Imagine driving along a busy street in the dim light of a rainy evening. Suddenly, you see from the corner of your eye a dark object moving toward you from the right. Due to the poor light and rain, it is hard to decide what this object is and where exactly it is moving. However, you will have to make a decision to know whether you should swerve, hit the brakes, or just keep driving. How much information do you need to make such a perceptual decision and how sure do you have to be to act upon it? If you do not make your decision fast enough, you run the risk of a collision. On the other hand, the sensory data are noisy and you may not want to react prematurely, but rather collect more reliable information about the ominous object (e.g., by directing attention to it). It seems there is a trade-off between speed and accuracy: The faster you decide, the less accurate is your decision, and, vice versa, the more information you gather to increase accuracy, the slower is your reaction. Moreover, the path of action you choose will depend on how confident you are in your perceptual decision. This everyday example shows that a complex cascade of events must occur in our brains between the registration of a stimulus by our sensory systems and the potential action that we take in response to this stimulus. A study by Rahnev et al. (1) in PNAS investigates the neural mechanism underlying this decision process. The results suggest distinct functional roles of different regions in the frontal lobe in the attentional selection of stimuli, in setting a criterion for the speed–accuracy tradeoff, and in confidence judgments.
The process by which the available sensory information is gathered and used to influence how we behave in the world is commonly referred to as perceptual decision making (2, 3). In addition to a “sensory system” that is concerned with stimulus representation, there is a “decision system” that integrates the sensory evidence and forms a decision variable. Finally, the “motor system” prepares and executes an action on the basis of the perceptual decision (2). In the above example, it is the decision system that accomplishes the difficult job of making a tradeoff between speed and accuracy and factoring in the confidence with which a perceptual decision is made. Typical experimental approaches emulate this situation by presenting stimuli embedded in noise and requiring the participant to discriminate between two possible stimuli (e.g., leftward or rightward motion).
The decision system is thought to involve brain regions downstream of sensory cortices, such as parietal and frontal regions. These regions integrate the evidence represented in sensory areas to compute a decision variable that forms the basis for response selection. The integration of information is commonly modeled as a diffusion-to-boundary process, by which sensory evidence for competing decision outcomes is accumulated over time (4) (Fig. 1). The speed of accumulation is modeled by a drift rate that is related to the amount of stimulus information but can also be enhanced by internal factors, such as attention. A decision is made when evidence accumulation reaches a critical point, a decision boundary. Decision speed is determined by the drift rate of evidence accumulation and the decision boundary. Importantly, the decision boundary is critical for the speed–accuracy tradeoff: The lower the decision boundary, the faster, and the higher the boundary, the more accurate is the decision. In addition, the confidence in the perceptual decision determines the course of action that is eventually taken and influences perceptual decisions in the future (5). How these “three pillars of choice behavior” (3), speed, accuracy, and confidence, are implemented in the brain has been an area of extensive research over the past decades (2, 3). In humans, the neural mechanisms of perceptual decision making have been illuminated in particular by functional magnetic resonance imaging (fMRI), pointing to a key role of frontal lobe regions in setting the criterion for the speed–accuracy tradeoff (6) and in encoding subjective decision confidence (7). However, to date, there is little direct evidence for a functional differentiation between frontal subregions in these processes.
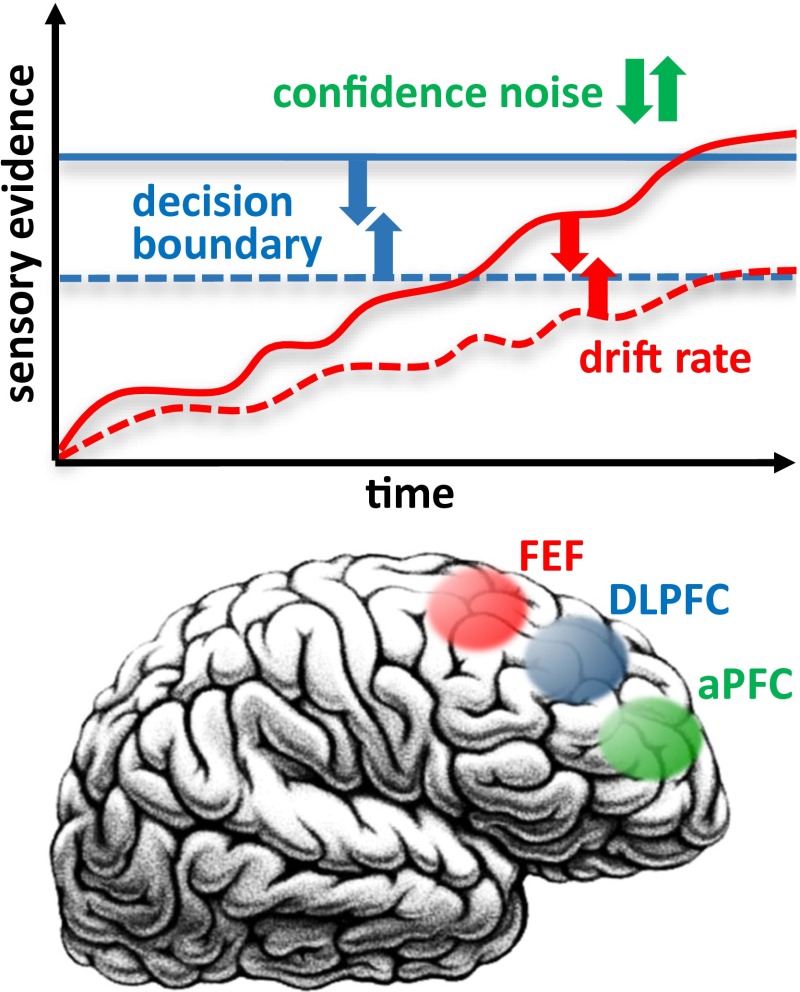
Fig. 1.
