Abstract
Clinical research activities at academic medical centers are challenging to oversee. Without effective research administration, a continually evolving set of regulatory and institutional requirements can detract investigator and study team attention away from a focus on scientific gain, study conduct, and patient safety. However, even when the need for research administration is recognized, there can be struggles over what form it should take. Central research administration may be viewed negatively, with individual groups preferring to maintain autonomy over processes. Conversely, a proliferation of individualized approaches across an institution can create inefficiencies or invite risk.
This article describes experiences establishing a unified research support office at the Duke University School of Medicine based on a framework of customer support. The Duke Office of Clinical Research was formed in 2012 with a vision that research administration at academic medical centers should help clinical investigators navigate the complex research environment and operationalize research ideas. The office provides an array of services that have received high satisfaction ratings. The authors describe the ongoing culture change necessary for success of the unified research support office. Lessons learned from implementation of the Duke Office of Clinical Research may serve as a model for other institutions undergoing a transition to unified research support.
Keywords: research administration, clinical research, academic medical center
Introduction
With direct access to patients and clinical investigators who fuel and test new ideas, academic medical centers (AMCs) play an important role in innovation that improves patient care.1 However, an increasingly complex environment for clinical research in healthcare delivery settings could threaten this path to discovery if inefficiencies are not addressed.2-4 Challenges facing clinical investigators include lack of time for research due to demands of clinical practice, disruption in established workflows associated with a transition to electronic medical records, insufficient infrastructure, and expanding complexities of data collection, government and institutional regulation, and contract negotiation.3,5-7 As summarized in a 2015 National Academies report, “continuing expansion of the federal regulatory system and its ever-growing requirements are diminishing the effectiveness of the nation's research investment by directing investigators’ time away from research and training toward overlapping and incongruent administrative matters.”8 Many associate an increase in administrative burden with discouraging clinicians from pursuing a career in research, resulting in a shortage that could jeopardize the clinical research enterprise.4,7,8 These challenges underscore the need for expert assistance from clinical research administrative support so clinical investigators can focus on science that will lead to improved patient care and health outcomes.
Compared with a traditional model in which the management of studies is performed in a coordinator/investigator unit responsible for all trial activities, there is a growing interest in whether consolidating research administration and support activities within a department or institution may contribute to more effective infrastructure for clinical research.9 However, barriers to implementing unified support systems may include lack of faculty support due to perceived loss of autonomy in research conduct.10 Detailed accounts are needed of transitions to unified research support offices, along with data to evaluate the potential benefits and limitations of such a model. This article reviews the Duke University School of Medicine experience with developing a centralized, support-oriented approach to managing clinical research.
Case Study: Duke Office of Clinical Research
Transition to a Unified Support Office
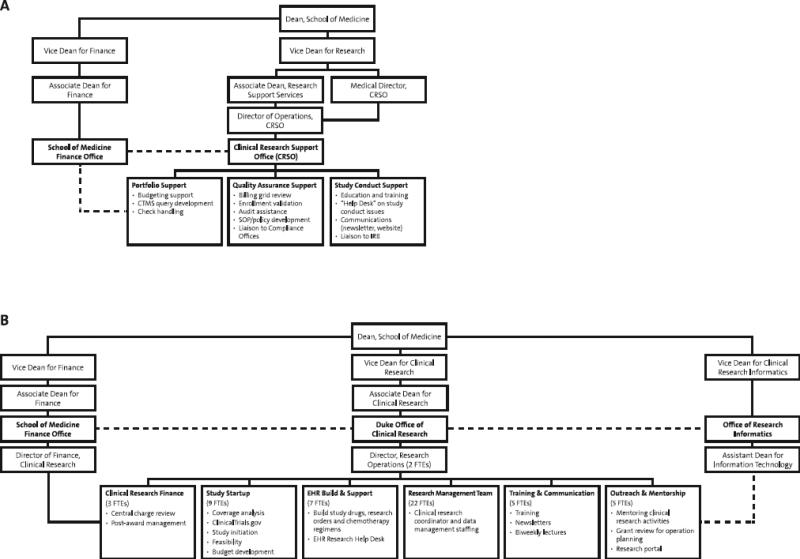
The transition to a unified research support office occurred gradually. Because of the proliferation of one-off approaches to institutional support for study teams, Duke created infrastructure and policy changes to provide a more consistent oversight approach for site-based clinical research. The changes were originally carried out under the Vice Dean for Research, who oversaw both clinical and basic research. Duke first created oversight bodies in the disease-based clinical departments and institutes. These Clinical Research Units (CRUs) were initially focused on addressing industry-sponsored trial oversight and management. Regardless of size, each CRU created a charter, named a director, research practice manager, and financial practice manager. The CRUs were supported by a narrowly scoped Clinical Research Support Office (Figure 2A); this central administration office pushed business processes and responsibility to the CRUs and served as a pass-through checkpoint. Although there was a marked benefit in oversight and compliance at the onset (e.g., improvements in clinical research billing, increased focus on training), with time this model became problematic. There was a lack of consistent processes across CRUs, given that each could establish policies and procedures unique to the history of the unit. These inconsistent policies (e.g., differences in clinical research training requirements, variations in feasibility review) created confusion for faculty performing research in multiple CRUs.
