Abstract
Including low penetrance genomic variants in population-based screening might enable personalization of screening intensity and follow up. The application of genomics in this way requires formal evaluation. Even if clinically beneficial, uptake would still depend on the attitudes of target populations. We developed a deliberative workshop on two hypothetical applications (in colorectal cancer and newborn screening) in which we applied stepped, neutrally-framed, information sets. Data were collected using nonparticipant observation, free-text comments by individual participants, and a structured survey. Qualitative data were transcribed and analyzed using thematic content analysis. Eight workshops were conducted with 170 individuals (120 colorectal cancer screening and 50 newborn screening for type 1 diabetes). The use of information sets promoted informed deliberation. In both contexts, attitudes appeared to be heavily informed by assessments of the likely validity of the test results and its personal and health care utility. Perceived benefits included the potential for early intervention, prevention, and closer monitoring while concerns related to costs, education needs regarding the probabilistic nature of risk, the potential for worry, and control of access to personal genomic information. Differences between the colorectal cancer and newborn screening groups appeared to reflect different assessments of potential personal utility, particularly regarding prevention.
Keywords: personalized medicine, screening, public health, evaluation, utility
Introduction
The sequencing of the human genome, and decreasing costs of sequencing technology, has led to forecasts that the use of genomic information to inform healthcare will soon be a reality, the notion of “personalized medicine” (PM). Genomic risk profiling—examining multiple low penetrance gene variants (Hawken et al. 2010; Yang et al. 2003)—offers the potential to produce a “personalized” risk assessment for conditions such as cancer (Michailidou et al. 2013) or heart disease (Di Angelantonio and Butterworth 2012), which, in most families, do not follow strongly Mendelian inheritance patterns (Burton et al. 2013). Although frameworks for the evaluation of evidence exist (Haddow and Palomaki 2003; Secretary’s Advisory Committee on Genetic Testing 2000; Teutsch et al. 2009), they are test oriented and do not examine issues of implementation in practice. Understanding how to ensure the effective implementation and appropriate use of genomic applications is an essential element of the research “translation cycle” (Khoury 2010).
Engaging target populations at an early stage in the development of any technology may help identify important issues related to implementation or acceptability (Auffray et al. 2011). Although they are usually intended to take a societal or policy perspective, health technology assessments are often limited by a lack of rigorous data on those “nontechnical” issues that may be important in implementation in practice (Potter et al. 2009). For example, Lehoux and Blume (2000) describe the Australian experience of a technology assessment process for cochlear implants that focused on the outcome of decibels of hearing loss restored but failed to appreciate the objections of the Deaf community to what they saw as a socially disruptive intervention. We suggest that, given the slow pace of development of genomic technologies for application in the general clinic, it would be valuable to generate broader insights on their use, to complement evaluations of clinical validity and clinical utility.
A particular challenge to this “anticipatory” approach, however, is that few people have had experience with genomic tests, limiting possibilities for “fully informed” engagement. For an emerging field like genomics in medicine, public engagement research must trade off participants’ personal experience against their representativeness: those most familiar with the personal impact of genomic tests and information—patients and families affected by genetic disorders—are not likely to be typical of the general population; those most “typical” of the general population are unlikely to have considered the potential personal impacts of genomic tests. In our program of research on the application of genomics in health care and public health, we have attempted to reconcile these trade-offs by recruiting participants directly from the community, typical of target populations, and facilitating meaningful consideration of issues despite lack of personal experience. One area of interest is the application of genomic technologies in public health programs, and the potential for genomic profiling within population-based screening has received recent attention (Khoury et al. 2004). We developed two scenarios where genomic profiling—while not imminent—could be fairly easily envisaged: cancer screening and newborn screening (NBS).
Cancer screening
Colorectal cancer (CRC) is a leading cause of cancer death in North America (American Cancer Society 2012; Canadian Cancer Society 2011; von Wagner et al. 2012); upwards of 1 million cases are newly diagnosed each year (Center et al. 2009; International Agency for Research on Cancer 2008). The risk of CRC includes a substantial heritable component (Dunlop et al. 2012; Hawken et al. 2010), largely consisting of numerous low penetrance variants (Hawken et al. 2010; Khoury et al. 2004). While these variants individually have limited predictive validity, cumulatively they could provide important information about disease susceptibility (Hawken et al. 2010; Khoury et al. 2004; Tenesa and Dunlop 2009). It is well established that screening to identify CRC in earlier stages improves survival rates at all levels of risk (de Jong et al. 2006; Walsh and Terdiman 2003). Assuming that meaningful accuracy could be demonstrated, genomic risk profiling could potentially reduce mortality by triaging individuals to different screening regimens: higher risk individuals receiving higher intensity of screening with the aim of reduced mortality through earlier detection of curable lesions, and lower risk individuals being spared unnecessarily frequent or invasive tests.
Newborn screening
Type 1 diabetes (T1D) is one of the most common chronic childhood diseases (Devendra et al. 2004) and shows a global increase in incidence (Catanzariti et al. 2009). In children, early symptoms may not be specific (e.g., thirst, blurred vision, abdominal pain), occasionally delaying clinical diagnosis until life threatening keto-acidotic coma necessitates emergency intervention. Consequently, there has been interest in identifying children who are at high risk to educate parents on the potential significance of otherwise nonspecific symptoms.
Even though T1D screening fails to meet the WHO screening criteria (Wilson and Jungner 1968), not least because there is no preventive intervention, a number of studies are exploring the potential for T1D susceptibility testing in infants, including The Environmental Determinants of Diabetes in the Young (TEDDY) trial (Hagopian et al. 2006), the Key Environmental Aspects of Type 1 Diabetes (KEA) study (Kerruish et al. 2007; Kerruish 2011), and the Environmental Triggers of Type 1 Diabetes (MIDIA) study (Aas et al. 2010). In addition, some states in the USA have piloted projects for newborn T1D screening (Hiraki et al. 2006; Ross 2003).
