Abstract
Qualitative and quantitative reviews of the neuroimaging literature show that overlapping brain regions support theory of mind (ToM) and autobiographical memory (AM). This overlap has been taken to suggest that individuals draw on past personal experiences to infer others’ mental states, but work with amnesic people shows that ToM does not always depend on AM. One variable that may determine the extent to which one relies on AM when inferring another’s thoughts and feelings during ToM is whether that individual is personally known. To test this possibility, participants were scanned with fMRI as they remembered past experiences in response to personal photos (‘AM’ condition) and imagined others’ experiences in response to photos of personally familiar (‘pToM’ condition) and unfamiliar (‘ToM’ condition) others. Spatiotemporal Partial Least Squares was used to identify the spatial and temporal characteristics of neural activation patterns associated with AM, pToM, and ToM. We found that the brain regions supporting pToM more closely resembled those supporting AM relative to ToM involving unfamiliar others, with the greatest degree of overlap within midline regions. A complementary finding was the observation of striking differences between pToM and ToM such that midline regions associated with AM predominated during pToM, whereas more lateral regions associated with social semantic memory predominated during ToM. Overall, this study demonstrates that ToM involves a dynamic interplay between AM and social semantic memory that is biased towards AM when a personally familiar other is the subject of the mental state inference.
Keywords: fMRI, self, other, partial least squares, medial prefrontal cortex, hippocampus
Humans possess the remarkable ability to decipher other people’s imperceptible mental states, including their thoughts, feelings, desires, and intentions. This ability, known as “theory of mind” (ToM), plays an important role in our social lives; it facilitates our capacity to communicate, cooperate, and empathize with others (Amodio & Frith, 2006). The question of how we attribute mental states to others has been a central pursuit in the field of social cognition. One possibility is that humans draw on their own past experiences to infer and simulate the mental states of other people (Buckner & Carroll, 2007; Corcoran, 2001; Gallagher & Frith, 2003). However, patient work shows that ToM does not always depend on the ability to remember past experiences via autobiographical memory (AM; Rosenbaum et al., 2007). One variable that may determine the extent to which one relies on AM to infer another’s mental state is one’s knowledge of that individual through shared personal experiences. The objective of the current study was to test whether different neural and cognitive mechanisms support mental state inferences of personally familiar versus unfamiliar others and how these abilities relate to AM.
Recent qualitative and quantitative reviews of the neuroimaging literature show that the brain regions supporting ToM are remarkably similar to those underlying AM (Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Spreng et al., 2009). These common regions include the medial prefrontal cortex (PFC), anterior cingulate cortex, posterior cingulate cortex, precuneus, temporal poles (TPs), superior temporal sulcus, and regions within the medial temporal lobes. This overlap has been taken to suggest that individuals draw on a personal repertoire of past experiences to infer, simulate, or project themselves into another person’s perspective (Buckner & Carroll, 2007; Corcoran, 2001; Gallagher & Frith, 2003). A study of ToM in amnesic people, however, provides contradictory evidence in showing that ToM does not always depend on AM (Rosenbaum et al., 2007). Despite severely compromised AM, two amnesic individuals were able to perform many of the same standard laboratory ToM tests that have been shown to activate the common set of regions revealed in the reviews of the neuroimaging literature. These tests included predicting a character’s false belief about the location of an object (False Belief Test; Stone et al., 1998), deciphering others’ thoughts and emotions based only on the eye region of their faces (Mind in the Eyes Test; Baron-Cohen et al., 2001), and identifying whether a character unintentionally said something hurtful to a second character as a result of not knowing certain information (Faux Pas Test; Baron-Cohen et al., 1999; Stone et al., 1998).
Findings of intact ToM in amnesic people with impaired AM suggest that ToM may be achieved via script-like, social knowledge – generic or semantic representations of the average person’s feelings and beliefs in a given situation. For example, to correctly identify whether something hurtful was said in the Faux Pas Test, participants likely rely on their knowledge of social etiquette rather than on specific instances of such occurrences in their own lives. This interpretation is supported by recent fMRI findings of greater activity within lateral frontal and temporal regions known to be involved in semantic memory during ToM when it is directly compared to AM. Conversely, the same studies show greater activity in midline regions, such as medial PFC and posterior cingulate cortex, during AM when it is directly compared to ToM (Rabin et al., 2010; Spreng & Grady, 2010; St. Jacques et al., 2011). In the study by Rabin and colleagues, additional activity in the right hippocampus was found to be specific to AM when participants were engaged in the initial search and construction of event details, whereas activity within right temporo-parietal junction (TPJ) was specific to ToM when participants were elaborating on event details. One variable that may determine the extent to which one relies on AM to infer another’s mental state is one’s knowledge of that individual through shared personal experiences. Given that the majority of ToM tasks employ strangers as targets (Baron-Cohen et al., 1999; Rabin et al., 2010; Spreng & Grady, 2010; St. Jacques et al., 2011; Stone et al., 1998), it remains unknown whether AM is more essential to ToM when personally known others are the subject of the mental state inference.
Several recent fMRI studies suggest that a personal relationship or the ability to identify with the target person in a ToM or perspective-taking task can modulate neural activity. In one study, imagining painful scenarios from one’s own perspective and the perspective of a loved one showed overlapping activity within the anterior cingulate cortex and anterior insula, whereas imagining the scenarios from a stranger’s perspective elicited greater activity in the superior frontal gyrus and right TPJ (Cheng et al., 2010). Separate work by Mitchell and colleagues shows that perceivers selectively engage in self-referential strategies supported by the ventral medial PFC when inferring the mental states of similar others but not when inferring the mental states of dissimilar others (Mitchell et al., 2006, 2005). Furthermore, a study by Perry and colleagues (2011) reported increased hippocampal activity when participants made mental state inferences about similar versus dissimilar others, but only when participants had a personal memory of the event involving the similar other. Taken together, these studies suggest that the strategy adopted to infer another person’s mental state depends on whether that person is personally known or is perceived as similar to oneself.
In the present study, we test the idea that AM and semantic memory interact in different ways during ToM depending on whether the target person is personally known or unknown. This idea extends from the transformation hypothesis, which suggests that there is a dynamic interplay between AM supported by the hippocampus and schematic versions of the original memory supported by the neocortex, which retain the gist of the event and related semantic information but lack the rich contextual details associated with the event (Winocur et al., 2010; see also Moscovitch et al., 2006, 2005; Rosenbaum et al., 2001; Spreng & Mar, 2012). To this end, we modified the paradigm used in a previous fMRI study (Rabin et al., 2010) to include a ToM condition that involves imagining events from the perspective of personally familiar others (i.e., relatives and close friends; pToM). Participants were scanned as they recollected past events in response to personal photos (‘AM’ condition) and imagined others’ experiences in response to photos of familiar (‘pToM’ condition) and unfamiliar (‘ToM’ condition) others. We predicted that familiarity with the target person would modulate the functional relationship between AM and ToM such that AM would show greater neural overlap with pToM than with ToM. Furthermore, we expected that relative to ToM, pToM would elicit greater activity within midline regions, including the hippocampus, suggesting some reliance on past autobiographical experiences. The opposite contrast was expected to show greater activity in lateral frontal and lateral temporal regions - a pattern that has been associated with accessing semantic knowledge (e.g., Martin & Chao, 2001). Nevertheless, these strategies are not viewed to be mutually exclusive, but rather, AM and semantic memory are believed to interact to support ToM (at least in healthy people), though one strategy is likely to dominate depending on one’s familiarity with the target person.
In the current study, the fMRI data were analyzed with Spatiotemporal Partial Least Squares (ST-PLS), a multivariate technique that identifies time-varying distributed patterns of activity that differentiate experimental conditions (McIntosh et al., 2004). Unlike univariate event-related analyses, ST-PLS does not make assumptions about the shape and time course of the hemodynamic response function (HRF). Moreover, ST-PLS can provide a more sensitive statistical assessment than univariate analyses (Lukic et al., 2002; Nichols & Holmes, 2002), as all voxels are analyzed in one single analytic step, thus eliminating the issue of multiple comparisons. In the present study, we focused on an early and late phase of event generation in order to capture differences that may exist during the construction and elaboration of events, respectively (Rabin et al., 2010).
