SUMMARY
Melanoma patients with oncogenic BRAFV600E mutation have poor prognoses. While the role of BRAFV600E in tumorigenesis is well established, its involvement in invasion that is clinically observed in melanoma patients, remains a topic of debate. Here we show that BRAFV600E melanoma cells have extensive invasion activity as assayed by degradation of extracellular matrix, and generation of F-actin and cortactin foci that mediate membrane protrusion. Inhibition of BRAFV600E blocks melanoma cell invasion. In a BRAFV600E-driven murine melanoma model or in patients’ tumor biopsies, cortactin foci decrease upon inhibitor treatment. In addition, genome-wide expression analysis shows that a number of invadopodia-related genes are down-regulated after BRAFV600E inhibition. Mechanistically, BRAFV600E induces phosphorylation of cortactin and the exocyst subunit Exo70 through ERK, which regulates actin dynamics and matrix metalloprotease secretion, respectively. Our results provide support for the role of BRAFV600E in metastasis, and suggest that inhibiting invasion is a potential therapeutic strategy against melanoma.
Keywords: oncogenic BRAF, invadopodia, tumor invasion
Graphical Abstract

INTRODUCTION
Malignant melanoma is well known for its aggressive metastasis, which accounts for most of patients’ deaths (Erdmann et al., 2013). Approximately 50% of melanomas harbor activating mutations in the BRAF protein kinase. The most common BRAF mutation is the substitution of valine at position 600 by glutamic acid (V600E), which leads to constitutive activation of the kinase and sustained activation of the RAS-RAF-MEK-ERK pathway (Davies et al., 2002; Wan et al., 2004).
While the role of BRAFV600E in driving melanomagenesis is well established, its role in melanoma invasion remains elusive, as conflicting evidence in the literature exists. In both cell culture and mouse models, oncogenic BRAF was reported to induce cancer cell invasion by activating the Rho family of GTPases (Makrodouli et al., 2011), down-regulation of PDE5A (Arozarena et al., 2011), and reorganization of actin cytoskeleton (Klein et al., 2008). Other studies, however, suggest that BRAF mutation alone does not induce metastasis, and proteins such as β-catenin act as a central mediator of tumor metastasis in BRAFV600E/PTEN−/− mouse model of melanoma (Damsky et al., 2011). In clinical studies, the frequency of BRAFV600E in metastatic melanomas is similar to primary melanomas (Casula et al., 2004; Colombino et al., 2012). In addition, BRAF or NRAS mutation status does not influence the clinical outcomes in patients with metastatic melanoma (Carlino et al., 2014). On the other hand, studies have shown that BRAFV600E is correlated to a lower overall patient survival rate compared to BRAF wild-type melanoma, which is similar to what has been observed in other types of cancer (Cho et al., 2006; Davies et al., 2002; Long et al., 2011; Menzies et al., 2012; Nikiforova et al., 2003; Roth et al., 2010; Ugurel et al., 2007; Van Cutsem et al., 2011; Yokota et al., 2011). Clearly, a more definitive study of the role of BRAFV600E in melanoma progression is needed.
Cancer cells initiate metastasis by invading through the extracellular matrix (ECM). To degrade the ECM, cells secrete metalloproteinases (MMPs) via actin-based membrane protrusions such as invadopodia (Hoshino et al., 2013b; Leong et al., 2014; Linder, 2007; McNiven, 2013; Murphy and Courtneidge, 2011; Paz et al., 2014; Yamaguchi, 2012). The formation of such invasion structures is controlled by signaling events that lead to phosphorylation of a number of proteins including cortactin, which, through N-WASP and the Arp2/3 complex, initiates the dynamic re-organization of the F-actin network (Bravo-Cordero et al., 2012; Hoshino et al., 2013a; Hoshino et al., 2013b). Secretion of MMPs also requires the proper function of the exocytosis machinery. The exocyst, an octameric protein complex consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84, mediates the docking of secretory vesicles to the plasma membrane during exocytosis (Wu and Guo, 2015; He and Guo, 2009; Hsu et al., 2004). Recent studies demonstrate that the exocyst is involved in MMP secretion and cell migration (Sakurai-Yageta M et al., 2008; Liu et al., 2009; Lu et al., 2013; Ren and Guo, 2012; Monteiro et al., 2013). The exocyst subunit Exo70 is a direct phospho-substrate of ERK, which plays an important role in MMP secretion in response to growth factor signaling (Ren and Guo, 2012).
In this study, we systematically investigated the role of BRAFV600E in promoting melanoma invasion using a number of in vitro and in vivo approaches. We demonstrate that BRAFV600E is involved in melanoma cell invasion. Inhibition of BRAFV600E significantly reduces the number of cortactin foci in a genetically engineered BRAF-driven mouse melanoma model and in melanoma patients’ tumor biopsies. Mechanistically, BRAFV600E promotes ERK-dependent phosphorylation of both cortactin and Exo70, which in turn regulates actin assembly and MMP secretion. Genome-wide expression analysis shows a number of invadopodia-related genes are regulated by BRAFV600E expression. Taken together, our study suggests that BRAFV600E plays an important role in melanoma invasion.
