DHCEP) in Shh-LIGHT2 cells. (B) 7-DHC–derived oxysterols inhibit Hedgehog pathway. Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with the indicated sterols either alone (white) or in combination with ShhN-conditioned medium (red). (C) 7-DHC levels in the medium after treatment with the indicated cylodextrins were quantified by tandem MS. (D) Scheme describing use of MCD to exchange cellular sterols. (E) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with ShhN-conditioned medium or 10 nM SAG1.5 and 1 mM MCD complexed with 100 μM cholesterol or 7-DHC.
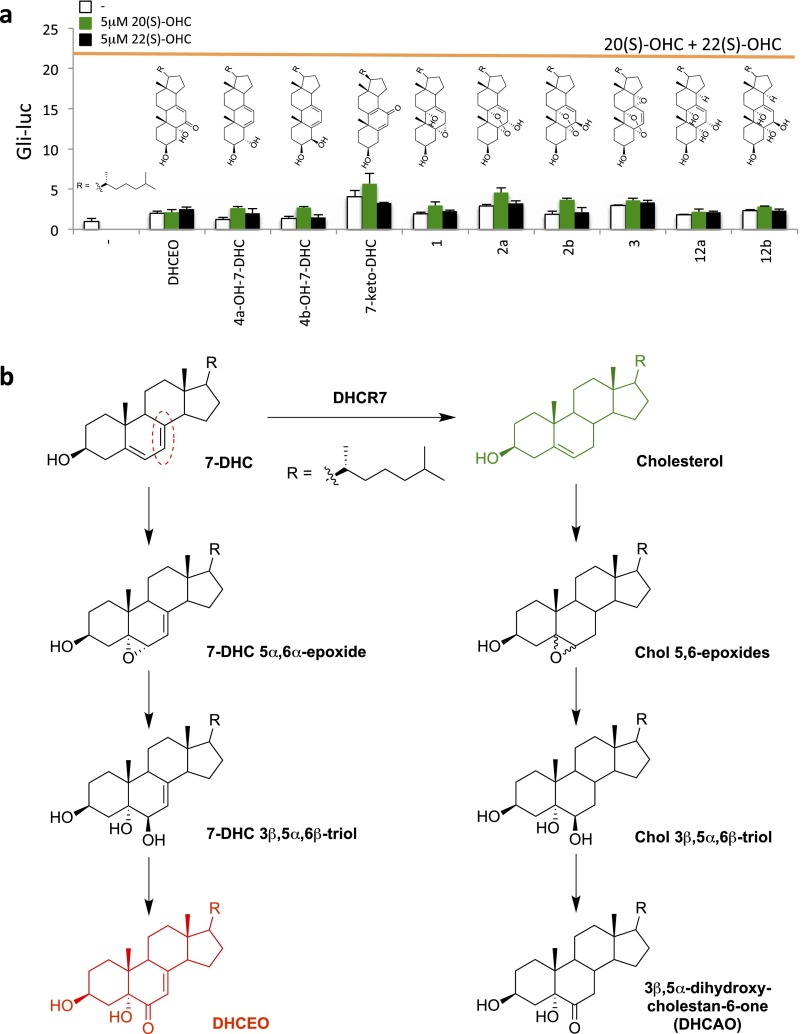
Other B-ring oxidized sterols derived from 7-DHC via enzymatic or free-radical–mediated oxidation have been identified as lipids that accumulate in SLOS animal models (33, 34), and we found that two of them antagonize ShhN treatment (Fig. 4B). One of these, another endoperoxide compound referred to as 2a (5α,9α-epidioxycholest-7-en-3β,6α-diol), does not accumulate in cells and tissues due to its rapid metabolism (35) and is likely not the endogenous inhibitory sterol. Importantly, compound 2b, which is the 6β-hydroxy isomer of compound 2a, did not show inhibitory activity (Fig. 4B), indicating a stereospecific mechanism of 2a action. The other sterol, DHCEO, is more abundant than any other oxysterol, including 24(S)-OHC, in Dhcr7-deficient cells as well as embryonic and newborn brain tissues (22, 36). The level of DHCEO is even higher in SLOS human fibroblasts (2% of 7-DHC) than in Dhcr7-deficient mouse brain (0.05% of 7-DHC) (34). Given the previously described ability of the side-chain oxysterols 20(S)-OHC and 22(S)-OHC to synergize in activating Smoothened (37), we also tested whether any of the 7-DHC derivatives would synergistically enhance Hedgehog reporter activity, and found that none did (Fig. S7A).
