Abstract
Transplantation of mesenchymal stem cells (MSCs) has emerged as a promising strategy for the treatment of myriad human disorders, including several neurological diseases. Superparamagnetic iron oxide nanoparticles (SPION) and fluorine nanoemulsion (19F) are characterized by low toxicity and good sensitivity, and, as such, are among the most frequently used cell-labeling agents. However, to date, their impact across the various populations of MSCs has not been comprehensively investigated. Thus, the impact of MRI tags (independent variable) has been set as a primary endpoint. The various populations of mouse MSCs in which the effect of tag was investigated consisted of 1) tissue of cell origin: bone marrow vs. adipose tissue; 2) age of donor: young vs. old; 3) cell culture conditions: hypoxic vs. normal vs. normal +ascorbic acid (AA); 4) exposure to acidosis: yes vs. no. The impact of those populations has been also analyzed and considered as secondary endpoints. The experimental readouts (dependent variables) included: 1) cell viability; 2) cell size; 3) cell doubling time; 4) colony formation; 5) efficiency of labeling; and 6) cell migration. We did not identify any impact of cell labeling for these investigated populations in any of the readouts. In addition, we found that the harsh microenvironment of injured tissue modeled by a culture of cells in a highly acidic environment has a profound effect on all readouts, and both age of donor and cell origin tissue also have a substantial influence on most of the readouts, while oxygen tension in the cell culture conditions has a smaller impact on MSCs. A detailed characterization of the factors that influence the quality of MSCs is vital to the proper pursuit of preclinical and clinical studies.
Keywords: mesenchymal stem cells, iron oxide, SPIO, 19F, age, bone marrow, adipose, cell size, ascorbic acid, hypoxia
Introduction
Recent advances in medicine have dramatically improved life expectancy, and while humans enjoy a longer average lifespan, this exacerbates issues related to the aging process, such as tissue wear, disease, and injury of all body organs. The central nervous system poses a particular challenge in this context, as it is the most challenging to repair. Stem cell-based regenerative medicine has emerged as an approach with which to treat otherwise incurable neurological disorders.
There are several types of stem cells, but mesenchymal stem cells have garnered the most clinical attention (Das et al. 2013, Wei et al. 2013). This is because of their abundance in adult tissues, simple derivation methods, outstanding safety, and high efficacy. The therapeutic activity of MSCs includes the production and release of trophic factors, immunomodulation, and contribution to tissue replacement as building blocks, in case of connective tissue diseases such as myopia (Janowski et al. 2015). While MSCs reside in each organ, they are particularly abundant and easily accessible in bone marrow and adipose tissue. Despite their mesenchymal origin, MSCs have been found to be highly therapeutic in animal models of neurological disorders, including stroke (Janowski et al. 2010, Vu et al. 2014). Moreover, MSCs were recently shown to differentiate toward neurons (Taran et al. 2014). While the therapeutic potential of MSCs is high, the efficacy in the treatment of disease depends on various factors, including safe and efficient cell delivery, donor characteristics, such as age, or the effect of the microenvironment following transplantation. Among various tested cell delivery routes, the intra-arterial route is very attractive due to minimal invasiveness (no necessity for burr hole and brain puncture), and possible selective targeting of a desired brain region. However, it was shown that the large size of MSCs (larger than blood cells) introduces the risk of complications following intraarterial injection (Janowski et al. 2013, Cui et al. 2015). It has also been shown that advancing donor age severely affects the therapeutic potential of MSCs, which may hamper autologous applications (Choudhery et al. 2014). Moreover, the harsh environment of injured tissues, including acidic conditions (Orlowski et al. 2011), may also potentially influence therapeutic potential of MSCs (Song et al. 2010). Hypoxic preconditioning of MSCs has already been reported to augment their therapeutic potential (Choi et al. 2014). The anti-oxidant effect of ascorbic acid (AA) has been found to positively affect cell viability (Stevanovic et al. 2014), ameliorate seizures and brain damage (Dong et al. 2013), and accelerate wound healing (Jagetia et al. 2007). Moreover, the addition of AA to media improved the quality of MSCs (Wei et al. 2014), and AA-treated cells facilitated wound healing (Muhammad et al., submitted).
Although proof-of-principle studies have shown the positive effects of MSCs, the application of non-invasive imaging, such as MRI, may further improve the accuracy and efficiency of cell targeting and facilitate the correlation of observed neurological and behavioral effects with the location/survival of transplanted cells (Janowski et al. 2012). Thus, there is urgent need to test labeling methods of MSCs to make them visible after transplantation, and MRI, due to the high resolution, topographical image capability, and wide clinical utility, is of special interest. There are various approaches, but the simplicity of cell tags, such as iron oxide and fluorine with commerically available (or, in the case of fluorine, clinically approved formulations), favors these types of tags as tools for clinical use.
Superparamagnetic Iron oxide nanoparticles (SPION) have been used long-term for cell labeling (Bulte et al. 1992), including a once-available clinical formulation of SPION (Endorem), which, after extensive testing was established as non toxic to stem cells (Kassis et al. 2010) and was successfully used in clinical studies (Karussis et al. 2010, Janowski et al. 2014). The withdrawal of Endorem from the market due to economical reasons had a negative impact on the field of regenerative medicine. Currently, there is only one FDA-approved formulation of SPION – Feraheme, registered for the treatment of anemia. However, that SPION is very resistant to stem cell incorporation, and a recent report about improving that incorporation process, by complexing with heparin and protamine (Thu et al. 2012), has not been reproduced in our laboratory. Another formulation of SPION: Molday ION Rhodamine (MIRB) produced by BioPAL (BioPAL) has been used in our laboratory for several years with great success for the very effcient labeling of glial-restricted precursors (GRPs) (Gorelik et al. 2012) or neural stem cells (Berman et al. 2011), and no detrimental effects were observed. We have heard, with a great enthusiasm, that BioPAL has started the procedure for FDA-approval of MIRB (personal communication), which also motivated us to select that formulation for our study.
While the major advantage of SPION is a very high sensitvity, enabling nearly single-cell detection, it is impractical in areas of high magnetic susceptibility artifacts, such as injured tissues. The “hot spot” fluorine (19F) MRI technique overcomes that limitation, but at a cost of lower sensitivity (Ruiz-Cabello et al. 2008). The interest in 19F MRI has been recently fueled by the availability of an FDA-approved 19F nanoemulsion (Ahrens et al. 2014).
