Abstract
Trinucleotide repeat (TNR) expansion beyond a certain threshold results in some 20 incurable neurodegenerative disorders where disease anticipation positively correlates with repeat length. Long TNRs typically display a bias toward further expansion during germinal transmission from parents to offspring, and then are highly unstable in somatic tissues of affected individuals. Understanding mechanisms of TNR instability will provide insights into disease pathogenesis. Previously, we showed that enhanced convergent transcription at long CAG repeat tracks induces TNR instability and cell death via ATR activation. Components of TC-NER (transcription-coupled nucleotide excision repair) and RNaseH enzymes that resolve RNA/DNA hybrids oppose cell death, whereas the MSH2 component of MMR (mismatch repair) enhances cell death. The exact role of the MMR pathway during convergent transcription-induced cell death at CAG repeats is not well understood. In this study, we show that siRNA knockdowns of MMR components—MSH2, MSH3, MLHI, PMS2, and PCNA—reduce DNA toxicity. Furthermore, knockdown of MSH2, MLH1, and PMS2 significantly reduces the frequency of ATR foci formation. These observations suggest that MMR proteins activate DNA toxicity by modulating ATR foci formation during convergent transcription.
Keywords: mismatch repair, convergent transcription, ATR DNA damage response, trinucleotide repeat instability, neurodegenerative diseases, cell death
Graphical abstract

Introduction
Trinucleotide repeats (TNRs) are a type of microsatellite sequence distributed in both coding and noncoding regions of the genome [1, 2]. TNRs are hypermutable, gaining or loosing repeat units at a high frequency. Variations in TNR tract length have been proposed to fine-tune gene expression and positively impact evolution [3, 4]. These important evolutionary benefits are balanced by negative effects that occur when repeats cross a length threshold beyond which they are biased towards expansion.
In humans, expanded repeats are the cause of neurodegenerative disorders [2, 5]. As many as 13 TNR diseases are caused by expansion of CAG repeats, as exemplified in Huntington disease (HD), myotonic dystrophy type 1 (DM1), dentatorubralpallidolusian atrophy (DRPLA), and seven different spinocerebellar ataxias (SCAs) [6, 7]. Repeat instability occurs not only in the germlines of affected families but also in many somatic tissues, where they exacerbate disease symptoms [6, 8-10]. By their ability to form intra-molecular secondary structures, expanded repeats evoke deleterious DNA damage responses via DNA metabolic pathways. Thus understanding mechanisms of repeat instability at expanded repeats is critical to any program of therapeutic intervention.
Studies of TNR instability in model organisms—bacteria, yeast, mammals, and human cells—shed light on the spectrum of DNA metabolic processes at play in expanded repeats, including replication, transcription, DNA repair, genome wide demethylation, and rereplication [11-19]. We previously showed that transcription across long CAG repeats induces repeat instability in human cells and defined the modulating role of DNA repair factors in this process [14, 20]. When we sought to study the role of convergent transcription in inducing repeat instability, we discovered a novel phenomenon, ‘DNA toxicity’—a synergistic increase in cell death following convergent transcription at expanded repeats [21, 22]. DNA toxicity could be a potential source of neuronal cell death seen in neurodegenerative patients. Interestingly, a high fraction of the human genome undergoes convergent transcription, indicative of the genome-wide impact of this process [23]. Additionally, convergent transcription-induced cell death is not restricted to CAG repeats. We showed that this process also inhibits cell growth and induces DNA toxicity across other expanded TNRs such as GAA, CGG, and CCTG, suggesting that convergent transcription could be a common cell death mechanism among TNR disorders [24].
Using small interfering RNA (siRNA), we previously showed during convergent transcription that ATR (ataxia-telangiectasia mutated [ATM] and Rad3 related) acts as the key mediator in the DNA damage response via its phosphorylated serine-428 residue [21, 24]. The mechanism of ATR recruitment and its biological role at expanded repeats during convergent transcription is not known. Additionally, we showed that DNA repair components—TC-NER, MMR, and R-loop resolution enzymes—are also important in modulating effects of convergent transcription-induced cell death [25]. In these studies, siRNA knockdown of single MMR component, MSH2, decreased the frequency of cell death, suggesting that the MMR pathway may play a role in inducing cell death during convergent transcription.
Ordinarily, MMR—a highly conserved pathway—corrects DNA mismatches or small insertions and deletions (indels) in the DNA [26-28]. In human and mammalian cells, the damage recognition step—of a mismatched base or an indel—is facilitated by two main complexes: MutSα (MSH2/MSH6) and MutSβ (MSH2/MSH3), which display some overlapping affinity to either substrate [28, 29]. After binding to DNA, the MutS complex recruits the MutL complex. MutLα (MLH1/PMS2) by means of its endonuclease activity is known to cleave the mismatch-containing strand, which is followed by strand extension and joining [29]. The importance of this process is exemplified by the occurrence of debilitating hereditary and sporadic cancers in humans, especially the microsatellite instability (MSI) disorders, where defective MMR fails to correct replication errors at repetitive sequences [26, 30].