Schematic depiction of the diffusion-to-boundary model of perceptual decision making and the relationship between parameters of the model and the functions of frontal brain regions as suggested by Rahnev et al. (1). The FEF is critical for the effect of attentional stimulus selection, which influences perceptual decision making by changing the drift rate in sensory evidence accumulation. DLPFC is involved in setting the criterion for the speed–accuracy tradeoff in perceptual decisions by shifting the decision boundary. aPFC influences confidence in perceptual decisions by modulating confidence noise. Brain image courtesy of Flickr/IsaacMao.
Rahnev et al. (1) set out to experimentally dissociate the contributions of different frontal lobe regions in perceptual decision making. Their reasoning was grounded in theories on the representation of action rules in frontal cortex (8). Action rules specify the relationship between a given condition and an action, and are therefore also relevant in typical perceptual decision-making tasks. An example of a simple action rule would be a stimulus-response mapping, such as “leftward motion–look left, rightward motion–look right.” The representation of action rules has been proposed to follow a gradient in the frontal lobe, with simple action rules being encoded in more posterior regions and increasingly abstract action rules being encoded in more anterior regions (8). Rahnev et al. (1) reasoned that the neural processes underlying perceptual decision making may follow a similar anterior–posterior gradient within the frontal lobe.
They devised an experiment that provided them with behavioral measures of both the criterion setting in terms of the speed-accuracy tradeoff and of observers’ decision confidence. In addition, they assessed the effects of attentional selection on decision speed. In a simple perceptual task, participants had to discriminate between two possible orientations of a noisy line grating. In the beginning of each trial, they were instructed to attend to one of two gratings presented on the screen and, critically, to prioritize either speed or accuracy (i.e., to respond either as fast or as accurately as possible). Shortly after stimulus presentation, they were prompted to report the orientation of either the attended or unattended grating and, thereafter, to rate their confidence in their decision. Participants performed the same task first in the fMRI scanner, which allowed the researchers to pinpoint in each individual the exact regions of interest involved in each aspect of decision making. Three individually defined regions (Fig. 1) involved in attentional selection, criterion setting, and confidence judgment, respectively, and an unrelated control region were then stimulated with transcranial magnetic stimulation (TMS) in separate sessions on the four following days.
The fMRI results confirmed the hypothesized anterior–posterior gradient. Activity during attentional selection was strongest in a posterior part of the frontal lobe, the frontal eye field (FEF). A more anterior region in dorsolateral prefrontal cortex (DLPFC) was most strongly activated during the perceptual judgment, whereas a nearby but even more anterior prefrontal cortex (aPFC) region showed stronger activations during both the perceptual decision and the subsequent confidence judgment compared with attentional selection. Critically, TMS to these three regions yielded changes in behavior that confirmed this functional differentiation. Disruption of FEF function by TMS reduced the enhancing effect of attentional selection on reaction times, whereas TMS over DLPFC specifically resulted in a smaller effect of prioritizing speed over accuracy. TMS over aPFC more strongly influenced participants’ ability to judge their own performance. These findings were further corroborated by relating the TMS results to a computational model parameterizing drift rate, decision boundary, and confidence noise (9). In a model simulation, changes in these three respective parameters had effects that closely resembled the effects of TMS to the FEF, DLPFC, and aPFC. Taken together, these results indicate differential roles for three subregions of the frontal lobe in perceptual decision making (Fig. 1): The FEF mediates the effect of attentional selection on perceptual decision making, likely by influencing the drift rate of evidence accumulation; DLPFC is involved in setting the criterion for perceptual decisions in terms of a speed–accuracy tradeoff; and aPFC plays a role in confidence judgments, possibly by modulating confidence noise.
What makes these findings compelling is that they are based on three lines of converging evidence from fMRI, TMS, and computational modeling within one study. Our knowledge of the functional differentiation within the frontal lobe so far has derived mainly from correlational studies, the majority of which have focused on a single one of a number of subprocesses of perceptual decision making. Successfully dissociating these processes within one experiment by providing not only correlational fMRI data but also concordant causal evidence from TMS thus marks a major step toward a mechanistic understanding of perceptual decision making. Conceptually, an important novel aspect of this study is that it relates perceptual decision making to other functions of frontal cortex. Earlier work on the functional differentiation between frontal subregions has focused on processes involved in cognitive control over actions. In particular, Rahnev et al. (1) motivated their study by the proposal that there is an anterior–posterior gradient in the frontal lobe such that more anterior regions are critical for progressively more abstract action rules (8) and later stages of the perception-action cycle (10).
