Abstract
Prenatal tobacco exposure (PTE) and prenatal stress exposure (PSE) each have been linked to externalizing behavior, although their effects generally have been considered in isolation. Here, we aimed to characterize the joint or interactive roles of PTE and PSE in early developmental pathways to behavioral disinhibition, a profile of cognitive and behavioral under-control that presages severe externalizing behavior. As part of a prospective, longitudinal study, 296 children were assessed at a mean age of 5 years. Exposures were assessed via repeated interviews across the prenatal period and bioassays of cotinine were obtained. Behavioral disinhibition was assessed using temperament measures in infancy, performance-based executive control tasks and measures of disruptive and inattentive behavior. PSE was associated with a higher probability of difficult temperament in infancy. Each exposure independently predicted poorer executive control at age 5 years. Difficult temperament and executive control difficulties in turn predicted elevated levels of disruptive behavior, although links from PTE and PSE to parent-reported attention problems were less robust. Children who experienced these prenatal exposures in conjunction with higher postnatal stress exposure showed the lowest executive control and highest levels of disruptive behavior. Findings highlight the compounding adverse impact of PTE and PSE on children’s behavioral trajectories. Given their high concordance, prenatal health campaigns should target these exposures in tandem.
Keywords: Prenatal tobacco exposure, prenatal stress, pregnancy smoking, externalizing, behavior problems
1. Introduction
There is growing recognition that the prenatal environment shapes health and disease across the lifespan (e.g., Barker, 1998). Two prenatal exposures that have been linked independently to externalizing behavior are maternal smoking and psychosocial stress during pregnancy. Often disregarded in studies of their impact, however, is the fact that prenatal tobacco exposure (PTE) often accompanies a high degree of stress. Women who smoke during pregnancy on average experience higher levels of financial stress, reduced social and partner support, more negative life events and trauma, and increased levels of psychological distress and anxiety (Bullock et al. 2001; Goedhart et al. 2009; Holtrop et al. 2010; Lynch et al. 2011). Moreover, high stress levels increase the likelihood of persistent smoking during pregnancy and are associated with reduced cessation self-efficacy (Weaver et al. 2008; Pickett et al. 2009; Prusakowski et al. 2011). PTE and prenatal stress exposure (PSE) therefore are intertwined and likely operate additively or interactively to shape children’s outcomes. A veridical understanding of the impact of these exposures demands consideration of their effects in the context of one another. Here, we aim to integrate measures of PTE and PSE and consider their mutual or interactive roles in shaping pathways to externalizing behavior.
Children with PTE are 2 to 4 times more likely to meet criteria for Oppositional Defiance Disorder and Conduct Disorder beginning in early childhood and also are more likely to exhibit substance use disorders and severe antisocial behavior in adolescence and adulthood (Wakschlag and Hans 2002; Gatzke-Kopp and Beauchaine 2007; Monshouwer and Huizink 2011; Ellis et al. 2012; Goldschmidt et al. 2012). Reported links to ADHD have been less consistent: while some studies have demonstrated associations between PTE and ADHD symptoms (Mick et al. 2002; e.g., Linnet et al. 2003), others suggest there is no association or that PTE predicts only comorbid ADHD and conduct problems as opposed to ADHD symptoms per se (Wakschlag et al. 2006a; Huijbregts et al. 2007; Ball et al. 2010). Studies using continuous outcome measures compliment those that have used clinical cut-offs, reporting elevated levels of aggression, impulsivity, hyperactivity and rule-breaking in preschoolers, children and adolescents with PTE relative to their non-exposed peers (Martin et al. 2006; Carter et al. 2008; Galera et al. 2011; Cornelius et al. 2012).
Similar to PTE, PSE has been linked to a higher likelihood of aggression, conduct disorder and ADHD in children and, more recently, to externalizing behavior in young adults (Talge et al. 2007; Li et al. 2010; Bekkhus et al. 2011; Blair et al. 2011; Glover 2011; Ronald et al. 2011; Grizenko et al. 2012; Class et al. 2014; Betts et al. 2015). In the Raine study, the number of maternal stressors experienced during pregnancy and PTE were two of the strongest predictors of psychiatric morbidity in early childhood, over and above several measures of postnatal psychosocial adversity (Robinson et al. 2008, 2011). Externalizing behavior problems were almost 2-fold more prevalent in children whose mothers reported 3 or more negative life events during pregnancy relative to those whose mothers reported fewer than 3 negative life events (Robinson et al., 2011).
Taken together, these studies suggest that PTE and PSE may contribute to the developmental origins of behavioral disinhibition, a risk phenotype that has been implicated in life-course persistent antisocial behavior and substance abuse (Tarter 2002; Tarter et al. 2003; Iacono et al. 2008; Zucker et al. 2011; Lester et al. 2012). Behavioral disinhibition is characterized by trait-like deficits in cognitive and behavioral self-control, which manifest as high levels of behavioral sensation seeking, impulsivity, aggression and early substance use initiation. Behavioral disinhibition is thought to reflect disruptions to prefrontal cognitive networks in the brain, including abnormal reactivity of thalamic and limbic neural structures that feed input to the prefrontal cortex or a deficiency in the prefrontal modulation of these inputs or both (Zucker et al. 2011). These neural networks modulate core executive control processes, which develop rapidly over the course of early childhood, allowing for greater goal-directed control over attentional resources (Clark et al. 2012; Wiebe et al. 2012).
There is accumulating evidence that children with PTE have impairments in executive control, including discrepancies in processing speed, sustained attention, response inhibition and working memory, relative to non-exposed controls in childhood and adolescence (Fried and Watkinson 2001; Willford et al. 2010; Mezzacappa et al. 2011; Cornelius et al. 2012). While research examining the impact of prenatal maternal stress on child executive control generally is lacking, one study of school-aged children reported dose-response reductions in working memory and inhibitory control performance with increasing maternal pregnancy-specific stress (Buss et al. 2011), while another found that sustained attention was reduced in boys, but not girls, whose mothers reported high levels of anxiety during pregnancy (Van den Bergh et al. 2006). Deficits in these core control processes have repeatedly been implicated in pathways to externalizing behavior, including ADHD, oppositional defiance and aggression (Hughes et al. 2000; Séguin et al. 2004; Hughes and Ensor 2008; Raaijmakers et al. 2008; Espy et al. 2011b). As youngsters approach kindergarten age and most children have gained some proficiency in executive control, delays or deficits in these key skills will likely become increasingly evident to caregivers in the form of behavior problems.
Less is known regarding the early features of behavioral disinhibition, although both theory and empirical work suggests that temperament may play an important role. Biological variations in infant temperament, evident as early as age 4 weeks, are thought to shape responses from others in the environment, exerting long-term influence on behavior and personality (Clark, 2002; Chess & Thomas, 1977). Difficult temperament in infancy, characterized by irregular feeding and sleeping patterns, high levels of withdrawal from environmental stimuli, difficulties adjusting to new experiences or routines, and intense expression of negative emotion, places children at increased risk of externalizing behavior problems later in childhood, particularly when paired with environmental stress (Sanson et al. 1993; Wright Guerin, Allen W. Gottfried, 1997; Fanti and Henrich 2010). PTE and PSE each have been linked to higher negative reactivity, higher activity levels and less adaptable temperament in infants (Martin et al. 2006; Wakschlag et al. 2006a; Willoughby et al. 2007; Carter et al. 2008; Pickett et al. 2008). Moreover, Lester et al., (2009) found evidence for a mediated pathway from prenatal substance exposure via early difficult temperament to behavioral disinhibition problems at age 7 years. It is possible, therefore, that there is an underlying heterotypic continuity to behavioral disinhibition that is evident even in the earliest phases of development among children with PTE and PSE.
Despite suggestions of parallel outcomes from PTE and PSE to behavioral disinhibition, most studies have treated the variance associated with one of these factors as ‘nuisance’ variance to be minimized through matching or statistical control. While this approach may help to isolate specific effects, it has limited applied value and does not elucidate potentially synergistic effects of these exposures. Further, studies often lack prospective, high quality measurement of one if not both of these exposures. As an exception, Roriguez and Bohlin (2005) found that self-reported smoking and maternal stress during pregnancy each accounted for significant variance in child ADHD symptoms at age 7 years. Their findings suggest that a combination of high prenatal stress and PTE may place children at ‘double jeopardy’ for later externalizing problems. Given that PTE and PSE often occur concurrently, there is a need for high-quality, prospective studies that jointly model the additive or interactive contributions of these prenatal exposures for children’s development over time.
Another important concern is the role of ongoing stress in the lives of children with adverse prenatal experiences. Research and theory suggest that the postnatal environment may amplify or exacerbate prenatal exposure effects. For instance, Fisher et al. (2011) found that prenatal substance exposure predicted neurobehavioral disinhibition in adolescence, but that early cumulative adversity also showed unique, additive effects. Similarly, Bekkhus et al. (2011) reported considerable stability (B = .86) in latent measures of pre- and postnatal maternal distress. There are conflicting findings as to whether prenatal, postnatal or cumulative effects are most important. Clearly, then, consideration of the postnatal environment will be important in pinpointing the role of exposure timing. Studies have also varied in their definition and approach to the measurement of stress during pregnancy (Nast et al. 2013). In this study, we drew upon decades of research in the developmental literature indicating that the effects of psychosocial stress are cumulative, where an increasing number of stressors increases the likelihood of poor functional outcome in a dose-response manner and the accumulation of these risk factors is a stronger predictor of cognitive and behavioral outcome than any single factor (Rutter 1979; Sameroff et al. 1987; Doan et al. 2012; Evans et al. 2013).