We judged that, by presenting hypothetical genomic profiling tests for familiar conditions, in the context of existing screening programs familiar to specific target populations, we would be able to compensate for lack of personal experience of genetic tests. The specific objectives of the present research were to explore, in participants drawn from the target populations for existing CRC and NBS programs, (i) general reactions to the idea of incorporating genomic risk profiling into routine screening activities, and (ii) the most important issues requiring consideration as these technologies are assessed and implemented in practice.
Materials and methods
Participants and recruitment
Participants were drawn from two locations in Canada: Ottawa, Ontario (ON) and St John’s, Newfoundland (NL). Ottawa is a large, mostly urban population with CRC incidence reflecting the Canadian average and overall relatively high socio-economic status, whereas St John’s is a smaller urban population with a high incidence of CRC (Green et al. 2007; Woods et al. 2005) and a broader socio-economic mix. Additionally, ON and NL have different newborn screening programs in terms of both organization and number of conditions included.
Participants were invited to one topic only, based on relevant demographics associated with existing screening programs. Thus, individuals were eligible for the CRC group if they were aged at least 50 (i.e., eligible for provincially funded CRC screening), or for the NBS group if they had children aged under five—and so had recent experience of NBS. Potential participants were excluded if they were unable to converse freely in English or French.
For both topics, individuals were recruited using random digit dialing (St. John’s) and in person via community-based groups and networks (Ottawa). Appropriate community groups of older people or parents of young children were identified through existing resources available through the municipality, and approaches to individuals were made in person during pre-arranged visits to the group or network. All participants were provided information about the study orally and eligibility confirmed. Upon confirmation of eligibility, written information was provided to those who expressed interest in taking part. At the workshop, participants were given additional copies of the information sheet and asked to confirm their willingness to take part before signing a consent form.
Public engagement methodology
Based on previous approaches to public engagement examining genomics in agriculture (Castle et al. 2005; Castle 2006; Castle and Finlay 2006), we developed a deliberative workshop consisting of three components: an information component comprising three information sets that provided a progressive release of information about genomic risk profiling and its potential implications; a deliberation component providing an opportunity for questions, discussion, and debate after each information set; and a data collection component using multiple approaches to capture participants’ reactions and attitudes. All workshops were facilitated by one researcher (B.J.W.), who has extensive experience of public attitudes research in the field of genetics and genomics.
Workshop format
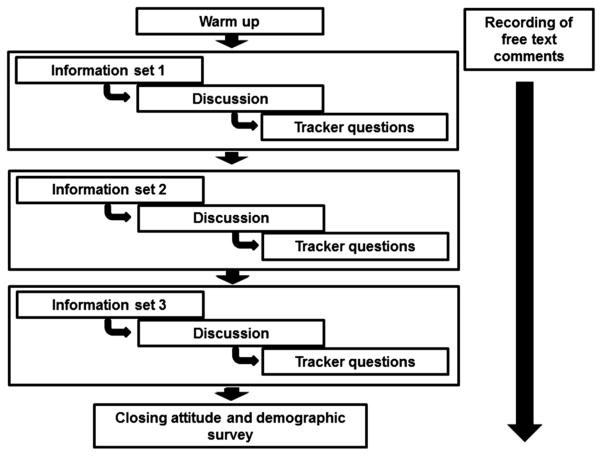
The workshop started with a series of warm-up questions, unrelated to the topic of discussion, to provide an orientation to the methodology. Following this, we worked through the information, deliberation, and data collection components in an iterative way (see Fig. 1).
Fig. 1.
Schematic of the workshop process.
Information component
Three information sets (Table 1) were developed that presented two “prototype” PM applications as case studies: genomic profiling for (i) CRC risk assessment in adults, or (ii) T1D risk assessment in newborns. The information sets were designed to encourage participants to rapidly “unpack” the personal implications of genomic profiling. The starting point was a description of the screening program (CRC or NBS) as currently implemented in the province (ON or NL). Given the recruitment approach, it assumed that the participants were at least aware of the program, so the description of the rationale for screening, the screening procedure, consent policy, etc. were intended as a reminder. The idea of a genomic profiling test as an additional component of the screening approach was then introduced. Given that there is no accepted scientific or medical terminology in this area (the term genomic profiling is also used to refer to tumour-specific analyses, for example), we adopted the term DNA risk test and explained it carefully. The intent was to communicate the central idea of genomic information being applicable to any individual as part of their routine health care and to clearly distinguish it from genetic tests applied to high risk individuals in clinical genetics settings.
Table 1.
Key points of information sets.
| Information set | Content |
|---|---|
| Set 1. The idea of genomic profiling | Description of colorectal cancer/newborn bloodspot screening program relevant to participant group—eligibility, process, and rationale. Idea of genomic profiling test (referred to as DNA risk test), examining variations in a person’s genome to assess a person’s risk level. How this could be used to triage people more accurately. Emphasis that the idea is to improve on current approaches, reducing unnecessary interventions and targeting interventions to those most at risk. |
| Set 2. The potential personal impacts of having a test | Potential advantages of knowing personal risk level: lifestyle choices, screening participation, attending promptly to early symptoms; personal utility of knowledge irrespective of potential for risk reduction. Potential disadvantages of knowing personal risk level: if higher risk, anxiety, depression, disease worry, reduced quality of life; if lower risk, failure to follow health advice, neglect of early symptoms. Potential for effects on other family members. Implications of third party (especially insurance company) access to test result recorded in medical chart, irrespective of whether person develops cancer or not. Idea that DNA results are “for life”. |
| Set 3. Reiteration of the nature of such a test, and its place in personal health management | Genomic profiling would be part of a broader set of risk assessment and screening tests, not a test in isolation. Reinforcement of the idea of “personalized” information on risk but not actual disease status. |
As with previous research using progressive information release (Castle et al. 2005; Castle and Culver 2006), information sets were developed to be understandable to participants, with a reasonable information load. Relevant experts (molecular and clinical genetics, epidemiology, ethics, family medicine, and psychology) collaborated to ensure that the descriptions were technically accurate and that the known risks, benefits, and issues were included. The information sets were pilot tested in small groups consistent with the target populations, to assess whether they provided adequate information for participants to form judgments about genomic risk profiling and to identify additional scientific or other information that should be included.