RESULTS
BRAFV600E is necessary for melanoma cell migration and invasion
To investigate the role of BRAFV600E in controlling melanoma cell invasion, we first inhibited BRAFV600E with either siRNA or the BRAF inhibitor PLX4720 in BRAFV600E-positive melanoma cells. WM3211 cell line with wild-type BRAF was included as a negative control. siRNA targeting both wild-type BRAF and BRAFV600E (“siBRAF”) (Poulikakos et al., 2011) effectively reduced the expression of BRAF in all four melanoma cell lines (Supplemental Figure 1A and 1B). While siBRAF did not affect the motility of WM3211 cells with wild-type BRAF, the motility was significantly inhibited in BRAFV600E-positive 1205Lu, WM35 and WM793 melanoma cell lines (Supplemental Figure 1C). Similar to siBRAF, the BRAF inhibitor PLX4720 blocked the migration of 1205Lu, WM35 and WM793 cells (Supplemental Figure 1D).
Next, we examined whether inhibition of BRAFV600E decreased the ability of melanoma cells to degrade ECM. 1205Lu and WM793 cell lines treated with DMSO or transfected with control Luciferase siRNA displayed a high level of gelatin degradation. In contrast, cells treated with PLX4720 or transfected with BRAF siRNA showed much less degradation (Figure 1A and 1B; Supplemental Figure 2A and 2B). MMP secretion mediates the degradation of the ECM (Murphy and Courtneidge, 2011). To test whether BRAFV600E regulates MMP secretion, we performed the zymography assay, which quantifies MMP activity by in-gel digestion of gelatin (Liu et al., 2008). 1205Lu cells were transfected with siBRAF or treated with PLX4720. Conditioned media were collected and analyzed on a gel containing gelatin. As shown in Figure 1C and D, both PLX4720 and siBRAF significantly reduced the activity of MMP2 in the media.
Figure 1. BRAFV600E is necessary for actin-based membrane protrusion formation and ECM degradation in human BRAFV600E melanoma cells.
(A) 1205Lu cells with different treatments were plated on cover slips coated with Alexa 568-labeled gelatin (red) for 12 hr. F-actin was stained with Alexa-488-phalloidin (green) and nuclei were stained with DAPI (blue). Areas of gelatin degradation were shown as black areas beneath the cells. Scale bars=10μm. (B) Quantification of Alexa 568-labeled gelatin degradation. n>150 from three independent experiments; “***”: p<0.001. (C) In-gel zymography analysis shows that BRAF inhibition suppresses MMP-2 secretion in 1205Lu cells. 1205Lu cells were transfected with siBRAF or treated with PLX4720 for 48 hr, and then incubated with serum free medium for 12 hr. Conditioned media were collected and analyzed on a gel containing gelatin. (D) Quantification of MMP-2 secretion from different groups of cells in (C). n=3; “***”: p<0.001. (E) 1205Lu cells with different treatments were plated on cover slips coated with gelatin for 5 hr. Cells were stained with cortactin (red), phalloidin (green) and DAPI (blue). Scale bars=10μm. (F) Quantification of percentage of cells with cortactin foci. n>150 from three independent experiments; “*”: p<0.05. (G) and (I) WM793 cells with different treatments were plated on cover slips coated with gelatin for 5 hr. Cells were stained with cortactin (blue), phalloidin (green) and MT1-MMP or TKS5 (red). Scale bars=10μm. (H) and (J) Quantification of percentage of cells with cortactin-MT1-MMP or cortactin-TKS5 foci. n>150 from three independent experiments; “*”: p<0.05, “***”: p<0.001.
Error bars: standard deviation, Kruskal-Wallis one-way ANOVA was performed in (B), (F), (H) and (J); Student’s t-test was performed in (D) using software R, version 2.14. See also Figure S1, S2, S3.
Cancer cells invasion also requires actin-based membrane protrusions that penetrate into the ECM (Friedl and Wolf, 2003; Linder, 2007; Murphy and Courtneidge, 2011; Nurnberg et al., 2011). These invasion sites are often enriched with F-actin and its regulators such as cortactin and Tks5, together with MMPs (Bowden et al., 1999; Eckert et al., 2011; Hoshino et al., 2013b). Inhibition of BRAFV600E with either siBRAF or PLX4720 significantly reduced the number of F-actin-cortactin foci (Figure 1E and 1F; Supplemental Figure 2C and 2D). The F-actin/cortactin foci were likely invadopodia, the actin-based membrane protrusions that penetrate into the ECM (Friedl and Wolf, 2003; Linder, 2007; Murphy and Courtneidge, 2011; Nurnberg et al., 2011). However, due to the fast actin dynamics and accumulative ECM degradation by MMPs, the F-actin/cortactin foci may not appear perfectly co-localized with the sites of ECM degradation. To further verify the invadopodia structures, we co-stained the cells for F-actin, cortactin, MT1-MMP or Tks5. As shown in Figure 1G to 1J, and Supplemental Figure 3, there were clear foci with co-localization of all the three proteins in control cells, suggesting the formation of invadopodia. Upon PLX4720 treatment, the number of such foci were significantly decreased.