Keeping in mind all the above-mentioned aspects related to successful stem cell therapy, we designed this study in such a way that enabled us to evaluate the effect of both SPION and 19F tags in various populations of mouse MSCs, and also see the effect size against the other independent variables that are highly relevant to the therapeutic utility of MSCs.
Materials and Methods
Experimental design
The investigated populations (nominal and dichotomous independent variables) of MSCs were: 1) cell labeling: SPION vs. 19F vs. non-labeled, 2) tissue of cell origin: bone marrow vs. adipose tissue; 3) age of donor: young vs. old; 4) cell culture conditions: hypoxic vs. normal vs. normal +AA; 5) exposure to acidosis: yes vs. no. The experimental readouts (continuous dependent variables) included: 1) cell viability; 2) cell size; 3) cell doubling time; 4) colony formation; 5) efficiency of labeling; and 6) cell migration. The experiments were triplicated. The experimental design is presented in Figure 1.
Figure 1.
A schematic presentation of the experimental design. The red circles point on primary endpoint, and black circles indicate secondary endpoints.
Cell derivation and culture
For cell culture studies, MSCs were derived from young (three months) and aged (20 months old) mice. Adipose-derived MSCs (ADMSC) were obtained from the abdominal fat of mice, as described previously (Meric et al. 2013). Approximately 1–2 g of adipose tissue was collected, minced, and digested by incubating with collagenase-I solution for 1 h at 37 °C. The digested adipose was then filtered with a 40 μm strainer, centrifuged for 15 min at 1300 rpm, and washed twice with 1 x phosphate-buffered saline (PBS). The cell number, size, and viability were determined by the Trypan-blue exclusion assay using a cytometer (Nexcelom, Biociences, USA). The isolated ADMSCs were suspended in DMEM-LG (Sigma, USA) containing 15% FBS (HyClone, USA), 100 U/ml penicillin (Sigma, USA), and 100 μg/ml streptomycin (Sigma, USA), seeded in a 25 cm2 flask at a cell density of 1×105 cells/cm2 and incubated at 37 °C and 5 % CO2. Bone marrow MSCs (BMMSC) were isolated from femurs, as described previously (Quittet et al. 2015). The medium used for BMMSCs was the same as that used for ADMSCs with the only difference being the 20% FBS used for BMSCs, otherwise cells were cultured in the identical conditions. The medium was changed every two-to-three days. Cells at the third passage were used for all the experiments in the study. All experiments were approved by the Johns Hopkins University Animal Care and Use Committee.
Ascorbic acid (AA) preconditioning of MSCs
AA preconditioning was performed by the addition of 250 μM L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma, USA) in the complete culture media containing FBS, from passage 1 to 3 (Potdar et al. 2010), whereas non-preconditioned MSCs were grown to the third passage with normal media, excluding AA, as reported previously.
Hypoxic preconditioning of MSCs
MSCs were preconditioned in hypoxic conditions while cultured in a 3% oxygen atmosphere from day 0 to passage 3 (Choi et al. 2014), whereas, non-preconditioned MSCs were grown under normal conditions and ambient oxygen, as described previously.
Labeling of MSCs with SPION
MSCs were labeled with SPION (MIRB, BioPAL) by following an already described procedure (Barczewska et al. 2013). Briefly, monolayer cultures grown in 24-well plates were washed with 1x PBS twice and labeled by supplementing 5ml of complete culture medium with 50 ul of MIRB (BioPAL, inc, USA). The cells were cultured overnight, and the next day, medium was discarded and cells were washed twice with 1x PBS. Fluorescent photomicrographs were acquired using a fluorescent microscope (ZEISS Observer Z.1 equipped with ORCA-Flash 4.0 CMOS camera; Hamamatsu) and labeling efficiency was assessed based on the percentage of red fluorescent MSCs.
Labeling of MSCs with 19F
MSCs were labeled with 19F, as described (Ribot et al. 2014). Briefly, monolayer cultures grown in 24-well plates were washed twice with 1x PBS and incubated overnight with 2ml of growth medium (without FBS) supplemented with 200ul of Cell Sense ATM DM-Green (Celsense, USA). The next day, the medium was discarded and cells were washed twice with 1x PBS. Fluorescent photomicrographs were acquired using a fluorescent microscope (ZEISS Observer Z.1 equipped with ORCA-Flash 4.0 CMOS camera; Hamamatsu) and labeling efficiency was calculated based on the percentage of green fluorescent cells. For subsequent assays, the cells were cultured in complete growth medium.
Exposure of MSCs to in vitro acidosis
MSCs were subjected to in vitro acidosis stress for 72 hours. The acidosis was induced by adjusting the pH of the complete culture media to 6.20, using 10 N HCl (Merck, Germany). The MSCs cultured in physiological pH were used as controls.
Assessment of cell viability and cell size
Cell viability and cell size were investigated using a cytometer (Nexcelom, Biosciences). Briefly, upon completion of the treatment, the cells were trypsinized, mixed in equal volume with Trypan blue (Sigma Aldrich, USA), and counted using a cytometer for automatic measurement of cell viability and size. Cell viability is expressed as a percent of counted cells, while cell size is presented in micrometers.
Cell doubling time
The cell proliferation rates for all the groups of MSCs were calculated as described previously (Choi et al. 2014) by seeding 1 × 105 cells/cm2 per well in 24-well culture plates with complete media. Cell viability was assayed on days 1, 3, 7, 10, and 14. A growth curve showing the number of viable cells over time was plotted for all the groups. Population doubling time (PDT) was calculated to further characterize the proliferative activity of MSCs. The number of cell doublings was calculated according to the formula n = (log10 Nh − log10 Ni)/log102 where Ni and Nh are the cell numbers at the beginning and at the end of the passage, respectively. PDT was calculated as the ratio of incubation period (days) divided by the number of cell doublings at each passage, and a mean PDT was determined. The doubling time is expressed in hours.
Colony-forming assay
The colony-forming assay was performed according to an already described procedure (Choudhery et al. 2014), and modified for the needs of our study, which included a seeding density of 1 × 104 cells per 25cm culture flask incubation in relevant conditions for 14 days, based on our experimental paradigm, as described in the independent variables section. On day 14, MSCs were washed twice with 1x PBS, cells were fixed with absolute methanol, and stained with 0.1% crystal violet for 60 minutes at room temperature. Then, cells were washed with water and colonies containing more than 35 MSCs were counted under the microscope. This outcome was expressed as integer depicting the absolute number of colonies.