In both case studies, care was taken to convey the idea that genomic profiling would be a risk test—not a diagnostic test—and to discuss the limitations of accurate prediction, the putative benefits and drawbacks, and the possible personal, family, and social implications.
Deliberation component
The deliberation component consisted of three periods of facilitated discussion, over the course of the workshop. Each discussion took place immediately following an information set and was semi-structured, insofar as discussion was framed against the backdrop of the preceding content, but it was flexible enough to allow participants to bring in new topics. Participants were encouraged to put forward opinions, and alternative or competing perspectives were sought to ensure broad coverage of the topics at hand. In lieu of any alternatives being presented by participants, differing perspectives were presented by the facilitator as talking points to encourage discussion and generate reflection by the group on these alternatives. There were three deliberation periods, each of which allowed participants to reflect and discuss the information presented.
Data collection component
We used four complementary approaches to collect data. For completeness, we describe each of these, but we report on the analysis of only two: participants’ individual free-text comments, and field notes captured during the deliberation component.
Free-text comments
Following orientation, but before the first information set, participants were instructed to:
Please write down everything that comes into your head when you hear the words DNA risk tests.
This question was repeated throughout the workshop as a way to encourage participants to record thoughts and reactions, although participants were encouraged to record thoughts as they occurred, irrespective of the stage of the workshop. These provided qualitative data through an approach resembling diary methods (Bowling 2004; Bryman 2004), i.e., cataloguing individual thoughts that may not be conveyed to the larger group. This was supplemented with detailed field notes and audio recording, where the participants consented, to capture discussion during each deliberation.
Other data
We captured data in three other ways, and the analyses are to be reported elsewhere. First, we posed two attitude questions after each deliberation component, to track how responses changed over the course of the workshop and in response to the information sets and group discussion. The first question asked whether the test in question should be funded by the province, and the second asked whether the participant would have the test personally or for a child. Finally, we asked participants to complete a post-workshop survey containing further attitude questions on the topic, personal or family experience with the condition discussed in the workshop, and demographic data.
Analysis
Here we report the analyses of the free-text individual comments and discussion. Transcripts were imported into Atlas.ti Qualitative Data Analysis (QDA) software (Scientific Software Development 2007) to assist with management and coding.
Two researchers (S.C. and S.N.) initially independently coded the data using the constant comparison method. This process of dual coding has been suggested as a qualitative comparator to traditionally quantitative notions of inter-rater reliability. While numerical approaches to validity have generally been resisted in qualitative studies in favour of standards of “credibility” (Morse et al. 2002; Murphy et al. 1998), empirical research has indicated the utility of such dual coding (Armstrong et al. 1997).
Data analysis followed a thematic analysis approach (Boyatzis 1998) in which textual data are coded and labeled in an inductive manner. As such, data analysis was iterative and ongoing in parallel to the conduct of workshops, allowing us to facilitate the discussion in light of emerging themes. This approach allowed for the revision, combination, or separation of codes in light of new data (Corbin and Strauss 2008; Strauss 1996). Each newly coded incident was compared to previous incidents to refine or revise the code (Fielding and Lee 1998). After an initial phase of open coding, individual codes were grouped into overarching themes or constructs through a process of data reduction. Consequently, the theme operates at a higher level than the immediate codes. Themes were then discussed with the larger team, with codes further reviewed and themes revised until a final set were derived. The study was approved by the Ottawa Hospital Research Ethics Board and the Newfoundland and Labrador Health Research Ethics Authority.
Results
Eight workshops were conducted with 170 individuals (120 participating in CRC workshops, 50 in NBS). Groups were predominantly female, white, and English speaking, although income and education levels varied between the groups and the two provinces (see Table 2).
Table 2.
Participant demographics (N = 170).*
| CRC
|
NBS/diabetes
|
|||
|---|---|---|---|---|
| ON | NL | ON | NL | |
| No. of participants | 46 | 74 | 19 | 31 |
| Female N (%) |
32 (70) | 48 (71) | 17 (94) | 15 (68) |
| No. of children mean (range) |
2.04 (0, 6) | 2.09 (0, 8) | 1.83 (1,3) | 1.73 (1, 3) |
| Age in years mean (range) |
71.3 (59, 88) | 62.2 (51, 79) | 34.8 (28, 43) | 35.5 (27, 48) |
| Married or living with partner N (%) |
25 (54) | 47 (69) | 46 (100) | 20 (91) |
| Undergraduate or higher level of education N (%) |
29 (63) | 30 (44) | 17 (94) | 11 (50) |
| Household income >$70 000 N (%) |
10 (24) | 25 (39) | 15 (88) | 14 (67) |
| White ethnicity N (%) |
46 (100) | 61 (92) | 17 (100) | 22 (100) |
| Language English or English/French bilingual N (%) |
44 (96) | 68 (100) | 17 (100) | 21 (100) |
Note: CRC, colorectal cancer; NBS, newborn screening; ON, Ontario; NL, Newfoundland.
Percentages expressed refer to those who responded to the question; missing data are excluded.
Overview
Participants varied in their receptivity to the potential for genomic risk profiling. Views ranged from an enthusiastic acceptance of genomic profiling as a good idea for risk assessment to a more cautious view that reflected ambivalence about learning risk information that might not be amenable to intervention, the irrevocable nature of the information, and the possibility for regret. No participants expressed outright dismissal of the possibility of genomic risk profiling within routine population screening, although participants within the NBS groups were notably more cautious. For those enthusiastic respondents, several comments reflected the idea that personal risk information has value in itself:
Knowing what your risk factor is for a health problem will allow you/your doctor to decide what preventative measures can or should be taken. [297, ON, CRC]
With potential health risks, I feel being proactive is better than being reactive. As scary as a false positive might be for a new parent, the more information you can have about a potential issue, the better. [384, NL, NBS]
For more cautious participants, parallels with traditional genetic testing, and the associated issues, were occasionally drawn.