Transient expression of BRAFV600E is sufficient to induce cell invasion
To further validate the role of BRAFV600E in melanoma cell invasion, we tested whether BRAFV600E is sufficient to promote the formation of actin-based protrusion and ECM degradation. BRAFV600E was expressed under the doxycycline (Dox)-inducible promoter in WM3211 cells that are wild-type for both BRAF and NRAS (Meerbrey et al., 2011) (henceforth termed as “WM3211-BRAFV600E cells”) (Supplemental Figure 4A and 4B). After 9 hours of Dox treatment, the percentage of WM3211-BRAFV600E cells with F-actin/cortactin foci was significantly higher than that of the WM3211 control cells (Figure 2A and 2B). Using fluorescence-labeled degradation assay, we further found that forced expression of BRAFV600E increased gelatin degradation by approximately 1.5 folds compared to control cells (Figure 2C and 2D).
Figure 2. Transient expression of BRAFV600E is sufficient to promote melanoma cell invasion.
(A) WM3211-BRAFV600E cells were pretreated with doxycycline or DMSO for 4 hr, and plated on cover slips coated with gelatin. The cells were still maintained in Dox except control. After 5 hr incubation, Cells were stained with cortactin (red), phalloidin (green) and DAPI (blue). Scale bars=10μm. (B) Quantification of the percentage of cells with cortactin foci. n>150 from three independent experiments; “*”: p<0.05. (C) WM3211-BRAFV600E cells were pretreated with doxycycline or DMSO for 4 hr, and then plated on cover slips coated with Alexa 568-labeled gelatin (red). The cells were maintained in Dox except control. After 12 hr incubation, cells were stained with F-actin (green) and nuclei (blue). Scale bars=10μm. (D) Quantification of Alexa 568-labeled gelatin degradation. n>150 from three independent experiments; “*”: p<0.05. (E) In-gel zymography analysis shows the effect of BRAFV600E on MMP-2 secretion in WM3211 cells. WM3211-BRAFV600E cells were treated with or without Dox for indicated times, and the conditioned media were collected and analyzed by in-gel zymography. β-actin was detected by Western blot. (F) Quantification of MMP-2 secretion from different groups of cells in (E). n=3; “***”: p<0.001. (G) In-gel zymography analysis shows that PLX4720 inhibits BRAFV600E-induced MMP-2 secretion in WM3211 cells. (H) Quantification of MMP-2 secretion from different groups of cells in (G). n=3; “***”: p<0.001.
Error bars: standard deviation. Student’s t-test was performed in (F) and (H) and Kruskal-Wallis one-way ANOVA was conducted in (B) and (D) using software R, version 2.14.
See also Figure S4.
To test whether BRAFV600E is sufficient to increase the secretion of MMPs, we measured MMP secretion in WM3211-BRAFV600E cells with or without Dox treatment using an in-gel zymography assay. WM3211-BRAFV600E cells displayed a more than 2-fold increase in MMP2 activity as compared to that of the control cells (Figure 2E and 2F). To further confirm that the increase in MMP2 activity was induced by BRAFV600E, we treated cells with PLX4720 in the presence of Dox. PLX4720 significantly inhibited the MMP-2 activity that was induced by BRAFV600E expression (Figure 2G and 2H). These data suggest that transient expression of BRAFV600E is sufficient to promote ECM degradation.
We also found that expression of BRAFV600E for 3 days induced cell growth arrest, and some cells exhibited the senescence-like phenotype (data not shown), similar to previous reports that BRAFV600E induces cell growth arrest and senescence as a cellular response to oncogenic BRAF (Damsky et al., 2015; Damsky et al., 2011; Dankort et al., 2009; Dhomen et al., 2009; Michaloglou et al., 2005; Vredeveld et al., 2012; Wajapeyee et al., 2008). This will be further discussed later.
BRAFV600E-mediated invasion is dependent on ERK phosphorylation of cortactin and Exo70
Next, we set out to understand how BRAFV600E promotes the assembly of protrusive actin structures and secretion of MMPs. Membrane protrusion is mediated by the recruitment and activation of actin regulatory proteins such as cortactin and Tks5 (Ayala et al., 2008; Seals et al., 2005). Cortactin can be phosphorylated at serine 418 (“S418”) by ERK, which is a key kinase in the RAF-MEK-ERK cascade (Kelley et al., 2010; Martinez-Quiles et al., 2004). We therefore examined the phosphorylation of cortactin in response to BRAFV600E. Forced expression of BRAFV600E increased phospho-ERK levels and the phosphorylation of cortactin by ERK at serine 418 (“S418”) in WM3211-BRAFV600E cells (Figure 3A). In addition, PLX4720 or siBRAF suppressed the phosphorylation of both ERK and cortactin in 1205Lu cells (Figure 3B). Furthermore, treatment of 1205Lu cells with two MEK inhibitors, U0126 or GSK1120212, significantly inhibited the phosphorylation of ERK and cortactin (Figure 3C and 3D). Fluorescence microscopy shows that these MEK inhibitors reduced the number of cells with cortactin foci by approximately 3 folds (Figure 3E to 3H). Similarly, drug treatments significantly reduced the number of foci with co-localization of F-actin, cortactin and Tks5, or those with co-localization of F-actin, cortactin and MT1-MMP (Supplemental Figure 5 and Supplemental Figure 6). These foci likely represent invadopodia.
Figure 3. Actin-based membrane protrusion formation in BRAFV600E cells is dependent on ERK.