Interestingly, recent studies indicate MMR-independent roles for both MutS and MutL proteins, in which ATR kinase is activated and recruited to methylation based DNA damage sites [31]. Also, MSH2 is shown to specifically bind and recruit ATR to nuclear foci during cisplatin treatment in human cells, which is independent of RPA, Rad17, and Mus8 proteins [32, 33]. Based on these observations, we hypothesized that the MMR proteins are involved in inducing cell death via ATR during convergent transcription. In this study, we show that knockdown of MSH2, MSH3, MLH1, PMS2, and PCNA during convergent transcription across CAG repeats caused less cell death. Additionally, knockdown of MSH2, MSH3, and MLH1 reduced the ATR foci formation at the expanded repeat, suggesting that the MMR pathway is involved in inducing cell death via ATR signaling. This is the first report showing an association of MMR proteins with ATR foci formation at CAG repeats during convergent transcription.
Materials and Methods
Cell Lines and Culture
DIT7 cells were derived from HT1080 cells via the intermediate cell line RS11, as described previously [21]. Briefly, RS11 cells express the rtTA protein under a pTRE-CMVmini promoter when induced with doxycycline. The rtTA protein is a fusion construct of reverse tetracycline repressor and HSV VP16 transcription activation domain. RS11 cells also express genes for RheoReceptor-1 and RheoActivator, under the control of the pNERB-X1 promoter when induced with RSL1. DIT7 cells are derivatives of RS11 cells that each carry a single integrated copy of an HPRT minigene with a CAG95 tract within its intron. Promoters pTRE-CMVmini and pNERB-X1 flank the minigene and drive sense and antisense transcription, respectively, across the HPRT minigene (Figure 1). An additional cell line, DIT7-R103, was derived from DIT7 by contraction of the repeat to 15 units. The DIT7 and DIT7-R103 cell lines were grown at 37°C with 5% CO2 in DMEM/F-12 medium (Gibco) supplemented with 10% fetal bovine serum (Hyclone, Thermo Scientific and 1% MEM nonessential amino acids (Gibco)). Cells were trypsinized using 0.25% trypsin-EDTA (Gibco) for passaging and further analysis.
Figure 1. Schematic of DIT7 and DIT7-R103 cell lines used to study the effects of convergent transcription-induced cell death.

DIT7 cells carry 95 units of CAG repeat tract and DIT7-R103 carries 15 units. Both CAG95 and CAG15 tracts were integrated within the center of 2.1kb long intron, as shown. The HPRT minigene was modified to contain promoters pTRE-pCMVmin and pNEBR-X1 at its ends. Expression of the modified HPRT minigene is regulatable in the sense direction by doxycycline and in the antisense direction by RSL1.
Experimental Outline and siRNA treatments
For a typical experiment to test convergent transcription-induced effects on long repeats, on day -1, 100,000 DIT7 or DIT7-R103 cells were plated in each well of a 6-well plate. After 24 hours (Day 0), inducers—doxycycline and RSL1—were added to the media at a concentration of 2 μg/ml and 500 nM, respectively. Because the half-life of doxycycline is 24 hours, from day 1 through 4, doxycycline was added daily at a concentration of 1 μg/ml without any additional RSL1. Viable and dead cells were counted on day 5.
For siRNA treatments, on day -3, 100,000 DIT7 or DIT7-R103 cells were plated in each well of a 6-well plate. On day -2, siRNAs at a final concentration of 200 nM were transfected into cells using Oligofectamine (Invitrogen), per the manufacturer's protocol. For single gene knockdowns, 100 nM target specific siRNA and 100 nM vimentin control siRNA were used for transfection. For double knockdowns, 100 nM of each siRNA was used to knockdown both target genes. In all cases, total siRNAs concentrations were 200 nM. siRNA sequences (Dharmacon Thermo Scientific) used in this study are listed in Table 1. After 48 hours (Day 0), a second round of siRNA treatment was administered, with the addition of inducers—doxycycline and RSL1—at the same concentrations stated above. Additional doxycycline was added to media from day 1 through day 4 at a concentration of 1 μg/ml. Knockdown efficiencies of target genes were evaluated on day 1, by isolating RNA and measuring percent knockdown by real time RT-PCR, as shown before [20]. In each case, at least 70% gene expression was lowered by targeted siRNA treatment.
