Abstract
Derangement of Rho-associated kinases (ROCKs) has been related to coronary artery disease and stroke. ROCK2, rather than ROCK1, plays a predominant role in vascular contractility. The present study aims to test (1) the associations between ROCK2 single nucleotide polymorphisms (SNPs) and arterial stiffness, and (2) the molecular mechanism accounting for their effects. Stiffness parameters including beta (β), elasticity modulus (Ep) and pulse wave velocity (PWV) were obtained by carotid ultrasonography. Seven tagging SNPs of ROCK2 were initially genotyped in 856 subjects and significant SNPs were replicated in another group of 527 subjects. Two SNPs in complete linkage disequilibrium were found to be significantly associated with arterial stiffness. The major alleles of rs978906 (A allele) and rs9808232 (C allele) were associated with stiffer arteries. SNP rs978906 was predicted to influence microRNA(miR)-1183 binding to ROCK2, while rs9808232 causes amino acid substitution. To determine their functional impact, plasmid constructs carrying different alleles of the significant SNPs were created. Compared to rs978906G-allele constructs, cells transfected with rs978906A-allele constructs had higher baseline luciferase activities and were less responsive to miR-1183 changes. Oxidized-low density lipoprotein (Ox-LDL) suppressed miR-1183 levels and increased ROCK2 protein amounts. For rs9808232, cells transfected with C-allele constructs had significantly higher ROCK activities than those with A-allele constructs. Leukocyte ROCK activities were further measured in 52 healthy subjects. The average ROCK activity was highest in human subjects with CC genotype at rs9808232, followed by those with AC and lowest in AA. Taken together, the present study showed that two functional SNPs of ROCK2 increase susceptibility of arterial stiffness in the Chinese population. Non-synonymous SNP rs9808232 influences ROCK2 activity, while 3' UTR SNP rs978906 affects the ROCK2 protein synthesis by interfering miR-1183 binding.
Keywords: ROCK2, Arterial stiffness, Polymorphisms, microRNAs
1. Introduction
Rho- associated coiled-coil containing protein kinases (ROCKs) play an important role in the pathophysiology of vascular diseases [1]. ROCK is a serine/threonine protein kinase that regulates the contraction, migration and proliferation of smooth muscle cells (SMCs) [1,2]. It can also affect vascular tone by suppressing endothelial nitric oxide synthase (eNOS) synthesis and activity [3,4]. Besides, up-regulation of the ROCK activity can cause endothelial dysfunction, vascular inflammation and platelet aggregation [5–7]. In human subjects, repressing ROCK activity by its inhibitor fasudil was proposed to be a potential target to treat hypertension, ischemic stroke, and myocardial infarction (MI) [8–10].
There are two isoforms of ROCKs, namely ROCK1 and ROCK2. These two isoforms share 65% homology in the amino acid sequences [11], but they are not functionally redundant. Previous studies have found that the two isoforms regulate different aspects of myosin activity [12]. ROCK2, but not ROCK1, plays a predominant role in SMC contractility [13]. Only ROCK2 participates in the signal cascades responsible for the up-regulation of adhesion molecules when endothelial cells are exposed to pro-inflammatory stimuli [14]. Furthermore, single nucleotide polymorphisms (SNPs) of the ROCK2 gene have been found to be associated with hypertension and coronary artery diseases [15,16]. All these findings support a pivotal role of ROCK2 in vascular diseases.
Arterial stiffness reflects the vascular compliance and is an independent predictor of cardiovascular risks [17]. A substantial variation of arterial stiffness is attributed to genetic factors with the estimated heritability between 0.18 and 0.66 [18,19]. Matrix metalloproteinase-9 (MMP-9) gene, NOS3 gene (encoded for eNOS), and genes involved in the inflammatory pathways have been found to be implicated in the pathogenesis of arterial stiffness [19]. Since ROCK2 can inhibit eNOS expression and enhance nuclear factor-κB (NF-κB) activity [3, 14], polymorphisms at the ROCK2 gene may confer a risk for arterial stiffening.
To delineate the genetic effect of ROCK2 on arterial stiffness, seven tagging SNPs were initially genotyped in 856 subjects. Significant SNPs in the screening dataset were further replicated in another group of 527 subjects. A series of cellular experiments and human studies was conducted to explore the molecular mechanism of the significant SNPs.
2. Materials and methods
2.1. Subjects
Two independent datasets were used in the genetic association study to screen and validate significant SNPs. The screening data comprised subjects from the unselected general population who participated in the ongoing cardiovascular genetic studies at the Kaohsiung Medical University Hospital (KMUH) [20]. After excluding subjects with a history of stroke or MI, a total of 856 participants were included. The validation data comprised 527 subjects with strong family history who were also recruited from the KMUH. A subject with strong family history was defined by the following two criteria: (1) the participant did not have a history of stroke or MI upon enrollment, and (2) he/or she has one first-degree relative with a documented MI or stroke history, or two second-degree relatives with MI or stroke. Blood samples from additional 52 subjects were used for the measurement of leukocyte ROCK activity.