Methods
Participants
Eighteen, right-handed, healthy women1 with normal or corrected-to-normal vision and no reported history of neurological or psychiatric illness participated in the study (mean age = 19.33, SD = 1.24; mean education = 13.32, SD = 1.13). All participants gave informed written consent in accordance with the ethics committees at York University and Baycrest. Participants received monetary compensation for their time.
Stimuli
A novel, real-world family photos test of mental state attributions was modified to include a ToM condition that involved personally known others (Rabin et al., 2010). Three conditions were developed for presentation to participants during scanning: personally experienced events (AM), events experienced by personally known others (pToM), and unknown events involving unfamiliar others (ToM). The AM condition consisted of 15 personal family photos of events that took place within the past 1 to 5 years. Thirteen of the eighteen participants appeared in each AM photo. Analyses confirmed that the presence or absence of participants in the AM photos did not affect the behavioural or fMRI results (see supplementary section). A relative or close friend of each participant collected the photos to reduce the likelihood that the events in the photos were rehearsed prior to scanning. The pToM condition consisted of 15 photos depicting specific events that had been experienced by family members and close friends but not by the participant him/herself. These photos were also collected by a relative or close friend of the participant to ensure that the photos were not seen prior to scanning and that photo selection was not biased in any way. The ToM condition consisted of 15 photos depicting unfamiliar people engaged in specific events that were matched to each AM photo by the experimenter according to theme (e.g., birthday party, picnic), scenery (e.g., indoor vs. outdoor), and time period from which the photo was taken. All photos were resized and converted to gray scale.
Two baseline conditions were included in this study. One baseline condition consisted of luminance judgments made in response to scrambled photos. Matlab was used to scramble the pixels of each AM, pToM, and ToM photo to produce a corresponding image matched in visual complexity and luminance. The second baseline condition consisted of an odd-even number judgment task. This second baseline was included because it has been shown that activity, particularly within the medial temporal lobe, can vary depending on the type of baseline employed (Stark & Squire, 2001).
E-Prime software (Psychology Software Tools, Pittsburgh, PA) was used for the presentation of stimuli as well as collection of reaction times and response data. Responses were made on an MR-compatible four-button response box.
Task
At the beginning of each run, participants viewed a set of instructions that corresponded to one of the three conditions (i.e., AM, pToM, or ToM). Each run contained five photos from one of the conditions. Each photo was presented for 20 seconds and was followed by three rating scales (see below). Trials were separated by a 2 second rest period during which a fixation cross was presented. There were three runs for each condition (for a total of 9 runs), which were presented in pseudorandom order. Each run lasted for 5 minutes and 4 seconds.
In the AM condition, participants were presented with their own photos and asked to recollect the event depicted in each photo in as much detail as possible. They were told to focus on what they were thinking and feeling at the time. For the pToM and ToM conditions, participants were presented with photos of other people and asked to generate a novel event for each photo while focusing on what one person in the photo might have been thinking and feeling at the time. Participants were specifically instructed to not draw on past experiences when generating these events.
Participants were instructed to press a specified button on the response box once an event had come to mind, whether remembered or imagined. This response time was recorded and indicated the end of an initial construction phase (i.e., searching/initial generation of the event) and the beginning of an elaboration phase. For the elaboration phase, participants were told to retrieve or generate as much detail as possible for the event. The elaboration phase continued until the photo was no longer present on the screen. All photos remained on the screen for 20 seconds, regardless of when responses were made, to maximize the recollection/generation of details associated with each event.
Following the presentation of each photo, participants rated the event they recollected/imagined on a number of dimensions known to influence neural activity, particularly within the medial temporal lobe. Three ratings scales were presented after each photo. The first rating scale differed for AM and pToM/ToM events. AM events were rated on the extent to which events were recollected or familiar (1 = don’t know event; 2 = familiar with event; 3 = remember event; Gardiner et al., 1998; Tulving, 1985). Participants were instructed to select “remember” if the event was specific to a time and place and they could re-experience it, to select “familiar with event” if the event was familiar to them, but they could not recall any specific contextual or other experiential details associated with the event, and “don’t know event” if they were unable to recall any aspect of the event. The imagined pToM and ToM events were rated for likeness to an actual memory (1 = exactly like a memory … 4 = nothing like a memory). The next two ratings scales were employed for all conditions. One scale assessed the amount of detail retrieved or imagined for each event (1 = not vivid…4 = very vivid) and the other scale assessed the spatial coherence of each event (contiguousness of the spatial context: 1 = fragmented scenes … 4 = continuous scene; Hassabis et al., 2007; not reported in the current study). After the rating scales, the corresponding scrambled photo was presented and participants were asked to indicate with a button press whether the photo was dark or light in brightness. The second baseline task followed and consisted of an odd-even number judgment task, in which participants were presented with a number from 1 to 9 and were asked to indicate with a button press whether the number was odd or even.
Immediately prior to the scan, a short training session was provided to ensure that participants fully understood the task instructions. The photos used in the training session were not used during the scan.
Post-scan Interview
Immediately following the scan, participants took part in an interview in which they viewed the same photos that had been presented in the scanner. To help prevent re-encoding or repeated retrieval of events in the scanner, participants were not told of the post-scan interview until after the scanning session. Participants were asked to think back to the events they generated in the scanner and to rate each event on the same three scales that were presented in the scanner. Post-scan ratings were collected in case a within-scanner rating was not provided. The photos with the highest vividness ratings (approximately half of all photos) were selected for a semi-structured interview in which participants described the events as they had been recollected or imagined in the scanner. The events were recorded and then transcribed for scoring. Narratives were scored using an adapted autobiographical interview scoring procedure described by Levine and colleagues (2002). Narratives were segmented into distinct details, which were classified as internal (including event-specific, temporal, perceptual, spatial, and thought/emotion details) or external (including semantic and generic, nonspecific details). Analyses of the post-scan narratives were conducted by a trained rater who achieved high interrater reliability on the autobiographical interview (intraclass correlation coefficient, two-way random effects model; Shrout & Fleiss, 1979) using a standard set of previously scored memories (Levine et al., 2002).
Data Acquisition
Brain imaging data were acquired with a Siemens Trio 3T magnet with a with a matrix 12-channel head coil. Anatomical scans were acquired using a T1-weighted volumetric MRI (TR = 2000 msec, TE = 2.63 msec, 160 oblique axial slices, 1.0 mm thick, FOV = 256 mm). Functional scans were acquired with a whole-head T2*-weighted EPI pulse sequence (TR =2000 msec, TE = 30 msec, flip angle = 70°, FOV = 200 mm, 64 × 64 acquisition matrix), consisting of 30 contiguous, 5-mm-thick axial slices. Physiological data (heart and respiration rate) were acquired during the scanning session. Stimuli were presented visually through a mirror mounted on a coil that reflected images from a projector located at the bottom of the scanner.
Images were reconstructed and preprocessed with AFNI (Cox, 1996). The initial 10 time points of each run, in which transient signal changes occur as brain magnetization reaches a steady state, were excluded. The data were first corrected for respiration and heart rate. Next, slice-timing was corrected to the first slice, followed by motion correction using a 3-D Fourier transform interpolation using a functional volume that minimized the amount of motion to less than 2 mm. The images were spatially normalized to the Montreal Neurological Institute (MNI) template and smoothed using an 8-mm Gaussian filter. The final voxel size was 4mm × 4mm × 4mm.
fMRI Data Analysis
For data analysis, we only included AM events that were successfully recollected (i.e., events rated as “remembered”), pToM and ToM events rated as novel (i.e., “nothing like a memory,” corresponding to a rating of 3 or 4), and AM, pToM, and ToM events rated as vividly recollected/imagined (i.e., vividness rating of 3 or 4), as vividness is known to influence activity in regions associated with the generation of AM and ToM events (Gilboa et al., 2004; Rabin et al., 2010). We relied on the ratings participants provided during the scan, as these were believed to be more reliable than post-scan ratings. However, when a within-scanner rating was missing, we used the rating provided during the post-scan interview (only 4 post-scan ratings were used: three vividness ratings for AM events and one rating indicating ‘likeness to an actual memory’ for a pToM event).