The specific aims of this study were to:
Describe the unique or interactive relations of PTE and PSE to infant temperament, executive control, and disruptive and inattentive dimensions of externalizing behavior in the late preschool period.
Test a sequenced developmental model, which links PTE and PSE indirectly to dimensions of externalizing behavior late in the preschool period via difficult infant temperament and poorer executive control.
Examine the roles of postnatal stress and postnatal tobacco exposure in these pathways.
Based on a behavioral disinhibition framework, we posited that PTE and cumulative PSE each would be linked to difficult temperament in infancy and that these temperamental difficulties would show developmental continuity, manifesting as deficits in executive control and higher levels of externalizing behavior in the late preschool period.
1. Method
2.1 Participants
Participants were recruited through fliers distributed at local obstetrics clinics in two sites: a 5-county rural area in Southern Illinois and a small city in Nebraska. Interested women were carefully screened via telephone interviews to assess study eligibility. Those who reported illegal substance use or alcohol use > 4 drinks on a single occasion were deemed ineligible. Mothers who reported smoking during pregnancy were over-sampled. Non-smokers were broadly matched to smokers for income to needs (t(291) = .91, p = .37) and ethnicity (χ2(4) = 6.39, p = .17) to reduce confounding of exposure with SES. Of the 380 women who met eligibility criteria and were enrolled in the study, 8 were later excluded from analysis because they reported high levels of alcohol or illegal drug use during interviews conducted at later time points. A further 8 infants were born at < 35 weeks GA and were excluded from analysis due to the known deleterious effects of preterm birth on the brain. Thus, 369 (5 sets of twins) infants and their mothers participated in the infant study. All participants were recruited before the 28th prenatal week and the majority were recruited prior to the 16th prenatal week.
Eighty-six percent (n = 293; 52% PTE) of the eligible participants from the original cohort participated in a follow-up study at a mean age of 5 years (SD = .27, range = 4.6 – 6 years). Reasons for non-participation included ineligibility due to neurodevelopmental conditions (e.g. Autism, TBI; n =14), death in the family (n = 4), child placement in state custody/adoption (n = 8), child age >72 months by enrollment (n = 2), or family relocation outside of the US (n = 1); loss to follow up (n = 29); declining to participate (n = 15); or repeated failure to attend scheduled appointments (n = 3). Families who did and did not participate in the follow-up did not differ in terms of proportion with PTE, χ2(1) = .11, p = .74; household income to needs, t(339) = 1.07 8, p = .28; maternal education, t(339) = .13, p = .89; child gestational age, t(339) = 1.58, p = .12 or child gender, χ2(1) = 2.80, p = .10.
Within the follow-up sample, mean maternal education was 13.45 (1.69) years. The ethnicity breakdown was 57% white, 28% African American, 13% Hispanic, 1% Native American, <1% were Asian, and 1% mixed race. The mean (SD) gestational age was 38.92 (1.30) weeks among PTE and 39.08 (1.23) weeks among non-exposed infants, t(290) = 1.12, p = .26. Mean birth weight was 3329.4g (485) in the PTE infants and 3399.3 (427) in the non-exposed infants, t(290) = 1.24, p = .21. On average, mothers who smoked during pregnancy reported smoking 4.14 (5.52) cigarettes per day (range = 0 – 35), although this average decreased by the third trimester to 3.42 (5.98) cigarettes. Within the smoking group, 14% reported smoking 10 or more cigarettes per day and 32% reported smoking at least 5 cigarettes per day. Mean alcohol intake during pregnancy was .03 (.07) drinks per day among smoking mothers and .01 (.01) drinks among non-smokers, t(291) = 5.36, p <.001.
2.2 Procedures
All study procedures were approved by university ethics boards and written, informed consent was obtained from mothers prior to participation. The prenatal and neonatal procedures have been described in detail elsewhere (Espy et al. 2011a). Briefly, mothers completed comprehensive timeline follow-back interviews (Sobell and Sobell 1992) to assess smoking in the preceding months as well as questionnaire measures of socio-familial background, depression and stress (see below) at 16 and /or 28 weeks of pregnancy. Mothers reported on infant temperament 4 weeks after giving birth.
Participants were re-contacted within 6 months of their children’s fifth birthdays and invited to participate in a home-based interview and a comprehensive laboratory-based assessment. During the home interview, measures of children’s family environments and socio-demographic backgrounds were obtained. Before or during the laboratory visit, mothers completed questionnaires to assess stress and child externalizing behavior, while the child completed computerized neuropsychological assessments in a separate room with a trained examiner. Children were offered regular breaks and snacks during the assessment, parents were financially compensated for their participation and children received a small toy.
2.3 Measures
2.3.1 Tobacco Exposure
Prenatal tobacco exposure (PTE) status was determined using a multi-step approach. If maternal reports of smoking status during screening, prenatal and postpartum interviews were consistent, then the exposure group status was retained. If the participant initially did not report smoking during pregnancy, but did report smoking during dates past the last menstrual period, then the child was re-classified as PTE. Cotinine assays from maternal urine samples collected during pregnancy and from infant meconium at birth were examined and compared with the self-report classifications. In two cases, cotinine levels were higher than 100 ng/ml even though mothers did not report smoking. These children were re-classified as PTE. Postnatal tobacco exposure was determined via maternal interview at 5 years. Mothers were asked how many smokers resided in the child’s home and children were classified as postnatally tobacco exposed if any smokers were present in the household.
2.3.2 Stress Exposure
Mothers reported on their household income, health insurance status, relationship status, household structure, education and household occupation during interviews conducted at 28 weeks post-menstrual age (prenatal time point) and at the 5-year follow-up point. Data from the interviews was imputed in SAS for 4.3% of cases who were missing one variable each at age 28 weeks. Additionally, The Life Stressors and Social Resources Inventory (Moos and Moos 1994) was administered at each time point. The scale consists of 200 items related to stress and social resources. In this study, scales assessing financial stress, home and neighborhood stress, family-related stress, friend and social relationship stress, and negative life events over the previous year were included. Data were multiply imputed for 3 cases who were missing single scales on the LISRES at the prenatal time point. Based on these measures collected at both the prenatal and 5-year time points, families were allocated 1 point for each of the following stressors from the interview and questionnaire measures: no caregiving support from a partner, maternal education < high school, unemployment for all caregivers, Medicaid status and household resident to room ratio > 1. A score of 1 was allocated for each LISRES scale with a score greater than 59, which indicates stress that is ‘well above average’ (Moos & Moos, 1994). In keeping with several studies that have used similar approaches (reviewed in Evans et al. 2013), a cumulative stress index was calculated for both time points by summing these scores. Stress scores were not computed for 2 cases at the prenatal time point and 3 at the 5 year time point due to missing scores on multiple stress variables.
2.3.3 Neonatal Temperament
The Early Infancy Temperament Questionnaire (EITQ; Medoff-Cooper et al. 1993) was administered at infant age 4 weeks. The EITQ is designed for children aged 1 – 4 months and comprises scales assessing the nine dimensions of temperament proposed by Thomas and Chess (1977), including: Activity level: The amount of physical movement; Rhythmicity: How regular or predictable the infant is in his/her routine; Approach/withdrawal: How positively the infant responds to new people or experiences; Adaptability: How well the child copes with changes to routine; Threshold: The infant’s level of emotional reactivity (e.g., loud, vigorous crying); Mood: The extent of positive or happy emotional expression; Persistence: How long the infant devotes attention to particular stimulus or persists with a particular behavior; Distractibility: How easily the infant can be distracted/soothed from negative stimuli; Sensitivity Sensory Threshold: How easily the infant reacts to stimuli. Statements such as, “Cries vigorously when sleepy” are rated by mothers on a 6 point scale from “almost never” to “always.” Test-retest reliability ranges from .48 −.87 over a 20 day period. In this sample, coefficient alphas were similar to those in the validation sample (.38 −.67). In their conceptualization of the temperament, Chess and Thomas considered constellations of these 9 scales to reflect 3 general classes of infant temperament: Easy, Slow to warm, and Difficult. Therefore, we did not use the individual scales from the EITQ, but instead used a latent profile analysis to identify clusters of children with different profiles of temperament.
2.3.4 Executive Control
Two measures were used to assess executive control at age 5 years. The Nebraska Barnyard (adapted from the Noisy Book task; Hughes, Dunn, & White, 1998) was programmed in Eprime and administered on a touch screen computer. During an initial training phase, children were presented with a 2 × 2 grid of colored buttons with pictures of familiar animals (cat, horse, pig and cow). The colors of the buttons corresponded to the animals (e.g. black for horse, pink for pig). As children pressed each button, it emitted the corresponding animal noise. Children completed a series of training trials to learn the button-animal associations. During subsequent test trials, the pictures were removed, leaving only the colored buttons. Children were required to press the buttons corresponding to increasing sequences of animal names. Thereafter, they were administered up to 3 trials at sequence lengths ranging from 2 to 8. If the child correctly completed the first two trials at a given sequence length, credit was automatically granted for the third trial. The task was discontinued after incorrect responses to 3 trials of a sequence length. The dependent variable was the number of correct trials.