The thought of DNA testing being a lifelong result makes one think more deeply about it. Do you want to live with some negative health issue, not knowing if it will affect you? The more this was discussed, the less sure it makes me feel. [377, NL, CRC]
This theme was particularly dominant within the discussion regarding genomic profiling for T1D, where a lack of preventive measures was viewed critically. For example:
But since Type 1 can’t be prevented with appropriate diet + exercise, there’s nothing you can do anyway—so why know? Just be informed so that you understand that certain illnesses could, in fact, be the first stage of diabetes.—[…]—If prevention was possible then this would be much more accepted. [147, ON, NBS]
A notable concern for parents was the potential for worry and possible negative effects of a high-risk result on parent–child bonding. While this was a particular concern that arose in the NBS workshops, it was also raised by parents in the CRC groups. One mother, in particular, discussed the implications of testing where a definitive status was lacking:
Before I had children I was tested for muscular dystrophy … and the test was not conclusive. Therefore every time I gave birth and it was a boy I was worried until he turned 5 and a new and better test was invented to test the boys. I can tell you it really affects your outlook. My daughter would not be tested and had 3 sons and I worried about them too. [106, ON, CRC]
From these discussions, four core issues emerged as requiring consideration before the implementation of genomic profiling technologies, each of which impacted on general receptivity to the technology: consideration of the target population; specific evidence requirements regarding the test; readiness of the health-care system for the technology; and third party access/use of information generated by the test.
Target population
This theme related to questions of whether genomic profiling should apply to the general population or only to individuals already identified as being at high risk—a key distinction between PM and standard genetic testing. The importance of this aspect was thrown into light by the differing potential for intervention in the examples used. For example, the lack of preventive treatment in the case of T1D was used as a reason for more targeted screening,
I think screening tests are useful but should be used cautiously. If there is already a family history, a DNA test should be done. But should all DNA risk tests be performed on all newborns? Maybe not, unless the disease/condition is extremely dangerous if not caught quickly. [233, NL, NBS]
Similarly, immediate strategies for intervention for colorectal cancer were used to support arguments for population-based approaches:
[Quoting information set] These tests would improve how we work out who most needs a colonoscopy. Do we know this for sure? If so then we should use them for everyone if not too expensive (what is too expensive?). [184, ON, CRC]
As the examples above illustrate, attitudes toward the relevant target populations for genomic risk profiling were tied together with questions of cost or disease severity. Put differently, attitudes toward a population or targeted approach were not driven solely by views on clinical utility for individuals. Importantly, some comments within the NBS groups suggested that screening would only be appropriate for individuals with a family history.
Evidence requirements regarding the test
As indicated above, expressions of support appeared to be contingent on evidence of benefit. Within this we identified three sub-themes: benefit was presumed, benefit was hoped for, and benefit was questioned. First, there was a set of comments that reflected a presumption that this technology would provide benefit, for example:
Will promote better use of resources. Will reduce risk of unnecessary testing of patients. Will reduce overall health care delivery costs. Will provide better health care delivery to patients. Knowledge assists health care providers focus on patient centered care. [197, NL, CRC]
The second sub-theme was more cautious—a hope, rather than assumption, that genomic profiling would provide benefit:
A new era—current practices are rather like a bludgeon. I’m hoping that DNA testing will be more precise …. [134, ON, CRC]
The final sub-theme was the most skeptical and questioned the benefit of the technology; in effect asking for evidence that these hypothetical tests would not only be technically accurate but also that a demonstration of clinical utility would be necessary.
I would also certainly like to know the pros and cons. The percentages regarding the risks. Also how important are the risks in coming up with a positive test towards positive outcomes re: a particular disease. [279, NL, CRC]
In each of these sub-themes, there were clear evidentiary assessments that participants saw as important: cost (and potential for cost savings in both the short or long term) or cost-effectiveness, clinical validity, and clinical utility. These reinforced the comments relating to the general receptivity to the idea of genomic profiling.
Health system readiness for this technology
Participants offered thoughts about the readiness of the health care system and needs of the public to adopt genomic profiling tests in practice. Almost all centered on the need for education, both in general,
There would be a huge public educational side to this whole aspect of DNA testing to avoid misconceptions, perhaps even legal cases arising—education for GP’s and psychologists too. [297, ON, CRC]
and, in particular, around understanding concepts of risk:
People would have to be really well educated about what “risk” means. Doctors would have to be well trained in how to explain the tests to their patients and how to work with their patients after a positive test. [286, ON CRC]
I really think that if DNA risk test [sic] are offered there needs to be really good communication about what it is/is not. Especially if there is some risk that is higher—then there really needs to be good communication about what the test result does and does not mean. [186, NL, NBS]
While expecting physicians to be knowledgeable, participants also indicated a perception that the incorporation of genomic information would require physicians to undergo further education themselves. Specifically, participants referred to two skill areas: the interpretation of risk information and the ability to counsel patients.
Third party access and use
Recognizing that concerns about the use of personal health information by insurance companies and other third parties are a consistent element of discourse around genetic tests, we sought to explicitly include discussion of third party access to genomic risk information to explore whether this form of risk test—as opposed to a diagnostic test—raised additional issues. We noted three distinct groups of comments. The first suggested that genomic risk information raised no more concerns than current practice, for example:
DNA is not much different with insurance co’s now because right now you can’t get insurance if you have a health risk after a certain age. Your health and family history is watched very closely now without DNA testing. [337, NL, CRC]
The second type of response indicated a level of genetic exceptionalism—that is, that genetic information was somehow special and needed to be treated differently. In some instances this also included an element of determinism, if not from the individual’s perspective but potentially in the way the information would be viewed by others, for example:
Employers might want to know your DNA to consider your long term health and decide if their educational training is worth it to the company! [123, ON, CRC]
The third set of responses suggested that concerns about third party access or use were misplaced. These arguments tended to be raised primarily in the CRC workshops, for example:
More attention should be paid to the personal benefits of DNA testing. Though insurance can cause great concern, this should not be considered as the major negative against the testing. [307, ON, CRC]
Personally, I would not be concerned about insurance companies and would rather know my health risks than making sure I have a life insurance policy. [213, NL, CRC emphasis in original]
Discussion
The findings of our workshops indicate diverse reactions to the idea of genomic risk profiling as part of screening, ranging from uncritically enthusiastic to cautious and skeptical, although no participants dismissed the idea out of hand. Previous studies of public attitudes to gene expression profiling in breast cancer treatment found support for PM technologies provided they were clinically meaningful and effective (Bombard et al. 2013). Our findings also indicate that participants’ support for genomic risk profiling as part of population screening would be contingent on evidence that the tests were accurate (clinically valid), and on their personal assessment of the usefulness of the information provided for clinical or personal decisions (clinical or personal utility). Moreover, while we identified a discourse in which genomic profiling was something exceptional that was to be approached cautiously, there was substantial discussion that took a view that this was just another medical test, particularly within the CRC groups.