The fMRI data were analyzed with ST-PLS (McIntosh et al., 2004). ST-PLS is a multivariate technique similar to principal components analysis that identifies time-varying distributed patterns of activity that differentiate experimental conditions. Note that unlike univariate event-related analyses, ST-PLS is not dependent upon assumptions about the shape and time course of the HRF. Moreover, it is more sensitive than univariate analysis (Lukic et al., 2002; Nichols & Holmes, 2002), as all voxels are analyzed in one single analytic step and therefore eliminates the need for post hoc correction due to multiple comparisons. For the current study, a 20-s temporal window was specified for each event (i.e., 10 TRs), and the onset of trials was specified at 2 seconds after stimulus onset.
Using singular value decomposition applied to the covariance matrix of task and functional activation, PLS extracts ranked latent variables (LV), or orthogonal patterns of brain activity, that express how well brain activity covaries with each condition. When applying PLS to event-related data, patterns of brain activity reliably related to task conditions are calculated for each post-stimulus TR for each LV (McIntosh et al., 2004). This provides information on the time course of activity associated with the experimental conditions. Each voxel is given a weight within each LV, known as a salience, that indicates how that voxel is related to the LV. A salience can be positive or negative, depending on whether the voxel shows a positive or negative correlation with the pattern identified by the LV. These salience values are then multiplied by the BOLD signal value in that voxel and summed across all voxels to derive an estimate of how robustly each participant displays that spatial pattern (known as a ‘brain score’). The brain score indicates how strongly individual subjects express the patterns of the LV.
PLS uses two different methods to test statistical significance. Permutation tests assess whether the effect represented in a given LV captured by the singular value, is sufficiently strong to be different from random noise. For the current experiment, 500 permutations were used. If the probability was less than .05, the LV was considered significant. To provide reliability measures of the contribution of each voxel to the LV, we used a bootstrap that resampled the data one hundred times to estimate the standard error of each voxel’s salience (Efron & Tibshirani, 1986). The ratio of each salience to its standard error (bootstrap ratio; BSR) was calculated and is roughly equivalent to a z-score. Peak voxels with a BSR of ±3, p < .001, and containing a minimum size of 5 voxels were considered reliable. The bootstrap also estimated the 95% confidence intervals for the mean brain scores in each condition. The confidence intervals provide estimates of whether activity in each condition is reliably different from other conditions as well as different from the overall mean.
We examined activity during an early phase (TRs 1 and 2, corresponding to activity 0 – 4 sec after stimulus onset) and a late phase (TRs 4 and 5, corresponding to activity 6 – 10 sec after stimulus onset) of event generation. The coordinates for the maximum of each cluster are reported in MNI space.
First, we computed a PLS analysis that included AM, pToM, and ToM along with the two baseline tasks (see supplementary material) to ensure consistency with previous findings. Subsequent analyses focused on identifying the relationship among the three experimental conditions and therefore we computed a PLS analysis that only included AM, pToM, and ToM. Because we had an a priori prediction regarding differences between pToM versus ToM and such an LV did not emerge (see results), we computed an additional PLS analysis that only included pToM and ToM.
Results
Behavioural Results
As mentioned above, only the following events were included in the analyses: AM events that were successfully recollected, pToM and ToM events rated as novel (i.e., different from a memory), and AM, pToM, and ToM events rated as vividly recollected/imagined. Events that did not have button response data distinguishing construction versus elaboration phases were also excluded. Therefore, each participant contributed an average of 12.39 AM events (SD = 2.0), 10.61 pToM events (SD = 3.0), and 10.22 ToM events (SD = 3.0) to the analysis (out of 15 possible events for each condition). A repeated measures ANOVA indicated that the length of the construction phase significantly differed across AM (M = 1799 msec, SD = 719 msec), pToM (M = 2066 msec, SD = 843 msec), and ToM events (M = 2341 msec, SD = 850 msec; F(2, 34)= 8.86, p = .001). Post-hoc tests were computed using a Bonferroni adjustment that maintained a family-wise error rate of < .05. Results indicated that AM construction took significantly less time than pToM, t(17) = −2.69, p = .015, and ToM construction, t(17) = −3.54, p = .003. There was no difference between the construction of pToM and ToM events, t(17) = −2.1, ns.
Post-Scan Interview
A repeated measures ANOVA indicated that the number of internal details generated in response to AM (M = 8.53, SD = 1.99), pToM (M = 6.13, SD = 1.58), and ToM photos (M = 6.16, SD = 1.85) differed significantly, F(2, 34) = 29.1, p < .0001. Post-hoc tests were computed using a Bonferroni adjustment that maintained a family-wise error rate of < .05. Results indicated that participants produced significantly more internal details in response to AM photos than pToM photos, t(17) = −5.76, p < .0001, and ToM photos, t(17) = −6.82, p < .0001. The number of internal details did not differ in response to pToM and ToM photos, t(17) = −.11, ns.
fMRI Results
PLS analysis that included AM, pToM, and ToM
The PLS analysis that included AM, pToM, and ToM identified two significant patterns of brain activity (i.e., LVs). The first LV showed a pattern contrasting AM and pToM with ToM (p < .005, explained variance = 61.3%; Figure 1A). The positive saliences listed in Table 1 and presented in warm colours in Fig. 1B correspond to greater activity during AM and pToM relative to ToM, whereas the negative saliences, presented in cool colours, correspond to greater activity during ToM relative to AM and pToM. We examined activity during an early phase and a late phase of event generation.
Figure 1.
Latent variable 1 (p < .005) depicts brain activity during AM and pToM vs. ToM. (A) A plot of design scores, indicating the amount of correlation between each task and the associated pattern of brain activity. (B) Depicts axial slices of the brain regions associated with AM and pToM (warm colours) or ToM (cool colours). Activity is shown during an early phase (TRs 1 and 2) and a late phase (TRs 4 and 5) of event generation. The functional maps are overlaid on the average anatomical image from all participants. Images follow neurological convention (left side of the brain is presented on the left).
Table 1.
