Abstract
While previous reports have demonstrated the efficacy of regulatory T cell therapy in the prevention of diabetes, systemic immunocompromise and Treg instability remain key safety concerns. Here we examined the influence of induced Treg (iTreg) cell therapy on anti-viral host defense and autoimmune T cell responses during acute viral infection in a murine model of autoimmune diabetes. Protective transfers of iTregs maintained IL-10 expression, and expanded in vivo and controlled diabetes, despite losing FoxP3 expression. Adoptive transfer of iTregs affected neither the primary anti-viral CD8 T cell response nor viral clearance, although a significant and sustained suppression of CD4 T cell responses was observed. Following acute viral clearance, iTregs transferred early suppressed both CD4 and CD8 T cell responses, which resulted in the reversion of diabetes. These observations indicate that iTregs suppress local autoimmune processes while preserving the immunocompetent host's ability to combat acute viral infection.
Keywords: Regulatory T cells, Diabetes, Viral infection, Stability, Safety, Therapy
1. Introduction
FoxP3+ regulatory T cells (Treg) are a class of suppressive T cells central to the induction and maintenance of immune tolerance [1–6]. In the steady state Tregs function by suppressing T-cell proliferation and cytokine responses directed against autoantigens and commensal bacteria. The ability of Tregs to maintain and restore tolerance makes them attractive candidates for the development of cellular therapies aimed at treating autoimmune disorders, such as type 1 diabetes (T1D). Although the key triggering events are still subject to debate, T1D is widely recognized as a chronic autoimmune disorder resulting from the destruction of insulin-producing beta cells of the pancreas [7–9]. In addition to the body of evidence generated with murine models, several key disease characteristics in humans implicate an autoimmune etiology in the development of T1D, including the presence of lymphocyte infiltration into the pancreas, antibodies reactive to islet cell proteins and various described genetic susceptibilities [10–13]. Murine models of T1D have indicated a deficiency in either frequency and/or function of Tregs as a parameter in the development of disease [14–18]. Furthermore the provision of functional Tregs (either adoptively transferred or in vivo induced) has been found to be protective [19], thus fueling the interest in Tregs as a promising T1D immunotherapeutic approach [20,21]. Early studies employing the NOD-SCID model found that transfer of total CD4+ CD25+ T cells taken from young pre-diabetic NOD mice conferred protection against diabetic onset [14,22,23]. Similarly, in immunocompetent NOD and rat models of T1D, transfer of Tregs was found to either block or reverse diabetes [20,21].
Clinical application of Treg therapy in the treatment of T1D is currently being investigated in both the U.S. and Poland. A phase I clinical trial (ClinicalTrials.gov Identifier NCT01210664), underway at the University of California San Francisco, is aimed at assessing the safety of autologous in vitro expanded Treg therapy in adults recently diagnosed with T1D. Additionally, a report detailing the effects of autologous Treg transfer in newly diagnosed children found that a single transfer of Tregs reduced insulin dependency and preserved C-peptide levels [24]. Although promising, it remains unclear whether such therapy will prove safe in the face of acute viral infection, and moreover whether the presence of exogenous Tregs would impair the host's ability to clear an acute viral infection. Likewise it remains to be determined whether infection with a viral pathogen would inhibit the ability of Treg therapy to exert its protective effect. This report investigates the stability and function of in vitro induced Tregs (iTregs) under the influence of acute viral infection and how therapeutic iTregs affect host defense against viral pathogens, while maintaining their ability to protect against T1D.
Global suppression of T cell responses has led to delayed disease progression in T1D trials; however, such approaches may render the patient sensitive to opportunistic infection, and as such current efforts are focused on more targeted therapeutic approaches [25–28]. Treg therapy is one such strategy currently under investigation in both murine models and clinical trials. Immunotherapeutic transfer of Tregs is aimed at restoring peripheral tolerance to beta cell antigens without ablating protective effector responses to foreign antigens. While classical immunosuppressive therapies have widely recognized off target effects, Treg therapy must also be evaluated for safety. Three scenarios in particular pose potential hazards including: outgrowth of contaminating effector cells, conversion of Tregs in vivo to T effectors (Teff), and global suppression through systemic cytokine release or antigen independent suppression of APC function.
Here the RIP-GP murine model of T1D was utilized to address questions of efficacy and safety of iTreg therapy in T1D and acute viral infection. RIP-GP transgenic mice express the lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP) under the control of the rat insulin promoter (RIP) resulting in the expression of the viral GP on the beta cells [29–31]. Infection of RIP-GP mice with LCMV breaks tolerance and induces T-cell mediated destruction of insulin producing beta cells [32]. The RIP-GP T1D model provides a unique opportunity to test the safety, stability and efficacy of Treg therapy in a worst-case scenario, where the transferred Tregs recognize both the beta cell autoantigen and viral antigen in an acute inflammatory model. We report that a single iTreg transfer leads to the reversion of diabetes despite the loss of FoxP3 expression in transferred iTregs during acute viral infection. While iTreg transfer leads to the early suppression of CD4 T effector responses, suppression of CD8 Teff responses was delayed and did not impact viral titers or clearance. These data suggest that despite the loss of FoxP3 expression, iTregs transferred into immunocompetent RIP-GP mice did not convert to Teff, provided protection from diabetes and did not interfere with host anti-viral defense.