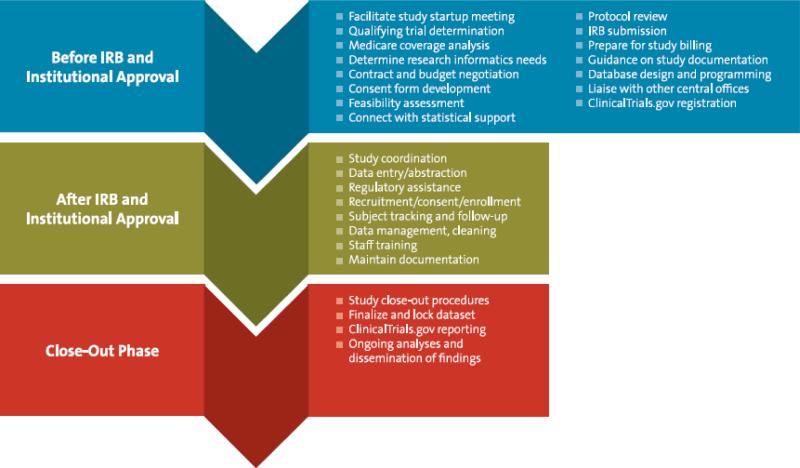
Figure 2.
Array of Services Offered by the Duke Office of Clinical Research
After years of mandates, poor customer service, faculty isolation, and inconsistent study management at the CRU level, the institution established the role of Vice Dean for Clinical Research to improve processes and communication involving the CRUs and central administration. Organizational changes included new reporting structures for the Institutional Review Board, Clinical Research Support Office, regulatory affairs (IND/IDE management), and research integrity. The key cultural change involved rebranding the Clinical Research Support Office by expanding the concept and scope of an established Research Management Team from the Duke University School of Nursing.11 The Research Management Team originally provided flexible, cost-efficient institutional support for research coordination, data management, and informatics. Its “study teams as my customer” approach served as a proof-of-concept model for what would become the new Duke Office of Clinical Research (DOCR; Figure 2B). In 2012, the Research Management Team's leader was installed as the director of DOCR to reinvigorate research support under the new office.
Key changes included bringing senior information technology expertise to bolster support for clinical research software applications and reporting,11 establishing a new position to liaise with contracts and finance departments, and creating designated staff to handle ClinicalTrials.gov registrations and reporting.12 DOCR increased from 14 to 54 full-time staff members over 3 years and now provides a broad range of support services (Figures 2B, 3). Some research support remains within the individual CRUs, which vary in size and scope, but the strengthened unified support office provides access to consistent navigation, tools, and training for the more than 700 clinical research coordinators and other research support staff throughout the institution. DOCR's mission is to improve patient care through outstanding clinical research that is supported by sustainable business processes and a well-trained clinical research workforce.