Table 1. Table shows sequences of siRNAs used in knocking down genes.
| Gene | siRNA sequence |
|---|---|
| Vimentin | GAAUGGUACAAAUCCAAGU |
| MSH2-1 | UCUGCAGAGUGUUGUGCUU |
| MSH2-2 | GGAGGUAAAUCAACAUAUA |
| MSH3-1 | AUACGCCGCUAGAAUUACA |
| MSH3-2 | GCAAGGAGUUAUGGAUUAA |
| MSH6-1 | GAAUACGAGUUGAAAUCUA |
| MSH6-2 | CGCCAUUGUUCGAGAUUUA |
| MLH1-1 | AUCAGGCAGGUUAGCAAGCUG |
| PMS2-1 | ACUGAUUUCCUUGCCAACUAGUAAA |
| PMS2-2 | GGCCAACCAUGAGACACAUCGCCAA |
| PCNA-1 | AAGCACCAAACCAGGAGAAAG |
Induction of Transcription
At all times, cells were grown and maintained in the absence of transcriptional inducers in complete medium. Under experimental conditions, transcription was induced on day 0 by adding inducers. For sense transcription of the HPRT minigene, 2 μg/mL doxycycline (Sigma Aldrich) was added on day 0, followed by the addition of 1 μg/mL of doxycycline for the next 5 days. For inducing antisense transcription, RSL1 (Rheoswitch ligand 1) (NEB) was added once to the media, to a final concentration of 500 nM.
Dead Cell Measurements
Percent dead cells were calculated by dividing number of nonadherent, or floating cells, by the total number of cells (the sum of adherent and nonadherent cells). We define adherent cells as viable cells, and nonadherent cells as dead cells [21]. We previously confirmed the viability of adherent cells by showing that >96% adherent cells do not incorporate propidium iodide (a dye retained in dead cells only) [21]. We did not adjust for the very small percent of dead cells present in the adherent population. On day 5, 100 μl of media containing floating cells was transferred to 10 mL isotonic media and number of cells were counted using a coulter cell counter. For counting adherent cells, cells were first trypsinized and then counted in the same way.
Immunofluorescence Microscopy
For immunofluorescence microscopy, DIT7 cells were grown on poly-L-lysine-coated coverslips in 6-well plates until they reached at least 50% confluence. The overall experimental outline for inducing transcription and siRNA-mediated gene knockdown was the same as described above. On day 4, cells were first fixed with methanol for 20 minutes at -20°C and then rehydrated by washing twice with 1× PBS. Then, cells were incubated in 70% ethanol for 10 minutes at 4°C followed by washing with 1× PBS. After the rehydration step, primary antibodies and 2% goat serum in PBS were added to cells and allowed to incubate at 4°C overnight. In this study, we used rabbit polyclonal ATR-S428P antibody to detect ATR. The next day, cells were washed once with 1× PBS and then incubated with secondary antibody and 2% goat serum for 1 hour at room temperature in the dark. In the last step, ProLong Gold antifade reagent with DAPI (4′, 6-diamidino-2-phenylindole; Molecular Probe) was mounted on cells and finally the coverslips were sealed with nail polish. Fluorescent signal was examined in a Nikon Eclipse TE 2000- U microscope. In each case, randomly selected fields from 3 independent cover slips were chosen, and a total of 1000 cells was counted for each. Results were summed to calculate percentage of positive fluorescent signal for each treatment.
Statistics
Statistical significance of means and standard deviations was carried out using Student's t test.
Results
Knockdown of MMR components reduces convergent transcription-induced cell death
In our earlier study, we constructed two human cell lines—DIT7 with 95 CAG units and DIT7-R103 with 15 CAG units—to establish the effects of convergent transcription on CAG repeat tracts [21]. The cytomegalovirus (CMV) promoter pTRE-CMV—doxycycline inducible—controls sense transcription from 5′ side and pNEBRX1 promoter—RSLI inducible—controls antisense transcription from 3′ side of the modified gene (Figure 1). Inducing transcription from both the sense and antisense promoters caused substantial cell death; however if MSH2 was knocked down during convergent transcription, cell death was significantly reduced. This result suggested that the MMR pathway might be involved in causing cell death during convergent transcription.
To delineate the role of the MMR pathway during convergent transcription, we knocked down individual MMR components and measured the effect on cell death in DIT7 cells. In most cases, we tested two different siRNAs to rule out off target effects, and real-time PCR was used to determine knockdown efficiency (Table 1). We tested the knockdown of the MutSα (MSH2 and MSH6) complex, which recognizes mismatched nucleotides; the MutSβ (MSH2 and MSH3) complex, which is responsible for recognizing IDLs (insertion-deletion loops); MutLα (MLH1 and PMS2), the second crucial component of the MMR pathway that is recruited by MutS complexes to DNA; and PCNA (proliferating cell nuclear antigen), a general DNA-clamp known to enable the localization of MSH3 and MSH6 to replication foci [28, 34]. As we showed before, and observe in this study, knockdown of MSH2 reduced the percentage of dead cells induced by convergent transcription across CAG95 repeats by 11 percent compared to vimentin control (Figure 2 and Table 2; P<0.05). Similarly, knockdown of MSH3 reduced cell death by at least 11 percent (Figure 2 and Table 2; P<0.01), but knockdown of MSH6 showed no statistical difference from vimentin control. These results suggested that the MutSβ complex—and not the MutSα complex—of MMR was responsible for initiating the cell death mechanisms during convergent transcription.