Co-ordinates of regions associated with AM and pToM vs. ToM (LV 1)
| Hemis | Region | BA | x | y | z | TR | BSR | vol. |
|---|---|---|---|---|---|---|---|---|
| AM and pToM > ToM during TR1 and TR2 | ||||||||
| R | Paracingulate Cortex* | 10 | 8 | 56 | 0 | 2 | 9.1 | 346 |
| R | Superior Frontal Gyrus | 6 | 4 | 12 | 48 | 1 | 5.8 | 11 |
| L | Middle Frontal Gyrus | 6 | −36 | 0 | 44 | 1 | 6.1 | 17 |
| L | Middle Frontal Gyrus | 8 | −32 | 20 | 40 | 2 | 6.5 | 37 |
| R | Middle Frontal Gyrus | 8 | 24 | 32 | 40 | 2 | 9.4 | 39 |
| R | Middle Frontal Gyrus | 8 | 28 | 24 | 36 | 1 | 5.3 | 9 |
| L | Cingulate Gyrus | 24 | −24 | −16 | 48 | 1 | 5.4 | 5 |
| R | Insula | 13 | 40 | −8 | 24 | 1 | 4.8 | 6 |
| L | Hippocampus | - | −28 | −20 | −12 | 2 | 4.8 | 8 |
| L | Parahippocampal Gyrus | 19 | −16 | −44 | −4 | 2 | 5.8 | 6 |
| L | Parahippocampal Gyrus | 36 | −24 | −44 | −12 | 1 | 5.9 | 12 |
| R | Parahippocampal Gyrus | 30 | 20 | −40 | −4 | 2 | 5.5 | 21 |
| R | Parahippocampal Gyrus | 28 | 28 | −24 | −16 | 2 | 5.1 | 9 |
| L | Parahippocampal Gyrus | 36 | −28 | −36 | −12 | 2 | 5.1 | 15 |
| R | PCC/RSC/Precuneus* | 23/30/31 | 8 | −60 | 28 | 1 | 8.3 | 84 |
| L | Precuneus* | 31 | −4 | −52 | 36 | 1 | 5.1 | 5 |
| L | Precuneus* | 31 | −8 | −52 | 36 | 2 | 15.2 | 517 |
| R | Precuneus | 19 | 36 | −72 | 40 | 2 | 7.6 | 98 |
| L | Middle Temporal Gyrus | 39 | −36 | −64 | 32 | 2 | 8.9 | 200 |
| R | Temporal Pole | 38 | 44 | 20 | −36 | 2 | 4.9 | 6 |
| L | Superior Temporal Gyrus | 22 | −48 | −20 | −8 | 2 | 5.4 | 7 |
| L | Superior Temporal Gyrus | 22 | −60 | 16 | −4 | 2 | 5.4 | 13 |
| R | Superior Temporal Gyrus | 22 | 64 | 4 | −8 | 2 | 5.4 | 6 |
| L | Cuneus | 18 | −8 | −80 | 36 | 2 | 4.7 | 10 |
| R | Cerebellum | - | 8 | −48 | −44 | 1 | 6.0 | 21 |
| R | Cerebellum | - | 40 | −60 | −52 | 2 | 5.3 | 10 |
| R | Cerebellum | - | 24 | −68 | −40 | 2 | 4.8 | 13 |
| R | Cerebellum | - | 8 | −48 | −48 | 2 | 5.3 | 6 |
| ToM > AM and pToM during TR1 and TR2 | ||||||||
| No regions | ||||||||
| AM and pToM > ToM during TR4 and TR5 | ||||||||
| R | Frontal Pole* | 10 | 12 | 56 | 8 | 4 | 7.5 | 674 |
| L | Paracingulate Cortex* | 32 | −8 | 32 | 0 | 5 | 8.1 | 543 |
| L | Middle Frontal Gyrus | 6 | −32 | 20 | 44 | 4 | 5.3 | 15 |
| R | Middle Frontal Gyrus | 8 | 28 | 32 | 40 | 5 | 7.2 | 72 |
| L | PCC/RSC/Precuneus* | 23/30/31 | −12 | −56 | 28 | 4 | 10.8 | 704 |
| L | PCC/RSC/Precuneus* | 23/30/23 | −4 | −44 | 28 | 5 | 9.8 | 749 |
| L | Precuneus | 39 | −40 | −68 | 36 | 5 | 5.9 | 142 |
| R | Middle Temporal Gyrus | 39 | 52 | −64 | 28 | 4 | 7.0 | 157 |
| L | Middle Temporal Gyrus | 21 | −60 | −16 | −12 | 4 | 5.2 | 25 |
| L | Inferior Temporal Gyrus | 21 | −60 | −8 | −16 | 5 | 4.0 | 8 |
| R | Thalamus | - | 32 | −32 | 0 | 4 | 4.5 | 13 |
| R | Cerebellum | - | 8 | −48 | −52 | 4 | 5.6 | 35 |
| ToM > AM and pToM during TR4 and TR5 | ||||||||
| L | Ventrolateral PFC | 44 | −48 | 12 | 16 | 5 | −4.96 | 27 |
| R | Ventrolateral PFC | 44 | 52 | 20 | 8 | 5 | −4.42 | 9 |
| L | Insula | 13 | −48 | 16 | 12 | 4 | −4.99 | 8 |
| L | Lingual Gyrus | - | −28 | −76 | 8 | 5 | −4.26 | 6 |
| R | Inferior Temporal Gyrus | 21 | 44 | −68 | 0 | 5t | −4.508 | 11 |
| L | Middle Occipital Gyrus | 19 | −36 | −84 | 8 | 4 | −4.5 | 12 |
| R | Middle Occipital Gyrus | 19 | 36 | −84 | 16 | 4 | −4.7 | 11 |
| R | Middle Occipital Gyrus | 37 | 48 | −72 | 8 | 4 | −4.5 | 6 |
MNI co-ordinates of the maximally activated voxel within each cluster are reported. AM = autobiographical memory condition; pToM = theory of mind condition involving personally known others; ToM = theory of mind condition involving unkown others; Hemis = hemisphere; BA = Brodmann area; Vol. = cluster volume in voxels; R = right; B= bilateral; L = left; PCC = posterior cingulate cortex; RSC = retrosplenial cortex; PFC = prefrontal cortex.
Activation in this region extended bilaterally.
During the early phase, all regions activated correlated with AM and pToM (vs. ToM) and included medial frontal, medial and lateral temporal, and medial parietal regions. During the late phase of event generation, AM and pToM continued to be supported by a similar network, but medial temporal lobe regions were no longer involved (see Table 1 and Figure 1B). The opposite contrast showed that ToM was associated with increased activity in bilateral ventrolateral PFC (BA 44), left insula, left lingual gyrus, right inferior temporal gyrus extending into middle occipital cortex, and bilateral occipital cortices (BA 19).
The second significant LV differentiated a set of brain regions supporting AM from a separate set of regions supporting pToM (p = .026, explained variance = 38.7%; Figure 2A). ToM was not significantly different from the mean and therefore did not contribute to this LV. The positive saliences, presented in warm colours, correspond to greater activity during AM, whereas the negative saliences, presented in cool colours, correspond to greater activity during pToM (See Table 2 for a list of regions and Figure 2B). During the early phase of event generation, the set of regions supporting AM included left cingulate gyrus (BA 32), right middle frontal gyrus (BA 46), left caudate, and right cerebellum, whereas left insula and right ventrolateral PFC activity emerged during pToM. Later in event generation, AM was associated with activity in the right posterior cingulate cortex/precuneus (BA 23/31), whereas pToM was associated with a much more extensive set of regions that included the right frontal pole (BA 10), bilateral dorsomedial PFC (BA 9), bilateral ventrolateral PFC, left insula, bilateral lateral temporal regions, left TP (BA 38), bilateral thalamus, and bilateral occipital regions.
Figure 2.
Latent variable 2 (p < .05) depicts brain activity during AM vs. pToM. (A) A plot of design scores, indicating the amount of correlation between each task and the associated pattern of brain activity. (B) Depicts axial slices of the brain regions associated with AM (warm colours) or pToM (cool colours). Activity is shown during an early phase (TRs 1 and 2) and a late phase (TRs 4 and 5) of event generation. The functional maps are overlaid on the average anatomical image from all participants. Images follow neurological convention (left side of the brain is presented on the left).
Table 2.