Cell labeling efficiency
Cell labeling efficiency was calculated as the percent of labeled cells and it concerns both types of labels. It was evaluated a day after removal of tags, which is relevant to in vivo application scenario. In case of acidosis labeling was performed prior to application of harsh environment, and it was investigated when cells were under acidic conditions to re-create conditions, when labeled cells after infusion reach the harsh, acidic environment of tissue injury, if they are expected to retain the label.
The fluorescent label incorporated to the MRI tags has been used to detect labeled cells. The amount of label in individual cells has not been investigated, as MSCs are to some extent heterogeneous with difference in size, thus such calculations being very time-consuming, would not guarantee to bring reasonable response.
In vitro scratch assay
In order to evaluate the migratory properties of MSCs, the in vitro central scratch model was used, as reported previously (Liang et al. 2007), with slight modifications. Briefly, MSCs seeded in density of 1 × 105 cells/cm2 per well in 24-well culture plates with relevant medium. When the monolayer reached about 80% confluence, two perpendicular sharp streaks were made through the center of a well with a sterile 1 ml pipette tip. The cells were washed with serum-free fresh DMEM-LG medium to remove the detached cells. After washing, cells were kept within their respective medium and conditions. After three days, the cells were washed twice with sterile 1 x PBS and fixed with 3.7% paraformaldehyde (Riedel-de Haën, USA) for 30 minutes. Fixed cells were stained with 0.1% crystal violet for 30 minutes and images were acquired randomly using a microscope. The colonization of the empty spaces on the scratched site of the plate was quantified using ImageJ software. This variable has been expressed as a percent of empty space, which has been colonized by cells.
Statistical Analysis
PROC MIXED (SAS) was used for statistical analysis. Univariate regression with a lowest means square (LMS) test was performed to investigate the statistical significance of particular independent variables. Multivariate regression was performed to assess the comparative contribution of independent variables to the variability of particular dependent variables.
Results
Independent variables
Cell labeling – primary endpoint
We did not identify any impact of cell labeling on the investigated populations in any of the readouts. (Tab. 1). Multivariate analysis showed that cell tagging was the smallest source of variability for all the readouts (Fig. 2).
Tab. 1.
The influence of cell labeling
| Dependent variable | Type III test | LSM | Difference in LSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Estimate | value | SE | estimate | value | SE | P | |
| Viability | 0.5 | 0.605 | 19F | 59.486 | 2.9179 | No difference | |||
| SPION | 59.805 | ||||||||
| No label: | 63.222 | ||||||||
| Cell size | 0.69 | 0.5008 | 19F | 13.526 | 0.2582 | No difference | |||
| SPION | 13.2181 | ||||||||
| No label: | 13.1125 | ||||||||
| Doubling time | 1 | 0.3699 | 19F | 14.819 | 0.4192 | No difference | |||
| SPION | 14.902 | ||||||||
| No label: | 14.138 | ||||||||
| Colony formation | 0.49 | 0.6139 | 19F | 14.069 | 0.9984 | No difference | |||
| SPION | 14.333 | ||||||||
| No label: | 15.388 | ||||||||
| Labeling efficiency | 1.47 | 0.2326 | 19F | 92.666 | 0.5789 | No difference | |||
| SPION | 93.930 | ||||||||
| No label: | 0 | ||||||||
| Cell migration | 0.22 | 0.8048 | 19F | 42.361 | 2.3923 | No difference | |||
| SPION | 42.444 | ||||||||
| No label: | 44.333 | ||||||||
Figure 2.
A graphic presentation of the distribution of variability among independent variables with annotated numbers (F values) that contributed to the particular dependent variables: A) cell viability; B) cell size; C) cell doubling time; D) colony formation; E) efficiency of cell labeling; and F) cell migration.
Secondary endpoints
Age of donor
We have shown that, while the age of the donor does not influence cell viability, mouse MSCs derived from older donors were bigger, characterized by a slower doubling time, with less propensity for colony formation, a lower labeling efficiency, and less intensive migration. Thus, the age of the donor has a very profound impact on the basic properties of mouse MSCs (Tab. 2).
Tab. 2.
The influence of the age of the cell donor
| Dependent variable | Type III test | LSM | Difference in LSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Estimate | value | SE | estimate | value | SE | P | |
| Viability | 2.17 | 0.1425 | Young: | 63.31 | 2.3705 | No difference | |||
| Old: | 58.37 | ||||||||
| Cell size | 157.72 | <.0001 | Young: | 11.863 | 0.160 | Young vs Old | 2.8435 | 0.2264 | <.0001 |
| Old: | 14.707 | ||||||||
| Doubling time | 41.8 | <.0001 | Young: | 13.1852 | 0.313 | Young vs Old | 2.8704 | 0.4438 | <.0001 |
| Old: | 16.055 | ||||||||
| Colony formation | 141.5 | <.0001 | Young: | 19.916 | 0.632 | Young vs Old | −10.638 | 0.894 | <.0001 |
| Old: | 9.2778 | ||||||||
| Labeling efficiency | 272.37 | <.0001 | Young: | 97.274 | 0.342 | Young vs Old | −7.9545 | 0.482 | <.0001 |
| Old: | 89.319 | 0.339 | |||||||
| Cell migration | 41.92 | <.0001 | Young: | 51.213 | 1.783 | Young vs Old | −16.333 | 2.522 | <.0001 |
| Old: | 34.879 | ||||||||
Cell culture conditions
The conditions in which cells were cultured, hypoxic vs. normal vs. normal + AA, influenced the cell size, doubling time, colony formation, and cell migration. Cells cultured with the addition of AA were significantly smaller, had a shorter doubling time, and had more propensity for colony formation than those cultured at hypoxic or normal conditions. Cells cultured with the addition of AA have also revealed the highest migratory activity, and cells cultured in hypoxic conditions displayed lower migration, but still significantly higher than that maintained in normal conditions. The viability and labeling efficiency were not affected by cell culture conditions (Tab. 3).
Tab. 3.