2. Materials and methods
2.1. Mice
C57BL/6 mice were purchased from the Jackson Laboratory and housed for two weeks prior to infection or spleen harvest. RIP-GP mice, which express the viral GP under the control of the rat insulin promoter, were bred in house [30]. Smarta mice, TCR transgenic for the dominant I-Ab-restricted LCMV GP61–80 peptide, and FoxP3 GFP reporter mice were bred in house and previously described [33,34]. Smarta mice were backcrossed onto FoxP3-GFP reporter background and screened for both reporter and TCR transgene expression prior to use. Blood glucose was measured weekly using the OneTouch Ultra system (LifeScan). Mice with blood glucose values >300 mg/dL were scored diabetic.
2.2. Ethics statement
The La Jolla Institute for Allergy and Immunology Institutional Animal Care and Use Committee (PHS assurance # A3779-01) approved all animal experimental protocols, adhering to the Guide for The Care and Use of Animals. The LIAI Department of Laboratory Animal Care is an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) International accredited unit.
2.3. Treg generation and adoptive transfer
CD4 T cells were isolated from the spleens of naive adult Smarta mice. Following mechanical disruption and RBC lysis, splenocytes were stained with rat monoclonal antibodies to B220, CD11c, CD11b, CD16/32, IA/IE and CD8 (BD Pharmingen). Labeled splenocytes were magnetically depleted (anti-rat IgG Dynal beads, Invitrogen) and CD4 T cells (B220−CD11c−CD11b−CD16/32−IA/IE−CD8−) negatively selected. Isolated Smarta CD4 T cells were cultured for 7 days in the presence of plate bound anti-CD3, 0.5 μg/ml soluble anti-CD28, 5 ng/mL recombinant TGFβ (Roche) and 100 U/mL IL-2 (Roche). At the conclusion of the culture cells were live sorted based on FoxP3 reporter expression and 1 × 106 FoxP3+ iTregs were transferred by tail vein injection into either RIP-GP or C57BL/6 mice as indicated.
2.4. Viral preparation, infections
LCMV Armstrong 53b strain was purified and quantified as previously described [35]. Mice were infected between 8 and 12 weeks of age with a single dose of 2 × 105 PFU of LCMV via intraperitoneal injection.
2.5. Flow cytometry and cytokine analysis
Fluorescently conjugated antibodies were purchased from BioLegend, eBiosciences or BD Pharmingen. For intracellular cytokine staining, cells were stimulated as indicated with PMA/Ionomycin (Calbiochem), GP61 or GP33 (Abgent) for 4 h in the presence of 5 μg/ml brefeldin A (Sigma-Aldrich), surface stained, fixed in 2% paraformaldehyde (Sigma-Aldrich), permeabilized and stained in 0.05% saponin (Sigma-Aldrich) buffer for indicated cytokines. Single cell suspensions were run on an LSR II or sorted with a FACS ARIA (BD Pharmingen). Data was analyzed with FlowJo (Tree Star) software. iTreg culture supernatants were collected for cytokine detection using a Bio-Plex Pro™ Mouse Cytokine 23-Plex assay kit (Bio-Rad, Cat. # M60-009RDPD) according to the manufacturer's instructions.
2.6. Statistics
Graphs were plotted and statistics were calculated with GraphPad Prism® (versions 4 and 5). Categorical data were analyzed using Fisher's exact test (Fig. 2). Kinetic analyses were assessed with a paired t-test (Fig. 3A). Comparisons between the different groups were performed with two-tailed unpaired non-parametric Mann–Whitney U tests (2 groups) or Kruskall–Wallis test followed by Dunn's multiple comparison tests (3–4 groups). Note that the latter test provides information for significance but does not yield exact P-values. Data are expressed as mean ± standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001.
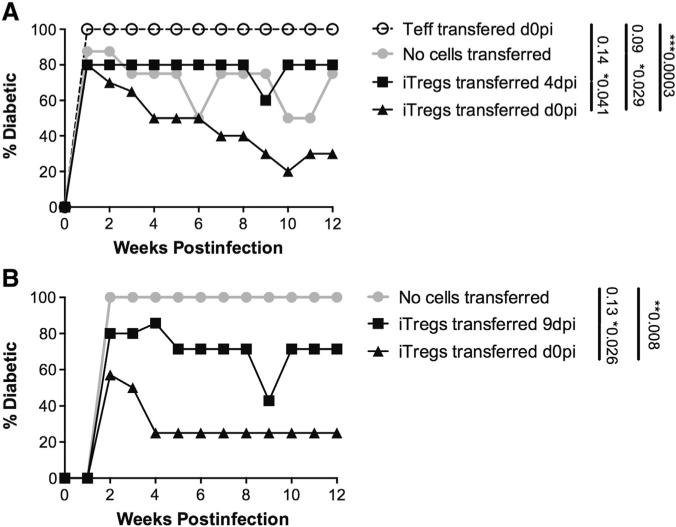
Figure 2.
