Abstract
Multiple mechanisms have emerged where the engulfment of whole live cells, leading to the formation of what are called ‘cell-in-cell’ structures, induces cell death. Entosis is one such mechanism that drives cell-in-cell formation during carcinogenesis and development. Curiously, entotic cells participate actively in their own engulfment, by invading into their hosts, and are then killed non-cell-autonomously. Here we review the mechanisms of entosis and entotic cell death and the consequences of entosis on cell populations.
Keywords: Entosis, Entotic cell death, Cannibalism, Cell-in-cell, Engulfment, Phagocytosis, Autophagy, LAP, Cell competition, Aneuploidy
Introduction
Programmed cell death is required for proper tissue development and homeostasis and inhibits the development of diseases such as cancer. In metazoans, cell death hollows the amniotic cavity [6], establishes tissue patterns [52], eliminates developmental organs [28], and sculpts complex tissue structures like fingers [32], mammary gland [39], muscle [23], brain [31], and retina [19]. Cell death maintains immune function by eliminating auto-reactive cells [50], and cell death maintains homeostasis by removing aged or damaged cells from adult tissues.
While physiologic cell death was once considered to occur only by apoptosis (Type I cell death), many ‘alternative’ forms of cell death have now also been identified that, like apoptosis, are controlled by genetic machinery and contribute to cell turnover [16]. For example, autophagic cell death (Type II cell death; e.g. autosis [35]) and programmed forms of necrosis (Type III cell death; e.g. necroptosis [8] and ferroptosis [9]) can eliminate cells during development [43] or carcinogenesis [24], and contribute to killing cells that are infected by viruses [40] or damaged by ischemia–reperfusion [33–35].
In addition to apoptotic, autophagic and necrotic forms of cell death, yet further mechanisms have emerged that may represent additional ways that cells can be eliminated. Among these, multiple mechanisms have been found where viable cells can become engulfed or cannibalized and then killed [46, 48, 63, 64]. These programs, termed entosis [12, 47], phagoptosis [3], suicidal emperipolesis [55], homotypic cell cannibalism [4], emperitosis [62], and cannibalism [38], appear to differ fundamentally from most classic forms of cell suicide, due to the requirement of engulfing cells for cell death execution. Like programmed forms of cell suicide, these cannibalistic programs may also contribute to cell turnover in normal or pathophysiologic contexts.
Cannibalistic cell death
The term ‘cannibalism’ generally describes non-cell-autonomous killing activity that targets viable cells of the same or related species. Cannibalism is an ancient strategy that is used as a stress response by even some unicellular organisms. For example, the sporulating bacterium Bacillus subtilis can cannibalize neighboring bacteria in response to starvation, by secreting lytic factors that induce cell rupture and the release of nutrients into the environment [17]. Similarly, the cellular slime mold Dictyostelium caveatum cannibalizes neighboring amoeba by engulfing and degrading them in order to obtain nutrients when bacteria are scarce [44, 59]. Dictyostelium amoebae also cannibalize neighboring cells when entering into an alternative starvation response called the sexual life cycle, where zygote giant cells engulf of up to hundreds of neighboring amoebae to form ‘macrocysts’, whose protective cell walls are constructed in part from recycled components of cannibalized cells [10, 45, 53].
Perhaps not unlike the cannibalization of individuals within ancestral single cell populations, the individual cells within the tissues of metazoan organisms can also cannibalize each other. While apoptotic cells are well known to be engulfed by phagocytic cells within such tissues (and in some cases phagocytosis is required for apoptosis execution [20, 51]), cannibalistic programs instead target viable cells, where engulfment does not respond to, but rather induces, cell death. Cell cannibalism in metazoan tissues can actually occur by a number of different molecular mechanisms. Some involve phagocytosis (see [3]), while others involve non-phagocytic mechanisms, such as suicidal emperipolesis [55], entosis [12], cell cannibalism [37], and others [63]. These mechanisms can be broadly characterized as heterotypic (occurring between different cell types) or homotypic (occurring between the same cell type), and lead to the formation of cell-in-cell structures [46, 63]. In this review we discuss the detailed molecular mechanism of one of these cell-in-cell processes, entosis, as well as the consequences of entosis on cell populations, and evidence that entosis occurs in vivo in normal and diseased contexts.