(A) WM3211-BRAFV600E cells were cultured with or without Dox for the indicated times, and cell lysates were analyzed by Western blot using antibodies against ERK1/2, phospho-ERK1/2 (“p-ERK1/2”), cortactin, phospho-cortactin (“p-CortactinS418”) and β-actin. (B–D) Cell lysates from 1205Lu cells with different treatments were analyzed by Western blot using antibodies against ERK1/2, phospho-ERK1/2, cortactin, phosphor-cortactin and β-actin. (E) and (G) 1205Lu cells were treated with GSK1120212 or U0126 for 24 hr, and then plated on cover slips coated with gelatin for 5 hr. Cells were stained with cortactin (red), phalloidin (green) and DAPI (blue). Scale bars=10μm. (F) and (H) Quantification of percentage of cells with cortactin foci. n>150 from three independent experiments; “*”: p<0.05.
Kruskal-Wallis one-way ANOVA was performed using software R, version 2.14.
See also Figure S5, S6.
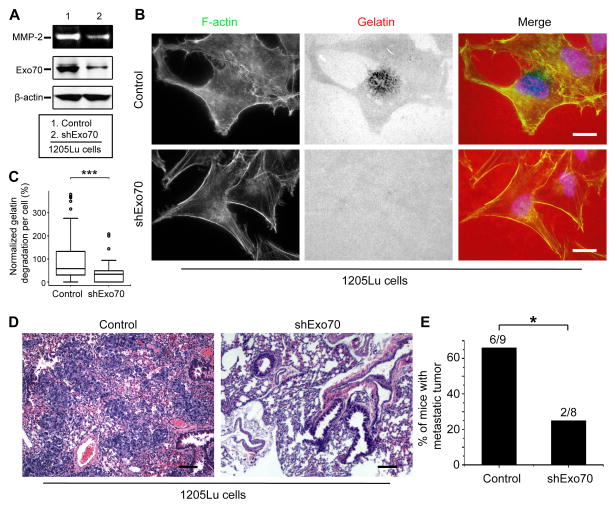
Previous studies have shown that the exocyst mediates MMP secretion during invasion (Sakurai-Yageta et al., 2008; Liu et al., 2009; Monteiro et al., 2013; Ren and Guo, 2012). The exocyst subunit Exo70 coordinates actin dynamics with exocytosis to control cell invasion (Liu et al., 2009). Importantly, ERK directly phosphorylates Exo70, thereby promoting exocytosis of MMPs (Ren and Guo, 2012). We therefore hypothesize that BRAFV600E regulates MMP2 secretion through Exo70. To test this hypothesis, we determined whether Exo70 is required for MMP2 activity in BRAFV600E-positive melanoma. Knockdown of Exo70 inhibited MMP2 secretion and gelatin degradation (Figure 4A–4C). Next, we examined the role of Exo70 in controlling tumor metastasis using a melanoma xenograft model. We generated 1205Lu stable cell lines expressing control shRNA or Exo70 shRNA and injected them into immunodeficient mice. Tumor metastasis was monitored over a period of 5 weeks. While the incidence of primary tumor was similar for each group, knockdown of Exo70 effectively suppressed lung metastasis of 1205Lu cells (Figure 4D and 4E).
Figure 4. Exo70 activity is required for the gelatin degradation and melanoma metastasis.
(A) In-gel zymography analysis showed that Exo70 knockdown inhibited MMP-2 secretion in 1205Lu cells. Cell lysates from indicated cells were analyzed by Western blot using anti-Exo70 and anti-β-actin antibodies. (B) The gelatin degradation assay was performed in 1205Lu cells which were transfected with Exo70 shRNA or vector control. Scale bars=10μm. (C) Quantification of Alexa 568-labeled gelatin degradation per cell. n>150 from three independent experiments; “***”: p<0.001. (D) Representative images show hematoxylin and eosin staining of metastatic tumor, scale bars=100μm. 1205LU cells stably expressed vector control or shExo70 were injected into nude mice, tissue samples were collected after 35 days. (E) Percentage of mice with metastasis tumors among the 2 different groups of mice. “*”: p<0.05.
Error bars: standard deviation. Student’s t-test was performed in (E), and Kruskal-Wallis one-way ANOVA was performed in (C) using software R, version 2.14.
We performed an immunoprecipitation assay to determine the level of Exo70 phosphorylation. Phosphorylation of Exo70 increased over 6 folds upon the forced expression of BRAFV600E in WM3211 cells, while the total expression level of Exo70 remained unchanged (Figure 5A). We further examined whether BRAFV600E promoted exocyst complex assembly, which is necessary for the tethering of secretory vesicles to the plasma membrane for exocytosis. As shown in Figure 5B and 5C, BRAFV600E promoted the association of Exo70 with Sec8, another component of the exocyst complex. In addition, both PLX4720 and siBRAF suppressed Exo70 phosphorylation and its interaction with Sec8 (Figure 5D–5F). Since Exo70 is a substrate of ERK, we also examined the effect of inhibition of BRAF-MEK-ERK pathway by a MEK inhibitor, U0126. As shown in Figure 5G–5I, after the treatment with U0126, phosphorylation of Exo70 decreased by approximately 6 folds, and the binding between Exo70 and Sec8 also decreased by more than 5 folds.
Figure 5. BRAFV600E promotes Exo70 phosphorylation and exocyst complex assembly.