Early iTreg transfer confers protection from diabetes in the RIP-GP model. Female RIP-GP mice were infected with LCMV Armstrong either 4 (A) or 9 days prior (B) to Treg transfer (LATE Treg transfer or on the same day as Treg transfer (EARLY Treg transfer) and monitored for blood glucose values (BGVs) on a weekly basis. Mice with BGVs in excess of 300 mg/dL were considered diabetic.
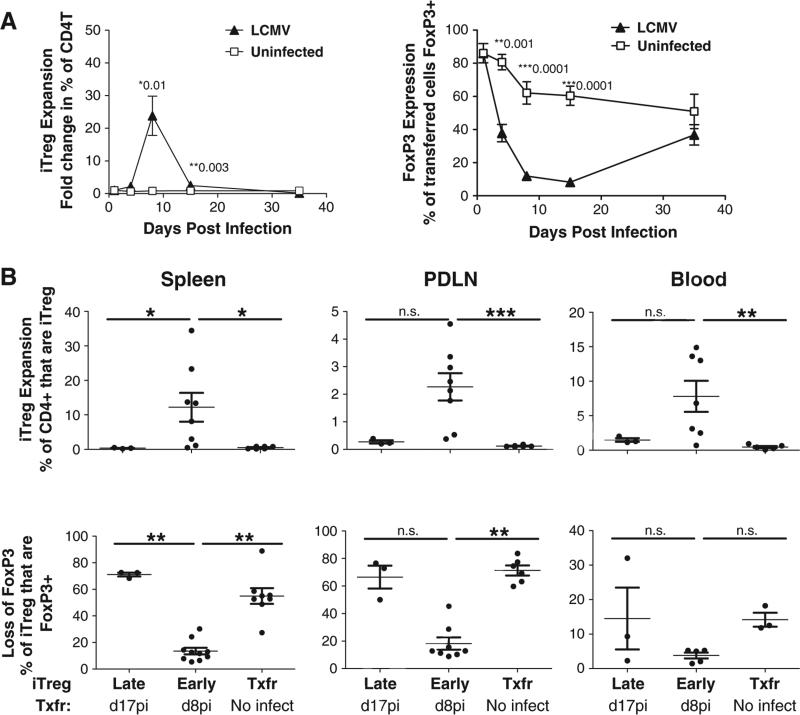
Figure 3.
Antigen specific FoxP3+ iTregs expand in vivo in response to LCMV Arm and transiently lose FoxP3 expression. (A) C57BL/6 iTreg expansion (left panel) in C57BL/6 mice is shown as the fold change in the percent of CD4T that are positive for the CD45.1 congenic marker. Loss of FoxP3 expression during LCMV infection is shown as the percent of transferred (CD45.1+) cells that are FoxP3+. Solid triangles indicate kinetics in LCMV infected C57BL/6. Open squares indicate kinetics in uninfected C57BL/6 mice. (B) iTreg expansion (upper three panels) and FoxP3 reporter expression (lower three panels) in transferred cells were monitored 8 days post transfer in female RIP-GP mice receiving iTregs LATE (9dpi) and EARLY (0dpi) versus mice that were not infected. iTregs were tracked by congenic marker, CD45.1, expression. Data are from 3 independent experiments.
