Abstract
Summary
Forearm fractures are common during growth. We studied bone strength in youth with a recent forearm fracture. In girls, suboptimal bone strength was associated with fractures. In boys, poor balance and physical inactivity may lead to fractures. Prospective studies will confirm these relationships and identify targets for prevention strategies.
Introduction
The etiology of pediatric forearm fractures is unclear. Thus, we examined distal radius bone strength, microstructure, and density in children and adolescents with a recent low- or moderate-energy forearm fracture and those without forearm fractures.
Methods
We assessed the non-dominant (controls) and non-fractured (cases) distal radius (7 % site) using high-resolution peripheral quantitative computed tomography (HR-pQCT) (Scanco Medical AG) in 270 participants (girls: cases n=47, controls n=61 and boys: cases n=88, controls n=74) aged 8–16 years. We assessed standard anthropometry, maturity, body composition (dual energy X-ray absorptiometry (DXA), Hologic QDR 4500 W) physical activity, and balance. We fit sex-specific logistic regression models for each bone outcome adjusting for maturity, ethnicity, height, and percent body fat.
Results
In girls, impaired bone strength (failure load, ultimate stress) and a high load-to-strength ratio were associated with low-energy fractures (odds ratios (OR) 2.8–4.3). Low total bone mineral density (Tt.BMD), bone volume ratio, trabecular thickness, and cortical BMD and thickness were also associated with low-energy fractures (ORs 2.0–7.0). In boys, low Tt.BMD, but not bone strength, was associated with low-energy fractures (OR=1.8). Boys with low-energy fractures had poor balance and higher percent body fat compared with controls (p<0.05). Boys with fractures (both types) were less active than controls (p<0.05).
Conclusions
Forearm fracture etiology appears to be sex-specific. In girls, deficits in bone strength are associated with fractures. In boys, a combination of poor balance, excess body fat, and low physical activity may lead to fractures. Prospective studies are needed to confirm these relationships and clarify targets for prevention strategies.
Keywords: Bone microstructure, Bone strength, Children, Forearm fracture, HR-pQCT
Introduction
The distal forearm is the most common site of fracture among children and adolescents, accounting for ~25 % of all fractures [1]. Boys suffer a disproportionately higher number of fractures compared with girls [1, 2]. Although reasons for this sex difference are not entirely clear, it may be due, in part, to boys’ higher bone turnover rate [3], a growth spurt of longer duration and a greater peak height velocity [4] compared with girls. In both sexes, the incidence of distal forearm fractures peaks in early to mid-puberty (10–12 years in girls, 13–15 years in boys) [1, 2, 4], a potentially transient period of bone fragility due to the lag time between peaks in linear growth and bone accrual [5]. Despite a decline in overall fracture incidence in recent years [6], the incidence of distal forearm fractures in children and adolescents has continued to rise [4]. Reasons for this increase are not fully understood, and thus, investigation of potential risk factors is warranted as children and adolescents who sustain forearm fractures are at risk for subsequent fractures not only during growth [7] but also later in life [8].
Deficits in bone strength—the “bottom” line in fracture prevention [9]—are thought to contribute to forearm fractures during growth [3]. Bone strength is a product of bone macro-and microstructure, bone material properties, and the amount of bone mineral [9]. To date, bone mass (or areal bone mineral density, aBMD), as measured with 2D dual energy X-ray absorptiometry (DXA), was the risk factor most often studied in relation to pediatric forearm fractures. Girls and boys with recent forearm fractures had significantly lower bone mineral content (BMC) and/or aBMD compared with same sex children without fractures [10–13]. This deficit in bone mass is apparent not only at the radius [10, 11] but also at other skeletal sites [10–12], suggesting potential systemic skeletal deficits. While DXA-based studies shed some light on the etiology of pediatric fractures, they are hampered by the well-documented limitations associated with this planar technology [14].
With more widespread use of 3D imaging tools such as peripheral quantitative computed tomography (pQCT) and high-resolution pQCT (HR-pQCT), it is becoming apparent that deficits in bone macro- and microstructure, and (volumetric) BMD may underpin lower bone strength in children with forearm fractures. For example, in a recent study that used pQCT to assess the forearm, fractures in children and youth aged 5–16 years were associated with lower cortical BMD and thickness at the radial shaft (20 % site) and concomitant deficits in bone strength, estimated with the polar strength strain index [12]. Similar deficits in HR-pQCT-derived cortical thickness and bone strength at the distal radius were also observed in girls and boys aged 8 to 15 years who sustained a mild trauma distal forearm fracture compared with non-fracture controls [15]. Such deficits in cortical bone microstructure in fracture cases supports the notion that a transient decrease in cortical thickness during early and mid-puberty, the period of peak linear growth, may result in a period of cortical bone fragility [16, 17].
Thus, in the present study, we aimed to assess whether there were deficits in bone strength, macro- and microstructure, and BMD in a cohort of Canadian children and adolescents with a recent history of forearm fracture. Additionally, we aimed to determine if the magnitude of such deficits, if apparent, varied according to the degree of trauma (i.e., low or moderate energy). Finally, we assessed the ability of HR-pQCT to discriminate between children and adolescents with and without a recent history of forearm fracture.
Participants and methods
Participants
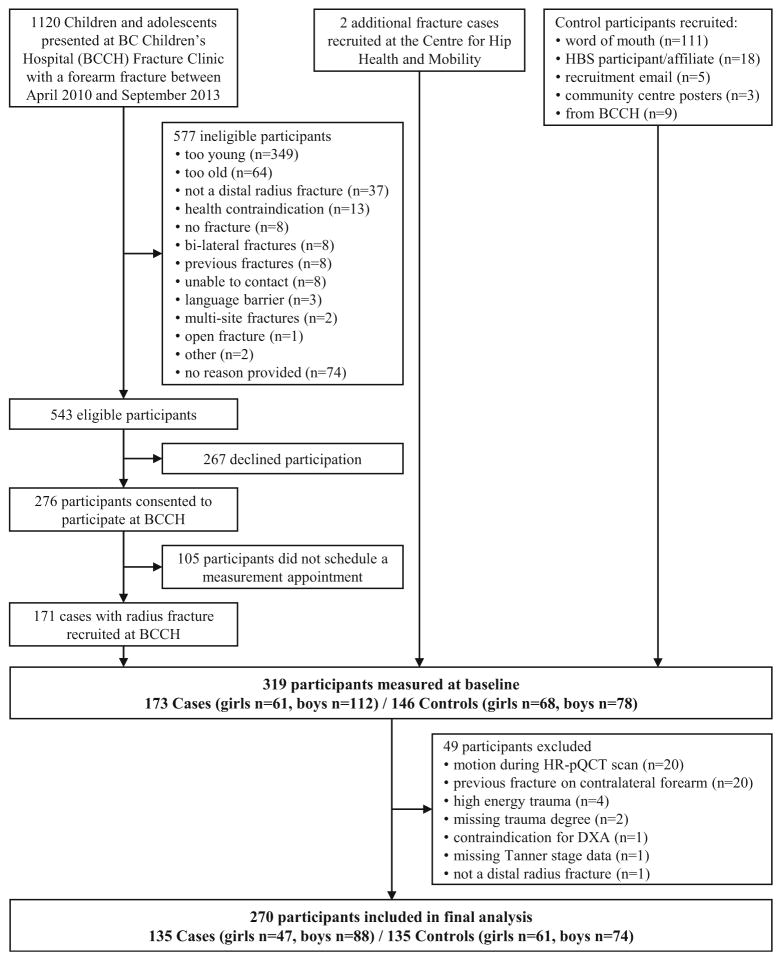
Between April 2010 and September 2013, 1120 children and adolescents presented to the BC Children’s Hospital (BCCH) Fracture Clinic with a forearm fracture (Fig. 1). We invited boys aged 9 to 15 years and girls aged 8 to 13 years who sustained a low- to moderate-energy distal radius fracture (with or without an ulnar fracture) and were otherwise healthy and could understand English to participate in our study. Prior to the start of each clinic, a research assistant reviewed patient charts to ascertain eligibility and confirmed potential participants with the attending orthopedic surgeon. The attending surgeon also reviewed patient radiographs to confirm all fractures. The research assistant then discussed the study with the patients and their families and obtained written informed consent from parents or guardians and participants 14 years of age and assent from participants younger than age 14.