Children also completed a Go/No-go task (Simpson and Riggs 2006; Wiebe et al. 2012), during which they were instructed to press a button when they saw a fish but to withhold responding when they saw a shark. After a brief training phase, 40 test trials were administered (75% fish and 25% sharks in random sequence), with stimuli presented for 1,500ms or until the child made a response. After each correct response to a fish, feedback was provided in the form of a fishing net and a bubbling sound for 250ms. If the child incorrectly responded to a shark, the computer showed a broken net with a buzzing sound. The inter-stimulus interval was 750ms. Children show significant improvement on this Go/no-go task between age 3 and 5 years and performance correlates with other measures of inhibitory control, supporting its validity (Wiebe et al., 2012). The dependent variable for the task was d’Prime, calculated by subtracting each child’s standardized probability of incorrectly responding to a shark from his/her standardized probability of correctly responding to a fish.
In previous studies, outcome variables from these executive control tasks have loaded onto a single executive control factor (Wiebe et al. 2011; Clark et al. 2014), suggesting that they tap a general executive control construct. In the interests of parsimony, task dependent variables were standardized to a mean of 50 and aggregated to create a single executive control score for each child.
2.3.5 Externalizing Behavior
Mothers completed the Multidimensional Assessment Profile of Disruptive Behavior (MAP-DB; Wakschlag et al. 2014) during the laboratory assessment at age 5 years, The MAP-DB is a developmentally-sensitive, dimensional measure designed to differentiate normative misbehavior from atypical patterns. Items are rated in terms of frequency over the past month using a 6-point scale from “never” to “many times each day.” Four scales generated from these ratings capture underlying dimensions of disruptive behavior – Temper Loss (α= .96), Noncompliance (α= .97), Aggression (α= .96), and Low Concern (α= .89).
Mothers also completed the ADHD scale from the Stonybrook Early Childhood Symptom Inventory (ECI; Gadow and Sprafkin 2000). The ADHD dimensional score was used in analyses for this study. The ECI is a DSM-based checklist designed for 3–6 year old children, where items such as, “runs about or climbs on things when asked not to do so,” are rated on a 4-point scale from 0- Never to 4 -Very often. ECI ratings demonstrate good discriminant validity when compared to chart diagnoses for ADHD and correlates significantly with comparable scales from other behavior checklists (Sprafkin et al. 2002). In the current sample, internal consistency was high (α = .92).
2.4 Statistical Methods
Analyses were completed in SAS and MPLUS in 5 major steps. In step 1, t-tests and Chi-squared tests were used to describe the overlap between PTE, PSE and postnatal stress. In step 2, we used latent profile analysis to distill meaningful classes of temperament in the sample based on the 9 temperament scales assessed with the EITQ. Thomas and Chess’ (1977) model, on which the EITQ is based, suggests that these 9 distinct dimensions of temperament should combine to form overarching temperament profiles. Given that latent profile analysis allows one to test whether the data meaningfully clusters into one or more underlying, categorical latent variables, it is an ideal technique for attempting to isolate these theorized temperament profiles. The best-fitting model for the number and structure of classes was chosen on the basis of statistical fit metrics, including Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), the Bootstrap Likelihood Ratio Test (BLRT) and the Lo-Mendell Rubin Likelihood Ratio Test (LMRT), as well as theoretical interpretability (Nylund et al. 2007). To avoid problems of local maxima, 500 sets of starting values were used in these models. The scale variances were allowed to vary freely within classes, as initial analyses showed that this resulted in higher entropy values.
In step 3, we turned to an analysis of the independent and interactive relations of PTE and cumulative PSE to each of our outcomes: infant temperament class, executive control scores, and externalizing behavior (Aim 1). Analyses were performed using multinomial or linear regression models, as appropriate. Maternal reported number of drinks per day during pregnancy was covaried in these models, given that alcohol consumption was associated with PTE. Interactions between PTE and PSE were tested by centering the cumulative PSE score, multiplying it with PTE, and entering it into the model simultaneous with main effects. A backward trimming approach was used to identify the most parsimonious models. Confirmatory factor analysis was performed on the externalizing behavior scales before proceeding to model building to identify more parsimonious, underlying constructs. Alternative factor structures were compared using the Satorra-Bentler scaled Chi-squared difference test (Asparouhov & Muthén, 2013).
In step 4, a full structural equation model was constructed in line with our aim of testing the continuity of behavioral disinhibition over time (Aim 2). That is, we examined whether relations of PTE and PSE to externalizing behavior were linked to executive control and associated externalizing behavior via infant temperament. The robust maximum likelihood estimator (MLR) was used, as it is less sensitive to violations of multivariate normality than maximum likelihood estimation, particularly in smaller samples (Kline, 2011). The Satorra-Bentler scaled Chi-squared difference test was used to compare models that included all direct paths to more parsimonious, nested models where direct paths were set to 0 (i.e., dropped from the model; Asparouhov & Muthén, 2013). The statistical significance of indirect path coefficients was further tested using bootstrapped 95% confidence intervals: a confidence interval that does not overlap with 0 indicates an indirect path of significant magnitude (Hayes 2009). Finally, in step 5, postnatal cumulative stress scores and postnatal tobacco exposure were added as additional predictors of the 5-year executive control and externalizing behavior outcomes (Aim 3).
3. Results
3.1 Overlap between Prenatal Tobacco and Stress Exposure
Table 1 shows the proportion of smoking and non-smoking mothers with high ratings for each stressor at the prenatal and 5-year assessment points. Overall, these findings suggest an overlap between PTE and PSE. Despite the fact that smoking and non-smoking groups were matched for income to needs, mothers who smoked during pregnancy were less likely to have completed high school. At the prenatal time point, mothers who smoked were more likely to report high numbers of negative live events in the preceding year and were more likely to report high levels of stress related to friends and social situations. Overall, cumulative stress scores were higher at both time points for mothers who had smoked prenatally and almost 60% of the smoking mothers reported experiencing 3 or more stressors during pregnancy. Within the PTE group, cumulative prenatal stress scores were weakly correlated with maternal reports of the number of cigarettes smoked per day per day during trimester 1 (r = .16, p = .05). Additionally, higher prenatal stress scores correlated with a lower likelihood of quitting by the second trimester of pregnancy, ρ = .20, p = .01.
Table 1.
Stressors Reported by Smoking and Non-Smoking Mothers During Pregnancy and at the 5-Year Follow-up
| % | Prenatal Time Point | 5 Year Time Point | ||||
|---|---|---|---|---|---|---|
| PTE | Non – PTE | p | PTE | Non – PTE | p | |
| Medicaid support | 84.42 | 82.01 | .58 | 78.43 | 78.42 | .99 |
| Unemployed | 6.49 | 5.76 | .79 | 14.38 | 10.79 | .36 |
| Household crowding | 5.19 | 5.04 | .95 | 11.18 | 8.63 | .47 |
| Low Maternal Education | 16.88 | 4.32 | <.001 | 11.76 | 4.32 | .02 |
| No caregiving partner | 14.29 | 9.35 | .19 | 41.18 | 28.78 | .02 |
| High financial stress | 35.06 | 29.71 | .33 | 27.27 | 23.02 | .40 |
| High home & neighborhood stress | 29.22 | 26.47 | .50 | 22.08 | 23.74 | .74 |
| High physical health stress | .65 | 1.45 | .60 | 2.60 | 2.88 | .88 |
| High family-related stress | 17.53 | 17.39 | .97 | 27.27 | 23.02 | .40 |
| High friend-related stress | 18.18 | 9.42 | .03 | 18.18 | 13.77 | .31 |
| High negative live events | 53.90 | 39.13 | .01 | 41.56 | 25.9 | <.01 |
| High cumulative stress | 56.58 | 38.85 | <.01 | 54.61 | 44.20 | .08 |
| M (SD) Cumulative stress score | 2.82 (1.73) | 2.26 (1.73) | <.01 | 3.02 (2.07) | 2.47 (1.73) | .01 |
Note. PTE: Prenatal tobacco exposure; Unemployed: No member of household currently employed; Household crowding: Resident to room ratio >1; Low maternal education: < High school education; High cumulative stress: > 3 stressors
3.2 Latent Profile Analysis of Infant Temperament
Prior to examining the relations of PTE and PSE to infant temperament, a latent profile analysis was performed to pare the EITQ scales down to meaningful classes that could be used in subsequent analyses. As shown in Table 2, although the AIC, BIC and BLRT values from the latent profile analysis favored a 4-class solution, the LMRT values favored a 3-class solution. Log likelihood value estimates for 20 final iterations suggested that there may have been some local maxima for the 4-class solution. Additionally, inspection of class means and variances for the 4-class solution indicated that two of the classes showed highly overlapping profiles. Based on the LMRT and on theoretical interpretability, the more parsimonious 3-class model was selected as the preferred solution.