Co-ordinates of regions associated with AM vs. pToM (LV 2)
| Hemis | Region | BA | x | y | z | TR | BSR | vol. |
|---|---|---|---|---|---|---|---|---|
| AM > pToM during TR1 and TR2 | ||||||||
| L | Cingulate Gyrus | 31 | −12 | 20 | 36 | 1 | 4.07 | 9 |
| R | Middle Frontal Gyrus | 46 | 52 | 44 | 8 | 2 | 4.08 | 6 |
| L | Caudate | - | −20 | 28 | 8 | 1 | 3.43 | 5 |
| R | Cerebellum | - | 24 | −68 | −48 | 1 | 5.38 | 14 |
| pToM > AM during TR1 and TR2 | ||||||||
| L | Insula | 13 | −40 | −4 | −4 | 1 | −4.24 | 5 |
| L | Insula | 13 | −32 | −16 | 20 | 1 | −4.15 | 5 |
| AM > pToM during TR4 and TR5 | ||||||||
| R | PCC/RSC | 30 | 16 | −56 | 20 | 5 | 4.74 | 8 |
| pToM > AM during TR4 and TR5 | ||||||||
| R | Frontal Pole | 10 | 20 | 64 | 8 | 4 | −4.32 | 14 |
| R | Dorsomedial PFC* | 9 | 8 | 44 | 32 | 5 | −7.58 | 549 |
| R | Dorsomedial PFC* | 9 | 8 | 36 | 40 | 4 | −5.68 | 123 |
| L | Superior Frontal Gyrus | 6 | −40 | 0 | 52 | 4 | −3.63 | 7 |
| L | Superior Frontal Gyrus | 6 | −8 | 4 | 52 | 4 | −5.96 | 81 |
| R | Middle Frontal Gyrus | 9 | 36 | 20 | 28 | 5 | −5.63 | 65 |
| L | Middle Frontal Gyrus | 9 | −52 | 12 | 36 | 5 | −4.58 | 15 |
| L | Middle Frontal Gyrus | 9 | −40 | 8 | 28 | 4 | −3.76 | 19 |
| L | Ventrolateral PFC | 47 | −56 | 16 | 16 | 5 | −5.10 | 73 |
| R | Ventrolateral PFC | 46/9 | 44 | 20 | 16 | 4 | −5.78 | 125 |
| R | Ventrolateral PFC | 44 | 36 | 16 | −28 | 5 | −4.42 | 19 |
| R | Cingulate Gyrus | 31 | 12 | −52 | 32 | 5 | −4.63 | 29 |
| R | Precentral Gyrus | 6 | 40 | 8 | 36 | 5 | −6.05 | 83 |
| L | Insula | 13 | −36 | −12 | 12 | 4 | −3.60 | 5 |
| L | Middle Temporal Gyrus | 21 | −56 | 4 | −28 | 4 | −4.08 | 5 |
| R | Middle Temporal Gyrus | 21 | 56 | 0 | −24 | 4 | −4.90 | 32 |
| R | Middle Temporal Gyrus | 21 | 64 | −32 | −4 | 4 | −3.87 | 11 |
| L | Superior Temporal Gyrus | 22 | −40 | −60 | 12 | 4 | −4.37 | 60 |
| R | Superior Temporal Gyrus | 22 | 40 | −60 | 12 | 4 | −5.47 | 138 |
| R | pSTS | 39 | 56 | −56 | 8 | 5 | −6.19 | 378 |
| L | Temporal Pole | 38 | −36 | 12 | −24 | 5 | −4.20 | 6 |
| L | Thalamus | - | −12 | −28 | −4 | 4 | −3.85 | 5 |
| R | Thalamus | - | 16 | −12 | −4 | 4 | −3.70 | 12 |
| L | Lingual Gyrus | 19 | −24 | −72 | 16 | 4 | −3.79 | 6 |
| L | Inferior Occipital Gyrus | 18 | −36 | −88 | 0 | 4 | −3.69 | 20 |
| R | Inferior Occipital Gyrus | 19 | 36 | −72 | −4 | 5 | −3.93 | 33 |
| R | Middle Occipital Gyrus | 18 | 24 | −88 | 20 | 4 | −4.23 | 25 |
MNI co-ordinates of the maximally activated voxel within each cluster are reported. AM = autobiographical memory; pToM = theory of mind involving personally known others; ToM = theory of mind involving unknown others; Hemis = hemisphere; BA = Brodmann area; Vol. = cluster volume in voxels; L = left; R = right; PCC = posterior cingulate cortex; RSC = retrosplenial cortex; PFC = prefrontal cortex; pSTS = posterior temporal sulcus.
Activation in this region extended bilaterally.
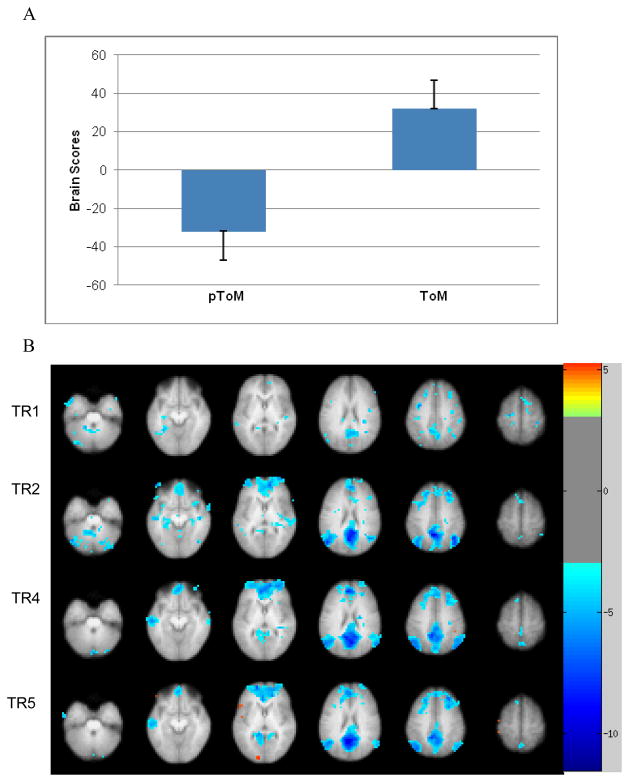
PLS analysis contrasting pToM with ToM
The PLS analysis that only included pToM and ToM revealed one significant LV that differentiated pToM from ToM (p < .0001, explained variance = 100%; Figure 3A). The positive saliences, presented in warm colours, correspond to greater activity during pToM, whereas the negative saliences, presented in cool colours, correspond to greater activity during ToM (See Table 3 for a list of regions and Figure 3B). During the early phase of event generation, all regions activated correlated with pToM including bilateral frontal pole/paracingulate cortex (BA 10/32), bilateral medial parietal cortex, bilateral hippocampus, bilateral lateral temporal cortex, bilateral TPs, and bilateral cerebellum. During the later phase of event generation, pToM continued to be associated with a very similar set of regions, however hippocampal activity was no longer present. In contrast, during the latter part of event generation, relative to pToM, ToM was associated with activity within left ventrolateral PFC (BA 44/45), left middle frontal gyrus (BA 6), left inferior temporal gyrus (BA 37), and left lingual gyrus extending into the superior occipital gyrus.
Figure 3.
Latent variable 3 (p < .0001) depicts brain activity during pToM vs. ToM. (A) A plot of design scores, indicating the amount of correlation between each task and the associated pattern of brain activity. (B) Depicts axial slices of the brain regions associated with pToM (cool warm colours) or ToM (cool colours). Activity is shown during an early phase (TRs 1 and 2) and a late phase (TRs 4 and 5) of event generation. The functional maps are overlaid on the average anatomical image from all participants. Images follow neurological convention (left side of the brain is presented on the left).
Table 3.
