Abstract
Background
Binge co-consumption of highly caffeinated energy drinks with alcohol (ethanol) has become a common practice among adolescents/young adults and has been associated with an increased incidence of hazardous behaviors. Animal models are critical in advancing our understanding the neurobehavioral consequences of this form of binge drinking. Surprisingly, virtually no work has explored caffeine and ethanol co-consumption or its long-term consequences in adolescent animals. The primary objective of the current study was to extend a previously established mouse model of voluntary binge caffeine and ethanol co-consumption to explore adolescent consumption and responses compared to adults.
Methods
Adolescent and adult male C57BL/6J mice had daily limited access to caffeine (0.03% w/v), ethanol (20% (v/v), a combined ethanol/caffeine solution, or water for 14 days via the binge-like drinking paradigm, Drinking-in-the-Dark. Home cage locomotor activity was measured during DID in a subset of mice. Following DID, all mice rested for 18 days so that adolescents reached adulthood, whereupon all mice underwent 7 days of continuous access two-bottle choice drinking for 10% (v/v) ethanol or water.
Results
Co-consumption with caffeine significantly increased ethanol intake and resultant blood ethanol concentrations in both adolescent and adult mice. In addition, adolescent mice exhibited a uniquely robust locomotor stimulant response to caffeine and ethanol co-consumption. Later ethanol intake and preference was not influenced, however, by prior fluid consumption history via DID.
Conclusion
Together with findings from the human literature, our results suggest that caffeine co-consumption may positively influence binge alcohol consumption in adolescents/young adults. Importantly, this age group may be particularly sensitive to the additive stimulant effects of caffeinated alcohol consumption, an effect which may be related to the high incidence of associated negative outcomes in this population. These observations are particularly concerning considering the heightened plasticity of the adolescent brain.
INTRODUCTION
A troubling trend in binge alcohol (ethanol) drinking has recently emerged with the common practice of consuming alcohol and highly caffeinated ‘energy drink’ mixed beverages. In response to numerous incidents concerning alcohol poisoning and physical harm associated with these beverages, the Food and Drug Administration issued a notice to companies producing pre-mixed caffeinated alcoholic beverages in 2010, essentially requiring them to remove these beverages from the market. However, both standard energy drinks (without alcohol) and liquor are widely available for individuals to mix, themselves. Therefore, this has not been a very effective regulatory measure. Alcohol-energy drink co-consumption has been associated with an increased risk for hazardous behavior such as drinking to the point of alcohol poisoning (CDC, 2012), driving under the influence (O’Brien et al., 2013; O’Brien et al., 2008; Thombs et al., 2010), sexual promiscuity (O’Brien et al., 2013), and aggression (Jones et al., 2012). Energy drinks contain many ingredients (e.g. sugars, amino acids, etc.), however caffeine is largely considered to be the primary psychoactive ingredient (Attwood et al., 2012; Giles et al., 2012; Peacock et al., 2013b).
A number of human laboratory studies have observed that caffeine/energy drinks can reduce motor and cognitive impairment induced by alcohol (Drake et al., 2003; Heinz et al., 2013) and may increase subjective stimulation (Ferreira et al., 2004a; Marczinski et al., 2012; Peacock et al., 2013a); effects which may be related to the associated harms noted above. However, it should be noted that these findings have not been consistently observed (Marczinski and Fillmore, 2006; Verster et al., 2012). These studies explored the consequences of acute caffeinated alcohol intoxication, which are becoming increasingly well documented, however the more protracted consequences of this type of consumption are not known as the practice of mixing energy drinks and alcohol is relatively new. As such, the potential public health impact of binge caffeine and alcohol co-consumption has likely not been fully realized and it is critical that we advance our understanding of this drug combination. Perhaps most importantly, the majority of these studies have been conducted with adolescents/young adults, highlighting that this age group may be at particularly high risk for consequences arising from caffeinated alcohol consumption.