Fig. 1.
Flow of participants through the study
Upon enrolment, the research assistant administered a fracture history questionnaire to participants and their parents/ guardians to assess the date, site, side, mode of injury, and fracture history. We excluded individuals with previous wrist fractures on the contralateral limb or bilateral wrist fractures (n=15). We categorized trauma degree as low, moderate, or high energy based on a modified version of Landin’s classification adapted from Clark et al. [18] (Table 1). Of the 543 eligible participants with recent fracture (cases), 267 declined participation and 105 consented to participate but did not schedule a measurement appointment. We recruited two additional fracture cases through word of mouth at the Centre for Hip Health and Mobility. Thus, we included 173 children and adolescents (61 girls, 112 boys; 32 % recruitment success) with a recent distal radius fracture in this analysis.
Table 1.
| Traumas related to low-energy fractures |
| Falling onto a non-resilient surface from standing height or less (<0.5 m) (e.g., from bed or couch) |
| Falling onto a resilient surface (e.g., rubber or sand) from 0.5 to 3 m |
| Injuries sustained during play including playground scuffles |
| Falling while moving at slow speed on a scooter, skateboard, skis, rollerblades, or skates (or similar) |
| Low-energy collision with a stationary or slowly moving object |
| Low-energy sport injuries (e.g., soccer, basketball, gymnastics) |
| Traumas related to moderate-energy fractures |
| Falling onto a non-resilient surface from 0.5 to 3 m (e.g., bunk bed, horseback) |
| Falling down stairs |
| Falling from a swing, slide, or monkey bars (or similar playground equipment) |
| Falling from a bicycle, falling while moving at moderate or fast speed on scooter, skateboard, skis, rollerblades, or skates (or similar) |
| Moderate-energy collision with a stationary or moving object while moving at moderate or fast speed (e.g., hockey, rugby) |
| Traumas related to high-energy fractures |
| Falling from a height exceeding 3 m |
| Traffic accidents |
| High-energy sport injuries |
| Being hit by a moving heavy object (>body weight) |
We used three methods to recruit our control group of same-age children and adolescents without a history of distal radius fracture. First, we asked fracture cases to distribute an information letter and consent form to any friends, siblings, or other relatives within the desired age range who had never sustained a forearm fracture. Second, we invited individuals from our previous Healthy Bones Study III [19] who met the inclusion criteria to participate. Third, we put up recruitment posters at local community centers and distributed recruitment emails at our research institute. Using these methods, we recruited 146 children and adolescents (68 girls, 78 boys) without a history of forearm fracture to serve as our comparison group (controls). Thus, our final study population included 319 children and adolescents (aged 8–16 years).
The University of British Columbia’s Clinical Research Ethics Board approved all study procedures, and we conducted the study in accordance with Declaration of Helsinki.
Anthropometry and body composition
We measured standing height (stretch stature) to the nearest 0.1 cm using a wall-mounted digital stadiometer (Seca Model 242; Seca, Hanover, MD, USA) and weight to the nearest 0.1 kg using an electronic scale (Seca Model 840; Seca). We then calculated body mass index (BMI, kg/m2). We measured non-dominant forearm girth (maximum circumference) and ulnar length (distance from the olecranon to the ulnar styloid process) to the nearest 0.1 cm. We used a Hologic QDR 4500 W bone densitometer (Hologic Inc, Waltham, MA, USA) with standard positioning for a total body scan to assess body composition (total body less head percent fat mass (% body fat) and bone mineral free lean mass (kg)). One of six technicians (all trained by the same International Society of Clinical Densitometry (ISCD)-certified technician) acquired scans and performed daily quality assurance scans. One of three trained technicians analyzed the DXA scans.
Health history, lifestyle and maturity
We administered a health history questionnaire to identify any medical condition(s) or use of medication(s) known to influence bone metabolism. We determined each participant’s ethnicity based on parents’ or grandparents’ place of birth [20]. We classified participants as “Asian” if both parents or three of four grandparents were born in East Asian (e.g., Hong Kong or China, Korea, Taiwan, Japan), Southeast Asian (e.g., Philippines, Vietnam), or South Asian (e.g., India) countries; non-Hispanic “white” if both parents or three of four grandparents were born in North America or Europe, or “other” if participants were of mixed ethnicity or if both parents or three of four grandparents were Hispanic, Oceanic, Black, or First Nations.
We assessed dietary calcium intake (mg/day) using a validated food frequency questionnaire [21] and leisure-time physical activity using a modified version of the Physical Activity Questionnaire for Children (PAQ-C) and Adolescents (PAQ-A) [22]. We calculated a general physical activity score (PA score) as an average of the PAQ-C/A items in a continuous range between 1 (low activity) and 5 (high activity). Participants self-reported their maturity status as per the method of Tanner [23]. Girls also completed a short questionnaire regarding age at menarche and use of oral contraceptives.
Muscle strength and balance
We assessed the maximum isometric grip strength (non-fractured arm in the fracture group; non-dominant arm in controls) using a hand-held grip dynamometer (Jamar Plus+ Digital Hand Dynamometer, Patterson Medical, Bolingbrook, IL, USA). Participants held the dynamometer at shoulder height with their elbow fully extended and arm parallel to the ground. The examiner then asked the participant to squeeze the dynamometer as hard as possible while lowering their arm in one smooth motion. Participants undertook three trials and we used the maximum grip strength value to the nearest 0.5 kg for analysis.
We also determined each participant’s balance using the balance subtest of the Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2) [24], which has been validated for use with children and adolescents in this age range [25]. The balance subtest involves nine tasks (seven static, two dynamic), three of which are performed first with eyes open, then with eyes closed. Example tasks include walking forward heel-to-toe and standing on preferred leg on a balance beam. We converted raw scores of each task to a point score using the BOT-2 Assist Software and then summed the point scores of each task to produce a total point score (range 0–37 points). We then adjusted scores for age and sex according to the manufacturer’s normative data (mean (SD) for the normative data 15 (5) points; range 1–35 points) [26].
High-resolution pQCT
We assessed bone microstructure at the 7 % site of the distal radius of non-fractured (cases) and non-dominant (controls) arm using HR-pQCT (XtremeCT, Scanco Medical, Brüttisellen, Switzerland; standard protocol 60 kVp, 900 μA, 100 ms integration time, 110 slices with an isotropic voxel size of 82 μm). The effective radiation dose for each HR-pQCTscan was 3 μSv. For fracture cases, we acquired the baseline scan within 3 months of plaster cast removal to minimize the effect of bone remodeling due to normal growth. The average time between injury and the HR-pQCT scan was 2.6(SD 0.9)months. We describe the scanning procedure in detail elsewhere [27]. Briefly, one of six technicians (all trained by an ISCD-certified technician) first immobilized each participant’s arm using an anatomically formed brace. The technician manually defined the scanning site using a 2D scout scan and placed the reference line at the end plate of the distal radius. Prior to analysis, the technician graded the quality of each scan [28], and scans with significant motion artifacts (grades 4 or 5) were excluded before analysis.