Fig. S7.
Lack of stimulatory activity of 7-DHC derivatives and formation mechanism of DHCEO. (A) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with the indicated sterols (described in ref. 33) alone (white) or in combination with 5 μM 20(S)-OHC (green) or 5 μM 22(S)-OHC (black). For comparison, luciferase induction obtained with 5 μM 20(S)-OHC plus 5 μM 22(S)-OHC (21.4-fold) is shown with the orange line. (B) DHCEO is proposed to form in multiple steps from 7-DHC analogous to a known biotransformation of cholesterol. Note that the double bond reduced by DHCR7 (circled in red) does not undergo a net reaction during DHCEO formation.
Based on the striking specificity of MCD in potentiating SAG1.5-mediated pathway activation (Fig. 3B), the endogenous inhibitor or its metabolic precursors should be preferentially extracted by MCD (but not by other cyclodextrins) from ShhN-responsive cells. We were unable to detect DHCEO by targeted liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis in any cyclodextrin extract (method as described in ref. 34). This could be either because of detection limitations, or because MCD does not extract DHCEO itself but, instead, a metabolic precursor essential for its production. Indeed, 7-DHC was efficiently extracted only by MCD and not other cyclodextrins (Fig. 4C), correlating with potentiation of SAG1.5 action. To further investigate a possible role for 7-DHC as a precursor of the endogenous sterol inhibitor, we prepared an inclusion complex of MCD with 7-DHC, which effectively exchanges the included sterol with cellular sterols (38) (Fig. 4D). We found that the MCD/7-DHC inclusion complex could neither rescue ShhN response nor potentiate SAG1.5 action (Fig. 4E), suggesting that 7-DHC exchange mediated by this inclusion complex is able to sustain the endogenous inhibitory sterol, and consistent with the possibility that this inhibitor is a 7-DHC derivative. In contrast, a MCD/cholesterol complex was even more efficacious than empty MCD in potentiating SAG1.5.
The 7-DHC Derivatives Target Smoothened at a Site Distinct from the CRD or the Cyclopamine Pocket.
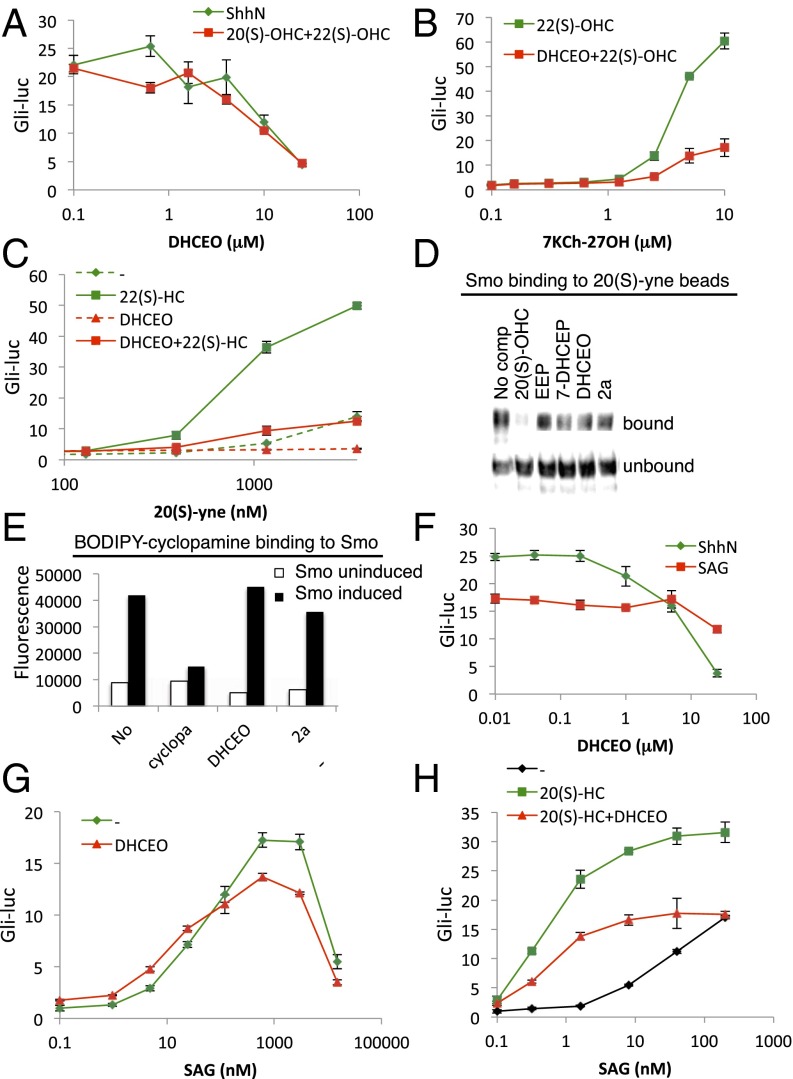