Preclinical studies in animals are vital to advance our understanding of neurobehavioral consequences of caffeinated alcohol consumption/intoxication. Rodent studies evaluating the effects of caffeine on alcohol intake have produced mixed findings. Systemic injections or intragastric infusions of caffeine prior to alcohol access increase alcohol intake at lower doses (5 mg/kg) and decrease intake at higher doses (10-20 mg/kg) (Kunin et al., 2000; Rezvani et al., 2013). When caffeine is added to an alcohol solution in voluntary drinking paradigms, however, caffeine has been observed to either have no effect on alcohol intake in mice (Fritz et al., 2014) or increase intake in female selectively bred alcohol preferring rats (Franklin et al., 2013). Perhaps the clearest findings thus far demonstrate that caffeine can significantly influence motor aspects of alcohol intoxication (Dar, 1988; El Yacoubi et al., 2003; Fritz et al., 2014; Hilbert et al., 2013; Kuribara et al., 1992). Some of these effects have also been observed with alcohol and energy drink combinations in mice (Ferreira et al., 2013; Ferreira et al., 2004b). For a mechanistic review of these effects, the interested reader is referred to López-Cruz et al. (2013). Furthermore, it was recently shown that repeated exposure to combined caffeine and alcohol produced a greater locomotor sensitization response than alcohol or caffeine alone (May et al., 2015). This suggests that the combination may more potently recruit neurobehavioral plasticity. Surprisingly, virtually all studies mentioned above were conducted with adult animals. Because the majority of concerning observations in human studies are seen in young adults and adolescents, increased research with adolescent animals is needed.
Recently, our lab added caffeine (at a concentration similar to standard energy drinks such as Red Bull©) to the 20% alcohol solution used in a mouse model of binge-like alcohol consumption, Drinking-in-the-Dark (DID). A markedly-altered intoxication state, appearing to mirror some of the key observations of human studies, was observed (Fritz et al., 2014). Consumption of the caffeinated alcohol solution completely reversed alcohol-induced sedation and antagonized ataxia while producing significant motor stimulation. However, alcohol-induced anxiolysis and impairment in recognition memory was unaltered by caffeine. These observations bolstered enthusiasm for a translationally-valid binge co-consumption animal model. This model allowed for a straightforward assessment of whether adolescent mice binge consume caffeinated alcohol solutions differently than adult animals and whether the motor response associated with caffeinated alcohol consumption is unique in adolescent mice. Furthermore, the model allowed for an evaluation of whether binge consumption of alcohol, caffeine, or a combined solution during adolescence influenced later 2-bottle choice alcohol intake in adulthood.
METHODS
Subjects
Adolescent (PND 21 ± 3) and adult (PND 56 ± 3) male C57BL/6J (B6) mice were ordered from the Jackson Laboratory (Bar Harbor, ME) and singly housed upon arrival. Mice were allowed to acclimate to the facility for 7 days prior to testing and were maintained on a 12-hour reverse light/dark cycle with lights OFF at 0800. Temperature and humidity were held constant near 20° C and 50%, respectively. Food and water were available ad libitum. Principles of laboratory animal care were followed and experiments were performed under a protocol approved by the IUPUI School of Science Institutional Animal Care and Use Committee.
Drinking Solutions
Ethanol (190 proof; Pharmco Inc., Brookfield, CT) was diluted to 20% (v/v) in tap water for the DID phase of the study and diluted to 10% (v/v) for the two-bottle choice phase. Caffeine (Sigma-Aldrich) was dissolved in tap water (0.03% w/v). The 0.03% concentration is roughly equivalent to that of standard energy drinks (e.g. Red Bull©) and was a concentration successfully used in our previously published work in this area (Fritz et al., 2014). For the combined ethanol and caffeine solution, 20% ethanol was mixed with caffeine at the 0.03% concentration.
Drinking-in-the-Dark
DID has been consistently validated as a binge-like drinking model as B6 mice will readily consume ethanol via DID to the point of behavioral intoxication and reliably reach blood ethanol concentrations in excess of 80 mg/dl in a short period of 2-4 hrs (Fritz et al., 2014; Linsenbardt et al., 2011; Rhodes et al., 2005; Rhodes et al., 2007). For detailed procedures concerning DID, please see Thiele et al. (2014). Beginning 3 hrs into the dark cycle (1100 hr), mice had their standard water bottles removed and replaced by a 10 ml tube fitted with a stainless steel double ball bearing sipper. These tubes were left on the cage for 2 hrs and then removed. The assigned test fluid was the only fluid available during the 2 hr DID period.