Table 2.
Fit Statistics for Different Models Tested in Latent Profile Analysis of Infant Temperament
| LL | AIC | BIC | Entropy | BLRT | BLRTp | LMRT | LMRp | |
|---|---|---|---|---|---|---|---|---|
| 1 class | −4801.84 | 9639.68 | 9709.977 | |||||
| 2 class | −4609.027 | 9274.055 | 9383.405 | .78 | 385.625 | <.001 | 379.204 | <.001 |
| 3 class | −4548.292 | 9172.583 | 932.987 | .72 | 121.472 | <.001 | 119.5 | .008 |
| 4 class | −4502.16 | 910.324 | 9287.781 | .75 | 92.259 | <.001 | 9.723 | .15 |
Note: LL: Log likelihood; AIC; Akaike’s Information Criterion (smaller is better); BIC: Bayesian Information Criterion; BLRT: Bootstrap likelihood ratio test; LMRT: Lo-Mendell Rubin Likelihood Ration Test (non-significant p indicates that the additional class is unecessary)
Figure 1 illustrates the mean EITQ scale scores for each of the temperament classes identified via latent profile analysis. Post-hoc Bonferroni-corrected contrasts indicated that class one (labeled Easy; 34%) was characterized by lower ratings for the majority of the scales. Class 2 (labeled Slow to Warm; 38%) showed a mixed profile, characterized by lower adaptability (p <.001), rhythmicity (p < .001) and higher negative mood (p <.001) and threshold (p <.001) than Class 1, but higher approachability (p < .001), rhythmicity (p = .02), adaptability (p < .001), threshold (p <.001) and distractibility (p <.001) and lower intensity (p <.001) than Class 3. Class 2 also was characterized by lower persistence scores than both of the other classes (p <.001). In addition to these distinctions from Class 2, Class 3 (labeled Difficult; 29%) showed higher ratings than Class 1 on all of the EITQ scales apart from Persistence. Children were assigned to temperament groups based on posterior probabilities from this latent profile analysis.
Figure 1.
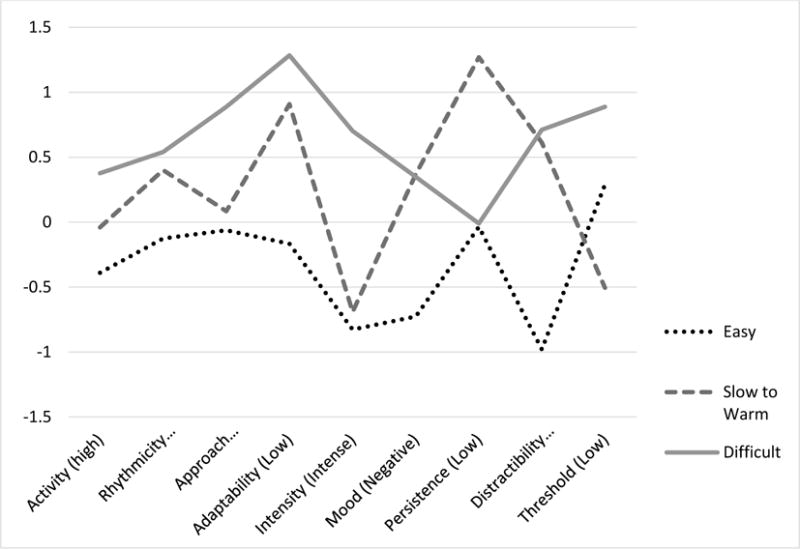
EITQ Scale Means for Three Temperament Classes Identified with Latent Profile Analysis.
Words in parentheses indicate the direction of the temperament scale.
Prenatal Tobacco Exposure, Prenatal Stress and Infant Temperament
A multinomial regression analysis including both PTE and PSE as independent variables indicated that each exposure reduced the probability of a child’s falling into the easy temperament class. Controlling for prenatal alcohol exposure, infants with PTE were 2.27 (CI = 1.23 – 4.19, p = .01) fold more likely to be classed as slow to warm as opposed to easy in temperament and 2.27 (CI = 1.58 – 4.33, p = .01) fold more likely to be classed as having difficult as opposed to easy temperament relative to infants without PTE. Similarly, each 1-point increase in the cumulative PSE score was associated with a 1.30 (CI = 1.09 – 1.56, p < .01) increase in the odds of difficult temperament class membership, although higher PSE did not increase the likelihood of membership in the slow to warm class, p = .24. Tests for interactions between PTE and PSE in relation to infant temperament were not significant and prenatal alcohol exposure did not predict temperament class membership in this model.
3.3 Prenatal Tobacco Exposure, Prenatal Stress and Executive Control at 5 Years
In the next phase of analysis, we examined the independent relations of PTE and PSE to executive control at the 5-year follow-up point. Findings showed that PTE predicted a 2.49 (SE = 1.06, p = .02) point reduction in the executive control composite score after accounting for age, alcohol exposure and PSE. In the same model, each additional prenatal stressor was associated with a .63 (SE = .15, p < .01) point decrease in executive control, whereas prenatal alcohol exposure was not significant. There were no significant interactions. Collectively, these prenatal exposures explained 14% of the variance in executive control at age 5 years.
3.4 Prenatal Tobacco Exposure, Prenatal Stress, and Externalizing Behavior at 5 Years
Given the high correlations among externalizing behavior scales (see Table 3), confirmatory factor analysis was used to determine whether these scales were underscored by one or more latent factors. The best fitting model was one with two correlated factors – a disruptive behavior factor, made up from scales from the MAP-DB, and an attention problems factor, made up of the scales from the ECI, χ2(7) = 18.33, p =.01, CFI = .99, RMSEA = .07. When these respective factors were regressed on PTE, PSE and prenatal alcohol exposure, PTE predicted elevated disruptive behavior, B = 3.50 (1.18), p < .01 and more attention problems, although only at a trend level for the latter, B = 2.09 (1.06), p = .06. Similarly, higher levels of PSE were associated with increased disruptive behavior, B = .76 (.31), p = .01, model χ2(19) = 36.56, p = .009, CFI = .98, RMSEA = .06, R2 = .03 – .07. Prenatal alcohol exposure was not related to either of the externalizing factors.
Table 3.
Correlations between Prenatal Tobacco Exposure, Cumulative Stress, and Behavioral Inhibition Measures across Early Childhood
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age at 5-year follow-up | |||||||||||||
| 2. PTE | −.12 | ||||||||||||
| 3. Postnatal tobacco exposure | −.12 | .56*** | |||||||||||
| 4. PSE | −.09 | .16** | .18** | ||||||||||
| 5. Postnatal stress exposure | −.05 | .14* | .24*** | .46*** | |||||||||
| 6. Prenatal Alcohol exposure | −.07 | .32*** | .13 | −.06 | −.09 | ||||||||
| 7. Slow to warm temperament | −.07 | .11 | .08 | −.01 | −.01 | .07 | |||||||
| 8. Difficult temperament | .01 | .07 | .09 | .18** | .17** | −.09 | −.49*** | ||||||
| 9. Executive control | .27*** | −.19** | −.22*** | −.23*** | −.28*** | −.05 | .06 | −.25*** | |||||
| 10. MAP-DB Temper loss | −.10 | .17** | .12* | .14* | .25*** | .05 | −.01 | .10 | −.20*** | ||||
| 11. MAP-DB Low concern | −.07 | .18 | .15 | .20*** | .25*** | −.01 | .06 | .09 | −.20*** | .72*** | |||
| 12. MAP-DB Non-compliance | −.13* | .23*** | .21*** | .15** | .20*** | .11 | .02 | .13* | −.21*** | .82*** | .75*** | ||
| 13. MAP-DB Aggression | −.08 | .20*** | .19** | .19** | .28*** | .06 | −.01 | .16** | −.26*** | .82*** | .86*** | .78*** | |
| 14. ECI Inattentive | −.13* | .23** | .21** | .19** | .23** | .11 | .13* | 0.02 | −.21** | .54** | .58** | .82** | .75** |
| 15. ECI Hyperactive/Impulsive | −.08 | .20** | .19** | .22** | .29** | .06 | .16** | −0.01 | −.26** | .51** | .53** | .82** | .86** |
p <.05
p <.01
p <.001;
PTE: Prenatal tobacco exposure, PSE; Prenatal stress exposure
3.5 Paths from Prenatal Stress and Tobacco Exposure to Externalizing Behavior
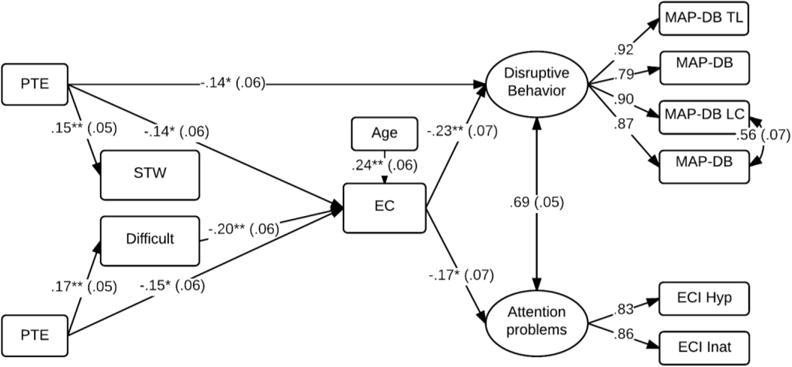
The above findings suggest compounding relations of PTE and PSE to markers of behavioral disinhibition across early childhood. As shown in Table 3, there were also significant correlations among the measures collected at different time points, suggesting continuity in the underlying pattern of behavioral disinhibition. Structural equation modeling was used to examine the pathways between these predictors over time. Note that prenatal alcohol exposure was not considered in this model as it was not a significant predictor in any of the initial models. Figure 3 illustrates the final model after trimming non-significant paths, χ2(45) = 62.44, p = .04, CFI = .99; RMSEA = .04, R2 = .02 – .17. Difficult temperament predicted a 3.75 (SE = 1.14, p < .01) point reduction executive control at the 5-year assessment point. However, the direct paths from PTE and PSE to executive control also remained significant and could not be removed from the model without a reduction in model fit. Poorer executive control was, in turn, associated with higher ratings of disruptive behavior and attention problems (p’s < .05). Although PTE continued to predict higher levels of disruptive behavior at a trend level of significance, all other direct pathways from PTE, PSE, and temperament to externalizing behavior could be dropped from the model without a reduction in model fit, χ2Δ = 6.22 (10), p = .77.