B-ring oxidized sterols appear to act downstream of Patched (Fig. 2B), at the level of Smoothened (Fig. 2 C and D). We further investigated the mechanism of action of these inhibitors in relation to two previously described ligand binding sites on Smoothened: the extracellular CRD and the cyclopamine pocket. We found that DHCEO antagonized activation by the 20(S)-OHC-plus-22(S)-OHC combination as potently as it antagonized ShhN, consistent with DHCEO action downstream of Patched and at the level of Smoothened (Fig. 5A). DHCEO also inhibited two other CRD agonists, 7-keto-27-hydroxycholesterol (7KCh-27OH) (18) and 20(S)-yne (39), in an apparently noncompetitive manner (Fig. 5 B and C). Consistent with the noncompetitive inhibition observed in these functional assays, neither ergosterol endoperoxide nor any of its mammalian analogs blocked Smoothened binding to the 20(S)-yne affinity resin, even at concentrations 10 times those sufficient to fully inhibit the functional cell-based reporter assay (Fig. 5D). DHCEO and 2a also failed to compete with BODIPY-cyclopamine for binding to Smoothened (Fig. 5E) and failed to antagonize SAG (Fig. 5F). Consistently, the dose–response curve of SAG was not altered in the presence of DHCEO (Fig. 5G), indicating that DHCEO does not act via the SAG/cyclopamine pocket.
Fig. 5.
DHCEO can block the action of CRD agonists but not SAG. (A–C) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with: (A) increasing concentrations of DHCEO in combination with ShhN-conditioned medium (green) or 5 μM 20(S)-OHC plus 5 μM 22(S)-OHC (red); (B) increasing concentrations of 7KCh-27OH in combination with 5 μM 22(S)-OHC (green) or 5 μM 22(S)-OHC plus 25 μM DHCEO (red); or (C) increasing concentrations of 20(S)-yne alone (dashed green) or in combination with 5 μM 22(S)-OHC (solid green), 25 μM DHCEO (dashed red), or 5 μM 22(S)-OHC plus 25 μM DHCEO (solid red). (D) Detergent-solubilized membranes from HEK293S-Smo cells were incubated with 20(S)-yne affinity resin in the presence of 50 μM 20(S)-OHC, 250 μM ergosterol endoperoxide (EEP), 250 μM 7-DHC endoperoxide (7-DHCEP), 250 μM DHCEO, or 100 μM 2a. After washing, bound protein was eluted and analyzed by immunoblotting. (E) Binding of 5 nM BODIPY-cyclopamine to membranes from tetracycline-inducible HEK293S cells expressing Smoothened was measured in the presence of 3 μM cyclopamine, 25 μM DHCEO, or 10 μM 2a. (F–H) Gli-luciferase activity in Shh-LIGHT2 cells was measured following treatment with increasing concentrations of DHCEO in combination with ShhN-conditioned medium (green) or 200 nM SAG (red); (G) increasing concentrations of SAG alone (green) or in combination with 25 μM DHCEO (red); or (H) increasing concentrations of SAG alone (black) or in combination with the indicated sterols.