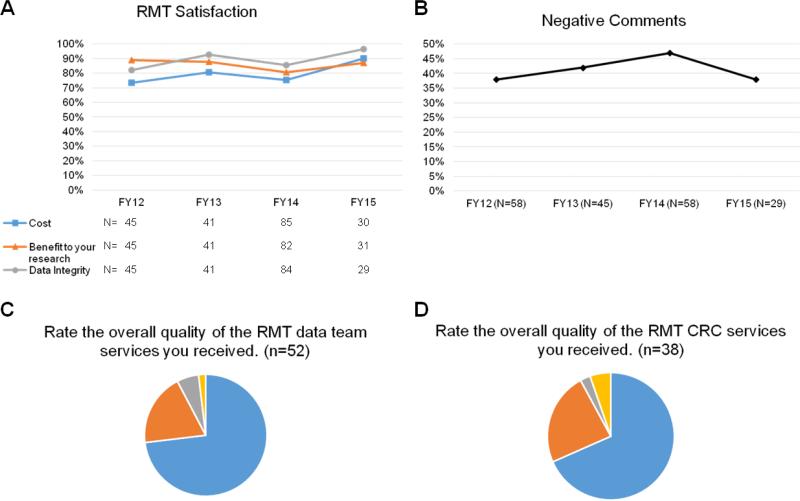
Figure 3. Satisfaction With DOCR Services as Assessed by (A, B) Annual Survey and (C, D) Survey After Support Completed by DOCR's Research Management Team.
Panel A shows quantitative data from the annual survey. For panel B, free-text comments entered during survey completion were assessed by a group of 8 DOCR staff to assign as positive or negative. Comments were anonymous and blinded to year. Panels C and D, respectively, show survey ratings after completion of DOCR data management and CRC support; data are from a one-year period from March 2015 to March 2016. CRC, clinical research coordinator; DOCR, Duke Office of Clinical Research; FY, fiscal year; RMT, Research Management Team.
Services
DOCR services span the stages of a clinical study and are supported by staff conducting a range of activities, including study startup, research builds in the electronic health record, and outreach and mentorship (Figure 2B, 3). Studies that will be billing any research costs through the Duke University Health System are required to have a study initiation meeting with DOCR to review the protocol for clinical and research-specific activities, identify needs for the electronic health record (e.g., order sets, billing calendar), review qualifying status and national coverage decisions, and identify funding for activities and coverage of related activities in alignment with health system financial policies. Use of all other DOCR services is voluntary.
During study planning, investigators may receive a free consultation (subsidized by the School of Medicine and Duke Clinical and Translational Science Award) to determine project needs, and an estimate for DOCR services is provided. In this way, DOCR is able to tailor support to the needs of the individual study and research team. For example, experienced and well-staffed study teams conducting industry-sponsored studies may need little assistance from DOCR. In contrast, an investigator-initiated NIH-funded study is likely to have a different set of needs. Aside from the initial study planning meeting, requests also may be submitted at any time through a central DOCR email address. DOCR publicizes its services on its website, through regular presentations, and via interactions with study teams (i.e., word of mouth).
As a unified research support office, DOCR is able to offer a wide range of services. Many DOCR staff are cross-trained, enabling an efficient infrastructure that can meet the objectives of multiple research programs. Some investigators have indicated that they would not have felt comfortable supporting full-time research administrative staff within a small group due to uncertainties of future funding. By providing partial effort with specific spheres of expertise, we hypothesize that investigators will be less likely to assign tasks to an employee with insufficient background (e.g., a coordinator assigned the additional role of data management). This DOCR shared service model allows researchers to more tightly manage labor expenditures by only paying for services when they are needed. The shared-effort coordinator and data management pools reduce pressure from investigators and provide job security for highly skilled staff, which allows the institution to develop and retain top talent. Workloads can be adjusted to prevent burnout, a common problem encountered with research coordinators in AMCs.13 Additionally, managing staff can relieve faculty from the burden of addressing any performance issues. Finally, this service can provide a quick stopgap in the event that a research team unexpectedly loses some of their staff during a study. This staffing model therefore benefits both the researchers and administration.
In general, requests for data management or clinical research coordinator effort are submitted via a Research Electronic Data Capture (REDCap) survey for that purpose. Requests also may come in via direct contact with a DOCR director, associate director, or Research Management Team member. A DOCR associate director contacts the study team to assess the effort needed, records the information in the DOCR effort database, and generates an agreement to send to the study team.