Figure 3.
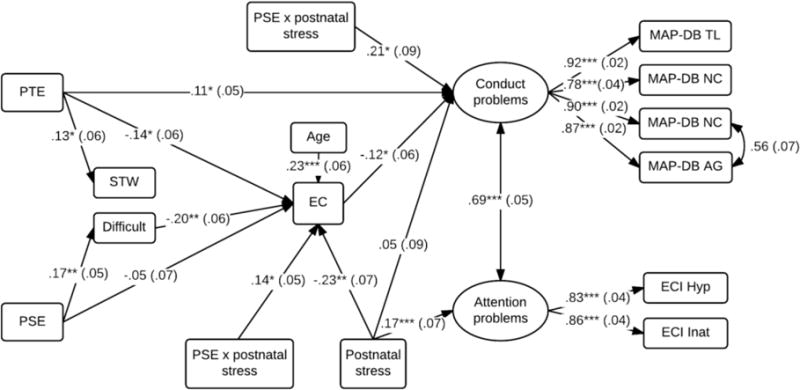
Pathways from PTE and PSE to child externalizing behavior
*p <.05, **p <.01, ***p <.001; PTE: Prenatal Tobacco Exposure. STW: Slow to warm temperament, Difficult: Difficult temperament. Standardized estimates are shown with SE in parentheses.
In addition to the direct paths illustrated in Figure 3, bootstrapped tests also revealed indirect paths from PTE to disruptive behavior [B = .57 (CI = .10 – 1.38)] and attention problems [B = .36 (CI = .01 – .90)] via executive control. Likewise, there were indirect paths from PSE to executive control via difficult temperament, [B = −.16 (CI = −.33 – −.05)], and to conduct problems [B = .21 (CI = .04 – .48)] via difficult temperament and executive control. Collectively, these findings indicate that PTE and PSE each presage a continuing pattern of behavioral disinhibition that manifests as difficult temperament in infancy, and lower executive control and higher levels of disruptive behavior by kindergarten age.
3.6 The Roles of Postnatal Tobacco Exposure and Psychosocial Stress
Although our analyses indicate independent relations of PTE and PSE to child behavioral disinhibition, there is a high degree of continuity in these adverse exposures. For descriptive purposes, we divided children into groups based on pre- and postnatal exposure levels. Of those who were PTE, 77% had mothers who reported household smoking at age 5 years. A further 22% of the sample were not PTE but were exposed to household smoking at age 5 years. As shown in Table 4, children exposed to tobacco both prenatally and up to the 5-year follow-up point on average performed lowest on the executive control measures and were rated by their mothers as having higher rates of noncompliance and higher levels of aggression relative to children with no exposure at either time point. In terms of stress exposure, Table 5 shows that children exposed to > 3 stressors at both time points showed lower levels of executive control, higher levels of low concern and higher levels of aggression relative to children exposed to fewer than 3 stressors at both follow-up points. Overall, then, these descriptive analyses suggest an additive effect of pre- and postnatal exposures when these exposures are considered individually, such that children exposed at both time points show the greatest levels of behavioral disinhibition.
Table 4.
Relations of Prenatal and Postnatal Tobacco Exposure to Markers of Child Behavioral Disinhibition
| 1. None (n =109) | Tobacco exposure | 4. Pre- & postnatal (n=118) | p | ||
|---|---|---|---|---|---|
| 2. Prenatal only (n = 35) | 3. Postnatal only (n=30) | ||||
| Executive Control | 52.85 (8.12)4 | 49.70 (7.92) | 48.67 (8.96) | 48.20 (8.27)1 | <.01 |
| MAP-DB Temper loss | 48.07 (8.51) | 51.18 (7.33) | 47.57 (8.44) | 51.88 (11.07) | .15 |
| MAP-DB Low Concern | 48.01 (8.73) | 50.24 (10.15) | 47.75 (7.52) | 51.91 (11.03) | .08 |
| MAP-DB Non-Compliance | 47.52 (8.69)4 | 49.20 (7.18) | 47.30 (9.42) | 53.04 (10.48)1 | <.01 |
| MAP-DB Aggression | 47.57 (7.21)4 | 49.06 (7.57) | 48.37 (6.95) | 52.37 (11.98)1 | .02 |
| ECI Inattentive | 50.28 (8.71) | 49.76 (8.71) | 51.24 (8.87) | 53.54 (10.67) | .05 |
| ECI Hyperactive/impulsive | 51.11 (10.36) | 53.38 (10.70) | 54.55 (9.05) | 54.97 (11.47) | .14 |
Note. Postnatal exposure based on maternal report of any household smoking at the 5-year follow-up point; 1 child missing exposure status at 5 years. Numbers indicate significant bonferroni-corrected group comparisons.
Table 5.
Relations of Prenatal and Postnatal Cumulative Stress Exposure to Markers of Child Behavioral Disinhibition
| 1. Low (n=105) | Cumulative stress exposure | 4. High pre- & postnatal (n=96) | p | ||
|---|---|---|---|---|---|
| 2. High prenatal only (n=41) | 3. High postnatal only (n =46) | ||||
| Executive Control | 51.37 (9.91)3, 4 | 48.30 (9.17)1 | 49.12 (8.05) | 46.22 (8.80)1 | <.001 |
| MAP-DB Temper loss | 47.64 (7.22) | 49.65 (1.82) | 51.21 (9.31) | 51.50 (11.16) | .09 |
| MAP-DB Low Concern | 47.36 (7.46)4 | 49.94 (11.53) | 49.57 (8.35) | 52.51 (11.38)1 | <.01 |
| MAP-DB Non-Compliance | 47.75 (7.11) | 49.25 (1.39) | 50.99 (9.92) | 51.66 (11.40) | .07 |
| MAP-DB Aggression | 47.14 (6.11)4 | 48.84 (9.62) | 50.49 (8.07) | 52.01 (11.45)1 | .01 |
| ECI Inattentive | 49.51 (7.36) | 51.37 (10.08) | 53.37 (9.28) | 52.46 (10.81) | .07 |
| ECI Hyperactive/impulsive | 51.01 (9.70) | 53.56 (11.81) | 54.30 (9.98) | 54.42 (11.01) | .20 |
Note: Children were classified as having high stress if they experienced 3 or more stressors. Numbers indicate significant bonferroni-corrected group comparisons.
In the final stage of analyses, we re-estimated the SEM, including exposure to postnatal smoking and postnatal cumulative stress as additional predictors of the 5-year outcomes. The final model is shown in Figure 3, with non-significant paths trimmed if they did not reduce model fit, χ2(76) = 87.56, p = .17, CFI = .99; RMSEA = .02, R2 = .02 – .22. Two interactions emerged as significant in this model. As described in Figure 4, model estimates revealed that children exposed to low levels of PSE and low levels of postnatal stress fared best on the executive control composite at age 5 years. Figure 5 shows that a combination of PTE and high postnatal stress predicted the highest levels of disruptive behavior. After taking postnatal stress exposure into account, there were no significant relations of postnatal tobacco exposure to any of the 5-year outcomes and all paths from this predictor could be dropped without reducing model fit, χ2Δ = 3.36 (3), p = .34. Unlike disruptive behavior, the only significant predictor of attention problems in the final model was postnatal stress.
Figure 4.