Mechanisms of Entosis
Entotic cell engulfment
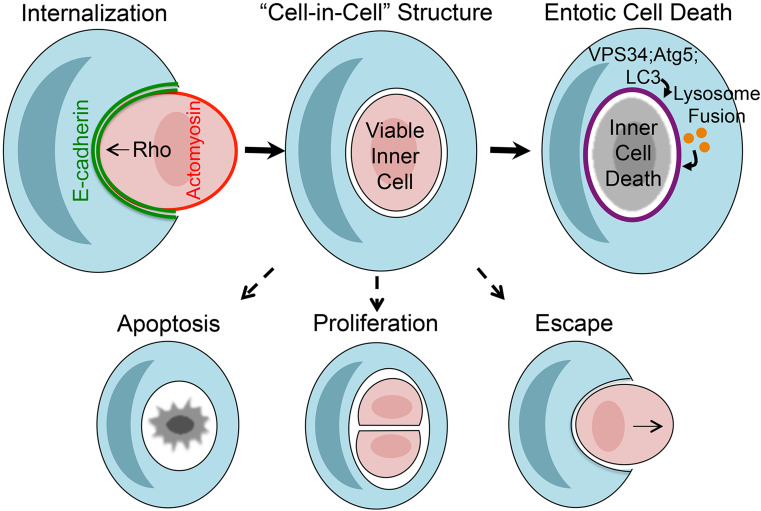
Entosis is triggered in culture by matrix detachment of adherent cells, similar to the apoptotic program of anoikis, although the processes are clearly distinct by functional studies [47]. The entosis engulfment mechanism, unlike phagocytosis, involves epithelial adherens junctions, composed of the cell–cell adhesion receptor E-cadherin and the adherens junction/cytoskeleton linker protein α-catenin, which are necessary [47, 61], and sufficient [56, 61], to mediate entosis in breast tumor cell populations. Entotic cells, unlike cells engulfed by phagocytosis, also play an active role in controlling their own uptake through RhoA-GTPase activity and the RhoA effector kinases Rho-kinases I and II (ROCKI/II) (Fig. 1) [47]. The overexpression of RhoA or ROCK I/II is sufficient to drive the uptake of epithelial cadherin-expressing cells [56], suggesting that entosis resembles more of a cell invasion activity leading to cell-in-cell formation rather than an engulfment per se [47]. Indeed internalizing entotic cells exhibit blebbing that is reminiscent of invading cells that utilize an amoeboid mode of motility [49]. This model is consistent with the localization patterns of actin and myosin during the entosis process, as actin and myosin heavy and light chains accumulate specifically within internalizing cells, at the cell cortex opposite the E-cadherin junctional interface, and do not accumulate within engulfing cells, as would occur during phagocytosis [49, 56, 60]. RhoA activity, measured with a FRET-based biosensor, and ROCK I and II, as well as the RhoA-regulated actin polymerizing formin mDia1, also accumulate specifically within internalizing entotic cells at the cell cortex colocalizing with actomyosin [49, 56]. The combined activities of a Rho-GTPase activating protein (GAP), p190-RhoGAP, which is recruited to E-cadherin-mediated cell–cell junctions and inhibits RhoA, and a Rho-guanine exchange factor (GEF) protein PDZ-RhoGEF, which recruits to the distal cortex of invading cells and activates RhoA, establish a zone of actomyosin contraction that is polarized in a manner to promote entotic cell uptake [49, 56]. Altogether, entosis appears to be a non-canonical engulfment mechanism, distinct from phagocytosis, that drives the ingestion of viable cells into their neighbors. While entosis leads to the formation of cannibalistic or cell-in-cell structures (in essence ‘engulfments’), the formation of such structures does not appear to involve classical ‘engulfing’ activity but is more reminiscent of a cell invasion (Fig. 1).
Fig. 1.