3. Results
3.1. Early iTreg transfer confers protection from diabetes in the RIP-GP model system
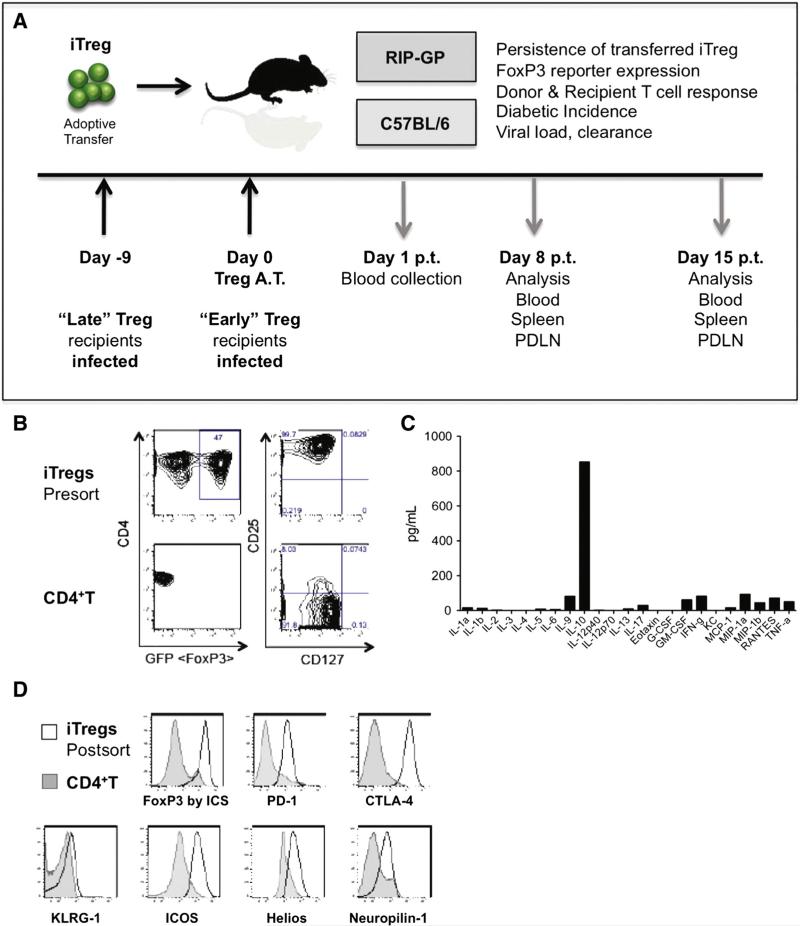
Multiple methods of Treg induction in vitro have been described [36–40]. Here FoxP3+ iTregs were generated from splenic CD4 T cells isolated from TCR transgenic, LCMV-specific (Smarta) mice (C57BL/6 background) crossed with congenic CD45.1 mice containing a GFP transgene under the control of the FoxP3 promoter [33,34]. Smarta TCR transgenic FoxP3/GFP reporter CD4 T cells were cultured in the presence of IL-2, TGF-β, anti-CD3 and anti-CD28 antibodies then rested for 3 days prior to sort. At the conclusion of the culture, iTregs were sorted based on high GFP (FoxP3) reporter expression in live cells prior to adoptive transfer into either wild-type (WT) C57BL/6 mice or RIP-GP mice (Fig. 1A). Pre-sort iTregs were uniformly CD25hiCD127− and nearly 50% expressed high levels of FoxP3/GFP (Fig. 1B). Cytokine expression analysis of the resting iTreg culture identified IL-10 as the most abundantly secreted cytokine (Fig. 1C). Post-sort iTregs highly expressed FoxP3 and other bona-fide Treg markers including PD-1, CTLA-4 and ICOS, had elevated levels of Helios and Neuropilin-1 and no significant difference in KLRG1 expression (Fig. 1D).
Figure 1.
Generation and characterization of induced FoxP3+ regulatory T cells. Negatively selected CD4+CD25−T cells were isolated from the spleens of LCMV-GP61–80 specific (Smarta) FoxP3-GFP reporter mice and cultured with α-CD3 and α-CD28 antibodies in the presence of rhIL-2 and TGFβ. (A) Overview of model system employed. (B) GFP reporter and surface marker expression of iTregs pre-sort (top) versus naïve CD4+ splenocytes (bottom). (C) iTreg culture supernatants analyzed by BioPlex. (D) Histograms demonstrate the surface expression phenotype of iTregs (black histogram) versus CD4+ splenocyte controls (grey filled histograms).
The ability of antigen-specific iTregs to protect against autoimmune T1D was tested by adoptive transfer of sorted (GFP/FoxP3+) iTregs into RIP-GP recipients (Fig. 2). While 80–90% of mice had blood glucose values (BGVs) greater than 300 mg/dL by week 2 post-infection, mice treated with iTregs experienced a steady decline in BGV, with only 20% of iTreg treated mice diabetic by week 10 post-infection, while 100% of mice receiving control CD4 Teff cells remained diabetic (Fig. 2A). While early transfer of iTregs was able to reverse diabetic incidence, transfer during acute viral infection (4 days post-infection, dpi) did not result in reversion (Fig. 2A). Likewise we found that iTregs transferred after viral clearance (9dpi) also did not result in reversion (Fig. 2B). Taken together, purified Smarta/FoxP3 Tregs protect from virally-induced diabetes when transferred early, at the time of viral infection, whereas when transferred at later time points iTregs do not confer protection from diabetes.
3.2. iTregs expand in vivo in response to LCMV Arm infection and transiently lose FoxP3 expression
To determine what factors may contribute to the protective effect observed with early transfer, iTreg trafficking and stability were examined in early (protective) versus late (not protective) iTreg transfer (Fig. 3). iTreg stability in response to acute LCMV infection was first examined in WT C57BL/6 mice. While iTregs (tracked by the expression of the congenic marker CD45.1) transferred into naïve mice did not expand, those transferred on the day of infection expanded between days 4 and 8 post-infection, with a 25-fold increase in the percent of transferred iTregs within the CD4 T cell compartment (Fig. 3A). However, an inverse correlation between iTreg expansion and FoxP3 expression was found, where at the peak of expansion, FoxP3 expression was dramatically suppressed as compared to their naïve counterparts (Fig. 3A). Although iTreg FoxP3 expression declined over time in naïve mice, a more rapid loss was observed in infected mice.