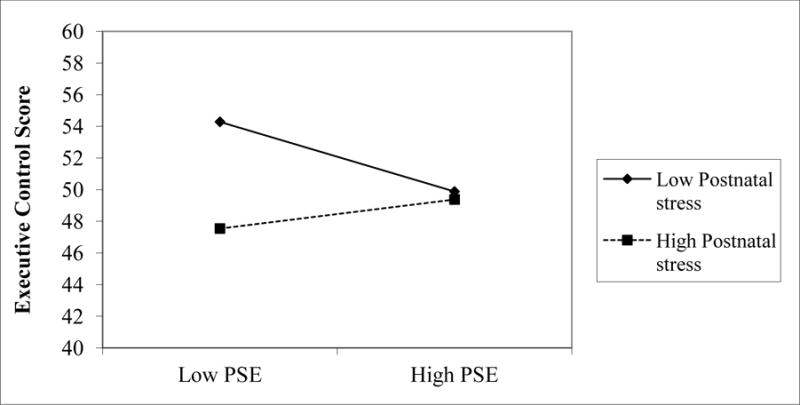
Executive control scores for children exposed to high (1SD above the mean) and low (1 SD below mean) stress pre- and postnatally, as estimated from SEM model parameters
Figure 5.
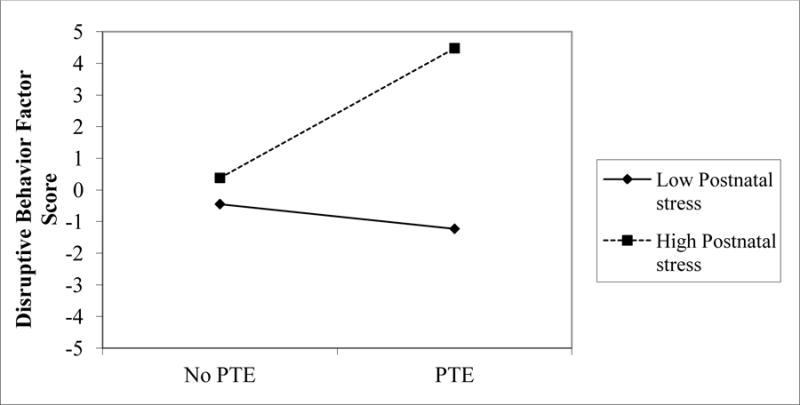
Disruptive behavior scores for children exposed to PTE and either high (1 SD above mean) or low (1SD below mean) postnatal stress as estimated from SEM model parameters
4. Discussion
Mothers who smoke during pregnancy often report high levels of stress, although studies that probe the overlap between these prenatal exposures and the developmental dynamics of their effects over time are limited. Drawing on a well-characterized, prospectively enrolled cohort, we aimed to describe the potentially synergistic relations of PTE and PSE to an unfolding pattern of child behavioral disinhibition. Findings provide support for the idea that PTE and PSE each presage a pattern of behavioral disinhibition in early childhood, characterized by difficult temperament, lower levels of executive control, and higher disruptive behavior, although the effects were less robust for inattentive/hyperactive behavior. Moreover, the effects of these prenatal exposures generally appear to be additive. Findings also suggest that the continuity in exposure to environmental stress over the course of early childhood amplifies behavioral disinhibition risk.
In keeping with previous research (e.g., Weaver et al. 2008), mothers who smoked during pregnancy reported higher levels of prenatal stress. They reported more negative life events and more stress related to friends and social situations and also were more likely to have dropped out of high school than mothers who did not smoke. They generally showed higher levels of stress related to social interactions and were less likely to have caregiving support from a partner by the 5 year time point, suggesting less access to social resources that might otherwise buffer the impact of stress. Across both study time points, mothers who smoked also were more likely to report that they faced multiple stressors relative to mothers who did not smoke. Smoking mothers who experienced more stressors during pregnancy also were less likely to quit smoking by the second trimester and generally reported smoking a greater number of cigarettes per day. It should of course be acknowledged that this cohort in general is characterized by high levels of psychosocial adversity, with approximately 80% meeting federal eligibility for food stamps, free lunch or Medicaid based on household income to needs. The independent effects of psychosocial stress may have been clearer had the cohort been more diverse in terms of SES. Nonetheless, findings indicate that even though objective income levels were similar for mothers who smoked vs. those who did not, mothers who smoke during pregnancy perceive their lives as more stressful than those who do not. They also are more likely to face numerous challenges and the higher the number of these stressors, the more likely they are to continue smoking through the full course of pregnancy.
Smoking during pregnancy and high levels of PSE were associated with a lower likelihood of easy infant temperament. Results from the latent profile analysis were consistent with temperament theory, suggesting that children can be clustered meaningfully based on their responses to others and the environment at this early age. The results also are interpretable according to Chess and Thomas’ classes of temperament, where the more ‘difficult’ class were less adaptable and rhythmic in their feeding and elimination cycles, more easily aroused and more intense in their expression of negative emotion than the other identified classes. Although our findings here are based on maternal report, other studies incorporating direct observations of infant neurobehavior have found early differences in temperament in children with PTE (Fried & Watkinson, 1987; Mansi et al., 2007; Sheutze & Eiden, 2007; Picone et al., 1985). While some studies suggest high levels of negative irritability and reactivity (e.g., Law, Stroud, & LaGasse, 2003), others report poorer visual attention and reduced auditory responsiveness (Fried, & Watkinson, 1987; Espy, Fang, Johnson, Stopp, & Wiebe, 2011). It is possible, therefore, that PTE may be related to two distinct profiles of temperament, the first characterized by high irritability and a low threshold for arousal and the second characterized by low attentiveness and reduced or typical arousal, reflected in our slow to warm and difficult classes. In contrast, prenatal stress was related only to an increased probability of difficult, rather than slow to warm temperament, which is consistent with studies noting higher negative reactivity in these infants (Blair et al., 2011; Bolten et al., 2013).
Infants who fell into the difficult temperament class, but not the slow to warm class, were more likely to exhibit lower executive control performance at age 5 years. Indeed, tests for indirect effects suggested continuity in the effect of PSE via difficult infant temperament. It is admittedly not possible from this study to isolate the underlying mechanism of continuity in the links between difficult temperament and executive control. On the one hand, maternal perceptions of difficult temperament and performance on measures of executive control may be joint outcomes of maternal representations of and reactions toward her infant, such that developmental continuity is driven by continuity in maternal-child representations and associated interactions. Alternatively, difficult temperament in the neonate may be indicative of underlying child neurophysiological dysregulation that manifests in different ways over time. Objective measures of temperament will be necessary to better disentangle these underlying mechanisms. Regardless of the mechanisms, these findings do suggest that maternal ratings of infant temperament as early as 4 weeks may offer some promise for the identification of infants who might be at risk for later executive difficulties. Furthermore, the relations between maternally-rated temperament and executive control were robust even after accounting for 5-year stress, indicating that a common link to postnatal psychosocial stress does not explain these associations.
The pathways from PTE and prenatal stress to reduced executive control performance are particularly concerning given the established importance of executive control for children’s school readiness (e.g., Blair & Razza, 2007; Clark, Sheffield, & Espy, 2011). As these children make the transition to school, their difficulties with these executive processes likely will take a toll on classroom learning and interactions with peers. Indeed, poorer executive control was associated with higher levels of disruptive behavior in our sample. Interestingly, the correlations between PTE and attention problems were weaker, a finding that is consistent with Wakschlag, Pickett et al. (2006b), who found links between PTE and ADHD only when they were comorbid with oppositional, aggressive behavior. There also was no relation of PSE to attention problems. In a genetic design study including mothers with and without IVF, PSE was associated with offspring antisocial behavior, although not symptoms of ADHD, after accounting for current maternal depression and anxiety (Rice et al. 2010). It is possible, then, that these prenatal exposures are differentially associated with a profile of increased disruptive behavior, as opposed to inattentive, ADHD-type symptoms.
There was evidence in this study for interactions between the prenatal and postnatal environment. Higher levels of prenatal stress were predictive of lower executive control when paired with higher levels of postnatal stress. Moreover, children who experienced both PTE and higher levels of postnatal stress showed the highest levels of disruptive behavior. This interaction between pre- and postnatal stress is consistent with a prenatal programming model, where an adverse prenatal environment triggers the methylation of genes that regulate physiological responses to stress in the developing fetus, thereby permanently altering the child’s ‘set point’ for homeostasis and level of reactivity to the postnatal environment (Beijers et al., 2014; Talge et al., 2007). Prenatal stress induction in pregnant dams, non-human primates and sheep leads to long-term alterations in hypothalamic-pituitary-adrenal axis functioning in offspring, as well as abnormal neurogenesis and synaptogenesis in the hippocampus and prefrontal cortex (Belnoue, Grosjean, Ladeveze, Arbous, & Koehl, 2013; Clarke, Wittwer, Abbott, & Schneider, 1994; Coulon, Wellman, Marjara, Janczak, & Zanella, 2013; Schneider et al, 1992, 2002; Zucchi et al., 2013). It is possible, then, that higher levels of prenatal stress increase the sensitivity of offspring to postnatal stressors, conferring greater vulnerability to behavioral disinhibition.