Mechanisms of entosis and entotic cell death. Top images: Matrix detachment of adherent cells triggers entosis, involving formation of E-cadherin and α-catenin-mediated cell junctions (green) between host (blue) and internalizing cells (red). RhoA and Rho kinase (ROCK) activity in internalizing cells leads to actomyosin accumulation at the cell cortex, which drives cell-in-cell formation, suggesting an active invasion–like process. Over time, most internalized cells undergo entotic cell death (top right), involving lipidation of the autophagy protein LC3 onto the entotic vacuole (requiring VPS34 and LC3 lipidation machinery (e.g. Atg5)), followed by lysosome fusion and death of internalized cells. Alternatively, some entotic cells undergo apoptosis, particularly when inhibited for macroautophagy, and others are observed to divide inside of their hosts, or to escape and return to the culture (bottom images)
Entotic cell death
Cells engulfed by entosis (‘entotic’ cells) primarily die, although some can divide inside of their host cell vacuoles, and can also escape altogether, emerging unharmed and capable of subsequent rounds of cell division (Fig. 1) [47]. That some engulfed cells can be completely rescued from death suggests that entotic cells are not engaged in a cell-autonomous death program prior to their engulfment. Indeed, entotic cells do not expose the ‘eat-me’ signal phosphatidylserine, which is common in dying cells for activation of phagocyte receptors and clearance by phagocytosis [47].
While most entotic cells eventually die after they are engulfed [13, 47], cell death occurs in the absence of cleavage of caspase-3, a hallmark of apoptosis, and dying entotic cells do not exhibit morphological features of apoptosis such as nuclear condensation and fragmentation [47]. Entotic cell death is also not blocked by overexpression of the apoptosis inhibitor Bcl-2, altogether supporting a model where apoptosis does not play a significant role in entotic cell engulfment or death [13, 47]. Engulfed entotic cells do exhibit some features of Type II, or autophagic cell death, as they accumulate autophagosomes and have high rates of autophagy flux [13]. But autophagy in this context appears to function to support cell survival, as the inhibition of autophagy within internalized cells induces apoptosis. Because engulfed entotic cells are deprived of nutrients and growth factors from media in the host cell vacuole, it is likely that nutrient starvation induces autophagy, and in the absence of autophagy-dependent nutrient recycling, engulfed cells undergo apoptosis [13]. This is consistent also with the induction of apoptosis of engulfed entotic cells by treatment with the lysosome inhibitor concanamycin A, which inhibits nutrient recovery by the autophagy pathway [13, 47].
As autophagy activity is required for entotic cell survival, it was therefore a surprising finding that entotic cell death also requires autophagy pathway proteins [13]. But it is autophagy pathway proteins acting within the engulfing cells that are required, in a non-cell-autonomous manner, for the death of engulfed cells [13]. Autophagy proteins in this context do not function in autophagosome formation, but instead to direct lipidation of the autophagy protein microtubule-associated protein 1A light chain 3 (LC3) onto the single-membrane vacuoles that harbor engulfed cells (Fig. 1) [13]. The core LC3 lipidation machinery that functions during autophagy (e.g. Atg5, Atg7), and the Beclin–Vps34 kinase, complex are required for LC3 lipidation in this context, but the autophagy pre-initiation kinase complex, composed of Ulk1/2 kinase and Fip200 and Atg13 adaptor proteins, is not required, genetically distinguishing entotic vacuole LC3 lipidation from canonical autophagy [13, 14]. The lipidation of LC3 onto entotic vacuoles appears to play a role to promote lysosome fusion, suggesting that entotic cell death is executed non-cell-autonomously by the autophagy pathway-dependent, lysosomal degradation of live engulfed cells (Fig. 1) [13, 14].
Biological functions of entosis
Entosis in cancer: pro- and anti-tumorigenic roles
Cell structures resembling those formed by entosis have been documented in a variety of human cancers, including breast, colon, liver, and pancreatic carcinoma, and others [21, 47, 54]. Similar to entotic structures in culture, engulfing cell structures from clinical breast tumor samples exhibit localization of the cell–cell adhesion protein β-catenin at the engulfing cell interface, and myosin light chain, phosphorylated at the ROCK I/II site (pMLC-S19), at the cortex of internalizing cells [47, 56]. And comparable to entotic cell structures, engulfed cell structures in human tumors sometimes involve complex, multiple cell relationships, such as ‘cell-in-cell-in-cell’ configurations [47]. Thus, while live tumor cell engulfments in human tumors have traditionally been referred to as ‘cell cannibalism’, and could form by several mechanisms, it is likely that at least in some tumors these cell structures form by entosis.