(A) Phosphorylation of endogenous Exo70. WM3211-BRAFV600E cells were treated with or without Dox for the indicated times. Exo70 was immunoprecipitated from the cell lysates using the anti-Exo70 antibody. The immunoprecipitation protein complexes were analyzed by SDS-PAGE and probed for anti-ERK1/2 phospho-substrate antibody. The total levels of Exo70 were used as a loading control. (B) WM3211-BRAFV600E cells were treated with or without Dox for the indicated times, Exo70 and Sec8 were immunoprecipitated from the cell lysates and analyzed by SDS-PAGE, and probed for Exo70 and Sec8. (C) Quantification of Exo70 and Sec8 binding in (B). “*”: p<0.05. (D) 1205Lu cells were treated with BRAF siRNA, Luciferase siRNA, DMSO or PLX4720. Exo70 was immunoprecipitated and analyzed as described in (A). (E) 1205Lu cells were treated with BRAF siRNA, Luciferase siRNA, DMSO or PLX4720. Exo70 and Sec8 were immunoprecipitated from the cell lysates and analyzed by SDS-PAGE, and probed for Exo70 and Sec8. (F) Quantification of Exo70 and Sec8 binding in (E). “**”: p<0.01. (G) 1205Lu cells were treated with U0126, and Exo70 was immunoprecipitated and analyzed as described in (A). (H) 1205Lu cells were treated with U0126. Exo70 and Sec8 were immunoprecipitated from the cell lysates and analyzed by SDS-PAGE, and probed for Exo70 and Sec8. (I) Quantification of Exo70 and Sec8 binding in (H). “**”: p<0.01.
Error bars, standard deviations. Student’s t-test was performed using software R, version 2.14.
BRAFV600E is necessary for invasion in a genetically engineered BRAF-driven mouse melanoma
To further investigate the role of BRAFV600E in vivo, we used a genetically engineered BRAF-driven mouse (iBIP mice, inducible BRAF INK/ARF PTEN) melanoma model (Kwong et al., 2015). As described previously, the iBIP mice were generated with a Tet-inducible human BRAFV600E transgene on a melanocyte-specific, Pten-null, and constitutive Cdkn2a-null background. Mice with melanoma were treated with PLX4720 for 14 days. Pre-treatment and on-treatment biopsies from the same mouse were collected and stained with antibodies against cortactin (Eckert et al., 2011; Leong et al., 2014; Lu et al., 2013). While the pre-treatment tumor cells contained abundant cortactin foci, treating the mice with PLX4720 significantly reduced the number of cortactin foci (Figure 6A and 6B). During the time course of the experiment, no acquired BRAFi resistance was observed in the iBIP system.
Figure 6. BRAFV600E is necessary for invasion in genetically engineered BRAF-driven mouse melanoma model.
(A) Representative images of sections of iBIP mouse tumors stained with cortactin (red) and DAPI (blue). The sections are longitudinal biopsies from the same tumor pre- and post-BRAF inhibition by PLX4720. Scale bars=50μm. (B) Quantification of cortactin foci in primary tumors. Three pairs of primary tumor biopsies from different mice were examined. Six field images were taken for each tumor sample. Student’s t-tests were performed using software R, version 2.14. “**”: p<0.01; “***”: p<0.001. (C–E) GSEA plots of the most significantly down regulated pathways after BRAF inhibition. Doxycycline was withdrawn from iBIP mice to induce BRAF extinction in established tumors, then analyzed by expression microarray. Genes decreasing over a time course of 90 days were analyzed by GSEA. (F) The heat map of microarray data showing the expression levels of the invadopodia-related gene set in iBIP mice after genetic BRAF inhibition.
To confirm the pharmacological effects and investigate the mechanism underlying BRAFV600E-induced invasion, we took advantage of the doxycycline (Dox)-controllable BRAFV600E transgene. With extinction of BRAFV600E expression upon doxycycline withdraw, a longitudinal time course of 42 samples were analyzed by gene expression microarray (GEO: GSE79972). In order to understand the role of invadopodia regulation in relation to global gene expression, we first generated a knowledge-based invadopodia gene set consisting of 38 genes (Murphy and Courtneidge, 2011). This includes regulatory kinases (e.g. RAC/RHO, PAKs), actin regulatory proteins (e.g. ARPC, WASL), and proteases (e.g. PLAUR, MMP9) that are implicated in different stages of invadopodia formation and function (Murphy and Courtneidge, 2011). Upon gene set enrichment analysis (GSEA) of genes that change over the full time course, we noted first that loss of BRAF activity resulted in significant down-regulation of ERK signaling, and of mitosis and cell cycle-related gene sets (Figure 6C–6E). These expected results serve as positive controls that lend confidence to the analysis (Kwong et al., 2012). Strikingly, the invadopodia gene set is significantly down-regulated on par with cell cycle genes, suggesting that invasion is one of the most prominent functions regulated by BRAFV600E in this model (Figure 6C–6E). Key genes including RHOA, PAK1, and PAK3 are down-regulated upon BRAF loss (Figure 6F), while others including RAC1, CDC42, and CTTN are down-regulated at later time points. These are consistent with our PLX4720 IHC results. These microarray analyses suggest that oncogenic BRAF is essential for invadopodia formation in vivo, and BRAFV600E inhibition modulates pathways that affect multiple stages of invasion.