Benefits from genomic risk profiling proposed by participants were the potential for early intervention, prevention, and closer monitoring, whereas concerns related to the (difficult) probabilistic nature of risk information and potential for worry. In the case of genomic risk profiling for T1D, this was reflected in comments regarding potential maternal anxiety created by high risk results and a negative impact on familial relationships, both of which have been reported as concerns within the literature around childhood genetic testing and screening (Grob 2008; Kerruish et al. 2007; Ross 2007). A further concern raised in both topic groups was control of access to the information generated by genomic tests.
This study had several important limitations. First, although we are confident that we reached saturation in the data obtained in the CRC workshops, we are less so for the NBS groups, in terms of capturing the entire spectrum of perspectives. This is partly because of a smaller absolute dataset (restricting seeking out “disconfirming data”), but also because barriers to access (lack of childcare and (or) available time) led to a somewhat homogeneous group of participants in terms of socio-economic status, dual parenting situation, and access to supportive resources. Second, the central assumption of the methodology was that participants inexperienced with genetic tests would be able to extrapolate from actual experience with screening. There is no way to know how reflective their responses to hypothetical PM applications would be if real tests were offered in real screening situations.
However, a strength of our approach has been the complementary data collection which, given that participants may have different communication preferences (oral or written), allowed us to obtain views that may not have been voiced had only one approach been taken. Moreover, the provision of a standardized educational component ensured a consistency of at least a basic level of information for participants.
The expressed views and focus on clinical validity and utility—i.e., the accuracy of the test in predicting the risk of cancer or diabetes, and the usefulness of these results to the individual—were key discussion points within both topic groups. This not only informed participants’ general receptivity to genomic risk profiling but also views regarding to whom the tests should be offered. More skeptical views regarding benefit—and specifically treatment or prevention—appeared to be associated with a perspective that genomic risk profiling should only be made available to subgroups such as those already deemed to be at high risk because of a family history. These elements of decision making are consistent with traditional screening criteria: that there should be an accepted treatment for patients with recognized disease, there should be a suitable test or examination, that there should be an agreed policy on whom to treat as patients, and that the test should be acceptable to the population (Wilson and Jungner 1968). They also overlap with existing approaches such as the ACCE framework, which indicates the need for assessments of analytic validity, clinical validity, clinical utility, and ethical, legal, and social issues (Secretary’s Advisory Committee on Genetic Testing 2000) for new tests. The present discussion may point to their continued utility as assessment criteria in a post-genomic era of personalized medicine.
A perspective that genomic risk profiling was just another medical test was underscored by the perceived ability to act on the information generated by genomic profiling, be it through treatment, prevention, or closer diagnosis.
Other studies of screening and genetic testing suggest that the ability of test results to inform decision making about prevention or therapeutic interventions is important (Andrykowski et al. 1996; Mesters et al. 2005; Ramsey et al. 2003; Rose et al. 2005; Wakefield et al. 2007; Walsh et al. 2012), and genetic exceptionalism may be reduced when active preventive strategies are available (Ross 2001; Saukko et al. 2006). Our findings are consistent with this, although require further examination given the stated limitations. Further studies exploring the potential mediating role of prevention or treatment options on attitudes toward genomic tests would be particularly beneficial.
We also note the importance of ensuring an adequate understanding of the nature, purpose, and evidence underpinning genomic risk profiling with a particular focus on test validity and utility. On the one hand, evaluations about test utility need to be supported by educational approaches that promote an accurate understanding of what information a “risk test” can provide and facilitate a critical approach to the personal implications of testing. This is important given concerns regarding “overdiagnosis”—or “patients in waiting”—which was related to the issue of parental anxiety in the T1D groups but may also be salient for CRC, where there is the potential risk of aggressive overtreatment of indolent cancers (Burton et al. 2013). Conceptions of risk will also be important to public acceptability (Burton et al. 2012)—public reactions to a potential reduction in screening on the basis of a low risk result need to be carefully considered. Moreover, the suggestion by some participants within the NBS groups that screening would only be appropriate for individuals with a family history may reflect a misconception that could have negative service provision implications should genomic risk profiling be implemented in practice—studies suggest that around 85% of newly-diagnosed patients with T1D have no family history (Atkinson and Eisenbarth 2001). This could indicate an important aspect of public education that would be necessary, although it may also reflect a more basic concern regarding the benefits to be gained from a risk profile for a disease that cannot, currently, be prevented.
Capable practitioners and effective interventions, such as decision aids, must also support informed decision making. Previous research has indicated that patients expected primary care physicians to have sufficient knowledge of genetics to recognize familial risk and make appropriate healthcare decisions, such as onward referral to specialists (Carroll et al. 2011; Miller et al. 2010; Vries et al. 2005). Participants in the present study indicated a similar expectation that health care professionals across the board will be engaged in the implementation of many types of PM applications, especially in the preventive medicine context. Confidence with genetic and genomic information was expected and has been shown to be important in this respect; a recent survey indicated that general practitioners who felt well-informed about genetics were over 10 times more likely to order a genomic risk profile (Haga et al. 2011). Educational interventions can improve physician knowledge of genetics and reported confidence in the management of individuals with genetic conditions (Carroll et al. 2009, 2011). However, it remains to be seen how best to educate and engage large numbers of physicians with respect to developments in genomic science and personalized medicine.