Each participant filled a self-administrated questionnaire which included demographic information and previous medical histories. Fasting blood glucose, total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), and triglyceride (TG) were measured by using standardized enzymatic procedures (Boehringer Mannheim, Germany). The coronary heart disease (CHD) risk at 10-year for each participant was predicted by Framingham Risk Score [21]. This study was approved by the KMUH Institutional Review Board and informed consent was given by each participant.
2.2. Measurement of arterial stiffness
Carotid stiffness was measured by an ultrasound with the echo tracking system (SSD-5500; Aloka, Tokyo, Japan) equipped with a 3–12-MHz linear array transducer and a vessel wall moving detector. Measurements were performed in the right common carotid artery 2 cm before bifurcation. The vessel movement detector system registered at least 10 consecutive cardiac cycles and the subsequent changes of arterial diameter. Five best visualized blood-intima boundaries were marked and selected for computation. This whole procedure was repeated three times and the average data were used for analysis. Three stiffness parameters, including beta (β), elasticity modulus (Ep) and pulse wave velocity (PWV) were calculated automatically [20]. In our lab, the intra-reader correlation coefficient was 0.98 in β and Ep and 0.92 in PWV.
2.3. SNP selection and genotyping
Tagging SNPs of ROCK2 were selected from the release 2.0 Phase II data of the HapMap Project (http://hapmap.ncbi.nlm.nih.gov/) using the Tagger Pairwise method [22]. Selection criteria were (1) r2 ≥ 0.8, (2) minor allele frequency (MAF) ≥ 5% in the Han-Chinese population, and (3) coverage of the entire ROCK2 genome plus 1000 base pairs (bps) up- and 1000 bps down-stream of the ROCK2 transcript. Seven tagging SNPs met the selection criteria and were genotyped initially in the 856 screening subjects. The significant SNP rs978906 was genotyped in 527 subjects in the second data to replicate the association.
Genomic DNA was extracted from the peripheral blood leukocytes using a commercial kit (Qiagen, Hilden, Germany). Genotyping was performed by the Applied Biosystems TaqMan technology with the ABI 7900 Real Time PCR System (Applied Biosystems, Foster City, CA). Fluorescence data were analyzed using its System SDS software version 1.2.3.
2.4. Bioinformatic prediction of SNP function
SNPinfo website (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc. htm) was used to predict which tagging SNP has a functional impact on ROCK2. For SNPs at the 3' untranslated region (3' UTR), three algorithms were used to identify possible microRNAs (miRs) that have binding sites where the significant SNPs are located. These algorithms were MicroCosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/), TargetScan (http://targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/).
2.5. Human ROCK2 3' UTR luciferase constructs
Double-stranded oligonucleotides containing 20 bps surrounding rs978906 were synthesized. Three reporter constructs were created: one with three tandem copies of the risk allele A (rs978906A-allele construct), the other with three tandem copies of protective allele G (rs978906G-allele construct), and another with three tandem copies of mutant sequencing (complete disruption of miR-1183 binding site). The constructs were then cloned into the pMIR-REPORT™ miRNA expression reporter vector (Applied Biosystems) using the restriction enzyme sites SpeI and MluI. The primer sets for constructs were shown in the Supplementary Table 1.
2.6. Luciferase assays and RNA interference
MiR-1183 was predicted to bind to ROCK2 3' UTR at the site where rs978906 is located. To test whether rs978906 can influence miR-1183 binding, human aortic smooth muscle cells (HASMCs; Cascade Biologics, Portland, OR) were transfected with either one of the pMIR-REPORT constructs (rs978906A-, rs978906G-allele constructs or mutant construct; 600 ng) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The pEGFP-C1 vector (100 ng) was co-transfected as the internal control. In the meantime, HASMCs were transfected with one of the following miRs: mimic control, inhibitor control, miR-1183 mimic (MH13324, mirVana®, Applied Biosystems), or antagomiR-1183 (MC13324). Three different doses (1 nM, 5 nM and 10 nM) of miR-1183 mimic and antagomiR-1183 were used. A constant dose of 5 nM was used for mimic control and inhibitor control. After transfection for 24 h, luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI). To determine the effect of oxidized-low density lipoprotein (ox-LDL) on ROCK2 expression, HASMCs carrying either one of the pMIR-REPORT constructs (rs978906A-, rs978906G-allele or mutant constructs) were incubated with or without 60 μg/ml ox-LDL. After treatment for 48 h, luciferase activity was measured as described.
2.7. Quantitative PCR (qPCR) and western blot
After HASMCs were transfected with mimic control, inhibitor control, miR-1183 mimic or antagomiR-1183 for 24 h, ROCK2 mRNA levels were measured by qPCR. Western blot was performed with rabbit anti-ROCK2 (1:1000; abcam, Cambridge, UK) and mouse anti-β-actin antibodies (1:10,000; Santa Cruz, Dallas, Texas). The detailed methods for qPCR and western blot were shown in the Supplementary materials.