Home Cage Locomotor Activity
Details concerning the exact monitors (Columbus Instruments Inc., Columbus, OH) used in this study were previously published (Linsenbardt and Boehm, 2012). Briefly, a subset of animals had their cages placed in the home cage monitors where they remained for the entire experiment. These monitors consist of metal bars with photocell beams that surround the cage and penetrate the cage’s wall. The interruption of intersecting beams records the animal’s position and the provided software translates these data into ambulatory activity. Home cage activity was recorded during each 2-4hr DID session.
Two-Bottle Choice Drinking
During this phase, mice had continuous, concurrent access to 10% ethanol (v/v) and water for 7 days. These fluids were presented in tubes identical to those used in DID. Volumes were measured daily and solutions were replaced 1 hr before lights out.
Procedure
Adolescent (n = 35; PND 28± 3 at initiation of study) and adult (n = 40; PND 63 ± 3 at initiation of study) mice underwent a 14 day DID procedure. Mice were assigned tap water (W), 20% ethanol in tap water (E), 0.03% caffeine in tap water (C), or combined ethanol and caffeine (EC) as their available fluid in DID. In a subset of these mice (n = 40), home cage activity was recorded each day while mice drank their assigned fluid during the DID session to evaluate motor responses to the consumption of each fluid. On days 7 and 14, the access period was extended from 2 to 4 hrs in the event that extension of the drinking session would elucidate group differences. Home cage activity monitoring was also extended to 4 hr on these days. Both the 2 and 4 hr durations are commonly used in DID and consistently produce binge-like ethanol intake in B6 mice (Thiele et al., 2014). Immediately following DID on day 14, periorbital blood samples were collected from all mice for analysis of BEC by an Analox AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). Blood samples were taken from W and C mice to control for the effects blood sampling and stress might have on later assessment of ethanol intake. Only samples from E and EC mice were analyzed. Following the final day of DID, all mice were allowed to rest for 18 days so that the adolescent group reached PND 60 ± 3, approximately the commonly defined age of adulthood in mice (Spear, 2000). At this point the ‘adult’ group was PND 95 ± 3. After this rest period, all mice underwent a continuous access, 7-day two-bottle choice drinking procedure for water and 10% ethanol. We elected to evaluate two-bottle choice drinking to determine whether these previous drinking histories in DID influenced later alcohol consumption and preference in a continuous access model; offering insight on whether the age at which the prior DID exposure occurred influenced preference for alcohol consumption potential when it is constantly available in adulthood.
Statistical Analysis
Data were analyzed by Analysis of Variance (ANOVA) with ‘fluid’ and ‘age’ as factors. ‘Fluid’ refers to the assigned DID fluid and ‘age’ refers to the age at which DID testing was initiated (adolescence or adulthood). Repeated measures ANOVAs also included day as the within-subjects factor. Analyses of fluid consumption data include all mice (both those housed in the home cage monitoring system and those that were not). The significance level was set at p < 0.05 and Newman-Keuls post-hoc statistics were run where applicable. All analyses were carried out with Statistica 7 software (Statsoft).