DOCR provides free guidance on study design, protocol writing, and institutional review board submission to all Duke residents and fellows. We believe these services help establish positive relationships with DOCR early and will ultimately lead to higher quality research conduct at the institution.
The theme of facilitation and navigation is central to DOCR services (Figure 3). It liaises with the CRUs and other institutional offices to shepherd studies throughout their life cycle. Close ties at the leadership level with the School of Medicine Finance Office, Office of Research Informatics, and Institutional Review Board, among others, help maintain productive partnerships (Figure 1). Parallel processes are initiated whenever possible to avoid delays; for example, an operational review can be conducted concurrent with institutional review board review and contract negotiation. Even where support services reside within other groups, DOCR strives to add value through coordination and communication. A communications team produces newsletters on relevant topics for faculty, research staff, and CRU leadership; topics are gathered from all research administrative offices. Furthermore, DOCR attends regular meetings with stakeholder groups to engage around initiatives, policies, or procedures that may involve multiple stakeholders.
Figure 1. Organization of the Initial Clinical Research Support Office (A) and the Subsequent Unified/Expanded Duke Office of Clinical Research (B).
The Duke Office of Clinical Research replaced the Clinical Research Support Office in 2012, offering added services and greater harmonization of administrative support. The Duke Office of Clinical Research liaises with multiple other central offices, as well as faculty, staff, and students across Duke Medicine and Duke University. Investigators and research teams are supported throughout study planning, conduct, and close-out. CRSO, Clinical Research Support Office; CTMS, clinical trial management system; EHR, electronic health record; FTE, full-time equivalent; IRB, institutional review board; SOP, standard operating procedure.
Operations
DOCR shares an administrative manager with the Office of Research Informatics, who assists with budgets and finance. Projects and effort are tracked in an internal database, and staffing is increased as needed to keep up with demand and prevent burnout. When DOCR rebooted, CRU staff were not absorbed, but they were eligible to apply for open DOCR positions. Turnover has been minimal within DOCR, though some staff do leave or go on to other positions within the institution. DOCR is a leader in developing staff and encourages staff to consider well-matched opportunities across Duke.
Training/Professional Development
An important facet of the unified support office is to contribute to the growth of a network of research professionals. In 2015, DOCR offered more than 300 classes on topics including human subjects research, study documentation, and data security. There were over 13,000 individual completions across the in-person, blended, and online courses. DOCR not only offers training, but also an opportunity for advancement that was not previously available to many clinical research staff. Skilled coordinators working under a single principal investigator may not envision a path for professional growth within the institution, and may feel pressured by the investigator to remain stagnant within a uniquely defined position. By harmonizing research support, a new avenue for career development is opened, and the institution can provide support and structure to assist future generations of research professionals in becoming leaders who are truly invested in team research. Potential for career progression also helps establish clinical research professionals as leading practitioners in the clinical research industry. In support of this, DOCR currently is leading a project to modernize all job descriptions for research staff in the School of Medicine. Job levels will be standardized across the institution and based on education, experience, and workforce competencies, and will allow for individual professional growth.14 In concert with this effort, human resources is conducting a market analysis of salaries. DOCR also launched a voluntary Research Professionals Network at Duke in 2014 that so far has more than 400 members. The network connects research professionals from across the institution to advocate for research careers, provide options for formal and informal education, and develop high standards in the research community.
Results
Efficiency
Although no specific efficiency goals were established when DOCR rebooted clinical research support, the office has been tracking data and monitoring improvements to establish ongoing metrics. When focusing on studies that were required to have a study initiation meeting with DOCR, decreases in the institutional review time are not yet demonstrated, but a decrease in the time from institutional approval until the first participant is consented has been observed (Table 1). By continuing to provide expert navigation and better prepare research teams, DOCR aims to continue to improve these timelines. Importantly, there has been a reduction in the percentage of these studies that close without consenting any patients (Table 2), which could signify a more effective review process that is selective of research likely to be successfully implemented. The overall number of clinical research studies, average number of patients accrued per enrolling study, and total number of patients enrolled across the institution increased over time; the types of studies remained constant (Table 3). During the period from 2011 to 2015, the percentage of patients seen in the health system who were enrolled in clinical research increased from 1% to 7%; this exceeded the goal of increasing enrollment by 5%.