Curiously, despite its inhibition of 20(S)-OHC activation, DHCEO does not completely block the synergy between 20(S)-OHC and SAG (39). We thus found that addition of 20(S)-OHC both increased the potency of SAG (reduced its EC50) and increased the maximum level of pathway activation achieved (Fig. 5H, compare green and black tracings); coadministration of DHCEO reversed the effect of 20(S)-OHC on the maximum level of pathway activation but not its effect on the increased potency of SAG (Fig. 5H, red and green tracings). Altogether, these results support the idea that DHCEO affects Smoothened activity without displacing any of these Smoothened agonists.
Discussion
The Hedgehog receptor, Patched, is a transporter-like protein thought to act by controlling an endogenous lipid that regulates Smoothened (5). Neither of the two distinct sites on Smo that are known to bind modulatory lipids, however, is likely to respond to Patched regulation. The extracellular CRD domain of Smoothened is not required for Patched action (18), and mutations affecting the cyclopamine pocket generally fail to disrupt regulation by Patched (15), suggesting that the Smoothened site responding to Patched regulation remains unknown, as does the identity of the molecule(s) that mediate this regulation. We therefore undertook an unbiased search for possible mediators of Smoothened regulation, using a cultured cell reporter assay to identify activities from various lipid extracts. By purifying and identifying the active lipid from Chlamydomonas, with an enriched source of modulatory activity in flagella, we identified oxidized sterols with modifications within the B-ring as a distinct class of Smoothened antagonists. Mammalian analogs of these B-ring oxysterols derive from the mammalian cholesterol synthesis intermediate 7-DHC, which due to the presence of conjugated double bonds within the B-ring make it subject to several enzymatic or nonenzymatic oxidation reactions that generate the antagonists we have identified.
Of critical importance, our finding that extraction with MCD potentiates the action of the Smoothened agonist SAG1.5 in pathway-responsive cultured cells provides evidence for an endogenous sterol antagonist. Our findings that 7-DHC is extracted specifically by MCD and that incorporation of 7-DHC but not cholesterol in an MCD inclusion complex can negate the potentiating effect of MCD makes 7-DHC or one of its derivatives a likely candidate for such an antagonist. Of note, whereas the potentiating effect of MCD on SAG1.5 action suggests the presence of an endogenous antagonist, SAG1.5 is nevertheless able to activate Smoothened in the presence of this endogenous antagonist, albeit to a more limited extent. This is consistent with the resistance of SAG1.5 to exogenously added B-ring oxysterols that are capable of fully inhibiting pathway activation by the Hedgehog signal.