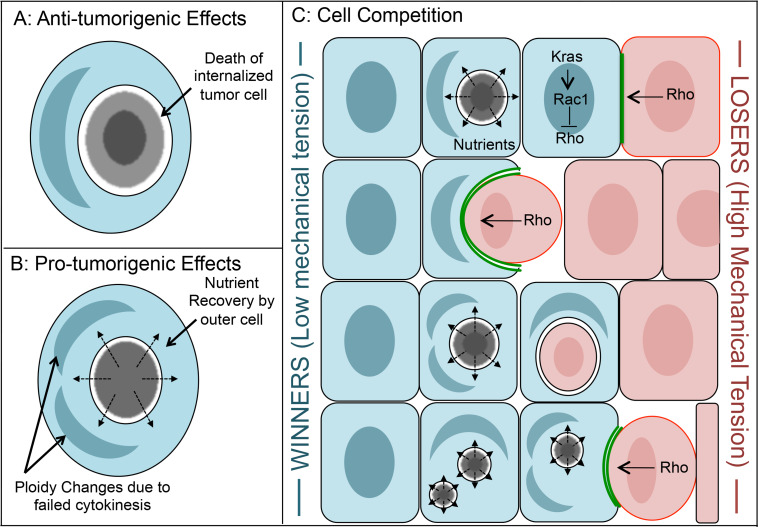
Entosis can limit the transformed growth of tumor cells cultured in soft agar, consistent with a potential role in tumor suppression. The treatment of entosis-competent breast tumor cells (MCF7) with an inhibitor of ROCKI/II (Y27632), significantly increases colony formation, and inhibits the appearance of entotic cell structures that are frequently observed under control conditions [47]. Remarkably, in soft agar entosis occurs between daughter cells arising from the first divisions that attempt to establish colony growth [47, 56]. The inhibition of entotic cell death by autophagy protein knockdown also increases the transformed growth of cells exhibiting high rates of entosis, suggesting that entosis may reduce transformed growth in part by inducing cell death (Fig. 2a) [13]. For breast tumor cells that do not express E-cadherin, the enforced expression of epithelial E- or P-cadherin is sufficient to induce entosis and entotic cell death in soft agar, and to suppress colony formation [56]. The treatment of such tumor cells with Y27632 blocks entosis and restores transformed growth, again consistent with a potential anti-tumorigenic role [56]. Given that entosis is triggered by matrix detachment, which also triggers the apoptotic program of anoikis that is evaded during metastasis [18], it is likely entosis acts as another tumor suppressive mechanism to prevent anchorage-independent growth (Fig. 2a).
Fig. 2.
Consequences of entosis. a Potential anti-tumorigenic effects of entosis involve the killing of internalized tumor cells. b Potential pro-tumorigenic effects of entosis involve the scavenging of nutrients by host cells, as well as ploidy changes that result from failed cytokinesis. c Model of entosis promoting competition between neighboring cells. Differences in deformability between cells can induce entotic cell engulfment in a population, as cells with high mechanical tension (losers, red) internalize into cells with lower mechanical tension (winners, blue) and undergo cell death. One pathway that can influence this competitive mechanism is the Kras pathway, where the activation of Rac1 by Kras reduces RhoA/ROCK-dependent actomyosin contractility and tension, rendering cells with Kras activation winners. Winner cells eliminate losers while becoming polyploid/aneuploid and also scavenging nutrients to support their own proliferation
But contrary to tumor suppression, entosis has also been shown to lead to the induction of aneuploidy, which is known to promote tumor progression [25, 27]. Entotic cells disrupt the division of engulfing cells by blocking the cleavage furrow, leading to frequent cytokinesis failure [25]. The resulting binucleate cells propagate gross aneuploidy [25], and are known to promote breast tumorigenesis [15], suggesting that while entosis leads to the death of engulfed cells, it may also promote the acquisition of tumorigenic properties by engulfing cells (Fig. 2b). Consistent with this, it was also recently reported that engulfing cells recover nutrients from digested entotic cells, and this form of nutrient scavenging can support cell proliferation and survival under conditions of starvation [26]. Therefore entosis could promote tumor progression by participating in multiple pro-tumor activities, by supporting the survival and proliferation of cells that also propagate gross aneuploidy (Fig. 2b). The reported correlations between homotypic cell-in-cell structures like those formed by entosis and high tumor grade [1, 25] as well as poor patient outcome [54] may indeed support the idea that this cell behavior promotes tumor progression.