Table 1.
Study Startup Metrics for Fiscal Years 2011-2015
| Fiscal year | Number of protocols approved | Median days from IRB submission to institutional approval | Median days from institutional approval to first participant consented | Median days from IRB submission to first participant consented |
|---|---|---|---|---|
| 2011 | 306 | 86 | 74 | 182 |
| 2012a | 352 | 93 | 69 | 180 |
| 2013b | 292 | 101 | 68 | 194 |
| 2014 | 296 | 92 | 52 | 161 |
| 2015 | 313 | 100 | 50 | 171 |
Note: Data are for clinical research studies that scheduled, ordered, or charged through the Duke University Health System.
Transition to unified research support office.
Electronic health record implemented at Duke.
Table 2.
Study Closure Metrics for Fiscal Years 2011-2015
| Fiscal year | Median days from institutional approval to study closurea | Number of protocols closed with no consent | Number of protocols closed (total)b | Percentage of studies closing with no consented patients |
|---|---|---|---|---|
| 2011 | 561 | 73 | 246 | 30% |
| 2012c | 449 | 67 | 323 | 21% |
| 2013d | 469 | 63 | 330 | 19% |
| 2014 | 592 | 68 | 393 | 17% |
| 2015 | 532 | 44 | 304 | 14% |
Note: Data are for research studies that scheduled, ordered, or charged through the Duke University Health System.
Shorter time from institutional approval to study closure is considered more efficient.
These protocols did not necessarily open in the fiscal year indicated.
Transition to unified research support office.
Electronic health record implemented at Duke.
Table 3.
Duke University School of Medicine Active Research Studies and Participants Enrolled for Fiscal Years 2011-2015
| Fiscal year |
Total active studies |
NIH- sponsored studies |
Other federal studies |
Industry- sponsored studies |
Internal studies |
Total enrolling studies |
Total patients enrolled on studies |
Average patients enrolled per enrolling study |
|---|---|---|---|---|---|---|---|---|
| 2011 | 4988 | 1114 | 844 | 1136 | 1894 | 2171 | 18371 | 8.5 |
| 2012a | 5362 | 1170 | 887 | 1195 | 2110 | 2187 | 18321 | 8.4 |
| 2013 | 5628 | 1230 | 933 | 1251 | 2214 | 2184 | 22707 | 10.4 |
| 2014 | 5749 | 1250 | 965 | 1265 | 2269 | 2167 | 24500 | 11.3 |
| 2015 | 5757 | 1239 | 1000 | 1292 | 2226 | 2104 | 24355 | 11.6 |
Transition to unified research support office.
One of the ways DOCR has increased efficiency is in the contracts process. A common complaint heard by School of Medicine leadership from faculty was how long it took for a contract to be negotiated and executed at Duke. DOCR hired a contracts liaison to research this issue and help navigate contracts through the process. After meeting with representatives from all CRUs, a common complaint emerged—the time from completion of negotiations to getting Duke's institutional signature was too long and not transparent. To better understand this issue, the contracts liaison reviewed the average turnaround times to get institutional signature, which was obtained in a separate central office. By moving the signature function to within DOCR, the average time to get institutional signature was reduced from 3.6 days to 0.12 days. While the original 3.6 days may not seem like a large delay, the improvement shows that there were inefficiencies in the process. DOCR streamlined required components and made it a priority to get the contracts signed within 24 hours of receipt. Additionally, DOCR provided frequent status updates if there was a contract that could not be signed within 24 hours and worked with the team to shepherd it through the process and get it approved for signature. This focus on turnaround time and customer service has eliminated the complaints regarding the long wait for institutional signature. The contracts liaison continues to identify other areas of the process that delay contract negotiation and execution and is working through each one to minimize inefficiencies and make the process as transparent and user-friendly for study teams as possible.
Compliance with results reporting in ClinicalTrials.gov is another area where DOCR has shown improvements. On-time reporting has increased from <50% to 90% to 100% compliance over the past year.