Given the high profile—although potentially over-hyped (Caulfield et al. 2013)—nature of genetics and insurance coverage, we expected our analysis of third party access would present the clearest evidence of views consistent with genetic exceptionalism; while we did see this, we also found indications of a considered approach in which insurance concerns were placed in the larger context of overall potential for benefit. Privacy of personal health information is a general concern (Ayatollahi et al. 2009; Eisenberg et al. 2005; Varga and Brookes 2008) and there is evidence of lower uptake of genetic testing in response to concerns about insurance (Armstrong et al. 2012; Keogh et al. 2009). Our study provided evidence that DNA-based profiling raised privacy worries, but we did not find extra concerns.
In conclusion, this study suggests that genomic profiling for CRC screening, and NBS for T1D may be useful examples for public engagement about PM applications in preventive medicine. We identified a full range of reactions to the idea, reflecting framing both as a “normal” medical test and also as “exceptional” because of the nature of genomic information. We noted concerns about the readiness of the health system to adopt this technology, particularly around education and understanding of the nature of risk information, and interpret these as indicating challenges for service provision to support informed decision making.
Acknowledgments
Silvia Visentin provided research administrative support for all aspects of this work. This study was funded by the Canadian Institutes of Health Research (ETG 92254). The members of the CIHR Emerging Team in Genomics in Screening are (PIs) Brenda Wilson, June Carroll, David Castle, Julian Little, Beth Potter, and (CIs) Judith Allanson, Denise Avard, Mario Cappelli, Tim Caulfield, Pranesh Chakraborty, Holly Etchegary, Jeremy Grimshaw, Louise Lemyre, Fiona Miller, Karin Morin, and George Wells.
Footnotes
This article is part of a Special Commemorative Issue marking the one-year anniversary of “Genomics: The Power and the Promise”.
Contributor Information
S.G. Nicholls, Department of Epidemiology & Community Medicine, University of Ottawa, Ottawa, ON, Canada
B.J. Wilson, Department of Epidemiology & Community Medicine, University of Ottawa, Ottawa, ON, Canada
S.M. Craigie, Department of Epidemiology & Community Medicine, University of Ottawa, Ottawa, ON, Canada
H. Etchegary, Clinical Epidemiology, Faculty of Medicine, Memorial University of Newfoundland, St. John’s, NL, Canada
D. Castle, Chair of Innovation in the Life Sciences, School of Social and Political Science, The University of Edinburgh, Edinburgh, UK
J.C. Carroll, Department of Family and Community Medicine, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada; Sydney G. Frankfort Chair in Family Medicine, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada
B.K. Potter, Department of Epidemiology & Community Medicine, University of Ottawa, Ottawa, ON, Canada
L. Lemyre, School of Psychology, University of Ottawa, Ottawa, ON, Canada; Institute of Population Health, University of Ottawa, Ottawa, ON, Canada
J. Little, Department of Epidemiology & Community Medicine, University of Ottawa, Ottawa, ON, Canada; Canada Research Chair in Human Genome Epidemiology, University of Ottawa, Ottawa, ON, Canada.
References
- Aas KK, Tambs K, Kise MS, Magnus P, Rønningen KS. Genetic testing of newborns for type 1 diabetes susceptibility: a prospective cohort study on effects on maternal mental health. BMC Med Genet. 2010;11:112. doi: 10.1186/1471-2350-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. [accessed 15 April 2013];Colorectal cancer. 2012 [Online.] Available from: http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/index.
- Andrykowski MA, Munn RK, Studts JL. Interest in learning of personal genetic risk for cancer: a general population survey. Prev Med. 1996;25:527–536. doi: 10.1006/pmed.1996.0086. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Gosling A, Weinman J, Marteau TM. The place of inter-rater reliability in qualitative research: an empirical study. Sociology. 1997;31(3):597–606. doi: 10.1177/0038038597031003015. [DOI] [Google Scholar]
- Armstrong K, Putt M, Halbert CH, Grande D, Schwartz JS, Liao K, et al. The influence of health care policies and health care system distrust on willingness to undergo genetic testing. Med Care. 2012;50:381–387. doi: 10.1097/MLR.0b013e31824d748b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/s0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- Auffray C, Caulfield T, Khoury MJ, Lupski JR, Schwab M, Veenstra T. Genome Medicine: past, present and future. Genome Med. 2011;3(6) doi: 10.1186/gm220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayatollahi H, Bath PA, Goodacre S. Accessibility versus confidentiality of information in the emergency department. Emerg Med J. 2009;26(12):857–860. doi: 10.1136/emj.2008.070557. [DOI] [PubMed] [Google Scholar]
- Bombard Y, Abelson J, Simeonov D, Gauvin FP. Citizens’ perspectives on personalized medicine: a qualitative public deliberation study. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2012.300. [Advance online publication.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. Research methods in health. Open University Press; Maidenhead, UK: 2004. [Google Scholar]
- Boyatzis RE. Transforming qualitative information. Sage Publications; Thousand Oaks, USA: 1998. [Google Scholar]
- Bryman A. Social research methods. Oxford University Press; Oxford, UK: 2004. [Google Scholar]