One of two trained technicians analyzed the HR-pQCT scans according to the manufacturer’s standard protocol [29, 30]. Briefly, a semiautomated segmentation method was used to trace the periosteal surface of radius. After this, a threshold-based algorithm separated the cortical and trabecular bone. With this standard morphologic analysis, we assessed a number of bone outcomes; total bone mineral density (Tt.BMD, mg HA/cm3), bone volume ratio (BV/TV), trabecular thickness (Tb.Th, mm), separation (Tb.Sp, mm), and number (Tb.N, mm−1). Additionally, we used an automated segmentation algorithm (Image Processing Language, Version 5.08b, Scanco Medical) [31, 32] to determine the total bone area (Tt.Ar, mm2) and cortical bone mineral density (Ct.BMD, mg HA/cm3), thickness (Ct.Th, mm), and porosity (Ct.Po, %). The short-term reproducibility in our lab is <3.8 % for all HR-pQCT standard analysis-derived parameters at the distal radius. Corresponding values for automated segmentation measures varied between 1.2 % (Ct.BMD) and 17.3 % (Ct.Po) [University of British Columbia Bone Health Research Group, unpublished data].
Finite element analysis
We used finite element analysis (FEA) to estimate participant-specific bone strength from HR-pQCT scans. We converted 3D HR-pQCT images to homogenous linear FE meshes using a voxel conversion approach and then solved the models using customized FE software (FEA software FAIM, Version 4.0) [33]. A uniaxial compression, corresponding to 1 % strain, was applied using a single homogeneous tissue modulus of 6829 MPa and a Poisson’s ratio of 0.3 to all elements [34]. The nodes at the top and bottom surfaces were unconstrained in x and y directions. FEA outcomes were failure load (Fload, N) and stiffness (kN/mm, calculated as a reaction force divided by an average bone cross-sectional area). Stiffness was used to evaluate the ultimate stress (UStress, MPa) [34]. We also calculated the load-to-strength ratio (Φ) as a ratio of the estimated fall load (fall load, N) applied to the outstretched arm after a fall from standing height [35] and FEA-based Fload.
Statistical analyses
We performed all analyses in Stata, version 10.0 (StataCorp, College Station, TX, USA). We conducted separate analyses for boys and girls due to known sex differences in the tempo and timing of maturation and growth [36]. We used parametric statistics as all data were normally distributed. First, we compared all descriptive outcomes (i.e., anthropometry, body composition, dietary calcium, physical activity, muscle strength, and balance) between fracture cases (low energy, moderate energy, and all fractures) and controls using a one-way ANOVA. For categorical outcomes (i.e., Tanner stage and ethnicity and fracture history), we used Pearson χ2 test. Second, to compare bone outcomes across groups, we fit multivariable linear regression models adjusting each bone variable for maturity, height, ethnicity, and % body fat. We adjusted for maturity and height due to the known influence of these variables on bone strength accrual [19, 37]. We adjusted for ethnicity as we previously documented ethnic differences in bone outcomes (by HR-pQCT) at the distal radius [20]. Finally, we adjusted for % body fat as it is known to be associated with forearm fractures in girls and boys [10, 11].
Third, to determine the association between fracture and bone microstructure, density, and strength, we fit logistic regression models for each bone outcome. For each model, we forced in Tanner stage to adjust for maturation and used a stepwise method to remove additional covariates (of those listed above) from the model if significance level was over 0.10. For each model, we report the odds ratio (OR) and the 95 % confidence interval (CI). We calculated receiver operator characteristics (ROC) area under the curve (AUC) based on logistic regression models to assess the ability of bone outcomes (by HR-pQCT) to discriminate between fracture cases and controls. We report AUC values for the maturity-adjusted model as well as for the final model where all covariates (determined using a stepwise method) were included in the regression model in addition to each HR-pQCT parameter. We set statistical significance at p<0.05 (two-tailed) for all tests.
Results
We excluded 49 participants (15.4 %) from our analysis for the following reasons: motion during HR-pQCT scan (n=20), previous wrist fracture on contralateral side that was not documented prior to enrolment (n=20), misclassification of trauma type or missing trauma degree (n=6), missing Tanner stage (participant chose not to answer, n=1), missing body composition data (DXA scan was contraindicated, n=1), and misclassification of fracture site (n=1). Therefore, we included 270 participants (girls n=108; cases n=47, controls n=61 and boys n=162; cases n=88, controls n=74) in our analysis.
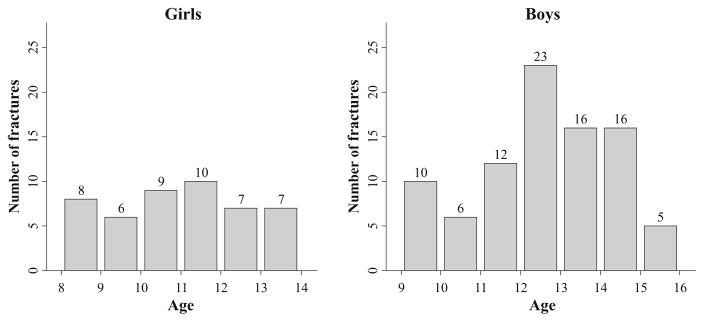
In the fracture cases, fracture prevalence was similar across the age range in girls. In boys, fracture prevalence was highest among 12-year-olds (Fig. 2). In both sexes, the majority of fractures occurred at the left radius (girls 57 %, boys 59 %), which was the non-dominant arm in 55 and 61 % of girls and boys, respectively. Thirty-eight percent of fractures were classified as low energy and 63 % as moderate energy. The distribution of low- and moderate-energy fractures did not differ between girls and boys. In girls, 23 % of fractures occurred during playground activities (compared with 7 % in boys), whereas in boys, the majority of fractures occurred during participation in team sports (47 % compared with 15 % in girls). In girls and boys, respectively, bicycle accidents accounted for 9 and 11 % of fractures; skiing and snowboarding 9 and 13 % of fractures; and skateboarding, scooter, and rollerblade accidents 9 and 8 % of fractures. Almost one quarter of our cases had previously broken bones: 8 girls and 18 boys had one previous fracture, 1 girl and 2 boys had two previous fractures, and 1 girl and 2 boys had three previous fractures (Table 2).
Fig. 2.
Prevalence of fractures across age (years) in girls (left) and boys (right)
Table 2.