Satisfaction
Satisfaction with DOCR services is assessed by an annual survey sent to individuals on the DOCR mailing list. The satisfaction rates have remained at approximately 75% or higher throughout DOCR's history (Figure 3A). Similarly, the proportion of negative comments received stayed relatively constant over time, with slightly more negative comments in fiscal year 2014, perhaps corresponding to frustrations associated with the implementation of the electronic health record across the health system (Figure 3B). In addition, satisfaction for Research Management Team services (data management and clinical research coordinator support) is assessed via email or phone call after one month, and then using a feedback survey sent after the effort has ended. Ratings for this support over a 1-year period have been very positive (Figure 3C, D). In fiscal year 2015, of individuals evaluating their optional study planning consultation (N=16) and grant development assistance (N=12), 94% and 100%, respectively, indicated they would be extremely or quite likely to use the service again.
DOCR aims to hire high-performing staff and match appropriately skilled and experienced individuals to study needs. If a performance issue or mismatch occurs, this can be corrected quickly for the study team with a replacement from DOCR, generally within 1 week. In this model, investigators and study teams do not have to spend time hiring or addressing performance issues themselves.
Cost
The operating budget of the narrowly scoped Clinical Research Support Office prior to transition to DOCR was approximately $916,000 in fiscal year 2012. In fiscal year 2015, DOCR had an operating budget of $2.68M, which included $905,000 covered by the CTSA grant. DOCR is paid for as part of the indirect cost rate for clinical research, which has not changed as a result of DOCR's inception. Only direct effort costs are charged to studies; there is no charge for overhead, management, facilitation, or consultation. In 2015, DOCR's Research Management Team had gross costs of $1.3M, all of which were covered by research grants or contracts. From fiscal year 2012 to 2015, the institution's costs for clinical research compliance and auditing (external to DOCR) remained virtually unchanged, from $2.48M to $2.45M, not accounting for inflation. This was in spite of a growing number of research studies and patients enrolled (Table 3). The percent gross profit margin for the School of Medicine as a whole was 10.3% in fiscal year 2012 and 6.5% in fiscal year 2015.
Discussion
Even when the need for research administration is recognized, there can be disagreements over the structure of support and responsibility for oversight. We have observed that the concept of “central” support can be met with a negative reaction—the perception being that these systems are heavy-handed and inflexible, consisting of top-down policy and bureaucracy. Conversely, in our experience supporting research teams, individualized approaches to research conduct throughout an institution may appear effective, or at least more expedient, but frequently lead to operational, regulatory, and compliance challenges.
In a transition to the unified support office, we observed that a culture change from within research administration may help reduce barriers to acceptance. To avoid creating infrastructure for the sake of infrastructure, it was essential to for DOCR to apply its efforts with the mindset of optimizing clinical research while also protecting human subjects from potential harm due to poor research conduct. Assessment and reassessment of existing administrative processes was essential to this process. Without thoughtful, multi-source input, development of institutional policies and procedures may distract faculty from research conduct and create a perception that administration is hampering research progress.
A focus throughout DOCR's growth and development was on building relationships with clinical investigators and study teams. Demonstrating advocacy for researchers by finding solutions that are compliant with policies and regulations while allowing the research to proceed expediently is the currency to support cultural change. Support from senior leadership within the institution was also critical throughout the office's transition. By assuming the role of facilitator, DOCR empowers staff within study teams to speak up when an issue needs to be addressed. This may not always be the case in decentralized structures where a principal investigator is also the supervisor of an administrator or coordinator.
Other consolidated units supporting clinical research at AMCs have been detailed in the literature, but those have been typically implemented at the level of a department or group of departments.15,16 This paper describes experiences implementing a clinical research support office for an entire AMC. As with DOCR, efficiency was described as a major goal of the previously published examples, though data to support the efficiency of such a model remain limited. In an analysis of high-performing clinical research teams at research centers by Retsch-Bogart and colleagues, characteristics included shared efficient processes, continuous process improvements, and a business-like approach to clinical research.17 All of these are approaches employed by DOCR.