The current standard of care for SLOS is cholesterol supplementation, which, in addition to increasing cholesterol levels, also provides feedback inhibition of sterol biosynthesis, reducing aberrant accumulation of cholesterol precursors (40). In various cultured cell or animal models of SLOS, both cholesterol deficiency and 7-DHC accumulation have been shown to contribute to the pathobiology of the disease (9, 10, 12–14, 22, 41, 42). Our findings suggest that the accumulation of 7-DHC derivatives in SLOS is likely to be responsible, at least in part, for the apparent reduction of Hedgehog pathway activity seen in this disease. The 7-DHC derivative DHCEO identified here as a Hedgehog pathway antagonist is detectable in normal brain but accumulates to 100-fold higher levels in SLOS—to an overall concentration of ∼3.5 μM—and may contribute to the disease phenotype (22, 36). DHCEO is proposed to form in a three-step process from 7-DHC based on a known biotransformation of cholesterol (34, 43, 44) (Fig. S7B). Although formation of the initial epoxide does not require enzyme activity, several known epoxide hydrolase enzymes could carry out the second reaction (45). There is also evidence for an enzymatic basis for the last step, but the specific enzyme has not yet been identified (34).
Other endogenous lipidic molecules proposed to directly modulate Smoothened activity include cholecalciferol (vitamin D3) (46) and the endocannabinoids (47). We and others have not been able to replicate the pathway inhibition or BODIPY-cyclopamine competition reported for cholecalciferol (48). Furthermore, vitamin D synthesis depends on UV radiation, not oxidation, and cholecalciferol does not accumulate in SLOS, ruling it out as the culprit for SLOS-associated Hedgehog-related phenotypes (49). Regarding endocannabinoids, of the dozen reported to inhibit Hedgehog pathway in mammalian cells, only anandamide (N-acylethanolamide 20:4) inhibited BODIPY-cyclopamine binding to Smoothened (47). None of the compounds tested inhibited ciliary accumulation of Smoothened, and most of them including anandamide blocked activation by SAG (46). These observations suggest a site of action for endocannabinoids that is downstream of mammalian Smoothened. A steroidal inhibitory activity was not reported in any extracts examined by these authors. We have found, however, that the high-temperature conditions of conventional saponification used in extract preparation destroys the Hedgehog pathway-inhibitory activity of ergosterol endoperoxide.
Regarding mechanism of B-ring oxysterol action, ergosterol endoperoxide antagonizes Hedgehog signaling downstream of Patched and disrupts Smoothened ciliary accumulation, consistent with a Smoothened-targeting effect. In addition, we find that the mammalian B-ring oxysterol DHCEO is bracketed pharmacologically by distinct classes of Smoothened ligands, as its antagonistic action prevails against pathway activation by CRD agonists but is surmounted by the cyclopamine pocket agonist, SAG. These observations, coupled with our binding competition studies, together constitute the basis of a model wherein antagonistic B-ring oxysterol derivatives of 7-DHC act directly on Smoothened, but at a site that is distinct from the Smoothened CRD and the cyclopamine pocket. Alternative ligand-binding sites are suggested by the general propensity of most GPCRs to interact with and in some cases be modulated by sterols (50, 51), and by the prediction of additional druggable cavities within the crystallographically determined structure of the Smoothened dimer (17). We have thus identified B-ring oxysterols as endogenous lipidic Smoothened modulators likely to play a role in the pathology of SLOS. Further investigation will be required to determine whether these compounds participate in Patched-mediated regulation of Smoothened in normal cells.
Methods
We carried out Shh signaling assays as described (18) in Shh-LIGHT2 cells, Smoothened−/− and Patched1−/− MEFs seeded into 96-well plates. Indirect immunofluorescence was performed as described (18) using methanol fixation. Sterol/MCD inclusion complexes (1:10 molar ratio) were prepared by adding 9% (wt/vol) MCD (Sigma; 332615; lot no. STBC2412V; 1.6–2.0 mol CH3 per unit anhydroglucose) to dried sterols, heating to 80 °C, and sonicating until a clear solution was achieved (52). Purification, chemical syntheses, LC-MS/MS, and binding assays are described in SI Methods.
SI Methods
Purification of Ergosterol Endoperoxide from Chlamydomonas.