To test whether ROCK2 and miR-1183 levels are altered during atherosclerotic process, HASMCs were incubated with or without 60 μg/ml ox-LDL. MiR-1183 levels were measured by qPCR using TaqMan microRNA assays (Applied Biosystems) with normalization to RNA-U6B. ROCK2 protein amount was quantified by western blot.
2.8. Human ROCK2 expression vector constructs
Two ROCK2 expression vectors (rs9808232C- and rs9808232A-allele constructs) were created. ROCK2/pCMV6 entry plasmid (major C allele at rs9808232) was purchased from ORIGENE (category no: RC217764) which contained the 4167 bps of ROCK2 cDNA open reading frame. To induce a single nucleotide C to A substitution at rs9808232 in mutant construct, wild type construct was used as a template for site-directed mutagenesis by QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The primers for mutagenesis were shown in Supplementary Table 1.
U2OS cells (human osteosarcoma cell line; ATCC) were transfected with pcDNA3 (negative control), vector with wild type construct (rs9808232C-allele), vector with mutant construct (rs9808232A-allele), or CAT-ROCK (positive control). The cell lysates were obtained 32 h after transfection, and proteins were used for the measurement of total ROCK activity.
2.9. Measurement of ROCK activity
Total ROCK activity was measured in (1) peripheral blood leukocyte from 52 healthy subjects, and (2) U2OS cells transfected with plasmid constructs. Total ROCK activity was assayed by measuring the amount of phospho-Thr853 in the myosin-binding subunit (MBS) of myosin light chain phosphatase (MLCP) as described elsewhere [23,24]. NIH 3T3 cell lysates under the stimulation of 10 μmol/l lysophosphatidic acid were used as a positive control to standardize the results of western blot. Equal amounts of cell extracts were subjected to 7.5% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with rabbit anti-phospho-specific Thr853-MBS polyclonal antibody (provided by Dr. James K. Liao), rabbit anti-MBS polyclonal antibody (Covance, Princeton, NJ), or anti-actin monoclonal antibody (Sigma-Aldrich). Bands were visualized using the ECL system and the band intensities were quantified using National Institutes of Health Image 1.61. ROCK activity was expressed as the ratio of phospho-Thr853-MBS (p-MBS) in each sample to p-MBS in each positive control divided by MBS in each sample per MBS in each positive control.
2.10. Statistical analysis
All statistical analyses were performed with SPSS statistical software (version 16.0). Genotype distributions were tested for Hardy–Weinberg equilibrium (HWE). Each stiffness parameter was adjusted for age, sex, diabetes, hypertension, hypercholesterolemia, smoking and body mass index (BMI) by multivariate regression. The adjusted stiffness parameters were used for genetic statistical analyses. ANOVA and Student's t-test were used to compare the means of each stiffness parameter across different genotypes. Linear regression was used to quantify the effect of risk genotype based on an additive model of inheritance. A nominal p value of 0.05 is considered statistically significant, and the significant SNPs were further tested in the validation data to reduce type I error.
The homogeneity test was used to compare the effect size (μm) of a SNP tested in both screening and validation datasets. The effect size (μm) represented the difference of stiffness parameters with one extra copy of risk allele of a significant SNP. The μm and the standard error (SE) of μm were obtained from the regression analysis of each dataset. Weight of each data was calculated by 1/SE2. Under the assumption of a constant genetic effect, μm should be homogeneous across different datasets. The formula to calculate the p value for the homogeneity test was described elsewhere [25]. A p value of greater than 0.05 indicated a constant genetic effect across two datasets; therefore, the two data can be combined to obtain an overall p value.
For the cellular experiments, Student's t-test or ANOVA was used to compare the variables between different treatment groups. Kruskal–Wallis test or Mann–Whitney U test was used to compare leukocyte ROCK activity among 52 subjects with different genotypes. A nominal p value less than 0.05 is considered statistically significant. All experiments were performed at least three times with technical duplicates in each sample.
3. Results
3.1. Genetic association studies
The demographic characteristics of the study subjects are shown in Table 1. The genotype distribution was in HWE for any of the seven SNPs. Five of the seven tested SNPs were significantly associated with at least one of the three stiffness parameters in the screening data (Supplementary Table 2). Among them, the non-synonymous SNP rs9808232 yielded the most significant results. Major allele C of rs9808232 exerted an additive effect to cause a detrimental effect on arterial stiffness. The average Ep was highest in the CC genotype of rs9808232, followed by AC and then lowest in AA (105.2 ± 31.7, 103.0 ± 30.8, and 98.1 ± 27.4 kPa, respectively). Consistent with the results in Ep, the C allele exerted a dose-dependent effect to have a higher value of β and PWV. The 3' UTR SNP rs978906 was also significantly associated with Ep (p = 0.031) and PWV (p = 0.038), and had a borderline significance for β (p = 0.063). Similar to rs9808232, the highest value of stiffness parameters was for major homozygote AA of rs978906, followed by AG, and then GG.