Co-ordinates of regions associated with pToM vs. ToM
| Hemis | Region | BA | x | y | z | TR | BSR | vol. |
|---|---|---|---|---|---|---|---|---|
| pToM > ToM during TR1 and TR2 | ||||||||
| R | Medial PFC* | 10 | 12 | 52 | 4 | 2 | −6.65 | 751 |
| R | Middle Frontal Gyrus | 8 | 28 | 20 | 40 | 1 | −5.52 | 19 |
| R | Middle Frontal Gyrus | 8/9 | 24 | 32 | 40 | 2 | −7.28 | 104 |
| L | Middle Frontal Gyrus | 8 | −28 | 12 | 44 | 2 | −5.70 | 61 |
| L | Superior Frontal Gyrus | 8 | 0 | 20 | 48 | 1 | −4.65 | 44 |
| L | Superior Frontal Gyrus | 8/9 | −12 | 32 | 48 | 2 | −3.86 | 9 |
| R | Cingulate gyrus | 12 | −12 | 28 | 2 | −3.75 | 14 | |
| L | Cingulate Gyrus | 24 | −4 | −16 | 44 | 1 | −3.19 | 6 |
| R | Inferior Frontal Gyrus | 6/9 | 44 | 0 | 32 | 1 | −3.95 | 11 |
| L | Inferior Frontal Gyrus | 6/9 | −36 | 4 | 36 | 1 | −6.43 | 61 |
| R | Postcentral Gyrus | 40 | 28 | −40 | 56 | 2 | −3.89 | 7 |
| R | Parahippocampal gyrus | 40 | −16 | −16 | 2 | −5.02 | 122 | |
| L | Parahippocampal gyrus | −36 | −16 | −24 | 1 | −4.22 | 11 | |
| R | Hippocampus | 28 | −24 | −16 | 2 | −4.95 | 99 | |
| L | Hippocampus | −24 | −28 | −12 | 2 | −3.97 | 35 | |
| R | PCC/RSC* | 23/31 | 8 | −60 | 28 | 1 | −6.88 | 230 |
| L | PCC/RSC* | 23/31 | −8 | −52 | 36 | 2 | −10.14 | 704 |
| RL | Middle Temporal Gyrus | 22 | 56 | −32 | 0 | 1 | −4.04 | 7 |
| L | Middle Temporal Gyrus | 22 | −56 | −32 | 4 | 2 | −4.14 | 5 |
| L | Middle Temporal Gyrus | 39 | −48 | −72 | 20 | 1 | −4.15 | 11 |
| L | Middle Temporal Gyrus | 39 | −48 | −68 | 20 | 2 | −7.73 | 285 |
| L | Middle Temporal Gyrus | 21/22 | −48 | −20 | −8 | 2 | −5.16 | 71 |
| L | Middle Temporal Gyrus | 22 | −48 | −44 | 0 | 2 | −4.53 | 27 |
| L | Inferior Temporal Gyrus | 20 | −48 | −52 | −12 | 1 | −3.81 | 7 |
| R | Superior Temporal Gyrus | 22 | 48 | −20 | 4 | 1 | −3.80 | 8 |
| R | Temporal Pole | 38 | 48 | 16 | −40 | 2 | −4.37 | 10 |
| L | Temporal Pole | 38 | −48 | 12 | −28 | 1 | −4.30 | 28 |
| L | Temporal Pole | 38 | −32 | 12 | −24 | 2 | −3.75 | 13 |
| R | Angular Gyrus | 39 | 36 | −60 | 32 | 1 | −5.49 | 51 |
| R | Angular Gyrus | 39 | 44 | −60 | 24 | 2 | −8.86 | 176 |
| L | Fusiform Gyrus | 37 | −32 | −44 | −16 | 1 | −5.29 | 17 |
| R | Insula | 13 | 40 | −8 | 24 | 1 | −4.44 | 22 |
| R | Middle Occipital Gyrus | 19 | 40 | −68 | −12 | 2 | −4.70 | 7 |
| R | Thalamus | 8 | −20 | 8 | 1 | −3.64 | 5 | |
| R | Cerebellum | 8 | −52 | −56 | 2 | −5.39 | 189 | |
| R | Cerebellum | 16 | −64 | −16 | 1 | −3.74 | 11 | |
| L | Cerebellum | −16 | −40 | −28 | 1 | −4.29 | 29 | |
| L | Cerebellum | −32 | −76 | −28 | 1 | −4.70 | 32 | |
| ToM > pToM during TR1 and TR2 | ||||||||
| No regions | ||||||||
| pToM > ToM during TR4 and TR5 | ||||||||
| L | Medial PFC* | 10 | −4 | 48 | 16 | 4 | −8.42 | 1035 |
| L | Medial PFC* | 10 | −12 | 56 | 8 | 5 | −8.42 | 1143 |
| R | Medial PFC* | 9 | 24 | 32 | 24 | 4 | −5.89 | 150 |
| L | PCC/Precuneus* | 23/31 | −12 | −56 | 28 | 4 | −10.44 | 1209 |
| L | PCC/Precuneus* | 23/31 | −4 | −56 | 20 | 5 | −9.98 | 873 |
| R | Middle Temporal Gyrus | 21 | 52 | −8 | −20 | 4 | −4.22 | 7 |
| L | Middle Temporal Gyrus | 21 | −56 | −20 | −12 | 4 | −5.57 | 89 |
| L | Middle Temporal Gyrus | 21 | −56 | −12 | −16 | 5 | −5.36 | 77 |
| R | Superior Temporal Gyrus | 39 | 56 | −64 | 24 | 4 | −7.61 | 224 |
| R | Superior Temporal Gyrus | 39 | 56 | −64 | 24 | 5 | −6.19 | 130 |
| L | Angular Gyrus | 39 | −40 | −72 | 36 | 5 | −6.70 | 204 |
| R | TP/Ventrolateral PFC | 38/47 | 32 | 12 | −16 | 4 | −3.84 | 5 |
| R | Cerebellum | 36 | −72 | −36 | 4 | −5.06 | 55 | |
| R | Cerebellum | 4 | −48 | −48 | 5 | −6.82 | 85 | |
| R | Cerebellum | 12 | −84 | −36 | 5 | −4.57 | 21 | |
| L | Cerebellum | 0 | −84 | −28 | 4 | −5.09 | 12 | |
| ToM > pToM during TR4 and TR5 | ||||||||
| L | Ventrolateral PFC | 44/45 | −52 | 16 | 8 | 5 | 3.32 | 7 |
| L | Middle Frontal Gyrus | 6 | −52 | 0 | 44 | 5 | 3.94 | 5 |
| L | Inferior Temporal Gyrus | 37 | −56 | −68 | −4 | 4 | 5.55 | 6 |
| L | Lingual Gyrus | 17 | −12 | −92 | 0 | 4 | 3.65 | 5 |
| L | Lingual/Superior Occipital Gyrus | −12 | −92 | 4 | 5 | 3.75 | 10 | |
MNI co-ordinates of the maximally activated voxel within each cluster are reported. pToM = theory of mind condition involving personally known others ; ToM = theory of mind condition involving unknown others; Hemis = hemisphere; BA = Brodmann area; Vol. = cluster volume in voxels; R = right; B= bilateral; L = left; PFC = prefrontal cortex; PCC = posterior cingulate cortex; RSC = retrosplenial cortex; TP = temporal pole.
Activation in this region extended bilaterally.
Discussion
In the present study, we tested whether different neural and cognitive mechanisms support mental state inferences of personally known versus unknown others and how these abilities relate to AM. Using ST-PLS, we replicated the finding that AM and ToM recruit a common pattern of activity that includes medial frontal, medial and lateral temporal, and medial parietal regions (Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Rabin et al., 2010; Spreng et al., 2009; Spreng & Grady, 2010, Spreng & Mar, 2012; see supplementary material) and that when AM and ToM are directly compared, midline regions predominate during AM and more lateral regions predominate during ToM. Unique to the current study was the finding that the pattern of activity associated with pToM involving familiar others resembled the pattern of activity associated with AM to a greater extent than the pattern associated with ToM involving unfamiliar others, with the greatest degree of overlap within midline regions. This finding suggests that personal experience with the target person in a ToM task influences the functional relationship between AM and ToM. A complementary result was the observation of striking neural differences between pToM and ToM, suggesting that participants relied on different cognitive mechanisms to carry out these two tasks despite identical task instructions.