Chlamydomonas reinhardtii strains 21gr (wild-type) and sterol mutants KD7 and KD21 obtained from the Chlamydomonas Resource Center (St. Paul, MN) were grown in Tris-acetate-phosphate medium at room temperature. Preparation of cell bodies and flagella from gametes were carried out as previously described (24). Lipids were extracted according to Folch et al. (53) and measured by charring (54). In initial experiments, the lipid extract was loaded onto a silica gel in a glass column and eluted with dichloromethane:methanol (95:5). Ten-milliliter fractions were collected, and an equal proportion of each fraction dried and sonicated in DMEM with 0.5% serum to assess their effect on Gli-luciferase reporter activity. The active fraction was loaded onto a second glass column filled with C18-functionalized silica gel and eluted, in order, with water:methanol (1:9), methanol, and methanol:dichloromethane (9:1). One-milliliter fractions were collected and assayed as above. The active fraction that eluted with methanol was finally loaded on a ZORBAX SB-Phenyl HPLC column (5 μm, 4.6 × 250 mm, 80 Å) coupled in-line to an LTQ-Orbitrap mass spectrometer (positive-mode electrospray ionization). Fractions collected in parallel were assayed as above.
For large-scale purification of the active species, 228 mg of Chlamydomonas Folch extract was loaded onto a Silica 12g Gold (20–40 μm) column and eluted at 30 mL/min using a hexane-ethyl acetate gradient with a CombiFlash Rf 200i instrument; 0.3% of each fraction was assayed. The active fraction was dried, resuspended in 80% (vol/vol) methanol, and loaded onto a C18 15.5g Gold column. Fractions were eluted at 30 mL/min using 80–100% (vol/vol) methanol gradient and 0.6% of each fraction was assayed. The active fraction was loaded on a ZORBAX SB-Phenyl HPLC column (5 μm, 9.4 × 250 mm, 80 Å) in four aliquots, and the fractions containing the active species from each of the four runs were pooled for NMR analysis.
Photooxidation of Ergosterol and 7-DHC.
Ergosterol or 7-DHC at 2 mg/mL was irradiated for 90 min with visible light in the presence of TTMA-rose bengal (17 μg/mL) and air in 30 mL of ethanol as described (55). Reaction products were monitored using TLC. Ergosterol endoperoxide or 7-DHC endoperoxide was purified by Flash chromatography using a hexane-ethyl acetate gradient as above.
LC-MS/MS Analysis of 7-DHC in the Medium of Cyclodextrin-Treated Cells.
NIH 3T3 cells were treated with DMEM or DMEM containing 1 mM of individual cyclodextrins overnight, and the media were collected. To 2 mL of each medium was added d7-7-DHC (25.8 ng) as the internal standard, and the sample was extracted by 5 mL of Folch solution (containing 1 mM, each, of butylated hydroxytoluene and triphenylphosphine) following a previously reported procedure (33). The extracts were dried under nitrogen and reconstituted in 200 μL of methanol. 7-DHC was then derivatized by addition of 20 μL of a stock solution of 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) (30 mg/mL in methylene chloride). After 30 min at room temperature, the resulting solution was blown dry and reconstituted in 200 μL of methanol. The d0- and d7-7-DHC-PTAD adducts was then analyzed as previously reported (56).
BODIPY-Cyclopamine and 20(S)-yne Affinity Matrix Binding Assays.
HEK293S-Smo cells were grown and induced to express Smoothened as described (37). Cells were harvested and membranes were prepared as described (57). The membrane pellet was resuspended in PBS containing 0.1% fatty acid-free BSA, 5 nM BODIPY-cyclopamine, and competitors as indicated. After incubation for 4 h at room temperature, samples were centrifuged at 18,000 × g for 15 min, washed three times for 15 min each, and analyzed by fluorimetry. 20(S)-yne affinity matrix was prepared as described (18) and used with HEK293S-Smo membranes prepared as above.
Acknowledgments
We thank P. Aronov for LC-MS; C. Liu for NMR; and K. Loh and J. Chen for critical reading of the manuscript. This work was supported in part by National Institutes of Health NIGMS Grant R01 CA157877 (to P.A.B.), NICHD Grant R01 HD064727 (to N.A.P.), and Grant K99 HD073270 (to L.X.). P.A.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604984113/-/DCSupplemental.