Practically, our findings point to the need for a multi-tiered approach in perinatal health that recognizes the co-occurrence of stress and substance use and considers their potentially additive impact on children’s development. Current approaches to tobacco cessation often focus on potential health risks to the developing fetus. However, mothers who continue to smoke through pregnancy may be psychologically vulnerable and may lack optimal social support networks to encourage smoking cessation. Perinatal health campaigns may benefit from attention to stress reduction techniques, emotional care, and the development of positive social supports in addition to encouraging smoking cessation. Prenatal meditation and relaxation techniques, as well as personalized nursing partnerships offer some promise in this regard (Chan, 2014; Olds et al, 1998).
Some caveats to the findings should be noted. First, while the study did show hypothesized relations between prenatal exposures and later neurobehavioral disinhibition, effect sizes were modest and the design is correlational. Clearly, pathways to disruptive behavior are complex and there are likely to be strong genetic and epigenetic mechanisms at play in these links (e.g., Grizenko et al., 2013). Second, we used a cumulative measure of self-reported stress at both time points, which does not provide insights into potentially unique effects of specific stressors. However, numerous studies have shown that the number of stressors in a child’s environment has as much, if not greater bearing, on risk for psychopathology (Sameroff et al. 1987; Evans et al. 2013). Therefore, the cumulative stress scores are consistent with our focus on an ecologically valid approach to prenatal risk. Finally, while we did covary for alcohol exposure, it is important to note that the sample was selected to mitigate the confounding effects of this exposure and therefore, findings related to alcohol may not be generalizable to other samples.
Notwithstanding these limitations, the study provides new insights into the ongoing relevance of prenatal adversity in pathways to behavioral disinhibition. Prenatal tobacco exposure and stress appear to confer additive vulnerability to an unfolding profile of behavioral disinhibition. The behavioral disinhibition phenotype is notoriously resistant to treatment and intervention once frank disorder is present and offers a potential inter-generational mode of transmission of risk through its relation to substance use and antisocial behavior. The study points to a need for high-quality prenatal care and support to families to try and mitigate the compounding effects of early adversity in the lives of young children, as well as for early intervention to prevent the emergence of behavioral disinhibition markers from escalating to severe and entrenched patterns.
Figure 2.
Pathways from PTE and PSE to child externalizing behavior
*p <.05, **p <.01, ***p <.001; PTE: Prenatal Tobacco Exposure. STW: Slow to warm temperament, Difficult: Difficult temperament. Standardized estimates are shown with SE in parentheses.
Highlights.
Despite their common co-occurrence, few studies have simultaneously modeled the roles of prenatal tobacco (PTE) and prenatal stress exposure (PSE) in externalizing behavior trajectories.
In a prospectively-enrolled sample, PTE and PSE each predicted a lower likelihood of easy infant temperament and lower executive control and higher levels of disruptive behavior at age 5 years.
There was developmental continuity in these relations: difficult temperament and lower executive control predicted higher levels of disruptive behavior.
Children with prenatal exposure also were more likely to face continued household stress, with results suggesting additive effects of pre- and postnatal adversity.
Studies should model the effects of PTE and PSE together, as their co-occurrence may compound risk for disruptive behavior trajectories.
Acknowledgments
This study was supported by NIDA grants R01DA014661 to Kimberly Andrews Espy and R01DA023653 to Kimberly Andrews Espy and Lauren Wakschlag. We wish to acknowledge Desiree M. de Jong, Caitlin T. O’Brien, Sandra A. Wiebe, and members of the Developmental Cognitive Neuroscience Laboratory for their assistance with study coordination, data collection and coding. We also wish to thank all the families who graciously gave their time to participate in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball SW, Gilman SE, Mick E, Fitzmaurice G, Ganz ML, Seidman LJ, et al. J Psychiatr Res [Internet] 15. Vol. 44. Elsevier Ltd; 2010. Revisiting the association between maternal smoking during pregnancy and ADHD; pp. 1058–62. Available from: http://dx.doi.org/10.1016/j.jpsychires.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–28. [PubMed] [Google Scholar]
- Bekkhus M, Rutter M, Barker ED, Borge AIH. The role of pre- and postnatal timing of family risk factors on child behavior at 36 months. J Abnorm Child Psychol. 2011;39:611–21. doi: 10.1007/s10802-010-9477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mennes M, Stevens V, Van Der Meere J, Börger N, Stiers P, et al. ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatr Res. 2006;59(1):78–82. doi: 10.1203/01.pdr.0000191143.75673.52. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Alati R. the Relationship Between Maternal Depressive, Anxious, and Stress Symptoms During Pregnancy and Adult Offspring Behavioral and Emotional Problems. Depress Anxiety [Internet] 2015 Jan;32:82–90. doi: 10.1002/da.22272. Available from: http://doi.wiley.com/10.1002/da.22272. [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman Ca, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress [Internet] 2011 Nov;14:644–51. doi: 10.3109/10253890.2011.594121. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21790468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock LF, Mears JL, Woodcock C, Record R. Retrospective study of the association of stress and smoking during pregnancy in rural women. Addict Behav [Internet] 2001;26:405–13. doi: 10.1016/s0306-4603(00)00118-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11436932. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years of age. Stress. 2011;14(6):665–76. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Paterson J, Gao W, Iusitini L. Maternal smoking during pregnancy and behaviour problems in a birth cohort of 2-year-old Pacific children in New Zealand. Early Hum Dev. 2008;84:59–66. doi: 10.1016/j.earlhumdev.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Clark CAC, Nelson JM, Garza J, Sheffield TD, Wiebe SA, Espy KA. Gaining control: Changing relations between executive control and processing speed and their relevance for mathematics achievement over course of the preschool period. Front Psychol. 2014 Feb;5:1–15. doi: 10.3389/fpsyg.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Sheffield TD, Chevalier N, Nelson JM, Wiebe SA, Espy KA. Charting Early Trajectories of Executive Control With the Shape School. Dev Psychol. 2012 doi: 10.1037/a0030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med [Internet] 2014;44:71–84. doi: 10.1017/S0033291713000780. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23591021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, De Genna NM, Larkby C. Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14-year-old offspring of teenage mothers. Matern Child Health J. 2012;16:694–705. doi: 10.1007/s10995-011-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan SN, Fuller-Rowell TE, Evans GW. Cumulative Risk and Adolescent’s Internalizing and Externalizing Problems: The Mediating Roles of Maternal Responsiveness and Self-Regulation. Dev Psychol. 2012;48(6):1529–39. doi: 10.1037/a0027815. [DOI] [PubMed] [Google Scholar]
- Ellis LEE, Widmayer A, Das S. Maternal smoking during pregnancy and self-reported delinquency by offspring. 2012 Jun;335:325–35. doi: 10.1002/cbm.1828. [DOI] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Dev Psychol. 2011a;47:153–6. doi: 10.1037/a0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Sheffield TD, Wiebe SA, Clark CAC, Moehr MJ. Executive control and dimensions of problem behaviors in preschool children. J Child Psychol Psychiatry Allied Discip. 2011b;52(1):33–46. doi: 10.1111/j.1469-7610.2010.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull [Internet] 2013;139(6):1342–96. doi: 10.1037/a0031808. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23566018. [DOI] [PubMed] [Google Scholar]
- Fanti KA, Henrich CC. Trajectories of pure and co-occurring internalizing and externalizing problems from age 2 to age 12: findings from the National Institute of Child Health and Human Development Study of Early Child Care. Dev Psychol. 2010;46(5):1159–75. doi: 10.1037/a0020659. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, et al. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Dev Psychopathol. 2011;23:777–88. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol [Internet] 2001 Sep;23(5):421–30. doi: 10.1016/s0892-0362(01)00160-x. Available from: http://linkinghub.elsevier.com/retrieve/pii/S089203620100160X. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. The Early Childhood Inventory – 4 Screening Manual. Stoney Brook, NY: Checkmate Plus; 2000. [Google Scholar]
- Galera C, Cote SM, Bouvard MP, Pingault J-B, Melchior M, Michel G, et al. Early Risk Factors for Hyperactivity-Impulsivity and Inattention Trajectories From Age 17 Months to 8 Years. Arch Gen Psychiatry. 2011;68(12):1267–75. doi: 10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine T. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum Dev. 2007;38(4):255–69. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. J Child Psychol Psychiatry Allied Discip. 2011;52:356–67. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Goedhart G, van der Wal MF, Cuijpers P, Bonsel GJ. Addict Behav [Internet] 4. Vol. 34. Elsevier Ltd; 2009. Psychosocial problems and continued smoking during pregnancy; pp. 403–6. Available from: http://dx.doi.org/10.1016/j.addbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Ph D, Cornelius MD, Ph D, Day NL, Ph D, et al. Prenatal Cigarette Smoke Exposure and Early Initiation of Multiple Substance Use. 2012;14(6):694–702. doi: 10.1093/ntr/ntr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Fortier ME, Zadorozny C, Thakur G, Schmitz N, Duval R, et al. Maternal stress during pregnancy, ADHD symptomatology in children and genotype: Gene-environment interaction. J Can Acad Child Adolesc Psychiatry. 2012 Feb;21:9–15. [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun Monogr. 2009 Dec;76:408–20. 2013. [Google Scholar]