RESULTS
DID Fluid Consumption
The weights of adolescent and adult mice throughout the 14-day DID procedure are presented in Figure 1A. A repeated measures ANOVA found a main effect of age [F(1,73) = 207.5, p < 0.001] with adolescent mice weighing significantly less than adults and a significant age × day interaction [F(13,949) = 25.2, p < 0.001] indicated that adolescent mice increased in weight over days. Prior to the initiation of the DID phase of the experiment, mice were counterbalanced for weight among the 4 fluid assignments within each age group (all p’s > 0.15). In addition, it was determined that mice housed in the home cage activity monitoring system expressed statistically similar fluid intake (p’s > 0.05), therefore all analyses of fluid intake data represent all mice in the study. An overall repeated measures analysis of total fluid intake was conducted for days 1-6 and 8-13, collectively. Because day 7 and day 14 were 4hr probe sessions, these days were analyzed separately. The repeated measures analysis of 2-hr DID data found main effects of age [F(1,67) = 14.3, p < 0.001] and fluid [F(3,67) = 5.3, p < 0.01] with adolescent mice consuming more total fluid per unit weight than adults (p < 0.001) and C and EC mice consuming more fluid than W mice (p’s < 0.01; Figures 1B,C). A main effect of day [F(11,737) = 3.9, p < 0.001] also indicate that mice generally increased their fluid intake over days. The analysis of fluid intake during the first 4hr probe on day 7 fluid intake also found main effects of age [F(1,67) = 8.9, p < 0.01] and fluid [F(3,67) = 5.4, p < 0.01] with adolescent mice consuming more fluid than adults and C mice consuming more fluid than all other groups (p’s < 0.05; Figures 1D,E). Adolescents again consumed more fluid than adults on day 14 [F(1,67) = 10.0, p < 0.01], however C mice consumed significantly more fluid [F(3,67) = 3.9, p < 0.05] than only W mice (p < 0.05).
Figure 1. Fluid intake (ml/kg) throughout the 14-day DID phase of the experiment.
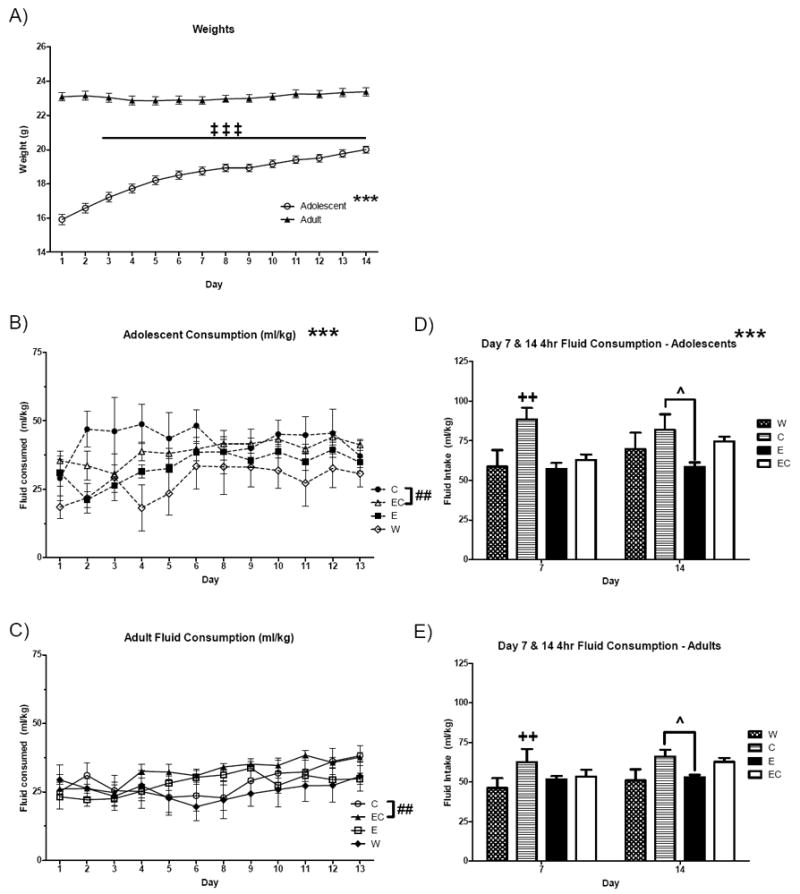
A) Weights of all adolescent (n = 35-40) and adult mice throughout 14 days of DID. B) Adolescent mice (n = 8-9) consumed more total fluid than C) adult mice (n = 10). D,E) Adolescent mice consumed more fluid than adults on both days 7 and 14 and C mice also consumed the most fluid on both days. p‡‡‡ < 0.001 Dunnett’s versus Day 1; p*** < 0.001 versus adults; p## < 0.01 versus W and E (main effect of fluid collapsed on Age); ++p < 0.01 versus all other fluids on day 7 (main effect of fluid collapsed on Age); ˆp < 0.05 versus E on day 14 (main effect of fluid collapsed on Age).