Entosis in cancer: cell competition
How can these opposing potential roles of entosis in cancer be reconciled? It is predicted that the opposing influences of entosis could promote competition between neighboring cells, where engulfed cells (losers), die at the expense of engulfing cells (winners), that benefit by acquiring nutrients (Fig. 2c). That entosis is mediated by E-cadherin uniquely poises this mechanism to allow neighboring cells to compete, as the junctions that neighboring cells form can directly mediate engulfment events that kill losers. Conceivably, a difference in Rho-pathway or actomyosin activity between neighbors could manifest as the engulfment and death of losers after the establishment of cell junctions (Fig. 2c).
Consistent with this hypothesis, we recently reported that entosis promotes competition between cells that have compatible cadherin expression and a mechanical differential, where winners have lower mechanical tension, or are more deformable (consistent with lower actomyosin), than losers [22, 57]. The co-culture of winner and loser cells, combined with repetitive entosis induction, progressively leads to the elimination of losers in a ROCK-dependent manner [57]. Winner cells also engulf losers in vivo in mixed xenograft tumors [57]. These findings suggest that human tumors with high cell-to-cell variability in mechanical tension are primed to engage in competition between individual cells by this mechanism. Intriguingly, the mechanical deformability of tumor cells is known to increase during tumor progression [65]. Entosis could contribute to this selection by promoting a form of cell competition.
Mechanical heterogeneity in tumors may be caused by genetic heterogeneity in pathways that control actomyosin. We have found that one pathway that controls whether cells are winners or losers during entosis is the Kras pathway, which signals through the Rac1-GTPase to inhibit Rho activity and thereby reduce tension by lowering actomyosin contractility [57]. Activation of Kras by expression of a constitutive-active mutant, or activation of Rac1, is sufficient to render cells winners over controls [57]. Moreover, Rac1 expression is required for Kras-induced winner cell status, and the loser status of Rac1-knockdown cells can be reversed by knocking-down the expression of ROCK I/II, which reduces myosin contraction and inhibits entotic cell uptake [57]. These results have several important implications. First, they suggest that tumor cells, for example with activation of Kras, could engulf non-tumor cells, which, while not yet demonstrated in vivo, could play a role in tumor cell dissemination through a tissue. Second, these results demonstrate that Rac1-depleted cells can be rendered winners, or engulfers, by the simultaneous depletion of ROCK I/II. As Rac1 is a key mediator of phagocytosis by controlling actin dynamics in engulfing cells, these data further distinguish the entosis and phagocytosis mechanisms. Third, these findings highlight that oncogene expression can induce winner status during entosis by downregulating contractile myosin, which lowers the setpoint of mechanical tension. Therefore, while the entosis mechanism overall may resemble a cell invasion-like activity on the part of internalizing cells, the gain of function that can initiate this process can occur within engulfing cells, as a lower setpoint of actomyosin activity can induce the invasion of control cells with higher contractility. In this manner, oncogene-expressing cells can induce control cells to drive their own uptake (Fig. 2). Together these findings demonstrate that entosis can promote a form of cell competition, where genetically distinguished winner cells can engulf, kill, and benefit from the death of losers.
Competition between individual cells is an ancient strategy that drives selection and fitness within cell populations. The concept of ‘cell competition’ originally derives from Drosophila genetics, where it was first discovered that mosaic tissues in development exhibit distinct patterns of cell death, where cells of ‘loser’ genotype (first noted for ribosome gene deletion [41]), undergo death at the expense of cells with ‘winner’ genotype (in this case wild-type cells). Cells overexpressing certain oncogenes (e.g. Myc) actually recognize wild-type cells as losers, and these ‘supercompetitor’ cells overpopulate tissues at the expense of wild-type cells that undergo cell death [7, 42]. The individual cells in mammalian tissues may similarly compete, as Myc-overexpressing cells out-compete wild-type cells in embryonic stem cell cultures and in vivo in the tissues of chimeric mice [5, 58]. Interestingly cell engulfment was reported to be required for winner cells to kill losers during cell competition in mosaic tissues in Drosophila [29], and some engulfments involving apparently viable target cells were identified during cell competition in mammalian stem cell cultures [5]. But cell engulfment in these contexts is thought to primarily clear dead cells that have undergone apoptosis [36]. Whether an entosis-like mechanism, where engulfment causes cell death, could contribute to competition between cells within normal tissues as occurs in human tumors is an important topic for future study.