Figure 2. The effect of siRNA knockdowns of MMR pathways genes on convergent transcription-induced cell death in DIT7 cells.

The frequencies of dead DIT7 cells generated by convergent transcription following siRNA-mediated knockdown are: vimentin (47%), MSH2-1 (42%), MSH2-2 (42%), MSH3-1 (40%), MSH3-2 (42%), MSH6-1 (45%), MSH6-2 (46%), MLH1-1 (33%), PMS2-1 (40%), PMS2-2 (37%) and PCNA-1 (42%). The sequences of the siRNAs are shown in Table 1. Percent dead cells were calculated by dividing the number of floating (nonadherent) cells by the total number of cells (adherent and nonadherent). Data are the average of 6 independent siRNA knockdown experiments each with 3 biological replicates. Error bars represent standard deviations in each case. P values are indicated: ★P<0.05, ★★P<0.01, ★★★P<0.001.
Table 2. Decrease in percentage of dead cells after siRNA treatment.
| siRNA treatment | Decrease in cell death %a | |
|---|---|---|
| DIT7 | DIT7-R103 | |
| Vimentin | 0 | 0 |
| MSH2-1 | 11 | 60 |
| MSH2-2 | 11 | 36 |
| MSH3-1 | 15 | 18 |
| MSH3-2 | 11 | 14 |
| MSH6-1 | 4 | 0 |
| MSH6-2 | 2 | -7 |
| MLH1-1 | 29 | 36 |
| PMS2-1 | 15 | 36 |
| PMS2-2 | 21 | 25 |
| PCNA-1 | 11 | 25 |
| MSH2-1 + MLH1-1 | 21 | 50 |
| MLH1-1 & PMS2-1 | 30 | 36 |
| MSH2-1 & PCNA-1 | 25 | 53 |
The percentage change in cell death after specific siRNA treatment was calculated as {[(% dead cells after vimentin siRNA) - (% dead cells after specific siRNA)] / (% dead cells after vimentin siRNA)} (100%)
Knockdown of MLH1 reduced the percentage of dead cells during convergent transcription by 25 percent (Figure 2 and Table 2; P<0.01) and PMS2 by more than 15 percent (Figure 2 and Table 2; P<0.01), indicating that both components of the MutLα complex were critical for inducing the cell death mechanisms at long repeats. And finally knockdown of PCNA in DIT7 cells showed an 11 percent decrease in cell death during convergent transcription (Figure 2 and Table 2; P<0.01), suggestive of a possible role for PCNA in convergent transcription induced-cell death.
We have observed that modest effects in DIT7 cells are mirrored by much more substantial effects in DIT7-R103 cells, for reasons we do not understand, but have previously speculated about [25]. We tested the effects of knockdown of MMR components when convergent transcription was induced at the small CAG15 repeats in DIT7-R103 cells. As with DIT7 cells, target-specific siRNAs were transfected into DIT7-R103 cells and the percentage of cell death was measured. Knockdown of MSH2 resulted in at least a 35 percent reduction in cell death (Figure 3 and Table 2; P<0.001) and knockdown of MSH3 had a 14 percent effect on cell death (Figure 3 and Table 2; P<0.05). Similar to the results in DIT7 cells, knockdown of MSH6 had a non-significant effect (Figure 3 and Table 3). Knockdown of MLH1 and PMS2 had a 36 and a 25 percent reduction in cell death (Figure 3 and Table 2; MLH1, P<0.001; PMS2, P<0.01), suggestive of a role of the MutLα complex in relaying the cell death signal during convergent transcription of CAG repeats. Finally, knockdown of PCNA also resulted in reduction of cell death by 25 percent (Figure 3 and Table 2; P<0.001). These results suggest a critical role of MMR components in relaying the cell death signal during convergent transcription through CAG repeats.
Figure 3. The effect of siRNA knockdowns of MMR pathways genes on convergent transcription-induced cell death in DIT7-R103 cells.

The frequencies of dead DIT7-R103 cells generated during convergent transcription following siRNA-mediated knockdown are: vimentin (28%), MSH2-1 (11%), MSH2-2 (18%), MSH3-1 (23%), MSH3-2 (24%), MSH6-1 (28%), MSH6-2 (30%), MLH1-1 (18%), PMS2-1 (18%), PMS2-2 (21%) and PCNA-1 (21%). Percentages of dead cells were calculated by dividing the number of floating (nonadherent) cells by the total number of cells (adherent and nonadherent). Data are the average of 6 independent siRNA knockdown experiments each with 3 biological replicates. Error bars represent standard deviations. P values are indicated: ★P<0.05, ★★P<0.01, ★★★P<0.001.