Caffeine and ethanol intake, specifically, were also analyzed between fluid groups over all 2hr DID sessions (days 1-6 and 8-13) as well as specifically for 4hr sessions on days 7 and 14. Adolescent mice were found to consume significantly higher doses of caffeine than adult mice throughout the 2hr DID sessions [F(1,33) = 26.4, p < 0.001] and an age × fluid interaction trending toward significance [F(1,33) = 3.5, p = 0.071] suggested adolescent C mice may have consumed slightly more caffeine than adult C mice (Figure 2A). In addition, mice were found to increase their caffeine intake over days [F(11,396) = 2.2, p < 0.05]. For ethanol intake during 2hr DID sessions, adolescent mice were also found to consume significantly higher doses of ethanol than adults [F(1,34) = 20.0, p < 0.001]. EC mice also generally consumed more ethanol than did E mice [F(1,34) = 12.6, p = 0.001] (Figure 2B). Again, a main effect of day indicated a general increase in ethanol intake across sessions [F(11,374) = 12.1, p < 0.001].
Figure 2. Ethanol and caffeine intake throughout the 14-day DID procedure.
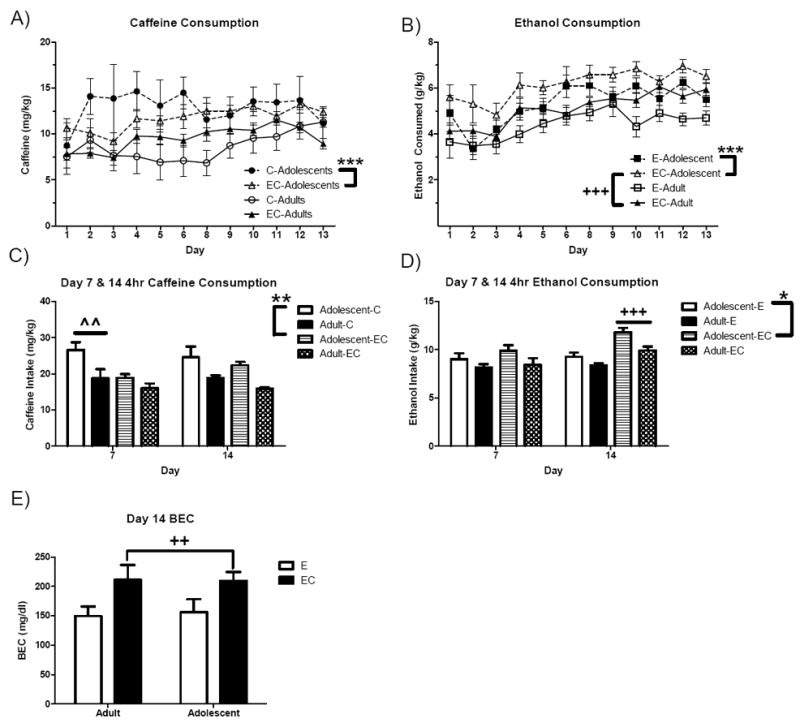
A) Caffeine and B) ethanol intake of adolescent and adult mice throughout the 2hr sessions of DID (n = 8-10). C) Caffeine and D) ethanol consumption during the 4-hr probes on days 7 and 14. E) Blood ethanol concentrations (BECs) of E and EC mice following DID drinking on Day 14. p* < 0.05, p** < 0.01, p*** < 0.001 versus adults; pˆˆ < 0.01 versus EC (main effect of fluid collapsed on Age); p++ < 0.01, p+++ < 0.001 versus E (main effect of fluid collapsed on Age).