Physiological role of entosis during embryo implantation
For implantation of the mouse embryo, epithelial cells in the lumen of the uterus have to be eliminated to allow the blastocyst to implant by contacting the stroma underneath. It was shown recently that uterine epithelial cells undergo entosis into trophectoderm cells of the blastocyst, to allow for implantation to occur [30]. Cell-in-cell structures formed between uterine epithelial and trophectoderm cells were imaged in vivo during implantation, and implantation failed in animals that were inhibited for ROCK activity. Moreover, entotic engulfments with uterine epithelial cells internalized into trophectoderm cells occurred in mixed cultures, where immunostaining clearly localized the cell junction protein β-catenin at engulfment interfaces [30]. Interestingly the death of the internalized uterine epithelial cells in this context also did not involve the cleavage of caspase-3, suggesting that a non-apoptotic death mechanism resembling entotic cell death could also be occurring in this context. Together these data provide the first evidence that entosis may control a critical stage of mammalian development, embryo implantation.
Conclusion
Here we have discussed the molecular mechanisms controlling cannibalistic cell behavior through one particular program, entosis. We emphasize that while entosis is one mechanism whereby viable cells can find themselves being cannibalized, it is not the only one. It is important to keep in mind in this field that the term ‘cannibalism’, while used to describe a specific molecular mechanism in some reports, is more commonly used to indicate simply the appearance of viable engulfed cells in tissues or in culture. While the term ‘cannibalism’ itself is suggestive of an active engulfment, several distinct mechanisms like entosis and suicidal emperipolesis [2], where internalizing cells exert major control over their own uptake, also result in the formation of cell structures with identical appearance. Therefore mechanistic studies in contexts where viable cell engulfments are observed are needed before conclusions can be made about how such cells become cannibalized. For entosis, while we [47] and others [49] have described this process as a cell invasion-like activity, this form of cannibalism can also be controlled by engulfing cells with altered setpoints of mechanical tension. One clear example discussed herein is cells expressing oncogenic Kras or activated Rac1 that preferentially cannibalize neighboring cells with higher setpoints of mechanical tension, through entosis [57]. While internalizing cells may do a large part of the ‘work’ associated with these entotic events, clearly oncogene expression within host cells can directly influence the ability of these cells to act as the ‘engulfers’, or ‘cannibals’, by this mechanism.
In future studies of entosis it will be important to identify additional inducers of this mechanism. To date, the only clear inducer of entosis in cell populations is detachment of cells from extracellular matrix, which, while likely relevant to induction of this mechanism in some cancers [47], may not be the only inducer of this process. Imbalances in mechanical tension [60], or perhaps differences in cell size [30], between neighboring cells may also be involved in inducing entosis. Whether other stresses to cell populations in addition to matrix detachment could contribute to the manifestation of such heterogeneities within a population is an important topic for future studies. We note here that several examples of ancestral cannibalistic cell behavior share nutrient starvation as a common inducer, as also pointed out by others [11]. As one clear consequence of entosis is the scavenging of nutrients by engulfing cells [26], it will be of importance to determine if forms of nutrient deprivation can also induce this process.
Acknowledgments
This article was Funded by a Grant from the National Cancer Institute (R01CA154649, to M.O.).