The influence of the cell culture conditions
| Dependent variable | Type III test | LSM | Difference in LSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Estimate | value | SE | estimate | value | SE | P | |
| Viability | 0.95 | 0.3866 | AA | 64 | 2.9118 | No difference | |||
| Hypoxic | 60.0278 | ||||||||
| Normal | 58.4861 | ||||||||
| Cell size | 6.35 | 0.0021 | AA | 12.6 | 0.2516 | Ascorbic vs Hypoxic | −0.805 | 0.355 | 0.0246 |
| Hypoxic | 13.4056 | Ascorbic vs Normal | −1.251 | 0.0005 | |||||
| Normal | 13.8514 | Hypoxic vs Normal | −0.445 | 0.2117 | |||||
| Doubling time | 11.32 | <.0001 | AA | 13.0833 | 0.4005 | Ascorbic vs Hypoxic | −2.097 | 0.566 | <.0003 |
| Hypoxic | 15.1806 | Ascorbic vs Normal | −2.513 | <.0001 | |||||
| Normal | 15.5972 | Hypoxic vs Normal | −0.416 | <.4627 | |||||
| Colony formation | 5.42 | 0.0051 | AA | 17.2083 | 0.9761 | Ascorbic vs Hypoxic | 4.1389 | 1.380 | 0.003 |
| Hypoxic | 13.0694 | Ascorbic vs Normal | 3.6944 | 0.008 | |||||
| Normal | 13.5139 | Hypoxic vs Normal | −0.444 | 0.7478 | |||||
| Labeling efficiency | 2.55 | 0.0814 | AA | 94.625 | 0.7038 | No difference | |||
| Hypoxic | 92.6735 | 0.6966 | |||||||
| Normal | 92.6875 | 0.7038 | |||||||
| Cell migration | 13.23 | <.0001 | AA | 50.9722 | 2.2586 | Ascorbic vs Hypoxic | 7.375 | 3.194 | 0.0219 |
| Hypoxic | 43.5972 | Ascorbic vs Normal | 16.402 | <.0001 | |||||
| Normal | 34.5694 | Hypoxic vs Normal | 9.0278 | 0.0052 | |||||
Tissue origin for derivation of MSCs
We have shown that, while cell origin does not influence cell viability, mouse MSCs derived from fat are characterized by a larger size, a faster doubling time, better propensity for colony formation, higher labeling efficiency, and higher migration. Thus, tissue origin has a very profound impact on the basic properties of mouse MSCs (Tab. 4).
Tab. 4.
The influence of the tissue of cell origin
| Dependent variable | Type III test | LSM | Difference in LSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Estimate | value | SE | estimate | value | SE | P | |
| Viability | 3.09 | 0.080 | ADMSC | 63.777 | 2.3655 | No difference | |||
| BMMSC | 57.898 | ||||||||
| Cell size | 26.4 | <.0001 | ADMSC | 14.009 | 0.1991 | ADMSC vs BMMSC | 1.4472 | 0.2815 | <.0001 |
| BMMSC | 12.562 | ||||||||
| Doubling time | 85.73 | <.0001 | ADMSC | 12.722 | 0.2899 | ADMSC vs BMMSC | −3.796 | 0.41 | <.0001 |
| BMMSC | 16.518 | ||||||||
| Colony formation | 58.35 | <.0001 | ADMSC | 18.5 | 0.7225 | ADMSC vs BMMSC | 7.8056 | 1.0218 | <.0001 |
| BMMSC | 10.694 | ||||||||
| Labeling efficiency | 10.8 | 0.0013 | ADMSC | 94.616 | 0.5582 | ADMSC vs BMMSC | 2.6025 | 0.7921 | 0.0013 |
| BMMSC | 92.013 | 0.562 | |||||||
| Cell migration | 14.05 | 0.0002 | ADMSC | 48.055 | 1.8897 | ADMSC vs BMMSC | 10.0185 | 2.6724 | 0.0002 |
| BMMSC | 38.037 | ||||||||
Acidosis
We have shown that acidosis has a profound influence on all the investigated readouts. It dramatically decreased cell viability, and revealed a profoundly negative impact on cell migration and colony formation, while cell size, cell doubling time, and labeling efficiency were also significantly affected, but the effect was relatively smaller than others (Tab. 5).
Tab. 5.
The influence of the cell exposure to acidosis
| Dependent variable | Type III test | LSM | Difference in LSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Estimate | value | SE | estimate | value | SE | P | |
| Viability | 4350.63 | <.0001 | Yes: | 36.7778 | 0.5159 | Yes vs No | 48.1204 | 0.7295 | <.0001 |
| No: | 84.8981 | ||||||||
| Cell size | 74.1 | <.0001 | Yes: | 14.3926 | 0.1819 | Yes vs No | −2.2139 | 0.2572 | <.0001 |
| No: | 12.1787 | ||||||||
| Doubling time | 94.31 | <.0001 | Yes: | 16.5833 | 0.2859 | Yes vs No | −3.9259 | 0.4043 | <.0001 |
| No: | 12.6574 | ||||||||
| Colony formation | 54.91 | <.0001 | Yes: | 10.787 | 0.7271 | Yes vs No | 7.6204 | 1.0283 | <.0001 |
| No: | 18.4074 | ||||||||
| Labeling efficiency | 6.7 | 0.0106 | Yes: | 92.2778 | 0.5697 | Yes vs No | 2.0784 | 0.8029 | 0.0106 |
| No: | 94.3562 | 0.5657 | |||||||
| Cell migration | 308.89 | <.0001 | Yes: | 27.537 | 1.248 | Yes vs No | 31.0185 | 1.7649 | <.0001 |
| No: | 58.5556 | ||||||||
The source of variability of dependent variables
It has been shown that acidosis was, by far, the most influential independent variable that affected cell viability, followed by cell origin and age of the donor, while cell culture conditions and cell labeling had the least influence (Fig. 2A). In contrast, cell size was mostly affected by the age of the donor, followed by acidosis and cell origin, while cell culture conditions had an even smaller impact, and cell labeling contributed only minimally (Fig. 2B). Acidosis was the most influential factor for cell doubling time, followed by cell origin and age of the donor. Again, cell culture conditions and cell labeling were the least influential (Fig. 2C). Colony formation was mostly affected by the age of the donor and cell origin, while the impact of acidosis was lower, with, again, the lowest impact from cell culture conditions and cell labeling (Fig. 2D). The efficiency of cell labeling was mostly affected by the age of the donor, with much less effect caused by cell origin and acidosis, while cell culture conditions and the type of label had a negligible effect (Fig. 2E). An example of labeled MSCs using both SPION and 19F tags is shown in figure 3. Finally, cell migration was mostly influenced by acidosis, and by the age of the donor. Cell origin and the conditions under which cells are grown had less impact, with a minimal effect of cell labeling on cell migration (Fig. 2F). Examples of cell migration in various MSC populations are presented in Figure 4.