Next the phenomenon of expansion coupled to reporter loss, observed in WT C57BL/6 mice, was investigated in the RIP-GP mouse where the viral GP serves as an autoantigen. A similar trend of expansion and loss of FoxP3 expression was found in the blood, spleen and pancreatic draining lymph nodes (PDLNs) of the RIP-GP recipients. Eight days post-transfer, iTreg expansion and FoxP3 expression were examined in the late versus early iTreg transfer in RIP-GP mice (Fig. 3B upper panels). Here we found that protective (early) iTreg transfer was associated with expansion of iTregs within the CD4 T cell compartment and concomitant loss of FoxP3 expression (Fig. 3B lower panels), as measured on day 8pi/post-transfer, compared to naïve mice receiving iTregs where a larger percent of transferred cells maintained their FoxP3 expression, but comprised a smaller percent of total CD4 T cells (Fig. 3B). Analysis of iTreg stability on day 8 post-transfer, revealed that iTregs transferred late maintained FoxP3 expression, but did not expand as compared to iTregs transferred early (Fig. 3B). Therefore, while the magnitude of expansion and loss of FoxP3 expression varied between spleen, peripheral blood and PDLNs, early transfer of iTregs was consistently associated with expansion and concomitant loss of FoxP3 expression, regardless of lymphoid compartment.
3.3. FoxP3 iTreg transfer does not significantly impair the acute anti-viral CD8 T cell response
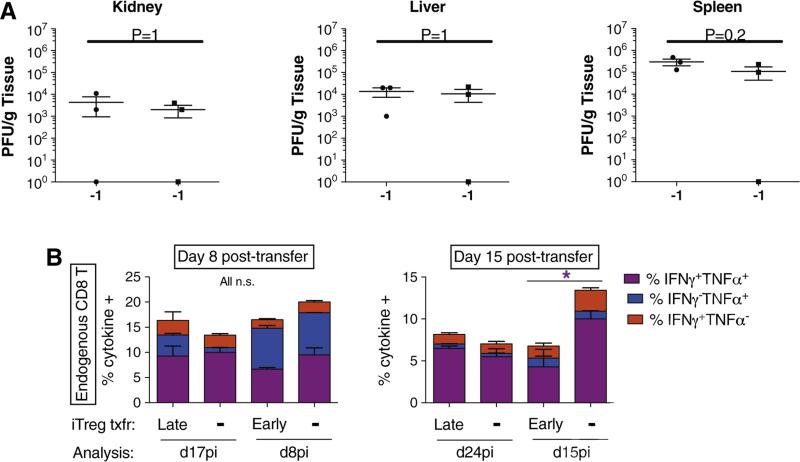
Next, we assessed whether or not the transfer of LCMV-specific iTregs at the time of LCMV infection affects clearance of the virus. We examined viral loads in the kidney, liver and spleen and observed no significant differences on day 6pi (Fig. 4A) and no difference in viral clearance at d8pi (data not shown). We then measured at the peak of iTreg expansion, i.e., day 8 post-transfer, the anti-LCMV endogenous CD8 T-cell response after in vitro restimulation with the immunodominant GP33–41 peptide and intracellular cytokine staining. Fig. 4B shows that compared to their respective non-transferred counterparts, neither early nor late iTreg transfer had an effect on IFN-γ- and TNF- single and double-producing CD8 T cells as observed in the spleen at day 8 post-transfer. Similar data were found in PDLNs and in the blood (not shown).
Figure 4.
FoxP3 iTreg transfer does not significantly affect the acute anti-viral CD8 T cell response. (A) Viral titers in the kidney, liver and spleen were monitored at day 6 and day 8 (data not shown) post early iTreg transfer/LCMV infection. No significant difference was observed between iTreg transferred mice and those not receiving iTregs. By day 8 post-infection/iTreg transfer virus was undetectable regardless of iTreg transfer (data not shown). (B) Anti-LCMV CD8 T cell responses were monitored 8 and 15 days post-iTreg transfer in RIP-GP mice. Cells recovered from the spleen were restimulated in vitro with GP33–41 peptide and assessed by ICCS for TNFα and IFNγ expression.
3.4. FoxP3 iTreg transfer impairs the endogenous CD4 T cell response during acute infection and diminishes CD8 T cell responses after viral clearance
We have shown that reversal of the course of diabetes upon early transfer of iTregs (concomitant to LCMV infection) could not be explained by an effect on the CD8 T-cell responses at day 8pi/post-transfer, or effects on viral titers or clearance. Therefore, we analyzed endogenous GP33–41 CD8 T-cell responses at a later time-point after viral clearance i.e., day 15 post-transfer, which corresponds to day 15pi for early transfers and day 24pi for late transfers. Here, we observed that the fraction of IFN-γ+TNF+ producing CD8 T cells was significantly decreased after early iTreg transfer (4.3 ± 2.1%) as compared to non-transferred animals (10 ± 1%) (Fig. 4B, right panel). In contrast, late iTreg transfer did not alter the proportions of cytokine-producing T cells (Fig. 4B, right panel). These results were similar in cells collected from PDLNs or blood compartments (not shown). Thus, early (protective) iTreg transfer differed from late (non-protective) iTregs in controlling the pro-inflammatory response of endogenous CD8 T cells. Specifically, only early and not late transferred iTregs were able to significantly reduce the frequency and number of CD8 Teff cells after viral clearance.