References
- 1.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 3.Roessler E, et al. Mutations in the human Sonic hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14(3):357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 4.Belloni E, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14(3):353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 5.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, et al. The role of ciliary trafficking in Hedgehog receptor signaling. Sci Signal. 2015;8(379):ra55. doi: 10.1126/scisignal.aaa5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley RL, et al. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: Does abnormal cholesterol metabolism affect the function of Sonic hedgehog? Am J Med Genet. 1996;66(4):478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Roux C, et al. Role of cholesterol in embryonic development. Am J Clin Nutr. 2000;71(5) Suppl:1270S–1279S. doi: 10.1093/ajcn/71.5.1270s. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MK, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33(4):508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 10.Blassberg R, Macrae JI, Briscoe J, Jacob J. Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet. 2016;25(4):693–705. doi: 10.1093/hmg/ddv507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell Biochem. 2010;51:439–466. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- 12.Gaoua W, Chevy F, Roux C, Wolf C. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: A model for antenatal growth retardation in the Smith-Lemli-Opitz syndrome. J Lipid Res. 1999;40(3):456–463. [PubMed] [Google Scholar]
- 13.Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: Implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51(11):3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Sheflin LG, Porter NA, Fliesler SJ. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim Biophys Acta. 2012;1821(6):877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharpe HJ, Wang W, Hannoush RN, de Sauvage FJ. Regulation of the oncoprotein Smoothened by small molecules. Nat Chem Biol. 2015;11(4):246–255. doi: 10.1038/nchembio.1776. [DOI] [PubMed] [Google Scholar]
- 16.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruat M, Hoch L, Faure H, Rognan D. Targeting of Smoothened for therapeutic gain. Trends Pharmacol Sci. 2014;35(5):237–246. doi: 10.1016/j.tips.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Myers BR, et al. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell. 2013;26(4):346–357. doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9(9):557–564. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachtergaele S, et al. Structure and function of the Smoothened extracellular domain in vertebrate Hedgehog signaling. elife. 2013;2:e01340. doi: 10.7554/eLife.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids—From mouse models to human diseases. FEBS J. 2012;279(9):1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, et al. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol Dis. 2012;45(3):923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nachury MV. How do cilia organize signalling cascades? Philos Trans R Soc Lond B Biol Sci. 2014;369(1650):20130465. doi: 10.1098/rstb.2013.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125(3):549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 26.Kim DS, et al. Anticomplementary activity of ergosterol peroxide from Naematoloma fasciculare and reassignment of NMR data. Arch Pharm Res. 1997;20(3):201–205. doi: 10.1007/BF02976145. [DOI] [PubMed] [Google Scholar]
- 27.Miller MB, Haubrich BA, Wang Q, Snell WJ, Nes WD. Evolutionarily conserved Delta(25(27))-olefin ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J Lipid Res. 2012;53(8):1636–1645. doi: 10.1194/jlr.M027482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank-Kamenetsky M, et al. Small-molecule modulators of Hedgehog signaling: Identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1(2):10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186(1-2):17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- 30.Kilsdonk EP, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 31.De Fabiani E, Caruso D, Cavaleri M, Galli Kienle M, Galli G. Cholesta-5,7,9(11)-trien-3 beta-ol found in plasma of patients with Smith-Lemli-Opitz syndrome indicates formation of sterol hydroperoxide. J Lipid Res. 1996;37(11):2280–2287. [PubMed] [Google Scholar]