The possibility of a link between frontal lobe functions in cognitive control and perceptual decision making is intriguing. However, care should be taken when interpreting the evidence for a functional differentiation in frontal cortex in decision making as reflecting a hierarchical organization. Hierarchical architectures are characterized by a dominance relationship between higher and lower levels, which may apply to the representation of abstract vs. concrete action rules in anterior vs. posterior regions, respectively (8). However, there is no obvious hierarchical relationship between stimulus selection, criterion setting, and confidence-related processes in perceptual decision making. Thus, the neurofunctional differentiation shown for these three subprocesses does not necessarily imply a hierarchical organization. Another important caveat for the interpretation of the findings is that the three steps in decision making examined in this experiment were temporally separated to dissect their respective neural substrates. However, perceptual decision making under natural conditions may not always follow such a sequential pattern. It is intuitive to assume that stimulus selection occurs prior to a perceptual decision and a subsequent confidence judgment that eventually leads to an action. However, there is also evidence to support the view that different processes of perceptual decision making might happen in parallel (2, 11). According to this view, the brain is processing sensory information to specify, in parallel, several potential actions that are currently available, and actions can, in turn, feed back into the perceptual decision-making process (11, 12).
In light of these considerations, the findings reported in the study by Rahnev et al. (1) may not provide unequivocal evidence for a hierarchical or serial organization of the processes underlying perceptual decision making. Still, they convincingly show a functional differentiation and are compatible with the notion of an anterior–posterior gradient in frontal cortical function. Moreover, they are highly relevant in the context of an ongoing debate as to whether lateral frontal cortex is broadly recruited by a range of different cognitive challenges or whether frontal subregions subserve different functions in cognitive control (13, 14). By providing strong support for a differential contribution of frontal subregions to the subprocesses of perceptual decision making, the study clearly speaks against the notion of lateral frontal cortex as a functionally homogeneous entity. Whether this functional differentiation is idiosyncratic to perceptual decision making or directly relates to other cognitive functions, such as cognitive control of actions, is an intriguing question for future research. The current study by Rahnev et al. (1) thus paves the way for new avenues of research that investigate the functional differentiation among frontal regions across cognitive domains. Moreover, the study will serve as a starting point for research into the relationship between the neural mechanisms of basic perceptual decisions and those neural mechanisms underlying other forms of decision making, such as value-based or social decisions (3, 9).
Acknowledgments
The author’s research is supported by Deutsche Forschungsgemeinschaft (DFG), Grants STE1430/6-2, STE1430/7-1, and GRK 1589/2.
Footnotes
The author declares no conflict of interest.
See companion article on page 6059.
References
- 1.Rahnev D, Nee DE, Riddle J, Larson AS, D’Esposito M. Causal evidence for frontal cortex organization for perceptual decision making. Proc Natl Acad Sci USA. 2016;113:6059–6064. doi: 10.1073/pnas.1522551113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9(6):467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- 3.Shadlen MN, Kiani R. Decision making as a window on cognition. Neuron. 2013;80(3):791–806. doi: 10.1016/j.neuron.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers D. Evidence for an accumulator model of psychophysical discrimination. Ergonomics. 1970;13(1):37–58. doi: 10.1080/00140137008931117. [DOI] [PubMed] [Google Scholar]
- 5.Guggenmos M, Wilbertz G, Hebart MN, Sterzer P. Mesolimbic confidence signals guide perceptual learning in the absence of external feedback. eLife. 2016;5:5. doi: 10.7554/eLife.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzlaff H, Bauer M, Maess B, Heekeren HR. Neural characterization of the speed-accuracy tradeoff in a perceptual decision-making task. J Neurosci. 2011;31(4):1254–1266. doi: 10.1523/JNEUROSCI.4000-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilgenstock R, Weiss T, Witte OW. You’d better think twice: Post-decision perceptual confidence. Neuroimage. 2014;99:323–331. doi: 10.1016/j.neuroimage.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Martino B, Fleming SM, Garrett N, Dolan RJ. Confidence in value-based choice. Nat Neurosci. 2013;16(1):105–110. doi: 10.1038/nn.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster JM, Bressler SL. Cognit activation: A mechanism enabling temporal integration in working memory. Trends Cogn Sci. 2012;16(4):207–218. doi: 10.1016/j.tics.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cisek P, Pastor-Bernier A. On the challenges and mechanisms of embodied decisions. Philos Trans R Soc Lond B Biol Sci. 2014;369(1655):20130479. doi: 10.1098/rstb.2013.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepora NF, Pezzulo G. Embodied choice: How action influences perceptual decision making. PLOS Comput Biol. 2015;11(4):e1004110. doi: 10.1371/journal.pcbi.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crittenden BM, Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb Cortex. 2014;24(2):532–540. doi: 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds JR, O’Reilly RC, Cohen JD, Braver TS. The function and organization of lateral prefrontal cortex: A test of competing hypotheses. PLoS One. 2012;7(2):e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]