Table 1.
Demographic characteristics of study participants.
| Screening data (N = 856) | Validation data (N = 527) | |||
|---|---|---|---|---|
| Age (year) | 51.2 ± 10.9 | 55.1 ± 9.23 | ||
| Men (%) | 48.0% | 46.9% | ||
| Hypertension (%) | 33.4% | 50.9%3 | ||
| Diabetes (%) | 8.9% | 16.1%3 | ||
| Hypercholesterolemia (%) | 24.9% | 48.2%3 | ||
| Current and ever smoker (%) | 19.4% | 25.8%3 | ||
| CHD risk at 10-year predicted by | ||||
| Framingham Risk Score | ||||
| Low risk/intermediate risk/high risk | 83.1%/15.2%/1.8% | 73.1%/21.6%/5.3%3 | ||
| Ep (kPa) | 97.3 ± 39.4 | 112.0 ± 48.43 | ||
| β | 7.5 ± 2.7 | 8.7 ± 3.73 | ||
| PWV (m/s) | 5.9 ± 1.1 | 6.3 ± 1.23 | ||
| ROCK2 tagged SNPs (3' to 5') | ||||
| SNP | Location | M/m | MM/Mm/mm (%) | MM/Mm/mm (%) |
| rs978906 | 3' UTR | A/G | 36.5/46.4/17.1 | 33.5/49.2/17.3 |
| rs1515223 | Intron 32 | C/T | 84.1/15.3/0.6 | NA |
| rs9808232 | Exon 10 | C/A | 36.1/47.1/16.9 | NA |
| rs10167277 | Intron 6 | A/T | 31.0/49.4/19.6 | NA |
| rs10168084 | Intron 6 | T/C | 36.9/46.8/16.3 | NA |
| rs12479227 | Intron 3 | C/T | 73.9/24.0/2.1 | NA |
| rs4669700 | Intron 3 | C/T | 30.0/49.6/20.4 | NA |
M/m: major/minor allele, MM/Mm/mm: Major homozygote/heterozygote/minor homozygote, CHD = coronary heart disease.
Variables are significantly different between two data (p < 0.05).
The LD map of the tested SNPs is shown in Supplementary Fig. 1. The two most significant SNPs (rs978906 and rs9808232) were in complete linkage disequilibrium (LD) (D' = 0.99 and r2 = 0.97). Haplotype analyses failed to find any significant result.
3.2. Validation data of genetic association studies
Since rs978906 and rs9808232 were in high LD, we only genotyped rs978906 in the 527 subjects in the validation data. The genotype distribution of rs978906 was similar between the screening and validation data (Table 1). Consistent with the screening data, the A allele of rs978906 was associated with a higher value of stiffness parameters in the validation data (Table 2). The effect sizes (μm) of the validation data were very similar to those in the screening data. For example, one extra copy of A allele at rs978906 was associated with an increment of 3.20 and 3.59 kPa in Ep in the screening and validation data respectively (homogeneity test p = 0.90). Similarly, the effect size was approximately 0.20 for β, and approximately 0.09 for PWV in both data (homogeneity test p = 0.71 and 0.95). Since the homogeneity test suggested that the two data had a consistent direction, they were combined to yield more reliable and significant results (p = 0.02, 0.03, and 0.02 for Ep, β, and PWV respectively).
Table 2.
The association between rs978906 and stiffness parameters.
| Phenotypes | Data | AA | AG | GG | Regression analysis (p value) | Δm (SE)a | Homogeneity test† |
|---|---|---|---|---|---|---|---|
| Adj Ep | Screening | 105.08 ± 31.62 | 102.38 ± 30.54 | 98.45 ± 27.17 | 0.03 | 3.20 (1.48) | |
| Validation | 108.02 ± 40.28 | 105.18 ± 44.19 | 100.55 ± 35.21 | 0.18 | 3.59 (2.65) | ||
| All | 106.12 ± 34.94 | 103.47 ± 36.47 | 99.25 ± 30.42 | 0.02 | 3.30 (1.36) | p = 0.90 | |
| Adj β | Screening | 8.03 ± 2.35 | 7.79 ± 2.14 | 7.64 ± 2.01 | 0.06 | 0.20 (0.11) | |
| Validation | 8.57 ± 3.09 | 8.43 ± 3.61 | 7.94 ± 2.37 | 0.17 | 0.29 (0.21) | ||
| All | 8.22 ± 2.64 | 8.04 ± 2.82 | 7.76 ± 2.16 | 0.03 | 0.22 (0.10) | p = 0.71 | |
| Adj PWV | Screening | 6.13 ± 0.85 | 6.05 ± 0.84 | 5.96 ± 0.82 | 0.04 | 0.09 (0.04) | |
| Validation | 6.21 ± 1.02 | 6.14 ± 1.07 | 6.04 ± 1.01 | 0.23 | 0.08 (0.07) | ||
| All | 6.16 ± 0.91 | 6.08 ± 0.94 | 5.99 ± 0.89 | 0.02 | 0.08 (0.04) | p = 0.95 |
The effect size (μm) represented the difference of stiffness parameters with one extra copy of risk A allele at rs978906.
p value < 0.05 denoted for heterogeneity in the genetic effect between two data.