As planned, caffeine and ethanol intake were also separately analyzed for days 7 and 14 to determine whether these 4-hr probes elicited any group differences not apparent in the standard 2-hr DID sessions. On day 7, adolescent [F(1,33) = 8.0, p < 0.01] and C mice [F(1,33) = 7.8, p < 0.01] consumed significantly higher doses of caffeine than adult and EC mice, respectively (Figure 2C). On day 14, adolescent mice again consumed more caffeine than adults [F(1,33) = 7.1, p < 0.05], however caffeine intake was equivalent between C and EC fluid conditions (p = 0.323). A significant age effect on ethanol intake during the probe on day 7 was found [F(1,34) = 4.1, p = 0.05] with adolescents consuming higher ethanol doses than adults (Figure 2D). Ethanol intake was statistically equivalent between fluid groups (p = 0.32). On day 14, adolescent mice again consumed significantly higher doses of ethanol than adults [F(1,34) = 12.3, p = 0.001], however this 4-hr probe found that EC mice of both age groups consumed significantly higher doses of ethanol than their respective E counterparts [F(1,34) = 27.0, p < 0.001]. The BEC analysis of samples collected after DID on day 14 revealed that EC mice reached significantly higher BECs than E mice [F(1,34) = 8.1, p < 0.01; Figure 2E], however there was no effect of age (p = 0.906) and no significant interaction (p = 0.842).
Home Cage Locomotor Activity During DID
An overall analysis of concurrent home cage activity throughout 2hr DID determined that fluid was a significant factor [F(1,32) = 15.7, p < 0.001], with EC and C mice being significantly more active than E and W groups (p’s < 0.001). A significant fluid × day interaction also indicated that this group separation occurred on days 9-13 [F(33,352) = 3.7, p < 0.001]. No main effect of age on home cage locomotor activity was found (p = 0.407), however, a significant age × day interaction [F(11,352) = 3.5, p < 0.001] indicated that adolescent mice generally registered fewer beam breaks than adults during DID on days 1-2 (p’s < 0.05). This observation may be due to the fact that adolescent mice were physically smaller than adults (Figure 1A), and as a result, their relative level of activity as recorded by the photocell beams of the monitors may be reflected differently than adults. In light of this, comparisons between the adult and adolescent groups may not accurately reflect fluid group differences. We therefore analyzed the effect of fluid on home cage locomotion within each age group separately.
Within adolescent mice, a main effect of fluid [F(3,16) = 10.5, p < 0.001; Figure 3A] indicated that EC mice were significantly more active during 2hr DID sessions than all other groups (p’s < 0.05). In addition, a statistical trend (p = 0.06) suggested C mice were more active than W and E mice. A significant fluid × day interaction [F(33,176) = 4.8, p < 0.001] indicated that these group differences emerged on days 9-13 (p’s < 0.05). Within adult mice, a main effect of fluid [F(3,16) = 7.0, p < 0.01; Figure 3C] indicated that C and EC mice were also significantly more active during DID than W and E mice (p’s < 0.05). However, C and EC adult mice did not differ in overall DID activity (p = 0.821).
Figure 3. Home cage locomotor activity during DID.
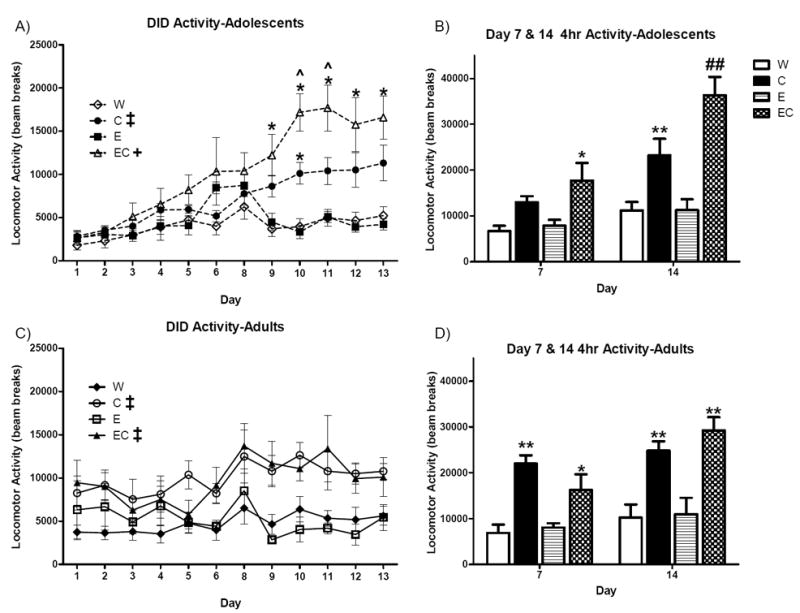
Activity was recorded during 2hr DID sessions for both A) adolescent and C) adult mice. Activity was also recorded during the 4-hr probe on days 7 and 14 B,D). p‡ < 0.05 main effect of fluid versus W and E; p+ < 0.05 main effect of fluid versus all other fluids; p* < 0.05, p < 0.01, p*** < 0.001 post-hocs versus W and E; pˆ < 0.05 post-hoc versus C; p## < 0.01 post-hoc versus all other fluids. n = 5.