Cortactin foci in BRAFV600E-positive melanoma patient’s clinical samples
To examine how oncogenic BRAF regulates invasion in human melanomas, we compared the cortactin foci in tumor biopsies derived from 6 BRAF-mutated, treatment-naïve melanoma patients and those derived from 7 wild-type BRAF treatment-naïve melanoma patients. As shown in Figure 7A and 7B, the number of cortactin foci in BRAFV600E patients’ tumor biopsies was significantly higher than that with wild-type BRAF. To determine whether BRAF inhibitor therapy decreased the number of cortactin foci in vivo, we examined paired pre-treatment and early on-treatment (2 weeks) tumor biopsies from three BRAFV600E-positive patients treated with vemurafenib. Vemurafenib significantly inhibited cortactin foci formation as exhibited in all three melanoma patients’ early on-treatment tumor biopsies (Figure 7C and 7D). The data suggest a direct association between oncogenic BRAF and invasion in human melanomas.
Figure 7. The number of cortactin foci in BRAFV600E melanoma patients decrease upon vemurafenib treatment.
(A) Representative images of human melanoma samples with wild type BRAF or human melanoma samples with BRAFV600E mutant stained for cortactin (red) and DAPI (blue). (B) Quantification of cortactin foci in human melanoma samples. n=6, six field pictures were taken for each melanoma sample. “***”: p<0.001. (C) Representative images of human melanoma samples before treatment or samples after 14 days vemurafenib treatment, scale bars=50μm. (D) Quantification of cortactin foci in patient melanoma samples. Pictures of five fields were taken for each melanoma sample. “***”: p<0.001.
Error bars, standard deviations. Student’s t-tests were performed using software R, version 2.14.
DISCUSSION
Tumor cell invasion constitutes the initial stage of cancer metastasis. Here we studied the role of oncogenic BRAF in melanoma cell invasion. Our data suggest that oncogenic BRAFV600E promotes melanoma cell invasion by stimulating the formation of actin structures and secretion of MMPs. Inhibiting BRAFV600E reduced the number of invasive activities, whereas transient expression of BRAFV600E promoted cell invasion. Activation of ERK by BRAFV600E stimulated the phosphorylation of cortactin and Exo70, which in turn promoted actin reorganization and MMP exocytosis. We further demonstrate that Exo70 knockdown inhibits melanomas metastasis in nude mice. Both pharmacological and genetic extinction of BRAF reduced cortactin foci in the iBIP mouse model. In BRAF-mutated melanoma patients’ tumor biopsies, the number of cortactin foci was significantly higher than that in patients with wild-type BRAF; the number of cortactin foci was significantly decreased in patients that received short-term vemurafenib therapy. All of our in vitro and in vivo studies indicate an intimate link between oncogenic BRAF and cell invasion. It was reported that the frequency of BRAF mutation in primary melanomas is comparable to that of metastatic melanomas (Colombino et al., 2012). However, it was also observed that BRAF mutation is associated with a worse outcome at a later tumor stage; the median survival rate of patients with BRAF-mutant metastatic melanoma is lower than that of patients with BRAF wild-type melanoma (Long et al., 2011; Menzies et al., 2012). The role of BRAF-mediated cell invasion observed in our study may contribute to these clinical observations.
We find that short-term expression of BRAFV600E is sufficient to promote invasion, whereas long-term expression of BRAFV600E induces growth arrest, which is reminiscent of the oncogene-induced senescence (OIS) documented previously (Damsky et al., 2015; Dhomen et al., 2009; Michaloglou et al., 2005; Vredeveld et al., 2012; Wajapeyee et al., 2008). Therefore, it is likely that OIS inhibits cell invasion phenotype shown at the early stage of BRAFV600E expression, which explains the lack of invasion observed in some studies. On the other hand, BRAF mutation may provide the critical background for rapid progression to metastatic melanoma once another oncogenic mutation such as loss of PTEN occurs (Damsky et al., 2011).
It was also shown that BRAF inhibitors promote tumor metastasis in RAS mutant or BRAF inhibitor-resistant melanoma cells (Sanchez-Laorden et al., 2014). As the MAPK pathway is re-activated in BRAF inhibitor-resistant cells, this study is consistent with our results, and further supports the role of RAF-MEK-ERK signaling in melanoma metastasis.
In summary, our studies demonstrate a role of BRAFV600E in regulating melanoma invasion. Our data warrant a further study of metastatic properties of melanoma and suggest that inhibiting invasion may be a therapeutic strategy for preventing melanoma progression.
EXPERIMENTAL PROCEDURES
Cell culture, inhibitor treatment and antibodies
Human melanoma cell lines were isolated from lesions defined by clinical and histological criteria. These cells were cultured in RPMI 1640 medium, 5% FBS (v/v). Transfection and RNAi experiments are included in the SUPPLEMENTAL INFORMATION. For inhibitor treatment, cells were incubated with 1 μM PLX4720, 1 μM U0126 or 100 nM GSK1120212 for 24 hr before proceeding to the invadopodia assay. Anti-cortactin antibody was purchased from Santa Cruz, Inc.; anti-TKS5 and anti-MT1-MMP antibody were purchased from Millipore; anti-phospho-cortactin antibody (S418) was a gift from Dr. Scott Weed (Kelley et al., 2010), and all the other antibodies were from Cell Signal, Inc.