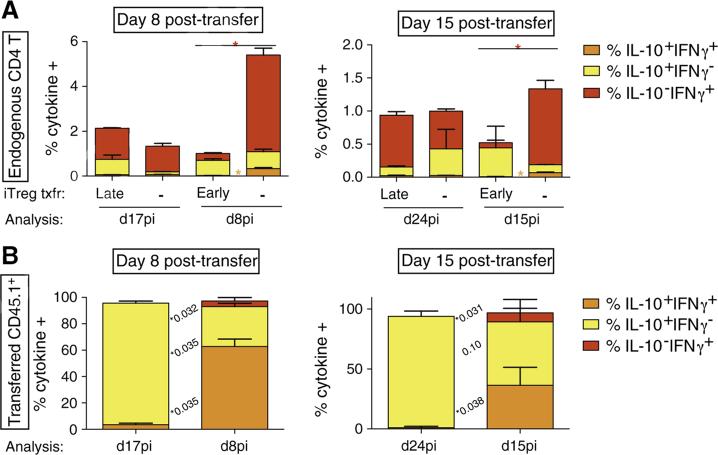
In parallel, we also assessed endogenous (CD45.1−) CD4 T-cell cytokine production upon restimulation with MHC class II-restricted GP61–80 peptide from LCMV. As early as 8 days post-transfer, we noticed a drastic reduction in the percentages of IFN-γ+TNF+-producing endogenous CD4 T cells after early iTreg transfer in comparison to non-transferred mice (not shown). The percentage of IFN-γ+IL-10− CD4 T cells after early iTreg transfer (0.3 ± 0.0%) was also diminished in comparison to non-transferred counterparts (4.3 ± 0.3%) (Fig. 5, left panel). Analyses at day 15 post-transfer further revealed a similar decline (Fig. 5, right panel) as well as a discernable but non-significant rise in the mean frequency of IL10+IFN-γ− endogenous CD4 T cells (0.44%) compared to non-transferred mice (0.12%) (Fig. 5, right panel, Fig. 6B). In contrast, endogenous CD4 T-cell responses upon late transfer of iTregs were not significantly altered for both time-points examined (Fig. 5). These findings are summarized in Fig. 5. Similar results were obtained from PDLN (data not included). As observed with endogenous CD8 T cell responses, early transfer of iTregs reduced the endogenous GP61–80 CD4 Teff cell responses. In vitro suppression data demonstrated that Smarta iTregs display an enhanced suppression of Smarta CD4 T effectors, as compared to P14 (TCR transgenic for a different LCMV GP epitope) CD8 T cells (Supplemental Fig. 1). These data are in line with previous reports of the differential effect of IL-10 on CD4 versus CD8 T cell responses, where IL-10 suppresses the proliferation of CD4 T cells, does not affect CD8 T cell proliferation, but suppresses the cytotoxic potential of CD8 T cells [41].
Figure 5.
Early FoxP3 iTreg transfer diminishes the activity of CD8 T at d15pi and CD4 T at both d8 and d15pi. Anti-LCMV CD4 T cell responses were monitored at days 8 (left) and 15 (right) post iTreg transfer in RIP-GP mice. Cells recovered from the spleen were restimulated in vitro with GP33 peptide and assessed by ICCS for TNFα and IFNγ expression. Data shown is from the spleen. Similar data was observed in the PLN and blood.
Figure 6.
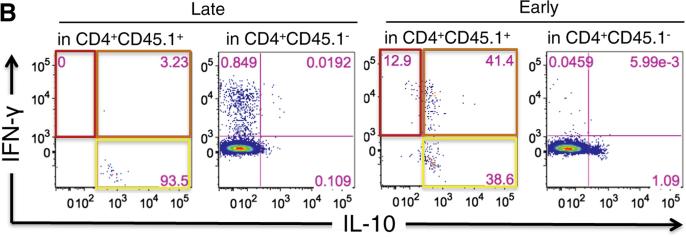
Early iTreg transfer results in an increase in the frequency of IL10+IFNγ+ iTregs. Transferred iTreg CD4 T cell responses were monitored 8 days post iTreg transfer (left) and 15 days post transfer (right). Cells recovered from the spleen were restimulated in vitro with GP61–80 peptide and assessed by ICCS for IL-10 and IFN-γ expression. Similar data was observed in the PDLN and blood. (A) Summarized data is shown. (B) Representative dot plots shown.