Descriptive, anthropometric, body composition, physical activity, and dietary characteristics in non-fracture controls, low- and moderate-energy fracture cases, and all fracture cases. Values are mean (SD) unless otherwise indicated
| Controls | Low energy | Moderate energy | All cases | p valuea | p valueb | p valuec | |
|---|---|---|---|---|---|---|---|
| Girls | |||||||
| Number of participants (n) | 61 | 22 | 25 | 47 | |||
| Age (years) | 11.0 (1.8) | 11.2 (1.8) | 10.8 (1.6) | 11.0 (1.7) | 0.577 | 0.620 | 0.990 |
| Ethnicity (Asian/White/other, n) | 7/43/11 | 0/18/4 | 5/16/4 | 5/34/8 | 0.246 | 0.584 | 0.978 |
| Height (cm) | 144.9 (11.9) | 152.5 (11.2) | 145.4 (11.4) | 148.7 (11.7) | 0.010 | 0.839 | 0.096 |
| Ulnar length (mm) | 228 (22) | 235 (20) | 228 (18) | 231 (19) | 0.198 | 0.957 | 0.461 |
| Weight (kg) | 39.9 (13.5) | 44.6 (8.9) | 39.6 (8.7) | 41.9 (9.1) | 0.111 | 0.914 | 0.376 |
| BMI (kg/m2) | 18.5 (3.5) | 19.1 (2.6) | 18.6 (2.7) | 18.8 (2.6) | 0.481 | 0.942 | 0.637 |
| Forearm girth (cm) | 20.4 (2.3) | 21.3 (1.3) | 20.5 (1.8) | 20.9 (1.6) | 0.082 | 0.821 | 0.235 |
| Body fat (%) | 26.1 (7.3) | 27.9 (7.9) | 28.7 (7.7) | 28.3 (7.7) | 0.339 | 0.150 | 0.130 |
| Lean mass (kg) | 25.5 (7.8) | 28.5 (5.9) | 24.7 (5.5) | 26.5 (5.9) | 0.088 | 0.607 | 0.493 |
| Tanner stage (stage I/II/III/IV/V, n) | 20/21/17/2/1 | 5/9/6/2/0 | 9/10/5/1/0 | 14/19/11/3/0 | 0.694 | 0.899 | 0.761 |
| Menarche (yes/no, n) | 15/46 | 3/19 | 2/23 | 5/42 | 0.285 | 0.079 | 0.064 |
| Age at menarche (years) | 12.4 (0.6) | 11.9 (0.5) | 12.8 (0.9) | 12.3 (0.8) | 0.180 | 0.510 | 0.548 |
| Previous fracture (yes/no, n) | 3/58 | 4/18 | 6/19 | 10/37 | 0.055 | 0.009 | 0.010 |
| Dietary calcium intake (mg/day) | 899 (536) | 823 (406) | 1097 (660) | 969 (567) | 0.572 | 0.129 | 0.517 |
| Physical activity score | 2.9 (0.6) | 2.7 (0.6) | 2.8 (0.6) | 2.7 (0.6) | 0.194 | 0.608 | 0.657 |
| BOT-2 balance score | 13.3 (4.6) | 11.5 (3.4) | 13.0 (4.8) | 12.3 (4.3) | 0.106 | 0.769 | 0.245 |
| Grip strength (kg) | 16.3 (5.3) | 18.5 (6.8) | 16.2 (4.4) | 17.2 (5.7) | 0.121 | 0.884 | 0.404 |
| Boys | |||||||
| Number of participants (n) | 74 | 29 | 59 | 88 | |||
| Age (years) | 12.7 (1.8) | 12.4 (1.7) | 12.8 (1.7) | 12.6 (1.7) | 0.397 | 0.782 | 0.855 |
| Ethnicity (Asian/White/other, n) | 10/52/12 | 10/14/5 | 13/39/7 | 23/53/12 | 0.043 | 0.387 | 0.139 |
| Height (cm) | 158.7 (14.0) | 156.5 (11.6) | 157.7 (12.1) | 157.3 (11.9) | 0.443 | 0.661 | 0.498 |
| Ulnar length (mm) | 254 (26) | 250 (19) | 254 (22) | 252 (21) | 0.414 | 0.885 | 0.629 |
| Weight (kg) | 49.6 (13.3) | 49.0 (11.9) | 48.2 (11.8) | 48.5 (11.8) | 0.838 | 0.540 | 0.582 |
| BMI (kg/m2) | 19.4 (3.2) | 19.9 (3.6) | 19.2 (2.9) | 19.4 (3.2) | 0.437 | 0.689 | 0.953 |
| Forearm girth (cm) | 22.5 (2.5) | 22.2 (1.8) | 22.3 (2.0) | 22.3 (1.9) | 0.507 | 0.540 | 0.447 |
| Body fat (%) | 20.3 (8.2) | 24.7 (11.5) | 21.8 (8.9) | 22.8 (9.8) | 0.028 | 0.360 | 0.093 |
| Lean mass (kg) | 35.1 (10.4) | 32.4 (7.5) | 33.3 (8.7) | 33.0 (8.2) | 0.177 | 0.262 | 0.146 |
| Tanner stage (stage I/II/III/IV/V, n) | 11/23/12/24/4 | 6/9/9/5/0 | 9/16/16/18/0 | 15/25/25/23/0 | 0.203 | 0.262 | 0.087 |
| Previous fracture (yes/no, n) | 7/67 | 6/23 | 16/43 | 22/66 | 0.123 | 0.007 | 0.010 |
| Dietary calcium intake (mg/day) | 1296 (739) | 1078 (587) | 1144 (640) | 1122 (620) | 0.143 | 0.201 | 0.105 |
| Physical activity score | 2.5 (0.5) | 2.3 (0.5) | 2.4 (0.5) | 2.4 (0.5) | 0.090 | 0.109 | 0.049 |
| BOT-2 balance score | 12.9 (3.9) | 10.9 (3.8) | 12.4 (3.9) | 11.9 (3.9) | 0.021 | 0.426 | 0.101 |
| Grip strength (kg) | 24.4 (8.5) | 22.2 (5.6) | 23.5 (7.0) | 23.1 (6.5) | 0.182 | 0.489 | 0.260 |
BMI body mass index, BOT-2 Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition
Controls vs. low-energy cases
Controls vs. moderate-energy cases
Controls vs. all cases
We provide descriptive characteristics of the cohort in Table 2. In girls, those with low-energy fractures were taller (mean difference; 95 % CI 7.6; 1.9–13.3 cm) compared with controls. Regardless of fracture type, fracture cases had 6.1-times more previous fractures than controls. All other descriptive outcomes were similar between fracture groups and controls.
In boys, ethnic distribution differed between the low-energy fracture cases and controls such that the proportion of white participants was smaller in the low-energy fracture group compared with controls. In addition, boys with low-energy fractures had higher % body fat (4.4 %, 0.3–9.1 %) than non-fracture controls and scored significantly lower on the balance test compared with controls (−2.0; −3.6–−0.3). Fractured boys, regardless of trauma type, had a lower physical activity score compared with controls (−0.2; −0.4–−0.02). Similar to girls, boys with fracture (all cases) had 3.5-times more previous fractures than controls.
Bone outcomes in girls
We summarize adjusted bone outcomes in fracture cases and controls in Table 3 and present results of the logistic regression in Table 4. In girls, low-energy fracture cases had lower Fload (−22.9 %) and UStress (−39.9 %) and higher fall load (2.1 %) and load-to-strength ratio (17.6 %) compared with non-fracture controls. In the trabecular compartment, girls with low-energy fractures had significantly lower Tt.BMD (−14.5 %), BV/TV (−10.4 %), and Tb.Th (−11.1 %) compared with controls. In the cortical compartment, girls with low-energy fractures also had lower Ct.BMD (−9.8 %) and Ct.Th (−21.1 %) than control girls. When we considered all fracture cases together, we observed similar trends, but the deficits in bone strength, microstructure, and BMD compared with controls were of smaller magnitude. However, we did not observe any differences in bone strength, microstructure, or BMD between girls with moderate-energy fractures and non-fracture controls.
Table 3.