Double knockdown of MMR proteins results in a similar reduction in cell death as single knockdown
In order to test whether MMR components act in the same pathway in modulating convergent transcription-induced cell death, we knocked down pairs of MMR components and measured cell death. Removal of both MSH2 and MLH1 from both DIT7 and DIT7-R103 cells resulted in a similar percentage of dead cell as their individual knockdown (Figure 2 and 3; Table 2), suggesting that the MutSβ and MutLα complexes of MMR work in the same pathway during convergent transcription-induced cell death. Next we asked if both components of the MutLα complex had similar roles during convergent transcription. By knocking down both MLH1 and PMS2, in DIT7 and DIT7-R103 cells, we observed a similar percentage of dead cells in double knockdown cells versus their single knockdown counterparts (Figure 2 and 3; Table 2), indicating that an intact MutLα complex was required in the observed cell death phenotype. Knockdown of both MSH2 and PCNA in DIT7 and DIT7-R103 cells shows an enhanced reduction in cell death (Figure 2 and 3, Table 2) suggesting that PCNA might have a role distinct from that of MMR components.
Knockdown of MSH2, MSH3 and MLH1 results in fewer ATR foci during convergent transcription
In our previous study we showed that in addition to RNAP II, ATRIP, and TOPBP1, convergent transcription also enriched ATR at CAG repeats. In addition ATR, p53, and CHK1 were phosphorylated, indicating that the ATR stress response pathway had been activated [21, 35]. Normally, an activated ATR pathway is expected to initially halt the cell cycle to allow repair of damaged DNA, but later trigger apoptosis if the damage is unrepairable [36, 37]. The source of ATR activation during convergent transcription is unknown. Because the appearance of phosphorylated ATR during convergent transcription preceded that of ATM, we hypothesized that ATR may be recruited and activated at a very early step during convergent transcription. Because MSH2 and MLH1 are known to activate ATR directly during alkylation, methylation and cisplatin damage [31-33], we hypothesized that MMR components may be responsible for activating ATR here. In order to test this hypothesis, we knocked down MSH2, MSH3, and MLH1 in DIT7 cells and measured the number of ATR-S428P fluorescent foci amongst DAPI stained nuclei during convergent transcription. We found that removing MSH2, MSH3, or MLH1 significantly reduced the ATR signal by more than 6 fold (Figure 4A and 4B), suggesting that MMR proteins participate in the activation of ATR stress response during convergent transcription.
Figure 4. Effects of siRNA knockdown of MMR pathway genes on the percentages of ATR-S428P positive cells.

(A) Representative images of ATR foci formed after vimentin and MSH2 knockdown in DIT7 cells. DAPI stained cells are shown on the left and ATR-S428P foci formed during convergent transcription are shown on the right (B) The percentages of cells positive for ATR-S428P staining were determined after knockdown of vimentin (30%), MSH2-1 (5%), MSH3-1 (3%) and MLH1 (4.5%). Data are the average of 1000 cells from each randomly selected field from 3 independent coverslips. Error bars represent standard deviations in each case. P values are indicated ★P<0.05, ★★P<0.01, ★★★P<0.001.
Discussion
Convergent transcription—induction of both sense and antisense transcription—across trinucleotide repeats (TNRs) is now recognized to stimulate repeat instability and DNA toxicity in human cells [21, 24, 25, 38]. The phenomenon of convergent transcription is a common occurrence in the human genome, where antisense transcription traverses 30% of human genes, including those genes that harbor CAG, CGG, and GAA repeats and are associated with TNR diseases [23, 39-45]. Antisense transcription may affect gene expression by modulating the efficiency of sense transcription, for instance, by head-on collision between RNAP II complexes; or by recruiting chromatin remodeling factors; or by generating double-stranded RNAs, which may regulate sense transcription [46, 47]. During convergent transcription at CAG repeats, as studied in the HPRT assay, the antisense transcript would allow formation of the more stable CTG hairpins in the untranscribed strand. Coupled with the CAG hairpins formed by sense transcription, convergent transcription might reasonably be expected to induce a stronger RNAP II stalling and an even stronger phenotype [38].
In our earlier studies, we found that convergent transcription-induced cell death proceeded by activation of ATR DNA damage response and that DNA repair factors—TC-NER, the MSH2 factor of MMR, and RNase (H1 & H1-2A)—modulated both repeat instability and cell death [21, 25]. In this study, we analyzed the role the MMR by examining other important proteins in this pathway. Loss of MSH2, MSH3, MLH1, PMS2, and PCNA lowered the percentage of cell death induced by convergent transcription. In addition, removal of MMR components—MSH2, MSH3, and MLH1—reduced the number of ATR foci formed during convergent transcription.