3.5. Early iTreg transfer results in an increase in LCMV-specific IL10+IFNγ+ CD4 T cells
Last, we evaluated the cytokine profile of the iTregs transferred early versus late, gating on cells expressing the congenic marker CD45.1. Eight days after being adoptively transferred, the majority of cells still produced IL-10 regardless of timing to LCMV infection (Fig. 6A, left panels), in keeping with the cytokine profile observed in total cell culture supernatants (Fig. 1C). However, protective iTregs displayed a significant increase regarding IL-10 and IFN-γ double production (62.8 ± 5.5% versus 3.5 ± 1.1% in non-protective iTregs transferred late) (Fig. 6A, left panels and Supplemental Fig. 2). This marked phenotype was also observed to a lesser extent at day 15 post-transfer, with mean frequencies of 36.4% (early) versus 1.1% (late) IFN-γ+IL-10+ cells (Fig. 6A, right panels; Fig. 6B).
While significant and sustained suppression of CD4 T cell responses was observed after iTreg transfer, iTregs neither affected the primary anti-viral CD8 T cell response nor acute viral load or clearance. Following the clearance of the acute infection, iTregs were able to suppress both CD4 and CD8 T cell responses, which resulted in the reversion of diabetes. Protective iTregs produced a mixed cytokine profile of IL-10, IFN-γ and TNF, but enhanced overall IL-10 expression in both transferred iTregs and endogenous anti-viral T cells.
4. Discussion
The salient finding of our current study is the discovery that induced regulatory Tregs are able to control virally-induced diabetes without compromising viral clearance. While induced Tregs were found to downregulate FoxP3 during acute viral infection, they maintained IL-10 expression and were able to suppress autoreactive CD4 and CD8 T cell responses resulting in the reversion of diabetes despite the loss of FoxP3. These data suggest that the modulation of iTreg profile in the face of systemic inflammation does not preclude the ability of iTregs to control aberrant autoimmune responses.
Early studies in the NOD murine model have demonstrated the efficacy of Treg therapeutic approaches in type 1 diabetes, illuminating the potential of Treg therapy in the amelioration of autoimmune diabetes, even in settings (such as NOD murine model) where the donor Tregs are derived from an individual with defects in peripheral tolerance induction or maintenance [14,19,21–23]. Despite the promise of these discoveries, more recent studies have sparked debate on the stability of Tregs in vivo and the safety profiles of Treg transfer approaches. Experiments conducted in lymphopenic settings suggested that the loss of FoxP3 expression results in the conversion of Treg to Teff in vivo [42–44]. One study in particular utilized FoxP3 lineage tracing mice, where even cells which have transiently expressed FoxP3 are labeled as “exFoxP3” cells [42]. Later reports have demonstrated in humans and mice that T cells can transiently express FoxP3, thus obscuring whether the exFoxP3 cells which accelerated T1D when subsequently transferred into lymphopenic (RAG KO) NOD mice, were exTregs, Teff or an outgrowth of Teff from a mixed pool of Teff and exTregs [45,46].
In contrast to the lymphopenic studies, models using immunocompetent mice have found that Tregs are relatively stable under steady-state conditions [47–49]. However infection and autoimmunity are far from steady state conditions and microbial infection has been documented to both promote and suppress Tregs [50–52]. We asked whether viral infection of an immunocompetent host would modulate therapeutic Treg function and vice versa, and whether the presence of transferred Tregs would impede the ability of the host to clear an acute viral infection.
Here we report that a single injection of antigen-specific iTregs at the time of infection can lead to the reversion of diabetic incidence, while at the same time not interfering with the host's ability to protect itself against viral assault. While significant and sustained suppression of CD4 T cell responses was observed, iTregs affected neither the primary anti-viral CD8 T cell response nor acute viral load or clearance from target organs. Following viral clearance, iTreg treated mice had diminished endogenous LCMV-GP specific CD4 and CD8 T cell responses, which resulted in the reversion of diabetes in the RIP-GP model. Protective iTreg transfers resulted in enhanced IL-10 expression in both transferred iTregs and endogenous anti-viral T cells. Collectively our data suggest that when iTregs expand in vivo during acute viral infection they lose FoxP3 expression, maintain IL-10 expression, and do not impede, nor aid in the clearance of an acute infection; however, after elimination of the virus, mice which received iTregs had diminished CD8 T responses long term. Taken together these data suggest that even in an acute model of viral infection and diabetic onset, where the self antigen is also the foreign antigen, iTreg therapy is both safe and efficacious.