Bone parameters of the distal radius in non-fractured controls and low- and moderate-energy fracture cases and all fracture cases. Values are mean (95 % CI) adjusted for maturity (Tanner stage), height, ethnicity, and % body fat
| Controls | Low energy | Moderate energy | All cases | p valueb | p valuec | p valued | |
|---|---|---|---|---|---|---|---|
| Girls | n=61 | n=22 | n=25 | n=47 | |||
| FLoad (N) | 1189 (1121, 1257) | 945 (829, 1060) | 1074 (968, 1180) | 1015 (937, 1094) | 0.002 | 0.113 | 0.005 |
| UStress (MPa) | 20.7 (18.9, 22.5) | 13.8 (10.8, 16.9) | 18.4 (15.6, 21.2) | 16.3 (14.2, 18.4) | 0.001 | 0.151 | 0.003 |
| Fall load (N)a | 2523 (2507, 2539) | 2576 (2550, 2603) | 2543 (2519, 2568) | 2559 (2540, 2577) | 0.001 | 0.091 | 0.002 |
| Load-to-strength ratio | 2.35 (2.20, 2.49) | 2.80 (2.55, 3.05) | 2.55 (2.32, 2.77) | 2.66 (2.49, 2.83) | 0.006 | 0.204 | 0.017 |
| Tt.Ar (mm2) | 203.4 (196.3, 210.5) | 215.4 (203.3, 227.5) | 199.8 (188.8, 210.8) | 206.9 (198.6, 215.1) | 0.081 | 0.806 | 0.411 |
| Tt.BMD (mg HA/cm3) | 232.1 (223.2, 241.0) | 200.8 (185.7, 215.9) | 220.7 (206.9, 234.5) | 211.6 (201.3, 222.0) | 0.001 | 0.142 | 0.005 |
| BV/TV | 0.142 (0.137, 0.148) | 0.128 (0.119, 0.137) | 0.135 (0.126, 0.144) | 0.132 (0.125, 0.138) | 0.032 | 0.220 | 0.042 |
| Tb.Th (mm) | 0.068 (0.066, 0.070) | 0.061 (0.057, 0.065) | 0.065 (0.062, 0.069) | 0.063 (0.061, 0.066) | 0.006 | 0.175 | 0.015 |
| Tb.Sp (mm) | 0.416 (0.404, 0.429) | 0.419 (0.398, 0.441) | 0.424 (0.404, 0.443) | 0.422 (0.407, 0.436) | 0.786 | 0.535 | 0.568 |
| Tb.N (mm−1) | 2.09 (2.04, 2.14) | 2.09 (2.01, 2.18) | 2.07 (1.99, 2.15) | 2.08 (2.02, 2.14) | 0.996 | 0.705 | 0.802 |
| Ct.BMD (mg HA/cm3) | 572.0 (556.7, 587.2) | 518.7 (492.7, 544.6) | 559.7 (536.0, 583.4) | 541.0 (523.2, 558.9) | 0.001 | 0.264 | 0.008 |
| Ct.Th (mm) | 0.54 (0.52, 0.57) | 0.44 (0.39, 0.49) | 0.51 (0.47, 0.55) | 0.48 (0.45, 0.51) | <0.001 | 0.101 | 0.001 |
| Ct.Po (%) | 3.53 (3.21, 3.84) | 3.07 (2.54, 3.61) | 3.39 (2.90, 3.88) | 3.25 (2.89, 3.60) | 0.145 | 0.746 | 0.296 |
| Boys | n=74 | n=29 | n=59 | n=88 | |||
| FLoad (N) | 1579 (1484, 1674) | 1417 (1264, 1570) | 1435 (1330, 1541) | 1429 (1343, 1516) | 0.080 | 0.101 | 0.047 |
| UStress (MPa) | 22.2 (20.4, 24.1) | 19.5 (16.5, 22.4) | 21.0 (18.9, 23.0) | 20.5 (18.8, 22.2) | 0.041 | 0.314 | 0.101 |
| Fall load (N)a | 2633 (2616, 2650) | 2646 (2618, 2673) | 2633 (2614, 2652) | 2637 (2622, 2653) | 0.309 | 0.934 | 0.617 |
| Load-to-strength ratio | 1.87 (1.75, 2.00) | 2.03 (1.84, 2.23) | 1.95 (1.82, 2.09) | 1.98 (1.87, 2.09) | 0.166 | 0.562 | 0.303 |
| Tt.Ar (mm2) | 254.4 (245.4, 263.4) | 255.1 (240.6, 269.5) | 245.3 (235.3, 255.3) | 248.4 (240.2, 256.7) | 0.599 | 0.282 | 0.557 |
| Tt.BMD (mg HA/cm3) | 244.8 (235.4, 254.1) | 231.0 (216.0, 246.1) | 236.4 (226.1, 246.8) | 234.7 (226.2, 243.2) | 0.076 | 0.386 | 0.158 |
| BV/TV | 0.154 (0.148, 0.160) | 0.149 (0.140, 0.159) | 0.148 (0.141, 0.155) | 0.148 (0.143, 0.154) | 0.280 | 0.220 | 0.164 |
| Tb.Th (mm) | 0.072 (0.069, 0.074) | 0.069 (0.065, 0.072) | 0.069 (0.066, 0.071) | 0.069 (0.067, 0.071) | 0.136 | 0.135 | 0.077 |
| Tb.Sp (mm) | 0.398 (0.387, 0.410) | 0.395 (0.377, 0.414) | 0.401 (0.388, 0.413) | 0.399 (0.389, 0.409) | 0.891 | 0.704 | 0.729 |
| Tb.N (mm−1) | 2.15 (2.11, 2.20) | 2.18 (2.10, 2.25) | 2.15 (2.10, 2.21) | 2.16 (2.12, 2.20) | 0.874 | 0.918 | 0.992 |
| Ct.BMD (mg HA/cm3) | 583.0 (570.0, 596.0) | 559.7 (538.8, 580.7) | 578.6 (564.2, 593.1) | 572.6 (560.6, 584.5) | 0.072 | 0.978 | 0.455 |
| Ct.Th (mm) | 0.60 (0.57, 0.64) | 0.54 (0.49, 0.59) | 0.57 (0.54, 0.61) | 0.56 (0.53, 0.59) | 0.037 | 0.442 | 0.142 |
| Ct.Po (%) | 4.59 (4.25, 4.94) | 4.70 (4.14, 5.26) | 4.03 (3.65, 4.42) | 4.25 (3.93, 4.57) | 0.954 | 0.029 | 0.096 |
Girls: n=108; controls n=61, low-energy fracture cases n=22, moderate-energy fracture cases n=25; boys: n=162; controls n=74, low-energy fracture cases n=29, moderate-energy fracture cases n=59
FLoad failure load, UStress ultimate stress, fall load estimated fall load, Tt.Ar total bone area, Tt.BMD total bone mineral density, BV/TV bone volume fraction, Tb.Th trabecular thickness, Tb.Sp trabecular separation, Tb.n trabecular number, Ct.BMD cortical BMD, Ct.Th cortical thickness, Ct.Po cortical porosity
Fall load not adjusted for height
Maturity, height, ethnicity, and % body fat adjusted difference between controls vs. low-energy cases
Maturity, height, ethnicity, and % body fat adjusted difference between controls vs. moderate-energy cases
Maturity, height, ethnicity, and % body fat adjusted difference between controls vs. all cases
Table 4.