A typical ATR activation requires RPA coated ssDNA or unligated nicks along with the presence of other activating factors such as ATRIP, 9-1-1 complexes, and TopBP1 [36, 48-55]. Activated ATR phosphorylates and activates other downstream proteins—Chk1, Nbs1, SMC1, and p53, leading to repair, transcriptional activation, cell cycle arrest and apoptosis [56]. Strikingly, both MutSα and MutLα complexes recruit ATR-ATRIP complex in an RPA-independent manner to O6-methyl-G/T mismatches. Here, activation of ATR downstream signaling molecules—p53, Chk1, Cdc25A, and SMC1—in response to alkylating damage was also dependent on both MutSα and MutLα complexes [31]. In fact, MSH2 is a major ATR-binding protein, which enables its recruitment and activation to cisplatin and N-methyl-N-nitrosurea-induced DNA damage, independent of RPA, Rad17, and Rad9-Hus1-Rad1 protein complexes [32, 57]. All these observations show the possibility that MMR proteins recruit and activate the ATR DNA damage-signaling pathway during convergent transcription.
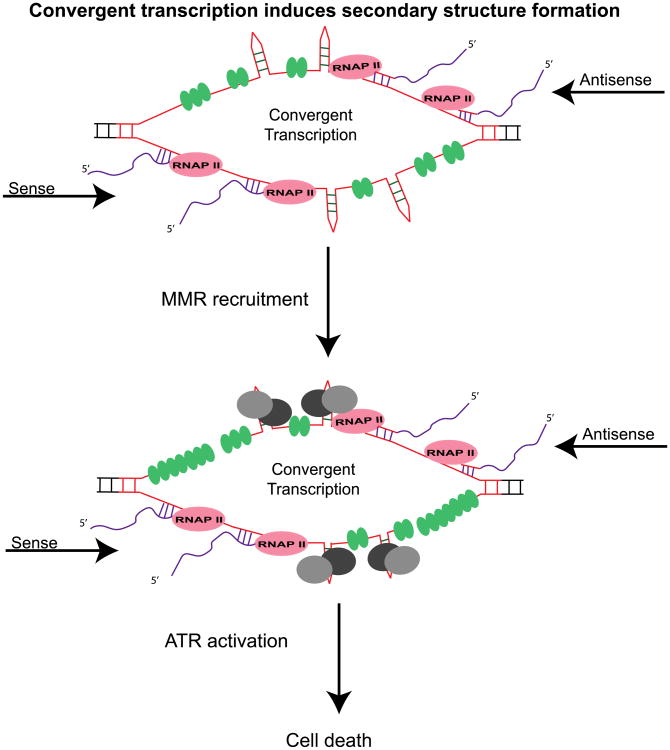
ATR recruitment and activation by MMR proteins during convergent transcription-induced cell death could occur in a number of ways. Our underlying assumption is that RNAP II complexes travelling from sense and antisense direction stall at long CAG repeats, creating a double-bubble (Figure 5) [58]. Unwound CAG/CTG repeats are known to form intramolecular secondary structures that are bound by the MutSβ complex (Figure 5) [59]. It may be that MutSβ not only binds and stabilizes these secondary structures, but also recruit the MutLα complex, and together these complexes directly recruit and activate ATR signaling [59, 60].
Figure 5. Speculative model of ATR induction and cell death by MMR during convergent transcription.
Convergent transcription through CAG repeats tract induces hairpins and stem-loop structure formation, thus stalling RNAP II. MMR proteins (grey and black circles) bind and stabilize these secondary structures to further stall RNAP II. MMR proteins directly recruit and activate ATR at a stalled convergent transcription bubble and cause cell death. Additional ATR activation could come from RPA (green oval circles) coated single-stranded DNA resulting from nicks generated by MMR's endonuclease activity.
The decrease in cell death and reduction in ATR foci by knockdown of MSH2, MSH3, MLH1, and PMS2 (Figure 2, 3, 4) support this possibility. These observations are fit with the data showing that MutSα and MutLα complex-mediated ATR activation upon alkylating and cisplatin DNA damage. This means that the presence of MutSβ and MutLα is conducive to DNA toxicity mechanisms via ATR at the double-bubble.
Another way to explain how MMR might modulate cell death via ATR signaling during convergent transcription is that the MMR pathway is the source of single-stranded nicks that allow bound RPA to activate ATR. MMR may create nicks in two ways. First, bound MutSβ and MutLα complexes stall RNAP II complex by stabilizing the intramolecular secondary structures. Then stalled RNAP II triggers TC-NER to initiate repair, which may leave behind nicks and ssDNA to be bound by RPA. Alternatively, when MutS recruits MutL to the stalled transcription complex, a traditional MMR pathway may be initiated to repair the intramolecular secondary structures. Here, MutLα cleaves adjacent strand by means of its endonuclease activity and introduces nicks in the adjacent ssDNA. RPA-bound single-strand ends are known precursors to ATR activation [36, 52].