In-gel zymography
The in-gel zymography assay for MMP-2 activity was performed as described previously (Liu et al., 2009). Briefly, the serum-free conditioned culture media was collected and concentrated. Samples were snap frozen three times in liquid nitrogen, mixed with loading buffer, and then separated on an 8% polyacrylamide/0.3% gelatin gel. The gel was washed three times in 2.5% Triton X-100 and then incubated in the MMP reaction buffer (50 mM of Tris-HCl, pH 8.0, and 5 mM of CaCl2) for 36 to 72 hours at 37 °C. After the reaction, the gel was stained with Coomassie Brilliant Blue and destained overnight with destaining buffer.
In situ zymography
The protocol used to perform in-situ zymography was adapted from the Mueller laboratory (Artym et al., 2009). In brief, AlexaFluor 568-conjugated gelatin was prepared by Alexa Fluor 568 protein labeling (Molecular Probes, Inc.). Coverslips were acid-washed and incubated with 50μg/ml of poly-L-Lysine (Sigma, Inc.) for 20 min, then cross-linked with 0.5% glutaraldehyde (Ted Pella, Inc.) for 15 min. The coverslips were then inverted onto 80μl drop of gelatin (0.2% gelatin and Alexa Fluor 568-labeled gelatin at an 7:1 ratio) for 10 min. After washing in PBS, the coverslips were quenched with 5 mg/ml of NaBH4 for 15 min followed by another wash with PBS. The coverslips were then incubated in the growth medium, after 2 hours, 2×104 cells were plated on the coverslips and incubated at 37 °C for 8 hours. The cells were then fixed and stained for immunofluorescence. Each experiment was repeated 3 times. Gelatin degradation was quantified using ImageJ software.
Immunofluorescence microscopy
For immunofluorescence assay, cells were fixed with 4% paraformaldehyde (PFA)/PBS for 15 min and then permeabilized in PBS containing 0.2% Triton X-100 for 5 min. The cells were pre-incubated in 5% BSA/PBS for 30 min, and then sequentially incubated with primary and secondary antibodies. Alexa-phalloidin (Molecular Probes, Inc.) was used for F-actin staining. The images were captured using a LEICA CTR6000 confocal microscope at 630x magnification. For tumor tissue immunofluorescence assay, paraffin sections of tumor samples were dewaxed and rehydrated in xylene and graded alcohols. After blocking in 10% BSA/PBST (0.5% Tween-20), the samples were incubated with primary antibody overnight at 4 °C, then incubated with the second antibody for 2 hr. Finally, the slides were stained in DAPI, and mounted with Slowfade mounting buffer. Cells were imaged with the Leica DM IRB microscope at 630x magnification.
Xenograft tumor model and iBIP mouse model
Xenografts were generated as described before (Lu et al., 2013). Tumors were palpable at approximately 1 week, and caliper measurements were obtained 2 times per week. Mice were sacrificed after 5 weeks, and all internal organs were harvested and embedded in paraffin, sectioned, stained with hematoxylin and eosin, and then imaged. Mice with cancer metastases were counted and statistical analysis was performed using Fisher’s exact test.
Generation and tumor genesis of iBIP mice was described previously (Kwong et al., 2015). The procedure is included in the Supplemental Information.
Statistical analysis
Data were analyzed for normality using Shapiro-Wilk normality test. Nonparametric data were analyzed with Kruskal-Wallis one-way ANOVA, followed by Dunn’s multiple comparison test. Data with a normal distribution were analyzed by a Student’s test. All statistical analyses were performed using software R, version 2.14.
Microarray analysis used pre-ranked gene set enrichment analysis (GSEA). Briefly, all genes were ranked by the Spearman correlation to the length of doxycycline withdrawal in order to prioritize genes with monotonic changes over time. The data were then run against the c5 gene ontology (GO) gene set including two additional gene sets, an ERK down-regulated gene set previously published (Kwong et al., 2015) and a newly generated, knowledge-based invadopodia gene set.
Supplementary Material
Highlights.
BRAFV600E is involved in promoting melanoma cell invasion
BRAFV600E induces the phosphorylation of cortactin and Exo70 through ERK
Inhibiting BRAFV600E reduces invasion-related gene expression in a BRAF mouse model
BRAFV600E inhibition reduces cortactin foci formation in melanoma patient samples
Acknowledgments
We are grateful to Dr. Scott A. Weed (West Virginia University) for antibodies and Yemin Lan (Drexel University) for assistance in statistical analyses. This work is supported by National Institutes of Health grant GM085146 to W.G., CA114046, CA25874 and CA174523 to X.X., and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and NIH CA025874, CA114046 and CA174523 to M.H.
Footnotes
Supplemental information includes four figures and some of the methods used in the study.
AUTHOR CONTRIBUTIONS
HL, WG and XX conceived the project, designed, and interpreted experiments. SL, GZ, YZ, LK, JF, LW, JM and MH participated in the design and interpretation of mice experiments. HL, SL, YZ, WZ and JZ performed the in vitro experiments. YH assisted with statistical analyses. CK, KS, MX, WX, GK, LS, PZ provided melanoma specimens or associated clinical data. HL and WG wrote the manuscript.