3.3. Functional test for SNP rs978906
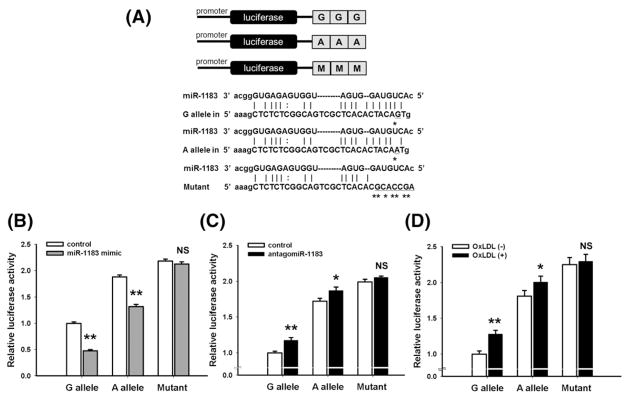
Both rs9808232 and rs978906 were predicted to have biological functions. Bioinformatic analyses implied that the 3' UTR SNP rs978906 is located at miR-1183 binding site (Fig. 1A). The G-allele of rs978906 was predicted to bind more strongly with miR-1183 than the A-allele. For HASMCs transfected with rs978906G-allele constructs, miR-1183 mimic reduced the luciferase activity by 52.4% in comparison to those transfected with control miR (Fig. 1B, p < 0.001). Similarly, miR-1183 mimic reduced the luciferase activity by 29.9% in rs978906A-allele constructs (Fig. 1B, p < 0.001). AntagomiR-1183 caused a greater increase of luciferase activity by 17.1% in G-allel than an increase by 8.4% in A-allele (Fig. 1C, p = 0.003 and 0.03 respectively). Similarly, ox-LDL treatment increased the luciferase activity by 27.9% in G-allele and 10.7% in A-allele (Fig. 1D, p = 0.0007 and 0.01 respectively). Mutant construct lost the miR-1183 binding site did not have any change under either one of following conditions: ox-LDL treatment or transfection of antagomiR-1183 or miR-1183 mimic. Taken together, miR-1183 mimic could knock down ROCK2 expression in both A- and G-allele constructs. AntagomiR-1183 and ox-LDL increased ROCK2 expression in both A- and G- allele constructs. Among the three conditions (control miR, mir-1183 mimic, and antagomiR-1183), cells carrying rs978906A-allele constructs had higher luciferase activities than those with G-allele constructs. Disruption of the miR-1183 binding site aborted the effects of miR-1183, antagomiR-1183 or ox-LDL on ROCK2 expression.
Fig. 1.
SNP rs978906 affects luciferase activities in HASMCs. (A) The upper plot shows the luciferase reporter with one construct carrying three copies of rs978906G-allele or rs978906A-allele or mutant sequence. The lower plot shows the theoretical miRNA:mRNA duplex pairing between miR-1183 and the ROCK2 3' UTR. The G, A and mutant alleles are highlighted with an asterisk (*). (B, C) Luciferase activities were measured in HASMCs expressing either A-allele, G-allele or mutant construct that were treated by control miR, miR-1183 mimic or antagomiR-1183. (D) After incubated with 60 μg/ml ox-LDL for 48 h, luciferase activities were measured in HASMCs transfected with either A-allele, G-allele or mutant construct. *p < 0.05, **p < 0.01, NS: non-significant compared between treatment group and control group.
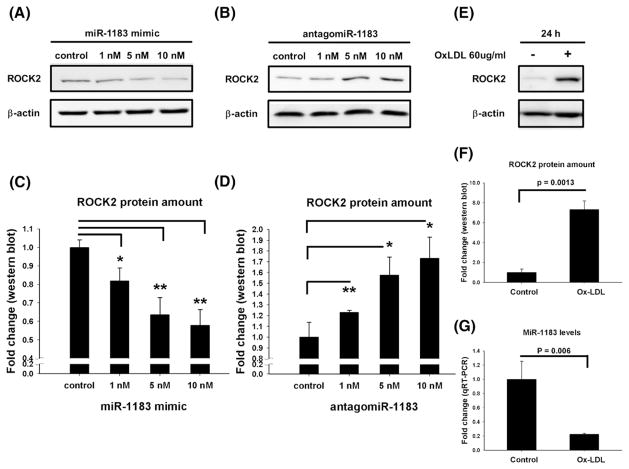
ROCK2 expression levels were then compared across HASMCs transfected with control miR, miR-1183 mimic or antagomiR-1183. There was no significant difference in mRNA levels (data not shown), but the ROCK2 protein amount differed among the treatment groups. MiR-1183 mimic dose-dependently decreased ROCK2 protein (Figs. 2A and C); while antogomiR-1183 significantly increased the ROCK2 protein levels (Figs. 2B and D). The ox-LDL effects on ROCK2 protein amounts and miR-1183 expression were also examined. Compared to the control group, ox-LDL substantially increased ROCK2 protein level by 7.32 fold (p = 0.0013, Figs. 2E and F) accompanied with a reduced miR-1183 level by 77.7% (p = 0.006, Fig. 2G).