References
- 1.Abodief WT, Dey P, Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology. 2006;17:304–305. doi: 10.1111/j.1365-2303.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 2.Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, Breen E, Allison AC, van Rooijen N, McGuffog C, Schlitt HJ, Bowen DG, McCaughan GW, Bertolino P. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci USA. 2011;108:16735–16740. doi: 10.1073/pnas.1112251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GC, Vilalta A, Fricker M. Phagoptosis—cell death by phagocytosis—plays central roles in physiology, host defense and pathology. Curr Mol Med. 2015;15(9):842–851. doi: 10.2174/156652401509151105130628. [DOI] [PubMed] [Google Scholar]
- 4.Cano CE, Sandi MJ, Hamidi T, Calvo EL, Turrini O, Bartholin L, Loncle C, Secq V, Garcia S, Lomberk G, Kroemer G, Urrutia R, Iovanna JL. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol Med. 2012;4:964–979. doi: 10.1002/emmm.201201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claveria C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- 6.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 7.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/S0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 8.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 9.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdos GW, Raper KB, Vogen LK. Mating types and macrocyst formation in Dictyostelium discoideum . Proc Natl Acad Sci USA. 1973;70:1828–1830. doi: 10.1073/pnas.70.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fais S, Fauvarque MO. TM9 and cannibalism: how to learn more about cancer by studying amoebae and invertebrates. Trends Mol Med. 2012;18:4–5. doi: 10.1016/j.molmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Florey O, Kim SE, Overholtzer M. Entosis: cell-in-cell formation that kills through entotic cell death. Curr Mol Med. 2015;15(9):861–866. doi: 10.2174/1566524015666151026100042. [DOI] [PubMed] [Google Scholar]
- 13.Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–1343. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012;22:374–380. doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Pastor JE. Cannibalism: a social behavior in sporulating Bacillus subtilis . FEMS Microbiol Rev. 2011;35:415–424. doi: 10.1111/j.1574-6976.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 18.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis–pathways to anchorage-independent growth in cancer. J Cell Sci. 2011;124:3189–3197. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 19.Hahn P, Lindsten T, Ying GS, Bennett J, Milam AH, Thompson CB, Dunaief JL. Proapoptotic bcl-2 family members, Bax and Bak, are essential for developmental photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2003;44:3598–3605. doi: 10.1167/iovs.02-1113. [DOI] [PubMed] [Google Scholar]
- 20.Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans . Nature. 2001;412:202–206. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Chen A, Wang T, Wang M, Ning X, He M, Hu Y, Yuan L, Li S, Wang Q, Liu H, Chen Z, Ren J, Sun Q. Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining. Oncotarget. 2015;6:20278–20287. doi: 10.18632/oncotarget.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Chen Z, Sun Q. Mammalian Cell Competitions. Curr Mol Med: Cell-in-cell Phenomena and their biomedical implications; 2015. [DOI] [PubMed] [Google Scholar]
- 23.Jacob DA, Ray T, Bengston CL, Lindsten T, Wu J, Thompson CB, Forger NG. The role of cell death in sexually dimorphic muscle development: male-specific muscles are retained in female bax/bak knockout mice. Dev Neurobiol. 2008;68:1303–1314. doi: 10.1002/dneu.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, Richardson AL, King RW, Cibas ES, Schnitt SJ, Brugge JS, Overholtzer M. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–330. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krajcovic M, Krishna S, Akkari L, Joyce JA, Overholtzer M. mTOR regulates phagosome and entotic vacuole fission. Mol Biol Cell. 2013;24:3736–3745. doi: 10.1091/mbc.E13-07-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krajcovic M, Overholtzer M. Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism. Cancer Res. 2012;72:1596–1601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11:358–365. doi: 10.1016/j.celrep.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsten T, Golden JA, Zong WX, Minarcik J, Harris MH, Thompson CB. The proapoptotic activities of bax and bak limit the size of the neural stem cell pool. J Neurosci. 2003;23:11112–11119. doi: 10.1523/JNEUROSCI.23-35-11112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/S1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Shoji-Kawata S, Sumpter RM, Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PG, Puyal J, Levine B. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lolo FN, Casas-Tinto S, Moreno E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell reports. 2012;2:526–539. doi: 10.1016/j.celrep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Lozupone F, Fais S. Cancer cell cannibalism: a primeval option to survive. Curr Mol Med. 2015;15(9):836–841. doi: 10.2174/1566524015666151026100916. [DOI] [PubMed] [Google Scholar]