- 32.Korade Z, Xu L, Mirnics K, Porter NA. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2013;36(1):113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52(10):1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, et al. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52(6):1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Korade Z, Rosado DA, Jr, Mirnics K, Porter NA. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J Lipid Res. 2013;54(4):1135–1143. doi: 10.1194/jlr.M035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meljon A, Watson GL, Wang Y, Shackleton CH, Griffiths WJ. Analysis by liquid chromatography-mass spectrometry of sterols and oxysterols in brain of the newborn Dhcr7(Δ3-5/T93M) mouse: A model of Smith-Lemli-Opitz syndrome. Biochem Pharmacol. 2013;86(1):43–55. doi: 10.1016/j.bcp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dwyer JR, et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282(12):8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 38.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768(6):1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachtergaele S, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8(2):211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan YM, et al. Effects of dietary cholesterol and simvastatin on cholesterol synthesis in Smith-Lemli-Opitz syndrome. Pediatr Res. 2009;65(6):681–685. doi: 10.1203/PDR.0b013e31819ea4eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correa-Cerro LS, et al. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum Mol Genet. 2006;15(6):839–851. doi: 10.1093/hmg/ddl003. [DOI] [PubMed] [Google Scholar]
- 42.Francis KR, et al. Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/β-catenin defects in neuronal cholesterol synthesis phenotypes. Nat Med. 2016;22(4):388–396. doi: 10.1038/nm.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watabe T, Sawahata T. Biotransformation of cholesterol to cholestane-3beta,5alpha,6beta-triol via cholesterol alpha-epoxide (5alpha,6alpha-epoxycholestan-3beta-ol) in bovine adrenal cortex. J Biol Chem. 1979;254(10):3854–3860. [PubMed] [Google Scholar]
- 44.Yamaguchi M, et al. 3 beta,5 alpha-Dihydroxycholestan-6-one exists in human blood. Biol Pharm Bull. 1997;20(9):1044–1046. doi: 10.1248/bpb.20.1044. [DOI] [PubMed] [Google Scholar]
- 45.Morisseau C. Role of epoxide hydrolases in lipid metabolism. Biochimie. 2013;95(1):91–95. doi: 10.1016/j.biochi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bijlsma MF, et al. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4(8):e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khaliullina H, Bilgin M, Sampaio JL, Shevchenko A, Eaton S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc Natl Acad Sci USA. 2015;112(11):3415–3420. doi: 10.1073/pnas.1416463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson CW, Chen MH, Chuang PT. Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS One. 2009;4(4):e5182. doi: 10.1371/journal.pone.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi M, et al. Vitamin D status in patients affected by Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2005;28(1):69–80. doi: 10.1007/s10545-005-3676-8. [DOI] [PubMed] [Google Scholar]
- 50.Prasanna X, Chattopadhyay A, Sengupta D. Cholesterol modulates the dimer interface of the β₂-adrenergic receptor via cholesterol occupancy sites. Biophys J. 2014;106(6):1290–1300. doi: 10.1016/j.bpj.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sengupta D, Chattopadhyay A. Molecular dynamics simulations of GPCR-cholesterol interaction: An emerging paradigm. Biochim Biophys Acta. 2015;1848(9):1775–1782. doi: 10.1016/j.bbamem.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34(42):13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- 53.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 54.Marsh JB, Weinstein DB. Simple charring method for determination of lipids. J Lipid Res. 1966;7(4):574–576. [PubMed] [Google Scholar]
- 55.Albro PW, Corbett JT, Schroeder JL. Doubly allylic hydroperoxide formed in the reaction between sterol 5,7-dienes and singlet oxygen. Photochem Photobiol. 1994;60(4):310–315. doi: 10.1111/j.1751-1097.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]
- 56.Liu W, et al. A highly sensitive method for analysis of 7-dehydrocholesterol for the study of Smith-Lemli-Opitz syndrome. J Lipid Res. 2014;55(2):329–337. doi: 10.1194/jlr.D043877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeBose-Boyd RA, et al. Transport-dependent proteolysis of SREBP: Relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell. 1999;99(7):703–712. doi: 10.1016/s0092-8674(00)81668-2. [DOI] [PubMed] [Google Scholar]