Conclusion
In this study we have shown that the MMR protein complexes, MutSβ and MutLα, act in one pathway and contribute to convergent transcription-induced cell death at CAG repeats. Additionally, we found that the absence of the MMR proteins lowered ATR foci formation. We hypothesize that MMR proteins induce cell death during convergent transcription via ATR signaling. This observation resolves the conundrum as to the origin of ATR signaling during convergent transcription.
Mechanistically, we speculate that during convergent transcription, when both sense and antisense transcription are induced in the CAG repeat tract, RNAP II polymerase induces formation of various secondary structures, including hairpins and slip-outs (Figure 5). The formation of these secondary structures and their stabilization by MutSβ, stall additional RNAP II complexes, thus creating a double-bubble structure [38]. MutSβ bound to these secondary structures either way directly recruit ATR or bind to the MutLα complex whose endonucleolytic activity generates enough RPA coated ssDNA to activate the ATR DNA-damage response.
Highlights.
Mismatch repair pathway enhance convergent transcription-induced cell death at CAG repeats
MutSβ and MutLα act in the same pathway to induce cell death during convergent transcription.
MSH2, MSH3 and MLH1 activate ATR at CAG repeats during convergent transcription.
Acknowledgments
We thank members of Wilson lab for helpful discussions. This project was supported by a grant from the NIH (GM38219) to J.H.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richard GF, Kerrest A, Dujon B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol Mol Biol Rev. 2008;72(4):686–727. doi: 10.1128/MMBR.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearson CE, Nichol Edamura K, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6(10):729–42. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- 3.Mittelman D, Wilson JH. Stress, genomes, and evolution. Cell Stress Chaperones. 2010;15(5):463–6. doi: 10.1007/s12192-010-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinces MD, et al. Unstable tandem repeats in promoters confer transcriptional evolvability. Science. 2009;324(5931):1213–6. doi: 10.1126/science.1170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 6.La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet. 2010;11(4):247–58. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6(10):743–55. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 8.Swami M, et al. Somatic expansion of the Huntington's disease CAG repeat in the brain is associated with an earlier age of disease onset. Hum Mol Genet. 2009;18(16):3039–47. doi: 10.1093/hmg/ddp242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Hubert L, Jr, Wilson JH. Transcription destabilizes triplet repeats. Mol Carcinog. 2009;48(4):350–61. doi: 10.1002/mc.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee N, S BA, W JH. Microsatellite repeats: canaries in the coal mine in Stress-induced mutagenesis. Springer; Ney York, NY: 2013. [Google Scholar]
- 11.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447(7147):932–40. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 12.McMurray CT. Mechanisms of trinucleotide repeat instability during human development. Nat Rev Genet. 2010;11(11):786–99. doi: 10.1038/nrg2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dion V. Tissue specificity in DNA repair: lessons from trinucleotide repeat instability. Trends Genet. 2014;30(6):220–9. doi: 10.1016/j.tig.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Dion V, Wilson JH. Transcription promotes contraction of CAG repeat tracts in human cells. Nat Struct Mol Biol. 2006;13(2):179–80. doi: 10.1038/nsmb1042. [DOI] [PubMed] [Google Scholar]
- 15.Bowater RP, et al. Transcription increases the deletion frequency of long CTG.CAG triplet repeats from plasmids in Escherichia coli. Nucleic Acids Res. 1997;25(14):2861–8. doi: 10.1093/nar/25.14.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierdl M, et al. Destabilization of simple repetitive DNA sequences by transcription in yeast. Genetics. 1996;143(2):713–21. doi: 10.1093/genetics/143.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovtun IV, et al. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447(7143):447–52. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubert L, Jr, et al. Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1. Hum Mol Genet. 2011;20(24):4822–30. doi: 10.1093/hmg/ddr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee N, et al. Environmental stress induces trinucleotide repeat mutagenesis in human cells. Proc Natl Acad Sci U S A. 2015;112(12):3764–9. doi: 10.1073/pnas.1421917112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Wilson JH. Diverse effects of individual mismatch repair components on transcription-induced CAG repeat instability in human cells. DNA Repair (Amst) 2009;8(8):878–85. doi: 10.1016/j.dnarep.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, et al. Convergent transcription through a long CAG tract destabilizes repeats and induces apoptosis. Mol Cell Biol. 2010;30(18):4435–51. doi: 10.1128/MCB.00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamori M, Pearson CE, Thornton CA. Bidirectional transcription stimulates expansion and contraction of expanded (CTG)*(CAG) repeats. Hum Mol Genet. 2011;20(3):580–8. doi: 10.1093/hmg/ddq501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama S, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309(5740):1564–6. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 24.Lin WY, Lin Y, Wilson JH. Convergent transcription through microsatellite repeat tracts induces cell death. Mol Biol Rep. 2014;41(9):5627–34. doi: 10.1007/s11033-014-3432-y. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Wilson JH. Nucleotide excision repair, mismatch repair, and R-loops modulate convergent transcription-induced cell death and repeat instability. PLoS One. 2012;7(10):e46807. doi: 10.1371/journal.pone.0046807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 27.Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281(41):30305–9. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer RR, et al. DNA mismatch repair: functions and mechanisms. Chem Rev. 2006;106(2):302–23. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 29.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–46. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 30.Muro Y, et al. DNA mismatch repair enzymes: Genetic defects and autoimmunity. Clin Chim Acta. 2015;442C:102–109. doi: 10.1016/j.cca.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22(4):501–10. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pabla N, et al. hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem. 2011;286(12):10411–8. doi: 10.1074/jbc.M110.210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam EA, Cortez D. ATR signalling: more than meeting at the fork. Biochem J. 2011;436(3):527–36. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AB, et al. Functional interaction of proliferating cell nuclear antigen with MSH2-MSH6 and MSH2-MSH3 complexes. J Biol Chem. 2000;275(47):36498–501. doi: 10.1074/jbc.C000513200. [DOI] [PubMed] [Google Scholar]
- 35.Ljungman M. The transcription stress response. Cell Cycle. 2007;6(18):2252–7. doi: 10.4161/cc.6.18.4751. [DOI] [PubMed] [Google Scholar]
- 36.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers K, et al. ATR and Chk1 suppress a caspase-3-dependent apoptotic response following DNA replication stress. PLoS Genet. 2009;5(1):e1000324. doi: 10.1371/journal.pgen.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y, Wilson JH. Transcription-induced DNA toxicity at trinucleotide repeats: double bubble is trouble. Cell Cycle. 2011;10(4):611–8. doi: 10.4161/cc.10.4.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung DW, et al. A natural antisense transcript at the Huntington's disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20(17):3467–77. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladd PD, et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16(24):3174–87. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 41.Moseley ML, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38(7):758–69. doi: 10.1038/ng1827. [DOI] [PubMed] [Google Scholar]
- 42.Wilburn B, et al. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington's disease-like 2 mice. Neuron. 2011;70(3):427–40. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho DH, et al. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell. 2005;20(3):483–9. doi: 10.1016/j.molcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Boque-Sastre R, et al. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1421197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler JS, Napierala M. Friedreich's ataxia - a case of aberrant transcription termination? Transcription. 2015:1–4. doi: 10.1080/21541264.2015.1026538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris KV, Vogt PK. Long antisense non-coding RNAs and their role in transcription and oncogenesis. Cell Cycle. 2010;9(13):2544–7. doi: 10.4161/cc.9.13.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beiter T, et al. Antisense transcription: a critical look in both directions. Cell Mol Life Sci. 2009;66(1):94–112. doi: 10.1007/s00018-008-8381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamper AM, et al. ATR kinase activation in G1 phase facilitates the repair of ionizing radiation-induced DNA damage. Nucleic Acids Res. 2013;41(22):10334–44. doi: 10.1093/nar/gkt833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33(5):547–58. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennarun G, et al. ATR contributes to telomere maintenance in human cells. Nucleic Acids Res. 2010;38(9):2955–63. doi: 10.1093/nar/gkp1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marteijn JA, et al. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15(7):465–81. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 52.Fanning E, Klimovich V, Nager AR. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 2006;34(15):4126–37. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortez D, et al. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294(5547):1713–6. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 54.Yang XH, Zou L. Recruitment of ATR-ATRIP, Rad17, and 9-1-1 complexes to DNA damage. Methods Enzymol. 2006;409:118–31. doi: 10.1016/S0076-6879(05)09007-5. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai A, et al. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124(5):943–55. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 56.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21(13):4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Qin J. MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci U S A. 2003;100(26):15387–92. doi: 10.1073/pnas.2536810100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salinas-Rios V, Belotserkovskii BP, Hanawalt PC. DNA slip-outs cause RNA polymerase II arrest in vitro: potential implications for genetic instability. Nucleic Acids Res. 2011;39(17):7444–54. doi: 10.1093/nar/gkr429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen BA, et al. (CAG)(n)-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat Struct Mol Biol. 2005;12(8):663–70. doi: 10.1038/nsmb965. [DOI] [PubMed] [Google Scholar]
- 60.Pearson CE, et al. Human MSH2 binds to trinucleotide repeat DNA structures associated with neurodegenerative diseases. Hum Mol Genet. 1997;6(7):1117–23. doi: 10.1093/hmg/6.7.1117. [DOI] [PubMed] [Google Scholar]