The current data set examines the safety, stability and efficacy of iTreg therapy in the RIP-GP model of T1D, an acute onset, virus induced model. The RIP-GP model provides a highly sensitive system with clear metrics to examine the effect of viral infection on iTreg therapy in an autoimmune model of T1D. The RIP-GP models a worst-case scenario where the self antigen is recognized by both the therapeutic iTregs and the cytotoxic T lymphophytes (CTLs) necessary for the clearance of the viral pathogen, but responsible for the induction of diabetes in the RIP-GP. Here if the transferred iTregs destabilized and adopted effector function then, one would have expected to see enhanced disease progression manifested as either increased percent of mice diabetic, decreased time to diabetic onset, elevated BGV in treated populations as compared to untreated or enhanced viral clearance; none of these were observed in the RIP-GP model. Likewise if iTreg transfer inhibited the hosts’ ability to combat acute viral infection, one would expect to see enhanced viral titers in target organs or delayed viral clearance; neither of which was observed. Instead iTreg transfer resulted in the early and sustained suppression of CD4 T cell responses.
The ability of the iTregs to control LCMV-induced T1D while not impacting acute viral clearance was due to the minimal effect on early CD8 T cell responses during acute systemic inflammation, while dramatically impacting both CD4 and CD8 T cell responses at later times post infection. The effect in vivo on both CD4 T and CD8 T cells at later times post infection, coupled with the transferred iTregs' ability to maintain expression of IL-10 likely contributed to the reversion in diabetes incidence observed at later times post infection. In fact, we found that T1D emerged normally in iTreg treated mice but reverted within four weeks post-treatment. This is a novel and unexpected aspect of the current study. Future studies are aimed at addressing what mediates the retraction from the target tissue as it may give us clues for rapid interventions during disease onset. While possibilities include islet regeneration, we favor a scenario where acute intra-islet inflammation was controlled prior to irreversible beta cell loss. In other words, iTregs acted on the ongoing infiltrating cell aggressors and reversed their action prior to the complete destruction of the beta cells. In their entirety these data suggest that even in an acute model of viral infection and diabetic onset, antigen specific iTreg therapy is both safe and efficacious.
Supplementary Material
Acknowledgments
We would like to thank Malina McClure and Priscilla Colby for excellent technical and administrative assistance. Expert assistance was also provided by Cheryl Kim, Kurt Van Gunst and Anthony José in the LIAI flow cytometry core.
Footnotes
This work was supported by NIH grants AI089624-03 and 5T32DK007044.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clim.2014.05.006.
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
References
- 1.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J. Exp. Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 7.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 8.Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 11.Maier LM, Wicker LS. Genetic susceptibility to type 1 diabetes. Curr. Opin. Immunol. 2005;17:601–608. doi: 10.1016/j.coi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Eaves IA, Wicker LS, Ghandour G, Lyons PA, Peterson LB, et al. Combining mouse congenic strains and microarray gene expression analyses to study a complex trait: the NOD model of type 1 diabetes. Genome Res. 2002;12:232–243. [PubMed] [Google Scholar]
- 13.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 15.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 16.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J. Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 17.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, et al. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 20.Lundsgaard D, Holm TL, Hornum L, Markholst H. In vivo control of diabetogenic T-cells by regulatory CD4+CD25+ T-cells expressing Foxp3. Diabetes. 2005;54:1040–1047. doi: 10.2337/diabetes.54.4.1040. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR alpha beta+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J. Immunol. 1998;161:2620–2628. [PubMed] [Google Scholar]
- 23.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J. Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 24.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, et al. Administration of CD4+CD25highCD127–regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin. Immunol. 2013;149:268–278. doi: 10.1016/j.clim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, et al. Teplizumab (anti-CD3 mAb) treatment preserves c-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keymeulen B, Candon S, Fafi-Kremer S, Ziegler A, Leruez-Ville M, et al. Transient Epstein–Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2010;115:1145–1155. doi: 10.1182/blood-2009-02-204875. [DOI] [PubMed] [Google Scholar]
- 28.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 31.Van Belle TL, Taylor P, von Herrath MG. Mouse models for type 1 diabetes. Drug Discov. Today Dis. Model. 2009;6:41–45. doi: 10.1016/j.ddmod.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holz A, Dyrberg T, Hagopian W, Homann D, von Herrath M, et al. Neither B lymphocytes nor antibodies directed against self antigens of the islets of Langerhans are required for development of virus-induced autoimmune diabetes. J. Immunol. 2000;165:5945–5953. doi: 10.4049/jimmunol.165.10.5945. [DOI] [PubMed] [Google Scholar]
- 33.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat. Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 37.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 38.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur. J. Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 39.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 40.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye Z, Huang H, Hao S, Xu S, Yu H, et al. IL-10 has a distinct immunoregulatory effect on naive and active T cell subsets. J. Interferon Cytokine Res. 2007;27:1031–1038. doi: 10.1089/jir.2006.0144. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 44.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 46.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Nguyen P, Liu W, Cheng C, Steeves M, et al. T cell receptor CDR3 sequence but not recognition characteristics distinguish autoreactive effector and Foxp3(+) regulatory T cells. Immunity. 2009;31:909–920. doi: 10.1016/j.immuni.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belkaid Y, Tarbell K. Regulatory T cells in the control of host– microorganism interactions (*) Annu. Rev. Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 51.Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.