Figure 3.
The representative images of mouse MSCs labeled with SPION (A, B,C) and 19F (D,E,F). The presented cells are ADMSC derived from young donor not exposed to acidosis cultured with AA addition, A and C – phase-contrast microscopy, B and E: fluorescent images, C and F: merged pictures. Scale bar=0.1 mm.
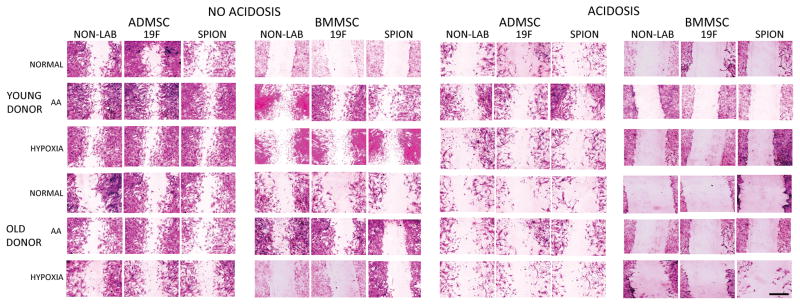
Figure 4.
Representative images of the in vitro scratch assay from all MSC investigated populations. Scale bar=1 mm.
Discussion
Here, we present an exhaustive in vitro study, in which we primarily assessed the potential influence of contemporary MRI cell-tracking agents on mouse mesenchymal stem cells. This characterization is of great relevance to regenerative medicine approaches that use MSCs, and is needed prior to using this technology for in vivo studies. We were specifically interested in extensive investigations using various populations of mouse MSCs, such as those derived from different tissues (fat or bone marrow), donors of different ages (young or old), different culture conditions (normal, normal with the addition of AA, or hypoxic), or those subjected to a very harsh acidic environment, mimicking an injured tissue microenvironment.
First, we showed that cell labeling has no impact on the basic properties of any population of mouse MSCs, even in a very harsh acidic environment; thus, cell labeling by MIRB and Cell Sense for proton and fluorine MRI, respectively, appears to be safe and can be safely used for in vivo neurological studies. We followed the range of SPION concentration used previously, which has been shown not to affect viability, but other potentially more sensitive parameters of toxicity were not investigated (Fan et al. 2013). It has also been previously shown that the SPION formulation, Resovist (not manufactured since 2009), does not compromise the differentiation of MSCs toward neural-like cells and the process of differentiation does not compromise labeling efficiency (Zhang et al. 2014). It is, to some extent, in line with our study, in which both agents were internalized equally efficiently, suggesting that the uptake process may be governed by a universal mechanism. Others also have not found a negative effect on the viability or the differentiation potential of primate (Addicott et al. 2011) or of human (He et al. 2014) MSCs, nor any negative effects on cell proliferation (Ren et al. 2011). We added more readouts to the evaluation package, including cell size, which is a very important safety parameter for intravascular infusions, especially for intra-arterial delivery to the CNS, and that feature has not been well-studied as yet.
In addition, the study revealed very important information about conditions that affect the basic properties of MSCs. The harsh acidic environment, as expected, had a dramatic impact on cells. It concerned mostly the variables that were measured just after the withdrawal from an acidic environment, such as cell viability and cell migration, while the outcomes that required further cell culture, such as evaluation of cell doubling time or colony formation, were less affected. This suggests the potential for loss and/or paralysis of transplanted mouse MSCs at injury sites; however the surviving cells might be capable of regaining function, provided that the environment becomes more favorable over time. This observation also calls for methods of cell engineering that would result in better resistance of stem cells to harsh conditions at cell injury sites, where they are expected to act.
Our study has shown that an advanced age of the donor negatively influences cell size, doubling time, colony formation, and cell migration. The study that evaluated adipose tissue-derived MSCs of human origin also showed greater proliferation of MSCs from young (below 30 years old), than from older patients (above 50 years old) (Yang et al. 2015). Faster proliferation was observed in MSCs derived from younger rats than from older rats (Wu et al. 2014). There was also a report that showed a lower yield of MSCs from older animals (Tarnowski et al. 2007). Others described impaired migration of MSCs derived from aged donors (Bustos et al. 2014). The results showed an increase in the doubling time for cells isolated from old vs. young donors. This was accompanied by a decrease in clonogenicity, while no changes were observed in cell phenotype (Li et al. 2014). In addition to differentiation, aging also impairs the responsiveness of MSCs to anoxia (Jiang et al. 2008). All the above-mentioned studies are in line with our findings of negative effects of the aging process on the quality of MSCs, and we are aware of only one study that failed to detect such difference (Gala et al. 2011). While the possibility of autografting is frequently mentioned among the main advantages of MSCs, the age-related impairment of their properties is a major blow to this concept. Thus, in the presence of such overwhelming evidence for an age-dependent loss of function, there is an urgent need for rejuvenation strategies. Indeed, promising results about the rejuvenation of MSCs have been observed after cell engineering based on the induction of TERT and MYOCD expression (Madonna et al. 2013), as well as up-regulation of miR-10a (Li et al. 2013). If there is a requirement for a scaffold, this could also be engineered by cytokine functionalization, which enables modulation of the aging-related p16 gene and enhances cell proliferation (Kang et al. 2012).