Odds ratios (OR) and 95 % confidence intervals (CI) for the association between fracture and HR-pQCT bone outcomes at the distal radius in girls and boys. Odds ratios are presented as per one SD decrease in each bone outcome
| Low-energy fracture vs controls
|
Moderate-energy fracture vs controls
|
All cases vs controls
|
||||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p | OR (95 % CI) | p | OR (95 % CI) | p | |
| Girls | ||||||
| FLoad | 4.30 (1.60, 11.52) | 0.004 | 2.06 (0.92, 4.63) | 0.079 | 2.63 (1.33, 5.20) | 0.005 |
| UStress | 3.60 (1.54, 8.39) | 0.003 | 1.52 (0.84, 2.77) | 0.166 | 2.10 (1.23, 3.59) | 0.006 |
| Fall loada | 0.23 (0.09, 0.61) | 0.003 | 0.45 (0.17, 1.21) | 0.113 | 0.34 (0.16, 0.70) | 0.003 |
| Load-to-strength ratio | 0.36 (0.16, 0.80) | 0.012 | 0.64 (0.35, 1.16) | 0.139 | 0.51 (0.30, 0.88) | 0.015 |
| Tt.Ar | 0.52 (0.22, 1.21) | 0.128 | 1.30 (0.60, 2.84) | 0.506 | 0.76 (0.40, 1.43) | 0.394 |
| Tt.BMD | 3.61 (1.54, 8.49) | 0.003 | 1.63 (0.88, 3.00) | 0.120 | 2.18 (1.27, 3.75) | 0.005 |
| BV/TV | 1.96 (1.02, 3.75) | 0.042 | 1.45 (0.85, 2.48) | 0.173 | 1.67 (1.05, 2.66) | 0.031 |
| Tb.Th | 2.41 (1.15, 5.07) | 0.020 | 1.63 (0.88, 3.01) | 0.122 | 1.88 (1.13, 3.13) | 0.015 |
| Tb.Sp | 0.96 (0.53, 1.73) | 0.896 | 0.79 (0.48, 1.29) | 0.349 | 0.87 (0.56, 1.34) | 0.522 |
| Tb.N | 0.95 (0.53, 1.71) | 0.862 | 1.22 (0.74, 2.03) | 0.439 | 1.08 (0.68, 1.69) | 0.755 |
| Ct.BMD | 4.10 (1.64, 10.27) | 0.003 | 1.37 (0.72, 2.62) | 0.336 | 2.12 (1.19, 3.75) | 0.010 |
| Ct.Th | 7.04 (2.20, 22.48) | 0.001 | 1.83 (0.85, 3.95) | 0.121 | 2.77 (1.43, 5.35) | 0.002 |
| Ct.Po | 1.56 (0.85, 2.89) | 0.153 | 1.25 (0.74, 2.11) | 0.412 | 1.34 (0.85, 2.10) | 0.208 |
| Boys | ||||||
| FLoad | 1.76 (0.96, 3.20) | 0.066 | 1.38 (0.90, 2.12) | 0.144 | 1.34 (0.92, 1.95) | 0.128 |
| UStress | 1.62 (0.95, 2.76) | 0.077 | 1.08 (0.75, 1.57) | 0.666 | 1.15 (0.82, 1.60) | 0.425 |
| Fall loada | 0.82 (0.44, 1.53) | 0.532 | 1.03 (0.61, 1.73) | 0.912 | 0.97 (0.62, 1.54) | 0.913 |
| Load-to-strength ratio | 0.83 (0.53, 1.31) | 0.431 | 0.88 (0.59, 1.33) | 0.548 | 0.85 (0.60, 1.22) | 0.385 |
| Tt.Ar | 0.84 (0.47, 1.50) | 0.564 | 1.31 (0.84, 2.05) | 0.238 | 1.17 (0.78, 1.74) | 0.442 |
| Tt.BMD | 1.81 (1.02, 3.22) | 0.044 | 1.12 (0.77, 1.63) | 0.543 | 1.18 (0.84, 1.66) | 0.350 |
| BV/TV | 1.49 (0.88, 2.50) | 0.134 | 1.26 (0.86, 1.84) | 0.236 | 1.22 (0.87, 1.72) | 0.241 |
| Tb.Th | 1.52 (0.89, 2.57) | 0.123 | 1.40 (0.92, 2.13) | 0.116 | 1.36 (0.94, 1.97) | 0.100 |
| Tb.Sp | 0.92 (0.55, 1.53) | 0.746 | 0.96 (0.67, 1.37) | 0.814 | 0.98 (0.71, 1.36) | 0.913 |
| Tb.N | 1.03 (0.62, 1.70) | 0.923 | 0.99 (0.69, 1.42) | 0.969 | 0.97 (0.70, 1.34) | 0.843 |
| Ct.BMD | 1.50 (0.88, 2.56) | 0.134 | 0.94 (0.66, 1.32) | 0.706 | 1.02 (0.74, 1.41) | 0.900 |
| Ct.Th | 1.82 (0.98, 3.36) | 0.058 | 1.10 (0.75, 1.61) | 0.626 | 1.18 (0.83, 1.68) | 0.350 |
| Ct.Po | 0.97 (0.60, 1.55) | 0.884 | 1.69 (1.08, 2.64) | 0.023 | 1.35 (0.94, 1.95) | 0.108 |
Girls: n=107; controls n=60, low-energy fracture cases n=22, moderate-energy fracture cases n=25; boys: n=158; controls n=70, low-energy fracture cases n=29, moderate-energy fracture cases n=59. Covariate odds ratio (OR) reported for one SD decrease
CI confidence interval, FLoad failure load, UStress ultimate stress, fall load estimated fall load, Tt.Ar total bone area, Tt.BMD total bone mineral density, BV/TV bone volume fraction, Tb.Th trabecular thickness, Tb.Sp trabecular separation, Tb.n trabecular number, Ct.BMD cortical BMD, Ct.Th cortical thickness, Ct.Po cortical porosity
Fall load not adjusted for height
In girls, all FEA measures of bone strength were associated with greater odds of fracture; lower Fload and UStress and higher fall load and load-to-strength ratio were associated with higher fracture risk (ORs 2.8–4.3). In addition, deficits in Tt.BMD, BV/TV, Tb.Th, Ct.BMD, and Ct.Th were significantly associated with forearm fracture (ORs 2.0–7.0). In girls, the maturity-adjusted AUC ranged from 0.58 to 0.74 when we considered only low-energy cases and controls and from 0.55 to 0.65 when including both fracture types. In the final regression models, the AUC ranged from 0.73 to 0.85 between both low energy cases and controls and all cases and controls.
Bone outcomes in boys
In boys, we observed lower UStress in low-energy fracture cases compared with non-fractured controls (−13.3 %) and lower FLoad in all fracture cases compared with controls (−10.0 %). In the cortical compartment, Ct.Th was lower (−11.2 %) among boys with low-energy fracture compared with non-fractured controls. In addition, boys with moderate-energy fracture had lower Ct.Po (−13.0 %) compared with controls. In the logistic regression analysis, none of the FEA parameters were associated with forearm fracture risk. However, lower Tt.BMD was associated with greater odds of low-energy fracture and lower cortical porosity was associated with greater odds of moderate-energy fracture. Maturity-adjusted AUC ranged from 0.62 to 0.65 when considering only low-energy cases and controls and from 0.57 to 0.61 when including low- and moderate-energy cases. AUC for the final models varied between 0.68 and 0.74 with low-energy fracture boys and controls and between 0.57 and 0.61 when comparing all fracture cases to controls.
Discussion
In this study, we advanced the current literature by examining differences in bone strength, BMD, and macro- and microstructure at the distal radius among Canadian girls and boys with and without a history of forearm fracture. We used state-of-the-art HR-pQCT technology to look beyond standard measures of bone mass to obtain a better understanding of skeletal deficits associated with forearm fracture risk during growth. Our findings suggest potential sex differences in risk factors for forearm fracture such that in girls, fractures (specifically those due to low-energy trauma) are associated with deficits in distal radius bone strength, BMD, and microstructure, whereas in boys, modifiable factors including poor balance, low levels of physical activity, and excess body fat may be associated with forearm fractures in addition to deficits in BMD.
Risk factors for forearm fractures in girls
The deficits in bone strength we observed in girls with low-energy fractures are similar to those previously reported by Farr and colleagues in their cross-sectional study of 223 children and adolescents aged 8–15 years with and without a distal forearm fracture [15]. In both studies, the load-to-strength ratio, an indicator of the biomechanical fracture threshold [38], was higher in girls with low-energy fractures compared with controls, whereas no differences were observed between girls with moderate-energy fractures and controls. Together, these findings support the notion that, in girls, the etiology of distal forearm fractures during growth differs according to trauma type [15]; skeletal deficits result in compromised bone strength that is unable to sustain loads associated with low-energy trauma, whereas forces related to moderate-energy trauma exceed the maximum load that normal (healthy) bone strength can withstand.