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods Mol Biol. 2009;522:211–219. doi: 10.1007/978-1-59745-413-1_15. [DOI] [PubMed] [Google Scholar]
- Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. Journal of Cell Science. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Current Opinion in Cell Biology. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino MS, Haydu LE, Kakavand H, Menzies AM, Hamilton AL, Yu B, Ng CC, Cooper WA, Thompson JF, Kefford RF, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. British Journal of Cancer. 2014;111:292–299. doi: 10.1038/bjc.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula M, Colombino M, Satta MP, Cossu A, Ascierto PA, Bianchi-Scarra G, Castiglia D, Budroni M, Rozzo C, Manca A, et al. BRAF gene is somatically mutated but does not make a major contribution to malignant melanoma susceptibility: the Italian Melanoma Intergroup Study. Journal of Clinical Oncology. 2004;22:286–292. doi: 10.1200/JCO.2004.07.112. [DOI] [PubMed] [Google Scholar]
- Cho NY, Choi M, Kim BH, Cho YM, Moon KC, Kang GH. BRAF and KRAS mutations in prostatic adenocarcinoma. International Journal of Cancer. 2006;119:1858–1862. doi: 10.1002/ijc.22071. [DOI] [PubMed] [Google Scholar]
- Colombino M, Capone M, Lissia A, Cossu A, Rubino C, De Giorgi V, Massi D, Fonsatti E, Staibano S, Nappi O, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. Journal of Clinical Oncology. 2012;30:2522–2529. doi: 10.1200/JCO.2011.41.2452. [DOI] [PubMed] [Google Scholar]
- Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, Erdelyi I, Platt JT, Huang L, Theodosakis N, et al. mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formation. Cancer Cell. 2015;27:41–56. doi: 10.1016/j.ccell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, et al. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, Bray F. International trends in the incidence of malignant melanoma 1953–2008--are recent generations at higher or lower risk? International Journal of Cancer. 2013;132:385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature Reviews Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Current Opinion in Cell Biology. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. Journal of Cell Science. 2013a;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Reports. 2013b;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. International Review of Cytology. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PloS One. 2010;5:e13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM, Spofford LS, Abel EV, Ortiz A, Aplin AE. B-RAF regulation of Rnd3 participates in actin cytoskeletal and focal adhesion organization. Molecular Biology of the Cell. 2008;19:498–508. doi: 10.1091/mbc.E07-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LN, Boland GM, Frederick DT, Helms TL, Akid AT, Miller JP, Jiang S, Cooper ZA, Song X, Seth S, et al. Co-clinical assessment identifies patterns of BRAF inhibitor resistance in melanoma. J Clin Invest. 2015;125:1459–1470. doi: 10.1172/JCI78954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Reports. 2014;8:1558–1570. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends in Cell Biology. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Yue P, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Molecular Biology of the Cell. 2009;20:3763–3771. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Zhu QF, Hao BS. Effect of the sera of rabbits fed with Tongxinluo on MMP-9 and TIMP-1 expression and secretion in U937 monocyte-derived macrophages. Journal of Southern Medical University. 2008;28:1703–1706. [PubMed] [Google Scholar]
- Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. Journal of Clinical Oncology. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- Lu H, Liu J, Liu S, Zeng J, Ding D, Carstens RP, Cong Y, Xu X, Guo W. Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Developmental Cell. 2013;27:560–573. doi: 10.1016/j.devcel.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrodouli E, Oikonomou E, Koc M, Andera L, Sasazuki T, Shirasawa S, Pintzas A. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Molecular Cancer. 2011;10:118. doi: 10.1186/1476-4598-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Molecular and Cellular Biology. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA. Breaking away: matrix remodeling from the leading edge. Trends in Cell Biology. 2013;23:16–21. doi: 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, Herschkowitz JI, Burrows AE, Ciccia A, Sun T, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies AM, Haydu LE, Visintin L, Carlino MS, Howle JR, Thompson JF, Kefford RF, Scolyer RA, Long GV. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clinical Cancer Research. 2012;18:3242–3249. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Monteiro P, Rosse C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. The Journal of Cell Biology. 2013;203:1063–1079. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature Reviews Molecular Cell Biology. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. The Journal of Clinical Endocrinology and Metabolism. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nature Reviews Cancer. 2011;11:177–187. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2014;33:4193–4202. doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Guo W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Developmental Cell. 2012;22:967–978. doi: 10.1016/j.devcel.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. Journal of Clinical Oncology. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. The Journal of Cell Biology. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Laorden B, Viros A, Girotti MR, Pedersen M, Saturno G, Zambon A, Niculescu-Duvaz D, Turajlic S, Hayes A, Gore M, et al. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Science Signaling. 2014;7:ra30. doi: 10.1126/scisignal.2004815. [DOI] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sharma VP, Eddy R, Entenberg D, Kai M, Gertler FB, Condeelis J. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Current Biology. 2013;23:2079–2089. doi: 10.1016/j.cub.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S, Thirumaran RK, Bloethner S, Gast A, Sucker A, Mueller-Berghaus J, Rittgen W, Hemminki K, Becker JC, Kumar R, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PloS One. 2007;2:e236. doi: 10.1371/journal.pone.0000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. Journal of Clinical Oncology. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D, McMahon M, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes & development. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Wu B, Guo W. The Exocyst at a Glance. J Cell Sci. 2015;128:2957–64. doi: 10.1242/jcs.156398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. European Journal of Cell Biology. 2012;91:902–907. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. British Journal of Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.