Although the negative impact of donor age observed by others and by us is well-established, there is little data about the rationale for the choice of tissue for the derivation of MSC. Here, we have shown the higher yield of colonies in ADMSCs compared to BMMSCs of mouse origin. The study on MSCs from miniature pigs was also favored the use of ADMSCs and showed that the expression of stemness genes was maintained in ADMSCs, but not in other sources (bone marrow, ear skin, abdominal skin, lung). Another observation from this study was that ADMSCs are characterized by the highest differentiation potential (Lee et al. 2015). A higher proliferation of rat (Lotfy et al. 2014) and human MSCs (Stern-Straeter et al. 2014) from ADMSCs than from BMMSC was also reported. Greater migration of ADMSCs compared to BMMSCs might be related to the higher expression of the chemokine CXCR4 receptor (Murakami et al. 2014). It has also been shown that BMMSCs were more affected by donor age, while this factor did not have any influence on the proliferation of ADMSCs (Beane et al. 2014). However, there are also studies reporting opposite findings. Higher proliferation was observed in BMMSCs vs. ADMSCs derived from sheep (Heidari et al. 2013), and higher clonogenicity was found in BMMSCs derived from goat (Mohamad-Fauzi et al. 2015). In another study, various isolation protocols from adipose tissue and bone marrow were compared, and it was shown that MSCs derived from bone marrow proliferated faster than ADMSCs, but they did not differ with regard to colony formation (Bortolotti et al. 2015). The variability of results may also depend on the variations in the cell derivation procedure, as well the culture protocols, which is typically the same for both cell populations, but may actually favor one of them. The experience and the time a particular researcher devoted to the isolation of MSC from a particular tissue may also affect the results, and even work as a self-fulfilling prophecy about the superiority of the previously selected/investigated cell source.
The assessment of independent variables and their impact on dependent variables revealed the quite important information that, in general, the age of the donor and cell origin (fat versus bone marrow) are responsible for more variability in most investigated readouts. Cell culture conditions, such as hypoxia or the addition of AA, had only a minor effect. However, the advantages of AA were also reported in the form of faster proliferation of ADMSCs and BMMSCs on metal-based biomaterials functionalized with AA (Marycz et al. 2013). The positive effect of AA on the proliferation of MSCs was found to occur through the enhancement of HGF expression (Bae et al. 2015). It has also been shown that AA mediates an increase of telomerase activity (Wei et al. 2012). Hypoxia was also shown to facilitate MSC proliferation (Grayson et al. 2006, Grayson et al. 2007, Dos Santos et al. 2010, Lindner et al. 2010, Nekanti et al. 2010, Drela et al. 2014, Li et al. 2015, Peng et al. 2015).
The issue of MSC size was never investigated as it was probably considered irrelevant, but now, with our recent findings that cell size strongly affects the safety of intra-arterial delivery (Janowski et al. 2013), this highlights the importance of this parameter. Although our present study has shown that, in general, the characteristics of ADMSCs are superior to bone marrow-derived counterparts, the larger size of ADMSCs may be a disadvantage as it could facilitate the formation of micro-infarcts after intra-arterial delivery to the brain or spinal cord; thus, in this case, BMMSCs may be more desirable. This observation is in line with another study, which showed that intravenous infusion of ADMSCs resulted in more thrombi formation compared to BMMSC; however, in that study, the cell size was not investigated, and rather, the presence of coagulation factors was emphasized (Shiratsuki et al. 2015). In this context, a method that enables MSC size to be controlled would be desirable, especially in a temporary manner.
Conclusion
The regression analysis across many independent and dependent variables is rarely used in experimental research, and is usually employed by large clinical studies. However, it provides a broader look at the observed phenomena and allows for more general conclusions than a comparison usually used in basic research conducted about one independent and one dependent variable per experimental paradigm. Here, we found that SPION and 19F nanoemulsions do not have a detrimental effect on any of the tested MSC populations at any investigated conditions; thus, these cell-labeling methods seem safe and their use seems justified for in vivo efficacy studies. In addition, we have shown that the “imprinted” qualities of mouse MSCs are tissue/donor/age-specific, and it is difficult to overcome by adjusting potentially more favorable cell culture conditions. If that observation proves true for human cells, it would be very important to use methods for cell engineering to rejuvenate MSCs, as the most desired and safe option is autografting and the prevalence of elderly patients may limit transplantation efficacy.
While we were able to make several important conclusions from this study, it must be emphasized that they may be limited to mouse MSCs, and, as such, be relevant only to preclinical studies. Clinical studies should be preceded by a detailed evaluation of human MSCs, including the effect of labeling with MRI contrast agents.
References
- Addicott B, Willman M, Rodriguez J, Padgett K, Han D, Berman D, Hare JM, Kenyon NS. Mesenchymal stem cell labeling and in vitro MR characterization at 1.5 T of new SPIO contrast agent: Molday ION Rhodamine-B. Contrast Media Mol Imaging. 2011;6(1):7–18. doi: 10.1002/cmmi.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72(6):1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Ryu H, Rhee KJ, Oh JE, Baik SK, Shim KY, Kong JH, Hyun SY, Pack HS, Im C, Shin HC, Kim YM, Kim HS, Eom YW, Lee JI. l-Ascorbic acid 2-phosphate and fibroblast growth factor-2 treatment maintains differentiation potential in bone marrow-derived mesenchymal stem cells through expression of hepatocyte growth factor. Growth Factors. 2015:1–8. doi: 10.3109/08977194.2015.1013628. [DOI] [PubMed] [Google Scholar]