Moving to a unified research support office involved an increase in cost to the institution, which was expected, and has been described with transitions at other institutions in association with increased services.16 However, during a period in which studies and enrollment increased, compliance costs remained stagnant. If DOCR can prevent compliance issues through training/education, consultation, and its suite of support services ensuring high-quality research, this will be of value to the institution. While some institutions approach shared support as a cost-savings measure, another view is to recognize research administrative support as an investment, with returns in the form of increased collaboration and retention of clinical investigators through the delivery of high-quality services.18
A limitation in our analysis is that formal satisfaction data are only available starting after the establishment of DOCR. Improvements in study startup times have only begun to be observed, and we will continue to monitor these and other study metrics to gain a better understanding of the effects of the support office. Rollout of a clinical research management system within the next year will provide access to more data for assessing changes in efficiency and return on investment for DOCR. It is unknown how long it would take to observe such changes. DOCR, along with CTSA administrators, is working on assessing common metrics, both within and across institutions.19 Currently, this remains challenging with varying systems to collect and analyze data. As new electronic systems (such as the clinical research management system) are deployed, these common data elements can be captured to standardize data capture across the institution for all studies.
Remaining Challenges
While significant strides have been made to improve clinical research oversight and operational support at Duke, there is much to do. In some areas, trust between faculty and administration must be re-established due to previously over-burdensome processes. Some investigators prefer to maintain their individualized approaches to conducting research. Having many of DOCR's services categorized as optional allows some flexibility while still providing overarching structure and support. Since the office evolved gradually, there is still some duplication with remaining CRU support, but this is expected to shift over time. Keeping focused on the ultimate goal of improving patient care and continuing to foster collaborations will help administration and faculty move forward as a team. Shifting a decades-long investigator/coordinator research culture to this team approach between central administration and a study group requires communication, trust, and a willingness to accept component responsibilities at all levels of a research organization. As the role of research professionals is increasingly professionalized, it should be more widely recognized that helping navigate the research environment is a valuable, high-level skill. In addition, DOCR, while successful in providing an increased range of services, must be mindful to grow responsibly and not just for the sake of growth. Finally, we must continue to share experiences to cultivate more effective research administration, particularly throughout the Clinical and Translational Science Award institutions, to transform the clinical trial enterprise.
Conclusion
The Duke Office of Clinical Research provides one example of how unified research support can be supported within a large AMC. Although unified research administration and support is often met with negative reactions initially, we have shown that it is possible to develop a team that cares and delivers excellent customer service to overcome this perception. The unified model has multiple advantages that can be realized through a shift in culture to refocus effort on the efficient and safe conduct of research that can lead to improved patient care.
Acknowledgements
The authors wish to thank Nancy Andrews, MD, PhD, Mitchelle Auton, MBA, MIS, Mark Bettger, MBA, L. Ebony Boulware, MD, Jill Boy, Ann Bradley, JD, Bruce Burnett, PhD, Robert Califf, MD, Elizabeth DeLong, PhD, Shelly Epps, John Falletta, MD, Gavin Foltz, JD, J. Scott Gibson, MBA, Leigh Goller, BS, Margaret Groves, JD, Chuck Kesler, Julie McCauley, Ross McKinney, MD, John Michnowicz, Rebbecca Moen, MBA, Billy Newton, Diane Oliver-Clapsaddle, MBA, Jody Power, MBA, Deborah Roth, MPH, Iain Sanderson, MB, Colleen Shannon, JD, Richard Sloane, MPH, H. Gilbert Smith, PhD, Ellen Steinour, Marissa Stroo, Geeta Swamy, MD, Tina Tyson, JD, and Lisa Varani, for supporting the vision and infrastructure for establishing a successful Duke Office of Clinical Research. Finally, we thank all the faculty and staff from Duke Health System Compliance Office, Office of Audit, Risk and Compliance, Regulatory Affairs, Duke Health Technology Solutions, Duke Medicine Information Security Office, Research Integrity Office, Office of Corporate Research Collaborations, Office of Research Administration, Institutional Review Board, Patient Revenue Management Organization, CRU leadership, School of Medicine Finance, Office of Research Informatics, and Duke Office of Clinical Research.
This paper was supported by the National Center for Advancing Translational Science of the NIH through Grant No. UL1TR001117. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: In the last three years, Mark Stacy, MD, received grant funding from the Michael J. Fox Foundation, NIH, Parkinson Study Group, and Pharma2B. He has served in a consultation capacity for Acorda, Genzyme, Eli Lilly, Lundbeck, Pfizer, ProStrakan, SK Life Science, and Vanda. He has also served on data management or protocol steering committees for Allergan, Biotie, Merz, Osmotica, and Revance. Lindsey L. Spangler, JD, owns stock in Allergan. None of the remaining authors has any potential conflicts of interest to disclose.