- Burton H, Sagoo GS, Pharoah P, Zimmern RL. Time to revisit Geoffrey Rose: strategies for prevention in the genomic era? Ital. J Public Health. 2012;9(4):e8665-1-e-9. doi: 10.2427/8665. [DOI] [Google Scholar]
- Burton H, Chowdhury S, Dent T, Hall A, Pashayan N, Pharoah P. Public health implications from COGS and potential for risk stratification and screening. Nat Genet. 2013;45(4):349–351. doi: 10.1038/ng.2582. [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Society. Canadian Cancer Statistics 2011. Canadian Cancer Society; Toronto, Ont: 2011. [Google Scholar]
- Carroll JC, Rideout AL, Wilson BJ, Allanson J, Blaine SM, Esplen MJ, et al. Genetic education for primary care providers. Improving attitudes, knowledge, and confidence. Can Fam Physician. 2009;55:e92–e99. [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Wilson BJ, Allanson J, Grimshaw J, Blaine SM, Meschino WS, et al. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam Pract. 2011;28(6):615–623. doi: 10.1093/fampra/cmr040. [DOI] [PubMed] [Google Scholar]
- Castle D. The balance between expertise and authority in citizen engagement about new biotechnology. Techné. 2006;9(3) doi: 10.5840/techne2006931. [DOI] [Google Scholar]
- Castle D, Culver K. Public engagement, public consultation, innovation and the market. Integr Assess J. 2006;6(2):137–152. [Google Scholar]
- Castle D, Finlay K. Consumers’ response to transgenic pork: the role of information. Asia-Pacific Advances in Consumer Research. 2006;7:283–284. [Google Scholar]
- Castle D, Finlay K, Clark S. Proactive consumer consultation: the effect of information provision on response to transgenic animals. Journal of Public Affairs. 2005;5(3–4):200–216. doi: 10.1002/pa.22. [DOI] [Google Scholar]
- Catanzariti L, Faulks K, Moon L, Waters A-M, Flack J, Craig ME. Australia’s national trends in the incidence of Type 1 diabetes in 0–14-year-olds, 2000–2006. Diabet Med. 2009;26(6):596–601. doi: 10.1111/j.1464-5491.2009.02737.x. [DOI] [PubMed] [Google Scholar]
- Caulfield T, Chandrasekharan S, Joly Y, Cook-Deegan R. Harm, hype and evidence: ELSI research and policy guidance. Genome Med. 2013;5(3):21. doi: 10.1186/gm425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- Corbin JM, Strauss AL. Basics of qualitative research. 3. Sage Publications; Thousand Oaks, USA: 2008. [Google Scholar]
- de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130(3):665–671. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Devendra D, Liu E, Eisenbarth G. Type 1 diabetes: recent developments. Br Med J. 2004;328:750–754. doi: 10.1136/bmj.328.7442.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Angelantonio E, Butterworth AS. Clinical utility of genetic variants for cardiovascular risk prediction: a futile exercise or insufficient data? Circ. Cardiovasc Genet. 2012;5(4):387–390. doi: 10.1161/CIRCGENETICS.112.964148. [DOI] [PubMed] [Google Scholar]
- Dunlop MG, Tenesa A, Farrington SM, Ballereau S, Brewster DH, Koessler T, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42 103 individuals. Gut. 2012;62:871–881. doi: 10.1136/gutjnl-2011-300537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ME, Swain C, Bearinger LH, Sieving RE, Resnick MD. Parental notification laws for minors’ access to contraception. What do parents say? Arch Pediatr Adolesc Med. 2005;159:120–125. doi: 10.1001/archpedi.159.2.120. [DOI] [PubMed] [Google Scholar]
- Fielding NG, Lee RL. Computer analysis and qualitative research. Sage Publications; London, UK: 1998. [Google Scholar]
- Green RC, Green JS, Buehler SK, Robb JD, Daftary D, Gallinger S, et al. Very high incidence of familial colorectal cancer in Newfoundland: a comparison with Ontario and 13 other population-based studies. Fam Cancer. 2007;6(1):53–62. doi: 10.1007/s10689-006-9104-x. [DOI] [PubMed] [Google Scholar]
- Grob R. Is my sick child healthy? Is my healthy child sick?: Changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc Sci Med. 2008;67:1056–1064. doi: 10.1016/j.socscimed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE. ACCE: a model process for evaluating data on emerging genetic tests. In: Khoury MJ, Little J, Burke W, editors. Human genome epidemiology: a scientific foundation for using genetic information to improve health and disease. Oxford University Press; New York: 2003. pp. 217–233. [Google Scholar]
- Haga SB, Carrig MM, O’Daniel JM, Orlando LA, Killeya-Jones LA, Ginsburg GS, et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J Gen Intern Med. 2011;26(8):834–840. doi: 10.1007/s11606-011-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian WA, Lernmark A, Rewers MJ, Simell OG, She JX, Ziegler AG, et al. TEDDY–The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann NY Acad Sci. 2006;1079:320–326. doi: 10.1196/annals.1375.049. [DOI] [PubMed] [Google Scholar]
- Hawken SJ, Greenwood CM, Hudson TJ, Kustra R, McLaughlin J, Yang Q, et al. The utility and predictive value of combinations of low penetrance genes for screening and risk prediction of colorectal cancer. Hum Genet. 2010;128(1):89–101. doi: 10.1007/s00439-010-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki S, Ormond KE, Kim K, Ross LF. Attitudes of genetic counselors towards expanding newborn screening and offering predictive genetic testing to children. Am J Med Genet. 2006;140(21):2312–2319. doi: 10.1002/ajmg.a.31485. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. [accessed 9 April 2013];Colorectal Cancer Incidence and Mortality Worldwide in 2008. 2008 [Online.] Available from: http://globocan.iarc.fr/factsheets/cancers/colorectal.asp.