- Barczewska M, Wojtkiewicz J, Habich A, Janowski M, Adamiak Z, Holak P, Matyjasik H, Bulte JW, Maksymowicz W, Walczak P. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One. 2013;8(9):e74658. doi: 10.1371/journal.pone.0074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014;9(12):e115963. doi: 10.1371/journal.pone.0115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn Reson Med. 2011;65(2):564–574. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti F, Ukovich L, Razban V, Martinelli V, Ruozi G, Pelos B, Dore F, Giacca M, Zacchigna S. In Vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Reports. 2015;4(3):332–339. doi: 10.1016/j.stemcr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte JW, De Jonge MW, Kamman RL, Go KG, Zuiderveen F, Blaauw B, Oosterbaan JA, The TH, de Leij L. Dextran-magnetite particles: contrast-enhanced MRI of blood-brain barrier disruption in a rat model. Magn Reson Med. 1992;23(2):215–223. doi: 10.1002/mrm.1910230203. [DOI] [PubMed] [Google Scholar]
- Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N, Mora AL, Rojas M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189(7):787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, Wan Safwani WK. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem Biophys Res Commun. 2014;448(2):218–224. doi: 10.1016/j.bbrc.2014.04.096. [DOI] [PubMed] [Google Scholar]
- Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, Janowski M, Walczak P, Boltze J, Lukomska B, Jolkkonen J. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Sundell IB, Koka PS. Adult mesenchymal stem cells and their potency in the cell-based therapy. J Stem Cells. 2013;8(1):1–16. [PubMed] [Google Scholar]
- Dong Y, Wang S, Zhang T, Zhao X, Liu X, Cao L, Chi Z. Ascorbic acid ameliorates seizures and brain damage in rats through inhibiting autophagy. Brain Res. 2013;1535:115–123. doi: 10.1016/j.brainres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223(1):27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- Drela K, Sarnowska A, Siedlecka P, Szablowska-Gadomska I, Wielgos M, Jurga M, Lukomska B, Domanska-Janik K. Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy. 2014;16(7):881–892. doi: 10.1016/j.jcyt.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Fan J, Tan Y, Jie L, Wu X, Yu R, Zhang M. Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res Ther. 2013;4(2):44. doi: 10.1186/scrt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gala K, Burdzinska A, Idziak M, Makula J, Paczek L. Characterization of bone-marrow-derived rat mesenchymal stem cells depending on donor age. Cell Biol Int. 2011;35(10):1055–1062. doi: 10.1042/CBI20100586. [DOI] [PubMed] [Google Scholar]
- Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, Gilad AA, Bar-Shir A, Kerr DA, Levchenko A, Bulte JW, Walczak P. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012;265(1):175–185. doi: 10.1148/radiol.12112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207(2):331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- He T, Wang Y, Xiang J, Zhang H. In vivo tracking of novel SPIO-Molday ION rhodamine-B-labeled human bone marrow-derived mesenchymal stem cells after lentivirus- mediated COX-2 silencing: a preliminary study. Curr Gene Ther. 2014;14(2):136–145. doi: 10.2174/1566523214666140408113900. [DOI] [PubMed] [Google Scholar]
- Heidari B, Shirazi A, Akhondi MM, Hassanpour H, Behzadi B, Naderi MM, Sarvari A, Borjian S. Comparison of proliferative and multilineage differentiation potential of sheep mesenchymal stem cells derived from bone marrow, liver, and adipose tissue. Avicenna J Med Biotechnol. 2013;5(2):104–117. [PMC free article] [PubMed] [Google Scholar]
- Jagetia GC, Rajanikant GK, Mallikarjun Rao KV. Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of gamma-radiation. Burns. 2007;33(4):484–494. doi: 10.1016/j.burns.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Janowski M, Bulte JW, Handa JT, Rini D, Walczak P. Using stem cells to prevent the progression of myopia - a concept. Stem Cells. 2015 doi: 10.1002/stem.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Bulte JW, Walczak P. Personalized nanomedicine advancements for stem cell tracking. Adv Drug Deliv Rev. 2012;64(13):1488–1507. doi: 10.1016/j.addr.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33(6):921–927. doi: 10.1038/jcbfm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem Cells Dev. 2010;19(1):5–16. doi: 10.1089/scd.2009.0271. [DOI] [PubMed] [Google Scholar]
- Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, Lukomska B, Roszkowski M. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One. 2014;9(2):e97631. doi: 10.1371/journal.pone.0097631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Kh Haider H, Ahmed RP, Idris NM, Salim A, Ashraf M. Transcriptional profiling of young and old mesenchymal stem cells in response to oxygen deprivation and reparability of the infarcted myocardium. J Mol Cell Cardiol. 2008;44(3):582–596. doi: 10.1016/j.yjmcc.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Sun L, Xiao Y, Li SH, Wu J, Guo J, Jiang SL, Yang L, Yau TM, Weisel RD, Radisic M, Li RK. Aged human cells rejuvenated by cytokine enhancement of biomaterials for surgical ventricular restoration. J Am Coll Cardiol. 2012;60(21):2237–2249. doi: 10.1016/j.jacc.2012.08.985. [DOI] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis I, Vaknin-Dembinsky A, Bulte J, Karussis D. Effects of supermagnetic iron oxide labeling on the major functional properties of human mesenchymal stem cells from multiple sclerosis patients. Int J Stem Cells. 2010;3(2):144–153. doi: 10.15283/ijsc.2010.3.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Lee J, Kim CL, Lee KS, Lee SH, Gu NY, Kim JM, Lee BC, Koo OJ, Song JY, Cha SH. Comparative studies on proliferation, molecular markers and differentiation potential of mesenchymal stem cells from various tissues (adipose, bone marrow, ear skin, abdominal skin, and lung) and maintenance of multipotency during serial passages in miniature pig. Res Vet Sci. 2015 doi: 10.1016/j.rvsc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Li J, Dong J, Zhang ZH, Zhang DC, You XY, Zhong Y, Chen MS, Liu SM. miR-10a restores human mesenchymal stem cell differentiation by repressing KLF4. J Cell Physiol. 2013;228(12):2324–2336. doi: 10.1002/jcp.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li L, Zhang Z, Jiang Z. Hypoxia promotes bone marrow-derived mesenchymal stem cell proliferation through apelin/APJ/autophagy pathway. Acta Biochim Biophys Sin (Shanghai) 2015 doi: 10.1093/abbs/gmv014. [DOI] [PubMed] [Google Scholar]
- Li Y, Charif N, Mainard D, Bensoussan D, Stoltz JF, de Isla N. Donor’s age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed Mater Eng. 2014;24(1 Suppl):47–52. doi: 10.3233/BME-140973. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lindner U, Kramer J, Beherends J, Fuchs F, Wendler NO, Rohwedel J, Schlenke P. Hypoxia enhances proliferation and attenuates differentiation capacity of human mesenchymal stromal cells - and prolongs their lifespan. J Stem Cells Regen Med. 2010;6(2):76. [PubMed] [Google Scholar]