Fig. 2.
MiR-1183 inhibited ROCK2 expression in HASMCs. (A, B) Western blot analysis of ROCK2 protein amount in HASMCs transfected with miR-1183 mimic or antagomiR-1183 in different doses. (C, D) Densitometric quantification of the protein expression levels of ROCK2 in HASMCs transfected with miR-1183 mimic or antagomiR-1183. (E, F) Western blot and quantification plot showed that ox-LDL treatment caused an increase in ROCK2 protein amount. (G) Ox-LDL treatment reduced miR-1183 mRNA levels. *p < 0.05, **p < 0.01.
3.4. Functional test for rs9808232
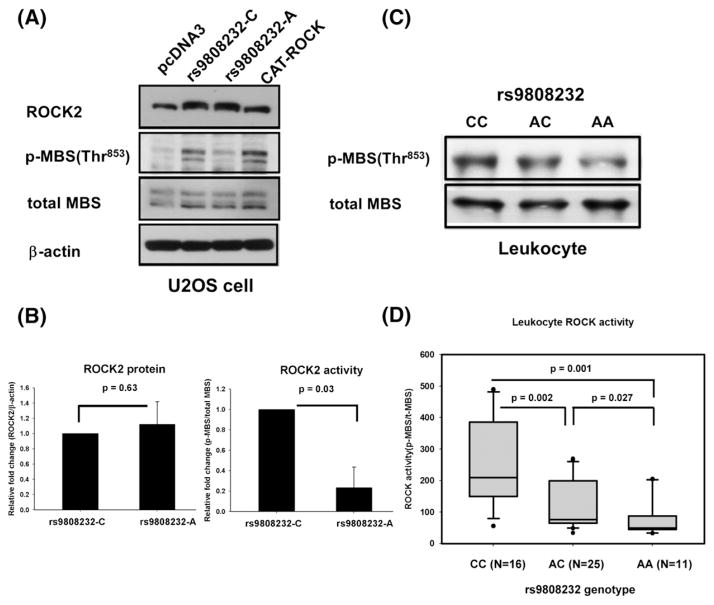
SNP rs9808232 (aka Thr431Asn, has been merged into rs2230774) may affect ROCK activity since it causes the amino acid substitution from threonine (Thr) to asparagine (Asn). ROCK2 protein amount and ROCK activity were measured in U2OS cells rather than HASMCs to facilitate the transfection efficacy of plasmids. The amount of ROCK2 protein was similar between U2OS cells transfected with rs9808232C- or rs9808232A-allele constructs (Figs. 3A and B). But, a greater amount of p-MBS was observed in cells transfected with the C-allele constructs (Figs. 3A and B). It suggested that substitution from C-allele to A-allele at rs9808232 caused a reduction in ROCK enzyme activity.
Fig. 3.
SNP rs9808232 affects total ROCK activity. (A) Western blot for ROCK2, p-MBS at Thr853, total-MBS and β-actin. U2OS cells were transefcted with pcDNA3 (negative control), wild type human ROCK2 expression vector (rs9808232C-allele constructs), mutant ROCK2 vector (rs9808232A-allele construct), or CAT-ROCK (positive control). Wild type construct was used as a template to induce C to A substitution at rs9808232 in the mutant construct by site-directed mutagenesis. (B) Densitometric quantification to compare the ROCK2 protein amount and ROCK activity (i.e. p-MBS at Thr853/total-MBS) between rs9808232-C and A allele constructs. (C, D) Representative blot data and quantitative analysis of leukocyte ROCK activity measured in 52 healthy persons with different genotypes at rs9808232.
The leukocyte ROCK activity was measured in 52 subjects (22 men and 30 women, age: 50.7 ± 7.5 years) to confirm the biological effect of rs9808232 in vivo. The total ROCK activity was significantly different among subjects with different rs9808232 genotypes (Figs. 3C and D). The average ROCK activity was highest in CC genotype, followed by AC and then lowest in AA (249.6 ± 137.2, 102.2 ± 82.9, and 79.4 ± 61.6 respectively). None of cardiovascular risk factors was associated with the ROCK activity in the peripheral blood leukocytes (Supplementary Table 3).
4. Discussion
The present study showed that ROCK2 polymorphisms were significantly associated with arterial stiffness in a Chinese population residing in Taiwan. The two significant SNPs (rs9808232 and rs978906) are in strong LD and both SNPs have functional consequences. Therefore, a person who carries the risk C-allele at rs9808232 will also carry the risk A-allele at rs978906. Non-synonymous SNP rs9808232 influences ROCK2 activity, while 3' UTR SNP rs978906 affects the ROCK2 protein synthesis by interfering miR-1183 binding. For rs9808232, the risk C-allele is associated with a higher ROCK activity and a stiffer carotid artery. The risk A-allele of rs978906 has an elevated ROCK2 expression level and a worse stiffness profile. Taken together, our study indicated that the ROCK2 polymorphisms can influence arterial stiffness by affecting ROCK2 levels and activity, which can eventually affect the risk of vascular diseases.