Compromised bone strength in girls with low-energy fractures was likely influenced by deficits in both cortical and trabecular bone quality. Specifically, a thinner, less dense cortex combined with thinner trabeculae (and associated lower trabecular bone volume, BV/TV) was associated with low-energy forearm fracture in girls. With the exception of the difference in cortical BMD observed in the present study, our results again align well with those of Farr and colleagues [15]. In particular, it appears that the magnitude of cortical thinning, which is believed to occur as a result of normal linear bone growth [39], may be larger in girls with low-trauma fractures compared with their non-fractured peers. During peak adolescent growth, the distal metaphysis contributes approximately 90 % to linear growth of the radius [40]. Shaping of the metaphyseal cortex that occurs during this period is associated with high rates of periosteal resorption and endocortical apposition [39]. However, as was estimated from cross-sectional pQCT data of the distal radius in 337 healthy children and adolescents (aged 6 to 18 years) [39], metaphyseal cortical thickness fails to increase to the extent needed during peak growth to ensure bone strength is adapted to mechanical challenges associated with a fall on an outstretched hand. Thus, this temporary cortical weakness may have occurred to a greater degree in girls who sustained a low-energy fracture compared with their non-fracture peers. Alternatively, as girls with low-energy fractures were taller compared with non-fractured controls (and girls with moderate-energy fractures), it may be that we captured these girls as they entered their period of peak linear growth. Prospective studies are needed to more accurately describe changes in cortical thickness during adolescent growth and how they influence fracture risk.
Interestingly, the lower cortical BMD we observed in girls with low-energy fractures was not associated with higher cortical porosity. As described by Parfitt [3], increased intracortical remodeling and a high demand for calcium during rapid pubertal growth leads to a transient increase in cortical porosity at the metaphysis of long bones and an associated decrease in bone strength. Further, timing of this transient period of increased porosity corresponds to peak incidence of distal forearm fractures in girls and boys [37]. However, as porosity was not associated with fracture risk in girls in the present study, or in the previous study by Farr and colleagues [15], it may be that this phenomenon occurs to a similar extent in all adolescents and is not an underlying risk factor for forearm fracture. It is also possible, however, that the resolution of HR-pQCT is insufficient to quantify small pores (<82 μm) that may be prevalent in children and adolescents.
We note that in girls, modifiable factors (e.g., % body fat, physical activity, and balance) did not discriminate between fracture cases and controls. Although poor balance was not a significant predictor of fracture in girls, we observed a trend for lower balance scores in girls with low-energy fractures compared with controls. Future trials may need to consider the greater variability in girls’ balance scores (compared with boys) in the design phase.
Risk factors for forearm fractures in boys
Whereas our findings in girls aligned well with the work of Farr and colleagues [15], they differed when we examined risk factors for fracture in boys. In a US study, boys with mild-energy trauma fractures demonstrated similar skeletal deficits to girls with fractures—low bone strength, smaller cortical area, a thinner cortex, lower trabecular number, and larger trabecular separation. Although we found lower UStress in low-energy fracture cases compared with non-fractured controls and lower Fload in boys with a fracture compared with non-fractured controls—outcomes that might have been driven by the thinner cortices observed in low-energy cases compared with controls—neither of these outcomes discriminated cases from controls in the logistic regression analysis. In fact, boys with low-energy fractures in our study demonstrated lower total BMD (only) compared with non-fracture controls. There are several possible explanations for the discrepancy in findings. First, our non-fracture control group included seven boys who reported a history of fracture at skeletal sites other than the distal radius. As current evidence suggests, children with a history of fracture at any site have systemic deficits in bone (mass) [10–12], inclusion of these participants in our analysis may have limited our ability to detect differences between fracture cases and controls. However, we note that when we performed the analysis with these participants excluded, the results did not differ (data not shown). Second, we observed considerably lower Fload and higher load-to-strength ratio in our study compared with those reported by Farr and colleagues [15]. This is attributed to differences in finite element analysis parameters between the two studies. Whereas we used a Young’s modulus of 6829 MPa as suggested by MacNeil and Boyd [34], Farr et al. used a modulus of 10 GPa and derived failure load from scaled models. This highlights the need to standardize HR-pQCT analysis protocols in pediatric studies. Third, we noted significant differences in several important modifiable factors between boys with and without low-energy fractures that were not observed in the Rochester sample. Specifically, boys with low-energy fractures had excess body fat and poor balance compared with their non-fracture peers. Overweight status was associated with a 33 % greater risk for forearm fracture in children and adolescents aged 3 to 19 years [11]. In this same cohort, overweight boys had impaired balance compared to their healthy weight peers, regardless of fracture history [25]. Poor balance may, in turn, lead to unintentional falls, which cause almost 50 % of all pediatric injury hospitalizations among children younger than 15 years of age [41]. Unfortunately, we were unable to determine whether excess fat mass in boys with low trauma fractures translated into a greater fall load, as our estimate of fall load incorporates only standing height [35]. Further study is needed to develop an estimation of fall load that accounts for higher loads associated with greater body weight due to excess fat mass.
Finally, we noted that boys with previous forearm fracture, regardless of trauma type, reported lower levels of physical activity than boys without fracture. When interpreted in the context of their body composition, it may be that although the majority of fractures in boys were sustained during participation in team sports, the generally lower levels of physical activity in this group may have influenced the accumulation of excess body fat. Together, these findings paint a picture of a group of boys who are generally less active, are more likely to be overweight, and may be at increased risk for falls and associated injuries.
Discriminative ability of HR-pQCT
Although HR-pQCT is currently used only as a research tool, there is interest in whether this 3D imaging device can be used as a diagnostic tool in clinical settings. AUC values in the present study (0.61 to 0.85) were slightly higher than those reported for pQCT-derived measures of BMD, bone geometry, and estimated bone strength as well as DXA-derived aBMD (0.63 to 0.65) [12]. Further, although we observed relatively high odds ratios (in girls), it should be noted that odds ratios are used to characterize population variation in risk and are not suited for evaluating classification accuracy at the individual level [42]. It would be of some interest in future studies to prospectively address the true predictive power of bone outcomes by HR-pQCT and the predictive ability of HR-pQCT outcomes compared with standard clinical measures of aBMD.
Limitations
We note several limitations of our study. First, due to the cross-sectional and retrospective (HR-pQCT scan acquired within 3 months of plaster removal) nature of the study, we are not able to calculate actual risk of forearm fractures. Second, although we accounted for ethnicity and maturity status in our analysis, we were not powered to evaluate whether associations between bone outcomes and fracture varied across ethnic or maturity groups. Maturity status affects cortical and trabecular bone macro- and microstructure across pubertal stages in both sexes [19], and thus, its effect on fracture risk should be examined in future trials. Third, we used self-report questionnaires to determine details of the fracture event, fracture history, physical activity, and dietary calcium intake. Thus, we acknowledge the potential influence of recall bias and note that in some cases we obtained limited information on fracture mechanism. Fourth, we focused our analysis of bone outcomes at the distal radius and did not assess bone mass or estimate bone strength at other clinically relevant sites. It is not clear, therefore, whether the skeletal deficits we observed, particularly in girls, are systemic, as reported in previous studies [10–12]. Additionally, the bone strength analyses were based on uniaxial compression, which may not accurately characterize the loading conditions during a fall on an outstretched hand [9]. Further study is required to improve the FEA to account for these different loading conditions. Also, FEA outcomes have only been validated in cadaver bone from older adults. Finally, due to limited recruitment success (32 %), our results may be subject to recruitment bias.
Conclusion
In summary, our findings suggest that skeletal deficits underpinning forearm fractures during growth may be sex-specific. In girls, low-energy forearm fractures are associated with compromised bone strength at the distal radius, whereas in boys, modifiable factors such as body composition, balance, and physical activity in addition to low BMD may play an important role in determining fracture risk. Interventions designed to target and ameliorate these risk factors support children’s overall health; our data suggest that these interventions may also counter a child’s predisposition to fracture. Long-term prospective studies and purposely designed intervention studies are needed to i) better characterize utility of HR-pQCT to assess fracture risk during growth, ii) determine whether risk factors for forearm fractures in girls and boys track across the key period of adolescent growth and into adulthood, and iii) whether interventions can offset risk factors and prevent fractures, in the future.