References
- 1.Dzau VJ, Yoediono Z, ElLaissi WF, Cho AH. Fostering innovation in medicine and health care: what must academic health centers do? Acad Med. 2013;88:1424–1429. doi: 10.1097/ACM.0b013e3182a32fc2. [DOI] [PubMed] [Google Scholar]
- 2.Califf RM. Clinical research sites—the underappreciated component of the clinical research system. JAMA. 2009;302:2025–2027. doi: 10.1001/jama.2009.1655. [DOI] [PubMed] [Google Scholar]
- 3.Feldman AM. Pursuing Excellence in Healthcare: Preserving America's Academic Medical Centers. CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- 4.Sung NS, Crowley WF, Jr., Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine . Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 6.Ennis C, Snyder D, Ainsworth T, Stacy M, Sanderson I. Utilization of the EPIC electronic health record system for clinical trials management at Duke University. Proceedings of the 2014 IEEE International Conference on Healthcare Informatics. 2014:216–219. [Google Scholar]
- 7.Schneider SL, Ness KK, Rockwell S, Shaver K, Brutkiewicz R. [April 14, 2016];Federal Demonstration Partnership 2012 Faculty Workload Survey. 2014 Apr; Available at http://sites.nationalacademies.org/cs/groups/pgasite/documents/webpage/pga_087667.pdf.
- 8.National Academies of Sciences . Engineering, and Medicine. Optimizing the Nation's Investment in Academic Research: A New Regulatory Framework for the 21st Century. National Academies Press; Washington, DC: 2015. [PubMed] [Google Scholar]
- 9.Rubin E, Lazar D. Clinical Trials Offices: What's New In Research Administration? Association of Academic Health Centers; Washington, DC: 2009. [Google Scholar]
- 10.Ginsberg B. The Fall of the Faculty: The Rise of the All-Administrative University and Why It Matters. Oxford University Press; New York: 2011. [Google Scholar]
- 11.Snyder DC, Epps S, Beresford HF, et al. Research management team (RMT): a model for research support services at Duke University. Clin Transl Sci. 2012;5:464–469. doi: 10.1111/cts.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Reilly EK, Hassell NJ, Snyder DC, et al. ClinicalTrials.gov reporting: strategies for success at an academic health center. Clin Transl Sci. 2015;8:48–51. doi: 10.1111/cts.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speicher LA, Fromell G, Avery S, et al. The critical need for academic health centers to assess the training, support, and career development requirements of clinical research coordinators: recommendations from the Clinical and Translational Science Award Research Coordinator Taskforce. Clin Transl Sci. 2012;5:470–475. doi: 10.1111/j.1752-8062.2012.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonstein S, Seltzer J, Li R, Silva H, Thomas Jones C, Daemen E. Moving from compliance to competency: a harmonized core competency for framework for the clinical research professional. Clinical Researcher. 2014 Jun;:17–23. [Google Scholar]
- 15.Croghan IT, Viker SD, Limper AH, et al. Developing a clinical trial unit to advance research in an academic institution. Contemp Clin Trials. 2015;45:270–276. doi: 10.1016/j.cct.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Early BJ, Huang DT, Callaway CW, et al. Multidisciplinary acute care research organization (MACRO): if you build it, they will come. J Trauma Acute Care Surg. 2013;75:106–109. doi: 10.1097/TA.0b013e318298779b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Retsch-Bogart GZ, Van Dalfsen JM, Marshall BC, et al. Highly effective cystic fibrosis clinical research teams: critical success factors. J Gen Intern Med. 2014;29(Suppl 3):S714–723. doi: 10.1007/s11606-014-2896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Squilla B, Steil A. Research shared services: a case study in implementation. National Council of University Research Administrators Magazine. 2016;48(2):37–39. [Google Scholar]
- 19.Rubio DM. Common metrics to assess the efficiency of clinical research. Eval Health Prof. 2013;36:432–446. doi: 10.1177/0163278713499586. [DOI] [PMC free article] [PubMed] [Google Scholar]