- Holtrop JS, Meghea C, Raffo JE, Biery L, Chartkoff SB, Roman L. Smoking among pregnant women with medicaid insurance: Are mental health factors related? Matern Child Health J. 2010;14:971–7. doi: 10.1007/s10995-009-0530-x. [DOI] [PubMed] [Google Scholar]
- Hughes C, Ensor R. Does executive function matter for preschoolers’ problem behaviors? J Abnorm Child Psychol. 2008;36(1):1–14. doi: 10.1007/s10802-007-9107-6. [DOI] [PubMed] [Google Scholar]
- Hughes C, White A, Sharpen J, Dunn J. Antisocial, angry, and unsympathetic: “hard-to-manage” preschoolers’ peer problems and possible cognitive influences. J Child Psychol Psychiatry. 2000;41(2):169–79. [PubMed] [Google Scholar]
- Huijbregts SCJ, Séguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. J Abnorm Child Psychol. 2007;35:203–15. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–48. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Lester BM, Bagner DM, Liu J, LaGasse LL, Seifer R, Bauer CR, et al. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics. 2009;124:1355–62. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Lin H, DeGarmo DS, Fisher Pa, Lagasse LL, Levine TP, et al. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug Alcohol Depend. 2012;126(1–2):80–6. doi: 10.1016/j.drugalcdep.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19:747–53. doi: 10.1007/s00787-010-0113-9. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Ph D, Obel C, Wisborg K, Sc DM, et al. Reviews and Overviews Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence. 2003 Jun;:1028–40. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Johnson KC, Kable Ja, Carroll J, Coles CD. Smoking in pregnancy and parenting stress: Maternal psychological symptoms and socioeconomic status as potential mediating variables. Nicotine Tob Res. 2011;13(7):532–9. doi: 10.1093/ntr/ntr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RP, Dombrowski S, Mullis C, Wisenbaker J, Huttenen M. Smoking during pregnancy: Association with childhood temperament, behavior, and academic performance. J Pediatr Psychol. 2006;31(5):490–500. doi: 10.1093/jpepsy/jsj041. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Carey WB, McDevitt S. The Early Infant Temperament Questionnaire. 1993;14(4):230–5. [PubMed] [Google Scholar]
- Mezzacappa E, Buckner JC, Earls F. Prenatal cigarette exposure and infant learning stimulation as predictors of cognitive control in childhood. Dev Sci [Internet] 2011 Jul;14(4):881–91. doi: 10.1111/j.1467-7687.2011.01038.x. [cited 2014 Dec 1] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3117204&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. J Am Acad Child Adolesc Psychiatry [Internet] 4. Vol. 41. American Academy of Child and Adolescent Psychiatry; 2002. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy; pp. 378–85. Available from: http://dx.doi.org/10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Monshouwer K, Huizink C. Prenatal Smoking Exposure and the Risk of Behavioral Problems and Substance Use in Adolescence: the TRAILS Study. 2011:342–50. doi: 10.1159/000334507. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. The Life Stressors and Social Resources Inventory. Odessa, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- Nast I, Bolten M, Meinlschmidt G, Hellhammer DH. How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr Perinat Epidemiol. 2013;27:313–22. doi: 10.1111/ppe.12051. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct Equ Model [Internet] 2007;14(4):535–69. Available from: http://www.tandfonline.com/doi/abs/10.1080/10705510701575396. [Google Scholar]
- Pickett KE, Wilkinson RG, Wakschlag LS. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. J Epidemiol Community Health. 2009;63:474–80. doi: 10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Wood C, Adamson J, D’Souza L, Wakschlag LS. Meaningful differences in maternal smoking behaviour during pregnancy: implications for infant behavioural vulnerability. J Epidemiol Community Health. 2008;62:318–24. doi: 10.1136/jech.2006.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusakowski MK, Shofer FS, Rhodes KV, Mills AM. Effect of depression and psychosocial stressors on cessation self-efficacy in mothers who smoke. Matern Child Health J. 2011;15:620–6. doi: 10.1007/s10995-010-0640-5. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MAJ, Smidts DP, Sergeant JA, Maassen GH, Posthumus JA, Van Engeland H, et al. Executive functions in preschool children with aggressive behavior: Impairments in inhibitory control. J Abnorm Child Psychol. 2008;36(7):1097–107. doi: 10.1007/s10802-008-9235-7. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2010;40:335–45. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Mattes E, Oddy WH, Pennell CE, van Eekelen A, McLean NJ, et al. Prenatal stress and risk of behavioral morbidity from age 2 to 14 years: The influence of the number, type, and timing of stressful life events. Dev Psychopathol. 2011;23:507–20. doi: 10.1017/S0954579411000241. [DOI] [PubMed] [Google Scholar]
- Robinson M, Oddy WH, Li J, Kendall GE, De Klerk NH, Silburn SR, et al. Pre- and postnatal influences on preschool mental health: A large-scale cohort study. J Child Psychol Psychiatry Allied Discip. 2008;49:1118–28. doi: 10.1111/j.1469-7610.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry Allied Discip. 2005;46:246–54. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJO. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol. 2011 Jan;1:1–8. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Protective factors in chidlren’s responses to stress and disadvantage. Ann Acad Med. 1979;8:324–338. [PubMed] [Google Scholar]
- Sameroff A, Seifer R, Zax M, Barocas R. Early indicators of developmental risk: Rochester Longitudinal Study. Schizophr Bull. 1987;13(3):383–94. doi: 10.1093/schbul/13.3.383. [DOI] [PubMed] [Google Scholar]
- Sanson A, Smart D, Prior M, Oberklaid F. Precursors of hyperactivity and aggression. J Am Acad Child Adolesc Psychiatry. 1993;32(6):1207–16. doi: 10.1097/00004583-199311000-00014. [DOI] [PubMed] [Google Scholar]
- Séguin JR, Nagin DS, Assaad J-M, Tremblay RE. Cognitive-neuropsychological function in chronic physical aggression and hyperactivity. J Abnorm Psychol [Internet] 2004;113(4):603–13. doi: 10.1037/0021-843X.113.4.603. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3283572&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A, Riggs KJ. Conditions under which children experience inhibitory difficulty with a “button-press” go/no-go task. J Exp Child Psychol. 2006;94:18–26. doi: 10.1016/j.jecp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Littenand RZ, Allen JP, editors. Meas alcohol Consum Psychosoc Biol methods. Totawa, N.J: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sprafkin J, Volpe RJ, Gadow KD, Nolan EE, Kelly K. J Am Acad Child Adolesc Psychiatry [Internet] 5. Vol. 41. American Academy of Child and Adolescent Psychiatry; 2002. A DSM-IV-referenced screening instrument for preschool children: the Early Childhood Inventory-4; pp. 604–12. Available from: http://dx.doi.org/10.1097/00004583-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry Allied Discip. 2007;48:245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE. Etiology of adolescent substance abuse: a developmental perspective. Am J Addict [Internet] 2002;11:171–91. doi: 10.1080/10550490290087965. Available from: http://onlinelibrary.wiley.com/doi/10.1080/10550490290087965/full. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius J, Pajer Ka, Vanyukov M, et al. Neurobehavior disinhibition in childhood predicts early age onset substance use disorder. Am J Psychiatry. 2003 Jun;160:1078–85. doi: 10.1176/appi.ajp.160.6.1078. 2003. [DOI] [PubMed] [Google Scholar]
- Thomas A, Chess S. Temperament and Development. New York: Brunner/Mazzel; 1977. [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi SW, Nichols SR, Kestler J, Burns JL, et al. J Am Acad Child Adolesc Psychiatry [Internet] 1. Vol. 53. Elsevier; 2014. Jan 1, Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior; pp. 82–96.e3. [cited 2015 Apr 6]; Available from: http://www.jaacap.com/article/S0890856713007818/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: a developmental framework. Dev Psychopathol. 2002 Oct;14:351–69. doi: 10.1017/s0954579402002092. 2000. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Pickett KE, Pine DS, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Dev. 2006a;77(4):893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R. J Am Acad Child Adolesc Psychiatry [Internet] 4. Vol. 45. The American Academy of Child and Adolescent Psychiatry; 2006b. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? pp. 461–7. Available from: http://dx.doi.org/10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
- Weaver K, Campbell R, Mermelstein R, Wakschlag L. Pregnancy smoking in context: the influence of multiple levels of stress. Nicotine Tob Res. 2008;10(6):1065–73. doi: 10.1080/14622200802087564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. J Exp Child Psychol. 2011;108:436–52. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield TD, Espy KA. Separating the Fish From the Sharks: A Longitudinal Study of Preschool Response Inhibition. Child Dev. 2012;83(4):1245–61. doi: 10.1111/j.1467-8624.2012.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willford JA, Chandler LS, L G, Day NL. NIH Public Access. Neurotoxicol Teratol. 2010;32(6):580–8. doi: 10.1016/j.ntt.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Greenberg M, Blair CB, Stifter C. Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy. 2007:273–301. [Google Scholar]
- Wright Guerin Allen W, Gottfried D. Difficult Temperament and Behaviour Problems: A Longitudinal Study from 1.5 to 12 Years. Int J Behav Dev. 1997;21(1):71–90. [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol-disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Dev Perspect. 2011;5(4):248–55. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