- Lotfy A, Salama M, Zahran F, Jones E, Badawy A, Sobh M. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells. 2014;7(2):135–142. doi: 10.15283/ijsc.2014.7.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna R, Taylor DA, Geng YJ, De Caterina R, Shelat H, Perin EC, Willerson JT. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ Res. 2013;113(7):902–914. doi: 10.1161/CIRCRESAHA.113.301690. [DOI] [PubMed] [Google Scholar]
- Marycz K, Smieszek A, Grzesiak J, Donesz-Sikorska A, Krzak-Ros J. Application of bone marrow and adipose-derived mesenchymal stem cells for testing the biocompatibility of metal-based biomaterials functionalized with ascorbic acid. Biomed Mater. 2013;8(6):065004. doi: 10.1088/1748-6041/8/6/065004. [DOI] [PubMed] [Google Scholar]
- Meric A, Yenigun A, Yenigun VB, Dogan R, Ozturan O. Comparison of chondrocytes produced from adipose tissue-derived stem cells and cartilage tissue. J Craniofac Surg. 2013;24(3):830–833. doi: 10.1097/SCS.0b013e3182902779. [DOI] [PubMed] [Google Scholar]
- Mohamad-Fauzi N, Ross PJ, Maga EA, Murray JD. Impact of source tissue and ex vivo expansion on the characterization of goat mesenchymal stem cells. J Anim Sci Biotechnol. 2015;6(1):1. doi: 10.1186/2049-1891-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Hayashi Y, Iohara K, Osako Y, Hirose Y, Nakashima M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/dentin Regeneration. Cell Transplant. 2014 doi: 10.3727/096368914X683502. [DOI] [PubMed] [Google Scholar]
- Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6(5):499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski P, Al-Senani F, Summers P, Byrne J, Noble JA, Ventikos Y. Towards treatment planning for the embolization of arteriovenous malformations of the brain: intranidal hemodynamics modeling. IEEE Trans Biomed Eng. 2011;58(7):1994–2001. doi: 10.1109/TBME.2011.2119317. [DOI] [PubMed] [Google Scholar]
- Peng L, Shu X, Lang C, Yu X. Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. Cytotechnology. 2015 doi: 10.1007/s10616-014-9818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potdar PD, D’Souza SB. Ascorbic acid induces in vitro proliferation of human subcutaneous adipose tissue derived mesenchymal stem cells with upregulation of embryonic stem cell pluripotency markers Oct4 and SOX 2. Hum Cell. 2010;23(4):152–155. doi: 10.1111/j.1749-0774.2010.00095.x. [DOI] [PubMed] [Google Scholar]
- Quittet MS, Touzani O, Sindji L, Cayon J, Fillesoye F, Toutain J, Divoux D, Marteau L, Lecocq M, Roussel S, Montero-Menei CN, Bernaudin M. Effects of mesenchymal stem cell therapy, in association with pharmacologically active microcarriers releasing VEGF, in an ischaemic stroke model in the rat. Acta Biomater. 2015;15:77–88. doi: 10.1016/j.actbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Ren Z, Wang J, Zou C, Guan Y, Zhang YA. Labeling of cynomolgus monkey bone marrow-derived mesenchymal stem cells for cell tracking by multimodality imaging. Sci China Life Sci. 2011;54(11):981–987. doi: 10.1007/s11427-011-4239-x. [DOI] [PubMed] [Google Scholar]
- Ribot EJ, Gaudet JM, Chen Y, Gilbert KM, Foster PJ. In vivo MR detection of fluorine-labeled human MSC using the bSSFP sequence. Int J Nanomedicine. 2014;9:1731–1739. doi: 10.2147/IJN.S59127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Cabello J, Walczak P, Kedziorek DA, Chacko VP, Schmieder AH, Wickline SA, Lanza GM, Bulte JW. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med. 2008;60(6):1506–1511. doi: 10.1002/mrm.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuki S, Terai S, Murata Y, Takami T, Yamamoto N, Fujisawa K, Burganova G, Quintanilha LF, Sakaida I. Enhanced survival of mice infused with bone marrow-derived as compared with adipose-derived mesenchymal stem cells. Hepatol Res. 2015 doi: 10.1111/hepr.12507. [DOI] [PubMed] [Google Scholar]
- Song H, Song BW, Cha MJ, Choi IG, Hwang KC. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. 2010;10(3):309–319. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- Stern-Straeter J, Bonaterra GA, Juritz S, Birk R, Goessler UR, Bieback K, Bugert P, Schultz J, Hormann K, Kinscherf R, Faber A. Evaluation of the effects of different culture media on the myogenic differentiation potential of adipose tissue- or bone marrow-derived human mesenchymal stem cells. Int J Mol Med. 2014;33(1):160–170. doi: 10.3892/ijmm.2013.1555. [DOI] [PubMed] [Google Scholar]
- Stevanovic M, Bracko I, Milenkovic M, Filipovic N, Nunic J, Filipic M, Uskokovic DP. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity. Acta Biomater. 2014;10(1):151–162. doi: 10.1016/j.actbio.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Taran R, Mamidi MK, Singh G, Dutta S, Parhar IS, John JP, Bhonde R, Pal R, Das AK. In vitro and in vivo neurogenic potential of mesenchymal stem cells isolated from different sources. J Biosci. 2014;39(1):157–169. doi: 10.1007/s12038-013-9409-5. [DOI] [PubMed] [Google Scholar]
- Tarnowski M, Koryciak-Komarska H, Czekaj P, Sebesta R, Czekaj TM, Urbanek K, Likus W, Malinowska-Kolodziej I, Plewka D, Nowaczyk-Dura G, Wiaderkiewicz R, Sieron AL. The comparison of multipotential for differentiation of progenitor mesenchymal-like stem cells obtained from livers of young and old rats. Folia Histochem Cytobiol. 2007;45(3):245–254. [PubMed] [Google Scholar]
- Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, Chaudhry A, Ren J, Varma NR, Arbab AS, Frank JA. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18(3):463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Liu X, Tao J, Wu R, Zhang P, Bian Y, Li Y, Fang F, Zhang Y. Effects of vitamin C on characteristics retaining of in vitro-cultured mouse adipose-derived stem cells. In Vitro Cell Dev Biol Anim. 2014;50(1):75–86. doi: 10.1007/s11626-013-9673-6. [DOI] [PubMed] [Google Scholar]
- Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, Liu Y, Zhang C, Shi S, Wang S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227(9):3216–3224. doi: 10.1002/jcp.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, Cooney DS, Lee WP, Brandacher G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30(4):122–127. doi: 10.1016/j.trim.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Kim KJ, Kim MK, Lee SJ, Ryu YH, Seo BF, Oh DY, Ahn ST, Lee HY, Rhie JW. The Stem Cell Potential and Multipotency of Human Adipose Tissue-Derived Stem Cells Vary by Cell Donor and Are Different from Those of Other Types of Stem Cells. Cells Tissues Organs. 2015 doi: 10.1159/000369969. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li J, Xie J. Efficient In vitro labeling rabbit bone marrow-derived mesenchymal stem cells with SPIO and differentiating into neural-like cells. Mol Cells. 2014;37(9):650–655. doi: 10.14348/molcells.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]