Acknowledgments
We greatly appreciate the valuable contributions of the participants and their families to our study. We are grateful to the research assistants from the Orthopedic Research Lab at BC Children’s Hospital (Rebecca Atkinson, Clint Hazen, and Michael Nitikman) for their assistance with participant recruitment; our research coordinator, Douglas Race, for his tireless efforts in contacting and scheduling participants and managing data collection; Danmei Liu, operations manager at the Centre for Hip Health and Mobility, for supervising all bone imaging and the imaging technicians and operations staff at the Centre for Hip Health and Mobility for their help with data collection. We received funding from the Canadian Institutes of Health Research (OBM-101391) for this study.
Footnotes
Conflicts of interest None.
Contributor Information
M. Määttä, Department of Orthopaedics, University of British Columbia, 3114-910 West 10th Avenue, Vancouver, BC V5Z 1M9, Canada. Centre for Hip Health and Mobility, Vancouver Coastal Health Research Institute, 2635 Laurel Street, Vancouver, BC V5Z 1M9, Canada
H. M. Macdonald, Department of Orthopaedics, University of British Columbia, 3114-910 West 10th Avenue, Vancouver, BC V5Z 1M9, Canada. Centre for Hip Health and Mobility, Vancouver Coastal Health Research Institute, 2635 Laurel Street, Vancouver, BC V5Z 1M9, Canada. Child & Family Research Institute, 950 West 28th Avenue, Vancouver, BC V5Z 4H4, Canada
K. Mulpuri, Department of Orthopaedics, University of British Columbia, 3114-910 West 10th Avenue, Vancouver, BC V5Z 1M9, Canada. Department of Orthopedic Surgery, British Columbia Children’s Hospital, 4480 Oak Street, Vancouver, BC V6H 3V4, Canada
H. A. McKay, Department of Orthopaedics, University of British Columbia, 3114-910 West 10th Avenue, Vancouver, BC V5Z 1M9, Canada. Centre for Hip Health and Mobility, Vancouver Coastal Health Research Institute, 2635 Laurel Street, Vancouver, BC V5Z 1M9, Canada. Department of Family Practice, University of British Columbia, 5950 University Boulevard, Vancouver, BC, Canada V6T 1Z3
References
- 1.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 2.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–81. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 3.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–98. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290:1479–85. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Fournier PE, Rizzoli R, Slosman DO, Theintz G, Bonjour JP. Asynchrony between the rates of standing height gain and bone mass accumulation during puberty. Osteoporos Int. 1997;7:525–32. doi: 10.1007/BF02652557. [DOI] [PubMed] [Google Scholar]
- 6.Mäyränpää MK, Mäkitie O, Kallio PE. Decreasing incidence and changing pattern of childhood fractures: a population-based study. J Bone Miner Res. 2010;25:2752–9. doi: 10.1002/jbmr.155. [DOI] [PubMed] [Google Scholar]
- 7.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–8. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 8.Amin S, Melton LJ, 3rd, Achenbach SJ, Atkinson EJ, Dekutoski MB, Kirmani S, Fischer PR, Khosla S. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, but not women. J Bone Miner Res. 2013;28:1751–9. doi: 10.1002/jbmr.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einhorn TA. Bone strength: the bottom line. Calcif Tissue Int. 1992;51:333–9. doi: 10.1007/BF00316875. [DOI] [PubMed] [Google Scholar]
- 10.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–8. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–15. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 12.Kalkwarf HJ, Laor T, Bean JA. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA) Osteoporos Int. 2011;22:607–16. doi: 10.1007/s00198-010-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–7. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Scheven E. Pediatric bone density and fracture. Curr Osteoporos Rep. 2007;5:128–34. doi: 10.1007/s11914-007-0028-7. [DOI] [PubMed] [Google Scholar]
- 15.Farr JN, Amin S, Melton LJ, 3rd, Kirmani S, McCready LK, Atkinson EJ, Müller R, Khosla S. Bone strength and structural deficits in children and adolescents with a distal forearm fracture due to mild trauma. J Bone Miner Res. 2014;29:590–9. doi: 10.1002/jbmr.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirmani S, Christen D, Van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Müller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25:1521–6. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 18.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23:173–9. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012;27:273–82. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Macdonald HM, Nettlefold L, McKay HA. A comparison of bone quality at the distal radius between Asian and white adolescents and young adults: an HR-pQCT study. J Bone Miner Res. 2013;28:2035–42. doi: 10.1002/jbmr.1939. [DOI] [PubMed] [Google Scholar]
- 21.Barr SI. Associations of social and demographic variables with calcium intakes of high school students. J Am Diet Assoc. 1994;94:260–6. 269. doi: 10.1016/0002-8223(94)90366-2. quiz 267–8. [DOI] [PubMed] [Google Scholar]
- 22.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29:1344–9. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Tanner JM. Foetus into man. Harvard Press; Cambridge: 1978. [Google Scholar]
- 24.Deitz JC, Kartin D, Kopp K. Review of the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2) Phys Occup Ther Pediatr. 2007;27:87–102. [PubMed] [Google Scholar]
- 25.Goulding A, Jones IE, Taylor RW, Piggot JM, Taylor D. Dynamic and static tests of balance and postural sway in boys: effects of previous wrist bone fractures and high adiposity. Gait Posture. 2003;17:136–41. doi: 10.1016/s0966-6362(02)00161-3. [DOI] [PubMed] [Google Scholar]
- 26.Bruininks RH, Bruininks BD. Bruininks-Oseretsky Test of Motor Proficiency. Pearson; Minneapolis: 2005. [Google Scholar]
- 27.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCTstudy. J Bone Miner Res. 2011;26:50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- 28.Pauchard Y, Liphardt AM, Macdonald HM, Hanley DA, Boyd SK. Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone. 2012;50:1304–10. doi: 10.1016/j.bone.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Laib A, Häuselmann HJ, Rüegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–37. [PubMed] [Google Scholar]
- 30.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29:1096–105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–90. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 33.MacNeil JA, Boyd SK. Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2008;30:792–9. doi: 10.1016/j.medengphy.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42:1203–13. doi: 10.1016/j.bone.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Chiu J, Robinovitch SN. Prediction of upper extremity impact forces during falls on the outstretched hand. J Biomech. 1998;31:1169–76. doi: 10.1016/s0021-9290(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 36.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A 6-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 37.Faulkner RA, Davison KS, Bailey DA, Mirwald RL, Baxter-Jones AD. Size-corrected BMD decreases during peak linear growth: implications for fracture incidence during adolescence. J Bone Miner Res. 2006;21:1864–70. doi: 10.1359/jbmr.060907. [DOI] [PubMed] [Google Scholar]
- 38.Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res. 2008;23:1541–7. doi: 10.1359/JBMR.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauch F, Neu C, Manz F, Schoenau E. The development of metaphyseal cortex—implications for distal radius fractures during growth. J Bone Miner Res. 2001;16:1547–55. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 40.Pritchett JW. Growth plate activity in the upper extremity. Clin Orthop Relat Res. 1991;268:235–42. [PubMed] [Google Scholar]
- 41.Forte T, McKeag AM, Keresteci M. National Trauma Registry: 2007 Injury Hospitalizations Highlights Report. Canadian Institute for Health Information; 2008. [Accessed 21 July 2014]. https://secure.cihi.ca/free_products/ntr_highlights_2007_en.pdf. [Google Scholar]
- 42.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]