Neural overlap between AM and pToM versus ToM
The results of the current study provide direct evidence that shared past experiences with the target person in a ToM task modulates the functional relationship between AM and ToM. This was evident in the first latent variable (LV1) of the PLS analysis that included all three experimental conditions. This LV, which accounted for the greatest amount of variance in the analysis, revealed that the pattern of activity supporting pToM shares more in common with AM than with ToM. The greatest degree of neural overlap observed between AM and pToM was found within bilateral medial PFC, medial parietal cortex, as well as hippocampus and related medial temporal lobe structures – regions previously associated with autobiographical recollection (Svoboda et al., 2006), self-referential processing (Amodio & Frith, 2006), and social relevance (Krienen et al., 2010).
The shared set of regions recruited during AM and pToM suggests a strategy of relying on past personal experiences when considering the mental states of personally known others. It may be the case that for each pToM photo, participants drew on personal semantic memories or general AMs (i.e., summaries of repeated events) that involved the close other in order to simulate and predict his or her mental state. This idea is in line with Buckner and Carroll’s (2007) self-projection hypothesis, which suggests that one draws on past experiences in order to project oneself into another person’s mind (see also Corcoran, 2001 and Gallagher & Frith, 2003). It is also consistent with other simulation theories, which suggest that individuals rely on their own thoughts and feelings to predict the mental states of close others. For example, using medial PFC activity as an index, Mitchell and colleagues have shown that perceivers use the self as a proxy only when the other is deemed similar to the self (Mitchell et al., 2006, 2005).
Although participants in the present study did not rate how similar they perceived themselves to the people depicted in the pToM photos, other research indicates that individuals tend to share similar values with family members and close friends (Mashek et al., 2003). However, more recent research has demonstrated that regions within the medial PFC respond more strongly when participants make judgments about friends compared to strangers, regardless of whether the other person is perceived as similar to the self (Krienen et al., 2010). This finding suggests that personal relevance (closeness), and not similarity is driving the neural overlap between AM and pToM. Lieberman (2012) proposed that we possess idiosyncratic theories (i.e., knowledge of unique characteristics) about ourselves as well as those who are close to us. These specific theories may have influenced how participants thought about themselves in the past during the AM condition and how they thought about close others during the pToM condition.
It is notable that participants in the present study were instructed not to refer to personal memories when generating pToM (and ToM) events, and only events rated by participants as different from a memory were included in the analysis. Because participants rarely rated pToM (and ToM) events as similar to a memory, it was difficult to determine if the neural overlap was modulated by the extent to which participants relied on specific AMs. Although it is unlikely that the pToM events themselves were replicas of specific past experiences, it remains possible that participants consciously recalled past experiences in response to the pToM photos, yet rated these events as ‘dissimilar to a memory’ in order to comply with the experimenter’s instruction to generate novel events. A more likely explanation is that previous episodes unintentionally influenced participants’ current social processing (e.g., Sheldon & Moscovitch, 2010; Greenberg et al., 2009). It has been suggested that the mere perception of a familiar individual is associated with the spontaneous retrieval of personal knowledge about that individual (i.e., personal traits, attitudes, biographical facts and episodic memories; Gobbini & Haxby, 2007), which is then used to successfully infer his or her mental state. In either case, the neural overlap may reflect the retrieval of past experiences in response to both AM and pToM photos, whether intentional or unintentional. A related alternative is that the overlap may result from constructive processes that are at play during both AM and pToM. In AM past episodes are reconstructed, whereas in pToM event details contained within AMs are flexibly recombined to generate novel events. This idea is captured by the “constructive-episodic-simulation hypothesis” proposed by Schacter and Addis (2007) to account for the neural overlap between AM and thinking about oneself in the future. The process of recombination is thought to rely on relational processes mediated by the hippocampus (Davachi, 2004; Eichenbaum, 2001; Ryan et al., 2000), which may be important for generating and/or binding episodic details for both real and imagined events (Rosenbaum et al., 2009).
It remains the case, however, that all three conditions are supported by a common set of regions, albeit to a lesser degree for ToM involving unfamiliar others. Thus, AM may be called upon to infer others’ mental states in general, though it may be less critical and used together with other strategies, such as semantic memory, when unfamiliar others are concerned.
Differences in ToM when it involves personally familiar vs. unfamiliar others
The finding that AM and pToM bear close resemblance in the regions that they recruit, along with the observation of differences between pToM and ToM, suggest that participants relied on different cognitive mechanisms to carry out the two ToM tasks. This finding is all the more remarkable given that the two ToM conditions involved identical task instructions and differed only in terms of familiarity with the target person and, in some cases the setting depicted in the photos. As expected, relative to ToM, pToM engaged midline regions that closely resemble those involved in AM during both the early and late phase of event generation likely due to reasons outlined above. In contrast, ToM versus pToM recruited a more lateral set of regions known to be involved in semantic memory during the late phase (Martin & Chao, 2001). The greater involvement of the left ventrolateral PFC during ToM along with lateral temporal activity (see supplementary section) may reflect participants’ reliance on social scripts and general knowledge about the world in order to infer the mental states of unfamiliar others. This may include rules for understanding how the average person is likely to experience and respond to different situations and events (rather than relying on specific AMs or idiosyncratic representations). That is, one does not need to know about an individual’s unique characteristics to make this type of judgment (Lieberman, 2012). Greater occipital activity was found during ToM versus pToM (and AM), which may reflect greater reliance on the visual information presented in the photos (e.g., facial expression) in order to construct the novel ToM events and to infer the mental states of unfamiliar others. Participants likely employ these types of strategies when carrying out standard laboratory tests of ToM. This idea is supported by the finding that patients with semantic dementia are impaired on a variety of laboratory tests of ToM (Duval et al., 2012).
Differences between AM and pToM
Despite the clear correspondence between AM and pToM, the second LV (LV2) in the analysis that included all three experimental conditions differentiated a set of regions supporting AM from a separate set of regions supporting pToM. This LV revealed few neural differences during the early phase of event generation, but as the events continued to unfold, more widespread differences emerged. The failure to find robust differences between AM and pToM during the early phase of event generation in LV2, along with the similarities revealed in LV1, suggest that the processes supporting AM and pToM are most similar during the initial construction of events. As mentioned above, it is possible that this early shared activity represents access to past experiences. However, in AM the recollection is conscious, whereas in pToM this process is likely unconscious or automatic given that participants were told to generate novel events without resorting to a specific past memory.
During the latter part of event generation, relative to pToM, AM was associated with greater activity within the right posterior cingulate cortex/precuneus (BA 23/31); at a slightly relaxed threshold (BSR > 2.4, p < .05), activity within the frontal pole (BA 10) and paracingulate cortex (BA 32) was also present. This pattern of activity likely reflects self-referential and visuospatial aspects of autobiographical remembering that have been identified in previous studies (Addis et al., 2004; Epstein, 2008; Fletcher et al., 1995; Gilboa et al., 2004; Northoff et al., 2006; Rosenbaum et al., 2004; Summerfield et al., 2009; Svoboda et al., 2006).
In contrast to AM, the regions engaged to a greater extent during pToM are consistent with those reported in the ToM literature and include the right frontal pole (BA 10), bilateral dorsomedial PFC (BA 9), bilateral ventrolateral PFC, left insula, bilateral lateral temporal regions, left TP (BA 38), and bilateral middle occipital cortex (BA 18/19; Amodio & Frith, 2006; Gallagher & Frith, 2003; Spreng et al., 2009). The involvement of the left ventrolateral PFC and lateral temporal regions suggest greater reliance on general semantic processing during pToM relative to AM (Martin & Chao, 2001). It is likely that imagining a novel event that has never occurred requires increased generative processing relative to retrieving a past event from memory (Addis et al., 2009). Furthermore, previous work has identified the anterior temporal cortex as a region important for representing social knowledge (for reviews, see Olson et al., 2007 and Simmons & Martin, 2009). The left TP, in particular, has been characterized as a storehouse for personal semantic memories and is thought to be responsible for linking high-level sensory representations, such as familiar faces, with semantic information (Olson et al., 2007).