- 38.Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini L, Malorni W, Fais S. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 39.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocarski ES, Guo H, Kaiser WJ. Necroptosis: the Trojan horse in cell autonomous antiviral host defense. Virology. 2015;479–480:160–166. doi: 10.1016/j.virol.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 42.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 43.Nelson C, Ambros V, Baehrecke EH. miR-14 regulates autophagy during developmental cell death by targeting ip3-kinase 2. Mol Cell. 2014;56:376–388. doi: 10.1016/j.molcel.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nizak C, Fitzhenry RJ, Kessin RH. Exploitation of other social amoebae by Dictyostelium caveatum . PLoS One. 2007;2:e212. doi: 10.1371/journal.pone.0000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Day DH, Keszei A. Signalling and sex in the social amoebozoans. Biol Rev Camb Philos Soc. 2012;87:313–329. doi: 10.1111/j.1469-185X.2011.00200.x. [DOI] [PubMed] [Google Scholar]
- 46.Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 47.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Overholtzer M, Wang X. Editorial: cell-in-cell: a century-old mystery comes to the table. Curr Mol Med. 2015;15(9):802–804. doi: 10.2174/1566524015999151026143028. [DOI] [PubMed] [Google Scholar]
- 49.Purvanov V, Holst M, Khan J, Baarlink C, Grosse R (2014) G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. Elife 3 [DOI] [PMC free article] [PubMed]
- 50.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in bak and bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 51.Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- 52.Rusconi JC, Hays R, Cagan RL. Programmed cell death and patterning in Drosophila. Cell Death Differ. 2000;7:1063–1070. doi: 10.1038/sj.cdd.4400767. [DOI] [PubMed] [Google Scholar]
- 53.Saga Y, Yanagisawa K. Macrocyst development in Dictyostelium discoideum. I. Induction of synchronous development by giant cells and biochemical analysis. J Cell Sci. 1982;55:341–352. doi: 10.1242/jcs.55.1.341. [DOI] [PubMed] [Google Scholar]
- 54.Schwegler M, Wirsing AM, Schenker HM, Ott L, Ries JM, Buttner-Herold M, Fietkau R, Putz F, Distel LV. Prognostic value of homotypic cell Internalization by nonprofessional phagocytic cancer cells. BioMed Res Int. 2015;2015:359392. doi: 10.1155/2015/359392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sierro F, Tay SS, Warren A, Le Couteur DG, McCaughan GW, Bowen DG, Bertolino P. Suicidal emperipolesis: a process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr Mol Med. 2015;15(9):819–827. doi: 10.2174/1566524015666151026102143. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q, Cibas ES, Huang H, Hodgson L, Overholtzer M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014;24:1288–1298. doi: 10.1038/cr.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, Robinson DN, Overholtzer M. Competition between human cells by entosis. Cell Res. 2014;24:1299–1310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villa del Campo C, Claveria C, Sierra R, Torres M. Cell competition promotes phenotypically silent cardiomyocyte replacement in the mammalian heart. Cell Rep. 2014;8:1741–1751. doi: 10.1016/j.celrep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Waddell DR, Duffy KT. Breakdown of self/nonself recognition in cannibalistic strains of the predatory slime mold, Dictyostelium caveatum . J Cell Biol. 1986;102:298–305. doi: 10.1083/jcb.102.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan Q, Liu J, Zheng Z, Zhu H, Chu X, Dong Z, Huang S, Du Q. Regulation of myosin activation during cell-cell contact formation by Par3-Lgl antagonism: entosis without matrix detachment. Mol Biol Cell. 2012;23:2076–2091. doi: 10.1091/mbc.E11-11-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Ning X, Chen A, Huang H, Ni C, Zhou C, Yu K, Lan S, Wang Q, Li S, Liu H, Wang X, Chen Z, Ma L, Sun Q. Impaired formation of homotypic cell-in-cell structures in human tumor cells lacking alpha-catenin expression. Sci Rep. 2015;5:12223. doi: 10.1038/srep12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, He MF, Chen YH, Wang MY, Yu XM, Bai J, Zhu HY, Wang YY, Zhao H, Mei Q, Nie J, Ma J, Wang JF, Wen Q, Ma L, Wang Y, Wang XN. Rapid reuptake of granzyme B leads to emperitosis: an apoptotic cell-in-cell death of immune killer cells inside tumor cells. Cell Death Dis. 2013;4:e856. doi: 10.1038/cddis.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X. Cell-in-cell phenomenon: a new Paradigm in life sciences. Curr Mol Med. 2015;15(9):810–818. doi: 10.2174/1566524015666151026095730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Wang X. Entosis and related forms of cell death within cells. Curr Mol Med. 2015;15(9):805–809. doi: 10.2174/1566524015666151026100339. [DOI] [PubMed] [Google Scholar]
- 65.Ward KA, Li WI, Zimmer S, Davis T. Viscoelastic properties of transformed cells: role in tumor cell progression and metastasis formation. Biorheology. 1991;28:301–313. doi: 10.3233/bir-1991-283-419. [DOI] [PubMed] [Google Scholar]