- Keogh LA, van Vliet CM, Studdert DM, Maskiell JA, Macrae FA, St John DJ, et al. Is uptake of genetic testing for colorectal cancer influenced by knowledge of insurance implications? Med. J Aust. 2009;191:255–258. doi: 10.5694/j.1326-5377.2009.tb02778.x. [DOI] [PubMed] [Google Scholar]
- Kerruish NJ. Parents’ experiences of newborn screening for genetic susceptibility to type 1 diabetes. J Med Ethics. 2011;37(6):348–353. doi: 10.1136/jme.2010.039206. [DOI] [PubMed] [Google Scholar]
- Kerruish NJ, Campbell-Stokes PL, Gray A, Merriman TR, Robertson SP, Taylor BJ. Maternal psychological reaction to newborn genetic screening for type 1 diabetes. Pediatrics. 2007;120(2):e324–e335. doi: 10.1542/peds.2006-1381. [DOI] [PubMed] [Google Scholar]
- Khoury MJ. Dealing with the evidence dilemma in genomics and personalized medicine. Clin Pharmacol Ther. 2010;87(6):635–638. doi: 10.1038/clpt.2010.4. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Yang Q, Gwinn M, Little J, Flanders DW. An epidemiologic assessment of genomic profiling for measuring susceptibility to common diseases and targeting interventions. Genet Med. 2004;6(1):38–47. doi: 10.1097/01.GIM.0000105751.71430.79. [DOI] [PubMed] [Google Scholar]
- Lehoux P, Blume S. Technology assessment and the sociopolitics of health technologies. Journal of Health Politics, Policy and Law. 2000;25(6):1083–1120. doi: 10.1215/03616878-25-6-1083. [DOI] [PubMed] [Google Scholar]
- Mesters I, Ausems A, de Vries H. General public’s knowledge, interest and information needs related to genetic cancer: an exploratory study. Eur J Cancer Prev. 2005;14:69–75. doi: 10.1097/00008469-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FA, Carroll JC, Wilson BJ, Bytautas JP, Allanson J, Cappelli M, et al. The primary care physician role in cancer genetics: a qualitative study of patient experience. Fam Pract. 2010;27(5):563–569. doi: 10.1093/fampra/cmq035. [DOI] [PubMed] [Google Scholar]
- Morse JM, Barrett M, Mayan M, Olsen K, Spiers J. Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Methods. 2002;1(2):13–22. [Google Scholar]
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess. 1998;2:1–274. [PubMed] [Google Scholar]
- Potter BK, Avard D, Entwistle V, Kennedy C, Chakraborty P, McGuire M, et al. Ethical, legal, and social issues in health technology assessment for prenatal/preconceptional and newborn screening: a workshop report. Public Health Genomics. 2009;12(1):4–10. doi: 10.1159/000153430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey SD, Wilson S, Spencer A, Geidzinska A, Newcomb P. Attitudes towards genetic screening for predisposition to colon cancer among cancer patients, their relatives and members of the community. Results of focus group interviews. Community Genet. 2003;6(1):29–36. doi: 10.1159/000069543. [DOI] [PubMed] [Google Scholar]
- Rose A, Peters N, Shea JA, Armstrong K. The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J Health Commun. 2005;10(4):309–321. doi: 10.1080/10810730590950039. [DOI] [PubMed] [Google Scholar]
- Ross LF. Genetic exceptionalism vs. paradigm shift: lessons from HIV. J Law Med Ethics. 2001;29:141–148. doi: 10.1111/j.1748-720X.2001.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Ross LF. Minimizing risks. The ethics of predictive diabetes mellitus screening research in newborns. Arch Pediatr Adolesc Med. 2003;157:89–95. doi: 10.1001/archpedi.157.1.89. [DOI] [PubMed] [Google Scholar]
- Ross LF. Against newborn screening for type 1 diabetes. Arch Pediatr Adolesc Med. 2007;161:616–617. doi: 10.1001/archpedi.161.6.616-c. [DOI] [PubMed] [Google Scholar]
- Saukko PM, Richards SH, Shepherd MH, Campbell JL. Are genetic tests exceptional? Lessons from a qualitative study on thrombophilia. Soc Sci Med. 2006;63(7):1947–1959. doi: 10.1016/j.socscimed.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Scientific Software Development. ATLAS/ti. Berlin: 2007. Version 5.2. 5.2 ed. [Google Scholar]
- Secretary’s Advisory Committee on Genetic Testing. Enhancing the oversight of genetic tests: recommendations of the SACGT. National Institutes of Health; Bethesda, Maryland: 2000. [Google Scholar]
- Strauss AL. Qualitative analysis for social scientists. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10:353–358. doi: 10.1038/nrg2574. [DOI] [PubMed] [Google Scholar]
- Teutsch SM, Bradley LA, Palomaki GE, Haddow JE, Piper M, Calonge N, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11(1):3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Brookes H. Factors influencing teen mothers’ enrollment and participation in prevention of mother-to-child HIV transmission services in Limpopo Province, South Africa. Qual Health Res. 2008;18(6):786–802. doi: 10.1177/1049732308318449. [DOI] [PubMed] [Google Scholar]
- von Wagner C, Good A, Smith SG, Wardle J. Responses to procedural information about colorectal cancer screening using faecal occult blood testing: the role of consideration of future consequences. Health Expect. 2012;15(2):176–186. doi: 10.1111/j.1369-7625.2011.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries H, Mesters I, van de Steeg H, Honing C. The general public’s information needs and perceptions regarding hereditary cancer: an application of the Integrated Change Model. Patient Educ Couns. 2005;56(2):154–165. doi: 10.1016/j.pec.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Wakefield CE, Kasparian NA, Meiser B, Homewood J, Kirk J, Tucker K. Attitudes toward genetic testing for cancer risk after genetic counseling and decision support: a qualitative comparison between hereditary cancer types. Genet Test. 2007;11(4):401–411. doi: 10.1089/gte.2007.0013. [DOI] [PubMed] [Google Scholar]
- Walsh JME, Terdiman JP. Colorectal cancer. Scientific review. J Am Med Assoc. 2003;289:1288–1296. doi: 10.1001/jama.289.10.1288. [DOI] [PubMed] [Google Scholar]
- Walsh J, Arora M, Hosenfeld C, Ladabaum U, Kuppermann M, Knight SJ. Preferences for genetic testing to identify hereditary colorectal cancer: perspectives of high-risk patients, community members, and clinicians. J Cancer Educ. 2012;27(1):112–119. doi: 10.1007/s13187-011-0286-z. [DOI] [PubMed] [Google Scholar]
- Wilson JMG, Jungner G. Principles and practice of screening for disease. WHO; Geneva, Switzerland: 1968. [Google Scholar]
- Woods MO, Hyde AJ, Curtis FK, Stuckless S, Green JS, Pollett AF, et al. High frequency of hereditary colorectal cancer in Newfoundland likely involves novel susceptibility genes. Clin Cancer Res. 2005;11(19 Pt 1):6853–6861. doi: 10.1158/1078-0432.CCR-05-0726. [DOI] [PubMed] [Google Scholar]
- Yang Q, Khoury MJ, Botto L, Friedman JM, Flanders WD. Improving the prediction of complex diseases by testing for multiple disease-susceptibility genes. Am J Hum Genet. 2003;72(3):636–649. doi: 10.1086/367923. [DOI] [PMC free article] [PubMed] [Google Scholar]