Our study is the first to demonstrate a significant association between ROCK2 polymorphisms and stiffness parameters. In coherent to our findings, Noma et al. found a positive correlation between leukocyte ROCK activity and PWV values in human subjects [26]. Our functional studies confirmed that the two LD SNPs (rs9808232 and rs978906) have individual biological effect. The feasibility of using these two SNPs as a marker of cardiovascular risks warrants further investigation. ROCK2 SNPs have been related to hypertension [15,27]; however, both our and another studies failed to find any association between blood pressure and ROCK2 polymorphisms [28].
Although rs9808232 has been related to several human diseases [15, 29,30], the present study is the first to demonstrate that this non-synonymous SNP could affect ROCK activity in vivo. The C to A substitution at rs9808232 leads to the amino acid change nearby the coiled-coil region of ROCK2 protein. The coiled-coil region acts like a hinge that opens up the hairpin structure of ROCK and exposes the kinase domain [31]. Our study showed that the C to A substitution at rs9808232 caused a lower ROCK activity in both U2OS cells and leukocytes of human subjects. SNP rs9808232 might affect the ROCK enzyme activity via influencing the function of coiled-coil region.
Another intriguing finding is that miR-1183 may modulate the atherogenic process by fine tuning the expression of ROCK2. The pivotal role of miRs in the cardiovascular diseases was just revealed recently [32,33]. Our study showed that ox-LDL increases the ROCK2 gene expression via reducing miR-1183 levels. We further demonstrated that two different alleles (A and G alleles) of the single nucleotide polymorphism (rs978906) at the 3' UTR could affect ROCK2 protein expression via interfering with miR-1183 binding. The A allele of rs978906 had reduced repression of miR-1183 resulting in significantly higher ROCK2 expression levels than the G allele. Therefore, people carrying the A allele are prone to arterial stiffness because their ROCK2 levels tend to be high, especially when miR-1183 is suppressed by ox-LDL.
The present study had several limitations. Since both the screening and validation subjects were recruited from Han Chinese residing in Taiwan, the association between ROCK2 SNPs and stiffness might not be generalized to other ethnic groups. According to a recent study investigating the population admixture and phylogenetic system of the Han Chinese residing in Taiwan [34], the genetic information is homogeneous among the Taiwanese subpopulations. The above evidence reduces the concern of spurious association due to population stratification. We did not apply the multiple testing corrections for the statistical analysis because the seven SNPs were in strong LD and the three stiffness parameters are intimately correlated. We acknowledged that the lack of significance in the validation cohort is likely to be due to insufficient power in a modest sample size. Our overall sample size provided a power of 89% with an alpha level of 0.05 according to the GWASpower/QT software for power estimation [35]. However, the power is only 55% for the validation data (N = 527) and 73% for the screening data (N = 856). From the biostatistics “central limit theorem”, the means of the point estimate (i.e. beta in the linear regression) are likely to maintain constant. However, the p value is the function of sample size and tends to increase while sample size decreases. The effect size (μm) between the screening and validation data remains constant. Furthermore, the cellular experiments confirmed biological functions for these two SNPs, which provide an additional line of evidence. Since there was no assay for ROCK2-specific activity, we could only measure the total ROCK activity in cells or humans subjects with ROCK2-deficient genetic background.
In conclusion, the present study identified two ROCK2 polymorphisms as genetic determinants of arterial stiffness. SNP rs9808232 leads to amino acid substitution and influences ROCK enzyme activity. SNP rs978906 is located in the miR-1183 binding site and its allele difference influences the miR-1183 effect on ROCK2 expression. Modulating miR-1183 levels might be a potential therapeutic strategy in ROCK2 related cardiovascular diseases.
Supplementary Material
Acknowledgments
Sources of finding
This work was supported by the following funding: Ministry of Science and Technology of the Republic of China (Taiwan, R.O.C. 101-2314-B-075A-013, 101-2628-B-075A-001-MY2, 101-2314-B-006-075-MY2, 101-2628-B-037-003-MY2 and 103-2314-B-075-076-MY3), National Health Research Institutes (Taiwan, R.O.C. NHRI-Ex101-10107PI), Academia Sinica (BM103010096), Kaohsiung Medical University Hospital (KMUH102-2T02), Kaohsiung Medical University Hospital (Aim for the Top 500 Universities grant KMU-DT103003, KMU-TP103C003), Ministry of Health and Welfare (Taiwan, R.O.C. MOHW103-TDU-B-211-113002), and Ministry of Education of the Republic of China (Taiwan, R.O.C. the Headquarters of University Advancement at the National Cheng Kung University).