Some of the differences observed between AM and pToM during the latter part of event generation may relate to differences between remembering actual events and constructing events from imagination. Therefore, the greater midline activity observed during AM versus pToM may reflect differences in the ‘realness’ of events. This interpretation converges with recent work showing greater medial PFC and posterior cingulate cortex activity during the recollection of personally experienced events relative to events that were previously imagined but not experienced (Summerfield et al., 2009). Based on these findings, it was suggested that a midline network of regions may help to distinguish actual experiences from imagined ones (see also Hassabis et al., 2007).
A related explanation to account for the differences between AM and pToM is that the conditions vary with respect to personal significance, with AM events most relevant, followed by pToM events. Therefore, it may be the combination of ‘realness’ and personal significance that explains the greater activity within medial frontal and parietal regions during AM versus pToM. Though conceivable, this was not reflected in hippocampal activity, which would have been expected to differ based on previous work showing that activity in this region is modulated by personal significance (Addis et al., 2004).
Alternatively, the increased midline activity, particularly the precuneus, during AM versus pToM may reflect the greater visual detail with which AM events were recollected. Although vividness was equated across all three conditions (using within-scanner rating scales), participants did, in fact, generate significantly more internal details for the AM versus pToM and ToM events during the post-scan interview. Therefore, it is possible that participants made their vividness ratings relative to events within a condition rather than across all conditions. If so, the increased number of details generated for the AM events may represent more vividly generated events.
Theoretical Implications
In the context of the current set of fMRI results, we suggest that the common core network supporting AM and ToM involving familiar and unfamiliar people may reflect an interplay between AM and semantic memory. This idea is consistent with the “transformation hypothesis,” whereby a dynamic interplay exists between episodic memory for vivid contextual details of a personally experienced event, supported by the hippocampus, and schematic versions of the original memory or gist of the event, supported by the neocortex (Winocur et al., 2010; see also Moscovitch et al., 2006, 2005 and Rosenbaum et al., 2001).
Similar to the transformation hypothesis, Spreng and Mar (2012) recently proposed that the shared network supporting AM and ToM reflects a distributed integration zone that provides a means for past personal experience to transform into social conceptual knowledge, knowledge that is then used to guide social processes and behaviour. Building on these ideas, we suggest that AM and semantic memory work together to support ToM abilities (at least in healthy people). The extent to which each memory system is involved will depend on whose mind one is inferring in addition to the processing demands of the task at hand and the social-perceptual cues that are available. ToM involving unfamiliar others, whether in the laboratory or real-world, is likely to rely more heavily on semantic or schematic memory, whereas ToM involving well-known others is more likely to rely on AM. The capacity to readily access semantic and autobiographical information about personally known others likely sets the stage for successful social interactions with people with whom we have close relationships. However, in the face of AM loss, the semantic memory system is likely sufficient for successful performance on standard laboratory measures of ToM (Rosenbaum et al., 2007) and possibly real-world ToM tests involving unfamiliar people, such as the one used here. It remains to be determined whether AM is critical for real-world ToM processes that involve personally familiar others. Taken together, the current results suggest that ToM is not solved by a single strategy but rather a flexible set of mechanisms that call upon autobiographical episodic memory and semantic memory representations to varying extents, depending, in part, on the level of familiarity with the subject of the mental state inference.
Conclusion
In sum, the current study offers a possible explanation for the neural correspondence consistently observed between AM and ToM. We suggest that there are multiple routes to ToM that involve some balance between autobiographical episodic memory and semantic memory among other processes. The particular strategy adopted likely depends on one’s relationship with the target person and the type of social knowledge gained through past experiences with that person. This in turn, interacts with one’s current goals and the cues or processing resources available to make the inference. Our findings suggest that individuals engaging in ToM more readily draw on past personal experiences when reasoning about the mental states of personally familiar others and on semantic memory or script-like social knowledge when inferring the mental states of unfamiliar others, though both types of processes are likely at play in healthy people. ToM tasks that involve familiar others may better reflect ToM abilities as they occur in the real world and highlight the need for more ecologically valid ToM paradigms.
Supplementary Material
Acknowledgments
This work was funded by a Sloan Research Fellowship, Canadian Institutes of Health Research (CIHR) New Investigator Award, and CIHR Operating Grant to R.S.R., and a CIHR Banting and Best Doctoral Award to J.S.R. Thank you to A. Gilboa, R. Mar, and J.B. Rich for their insightful comments and advice.
Footnotes
The current dataset only included women, as there is evidence that AM (Andreano & Cahill, 2009), and possibly ToM (Baron-Cohen et al., 1997), differ across sex. Although beyond the scope of the current study, sex differences in brain networks underlying AM and ToM is an important topic for future research.
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psyc. 1997;38:813–22. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autistic brain: An fMRI study. Eur J Neurosci. 1999;11:1891–1999. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen CY, Lin CP, Chou KH, Decety J. Love hurts: an fMRI study. Neuroimage. 2010;51:923–929. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Corcoran R. Theory of mind and schizophrenia. In: Corrigan PW, Penn DL, editors. Social Cognition and Schizophrenia. 1. American Psychological Association; Washington, DC: 2001. pp. 149–174. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davachi L. The ensemble that plays together, stays together. Hippocampus. 2004;14:1–3. doi: 10.1002/hipo.20004. [DOI] [PubMed] [Google Scholar]
- Duval C, Bejanin A, Piolino P, Laisney M, de La Sayette V, Belliard S, Eustache F, Desgranges B. Theory of mind impairments in patients with semantic dementia. Brain. 2012;135:228–41. doi: 10.1093/brain/awr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75. [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye-precuneus activation in memory related imagery. NeuroImage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Ramponi C, Richardson-Klavehn A. Experiences of remembering, knowing, and guessing. Conscious Cogn. 1998;7:1–26. doi: 10.1006/ccog.1997.0321. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Keane MM, Ryan L, Verfaellie M. Impaired category fluency in medial temporal lobe amnesia: the role of episodic memory. J Neurosci. 2009;29:10900–10908. doi: 10.1523/JNEUROSCI.1202-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Lieberman MD. Self-knowledge: From philosophy to neuroscience to psychology. In: Vazire S, Wilson TD, editors. Handbook of Self-knowledge. New York: Guilford; 2012. pp. 63–76. [Google Scholar]
- Lukic AS, Wernick MN, Strother SC. An evaluation of methods for detecting brain activations from functional neuroimages. Artif Intell Med. 2002;25:69–88. doi: 10.1016/s0933-3657(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Mashek D, Aron A, Boncimino M. Confusions of self with close others. Pers Soc Psychol Bull. 2003;29:382–392. doi: 10.1177/0146167202250220. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau W, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady CL, McAndrews MP, Winocur G, Nadel L. Functional neuroanatomy of remote episodic (autobiographical), semantic and spatial memory in humans as determined by lesion and functional neuroimaging studies: A unified account based on Multiple Trace Theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Perry D, Hendler T, Shamay-Tsoory SG. Projecting memories: The role of the hippocampus in emotional mentalizing. Neuroimage. 2011;54:1669–1676. doi: 10.1016/j.neuroimage.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and unique neural correlates of autobiographical memory and theory of mind. J Cogn Neurosci. 2010;22:1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa AG, Levine B, Winocur G, Moscovitch M. Amnesia as impairment of detail generation and binding: Evidence from person, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47:2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Moscovitch M. New views on old memories: Re-evaluating the role of the hippocampal complex. Behav Brain Res. 2001;127:183–197. doi: 10.1016/s0166-4328(01)00363-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon SAM, Moscovitch M. Recollective performance advantages for implicit memory tasks. Memory. 2010;18:681–697. doi: 10.1080/09658211.2010.499876. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsycho Soc. 2009;15:645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection and theory-of-mind and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA. I remember you: A role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res. 2012;1428:43–50. doi: 10.1016/j.brainres.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim A. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J Cogn Neurosci. 2011;23:1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48:2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.