The authors thank the bioinformatic consultation provided by Dr. Edward Hsi, scientific advice from Dr. Chung Y. Hsu and technique help from Dr. Ku-Chung Chen, Ms. I-Wen Wang, and Ms. Hsin-Yun Cheng.
Abbreviations
- ROCKs
Rho- associated protein kinases
- SMCs
smooth muscle cells
- eNOS
endothelial nitric oxide synthase
- MI
myocardial infarction
- SNP
single nucleotide polymorphism
- MMP-9
matrix metalloproteinase-9
- NF-κB
nuclear factor-κB
- Ep
elasticity modulus
- PWV
pulse wave velocity
- MAF
minor allelefrequency
- 3' UTR
3' untranslated region
- miRs
microRNAs
- HWE
Hardy–Weinberg equilibrium
- BMI
body mass index
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2014.11.023.
References
- 1.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–34. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009;15:3108–15. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 4.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–7. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SA, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J Biol Chem. 2004;279:42331–6. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- 6.Noma K, Kihara Y, Higashi Y. Striking crosstalk of ROCK signaling with endothelial function. J Cardiol. 2012;60:1–6. doi: 10.1016/j.jjcc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Wang QM, Liao JK. ROCKs as immunomodulators of stroke. Expert Opin Ther Targets. 2012;16:1013–25. doi: 10.1517/14728222.2012.715149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–10. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 9.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–9. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 10.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–7. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–93. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–53. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–40. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–42. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–47. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Cao Y, Cui G, Li Z, Sun J, Zhang L, et al. Association analysis of polymorphisms in ROCK2 with cardiovascular disease in a Chinese population. PLoS One. 2013;8:e53905. doi: 10.1371/journal.pone.0053905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juo SH, Rundek T, Lin HF, Cheng R, Lan MY, Huang JS, et al. Heritability of carotid artery distensibility in Hispanics: the Northern Manhattan Family Study. Stroke. 2005;36:2357–61. doi: 10.1161/01.STR.0000185926.05011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacolley P, Challande P, Osborne-Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res. 2009;81:637–48. doi: 10.1093/cvr/cvn353. [DOI] [PubMed] [Google Scholar]
- 20.Lin HF, Liu CK, Liao YC, Lin RT, Chen CS, Juo SH. The risk of the metabolic syndrome on carotid thickness and stiffness: sex and age specific effects. Atherosclerosis. 2010;210:155–9. doi: 10.1016/j.atherosclerosis.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–4. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–8. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–24. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juo SH, Wyszynski DF, Beaty TH, Huang HY, Bailey-Wilson JE. Mild association between the A/G polymorphism in the promoter of the apolipoprotein A-I gene and apolipoprotein A-I levels: a meta-analysis. Am J Med Genet. 1999;82:235–41. [PubMed] [Google Scholar]
- 26.Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, et al. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankinen T, Church T, Rice T, Markward N, Blair SN, Bouchard C. A major haplotype block at the rho-associated kinase 2 locus is associated with a lower risk of hypertension in a recessive manner: the HYPGENE study. Hypertens Res. 2008;31:1651–7. doi: 10.1291/hypres.31.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q, Wang L, Yang W, Chen S, Huang J, Fan Z, et al. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics. 2008;18:459–66. doi: 10.1097/FPC.0b013e3282f97fb2. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, et al. Association of genetic variants with chronic kidney disease in individuals with different lipid profiles. Int J Mol Med. 2009;24:233–46. doi: 10.3892/ijmm_00000226. [DOI] [PubMed] [Google Scholar]
- 30.Kalender ME, Demiryurek S, Oztuzcu S, Kizilyer A, Demiryurek AT, Sevinc A, et al. Association between the Thr431Asn polymorphism of the ROCK2 gene and risk of developing metastases of breast cancer. Oncol Res. 2010;18:583–91. doi: 10.3727/096504010x12767359113767. [DOI] [PubMed] [Google Scholar]
- 31.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–98. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 32.Liao YC, Wang YS, Guo YC, Lin WL, Chang MH, Hank Juo SH. Let-7g improves multiple endothelial functions through targeting TGF-beta and SIRT-1 signaling. J Am Coll Cardiol. 2014;63(16):1685–94. doi: 10.1016/j.jacc.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 33.Chen KC, Juo SH. MicroRNAs in atherosclerosis. Kaohsiung J Med Sci. 2012;28:631–40. doi: 10.1016/j.kjms.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang HC, Lin CH, Hsu CL, Hung SI, Wu JY, Pan WH, et al. A comparison of major histocompatibility complex SNPs in Han Chinese residing in Taiwan and Caucasians. J Biomed Sci. 2006;13:489–98. doi: 10.1007/s11373-006-9077-7. [DOI] [PubMed] [Google Scholar]
- 35.Feng S, Wang S, Chen CC, Lan L. GWAPower: a statistical power calculation software for genome-wide association studies with quantitative traits. BMC Genet. 2011;12:12. doi: 10.1186/1471-2156-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.