Specific analyses of home cage activity were also conducted for the four hour probes on day 7 and day 14. Within adolescent mice, a main effect of fluid on day 7 [F(3,16) = 5.3, p < 0.05 Figure 3B] indicated that EC mice were significantly more active than W and E mice (p’s < 0.05). A main effect of fluid on day 14 [F(3,16) = 15.0, p < 0.001] demonstrated that EC and C mice were significantly more active than W and E mice (p’s < 0.01). In addition, EC mice were significantly more active than C mice (p < 0.01). In adults, EC and C mice were significantly more active than E and W mice on both day 7 [F(3,16) = 10.5, p < 0.001; Figure 3D] and day 14 [F(3,16) = 11.0, p < 0.001] (post-hoc p’s < 0.01). The home cage locomotion of adult EC and C mice did not significantly differ during either 4-hr probe (p’s > 0.05). Drinking and BEC data on days 7 and 14, specifically, from the subset of mice that were housed in the home cage monitoring system are presented in Table 1. As can be seen, these values reflect the overall group means and overall trends of the overall group data.
Table 1.
Caffeine, ethanol intake, and BEC on Day 7 & 14 of mice housed in the locomotor activity monitors.
| Day 7 Caffeine Intake (mg/kg) | Day 14 Caffeine Intake (mg/kg) | Day 7 Ethanol Intake (g/kg) | Day 14 Ethanol Intake (g/kg) | Day 14 BEC (mg/dl) | |
|---|---|---|---|---|---|
| Adolescents | C: 27.9 ± 3.4 | C: 21.0 ± 3.9 | E: 8.5 ± 1.0 | E: 8.9 ± 0.7 | E: 186.4 ± 27.7 |
| EC: 18.9 ± 1.9 | EC: 22.2 ± 1.2 | EC: 10.0 ± 1.0 | EC: 11.7 ± 0.6 | EC: 240.4 ± 12.7 | |
| Adults | C: 20.0 ± 3.4 | C: 20.6 ± 1.7 | E: 8.6 ± 0.2 | E: 8.3 ± 0.4 | E: 162.8 ± 24.3 |
| EC: 12.6 ± 0.5 | EC: 18.3 ± 1.1 | EC: 6.6 ± 0.3 | EC: 9.6 ± 0.6 | EC: 216.4 ± 30.2 |
Two-Bottle Choice Ethanol Consumption
Following DID, mice were left undisturbed for 18 days, allowing the adolescent mice to mature into adulthood. After this rest period, all mice underwent continuous two-bottle choice drinking for ethanol and water for 7 days. A repeated measures ANOVA found a main effect of age [F(1,67) = 12.0, p < 0.001; Figures 4A,C] on ethanol intake with post hoc testing revealing that mice initiating testing in adolescence consumed higher doses of ethanol across the two-bottle choice phase (p < 0.01). This finding also held consistent with ethanol preference across two-bottle choice with a main effect of age [F(1,67) = 5.8, p < 0.05; Figures; 4B,D] indicating the mice previously tested as adolescents generally demonstrated greater ethanol preference. Previous fluid exposure was not a significant factor in either of these analyses (p’s > 0.2).
Figure 4. Two-bottle choice assessment of ethanol intake and preference 18 days following DID.
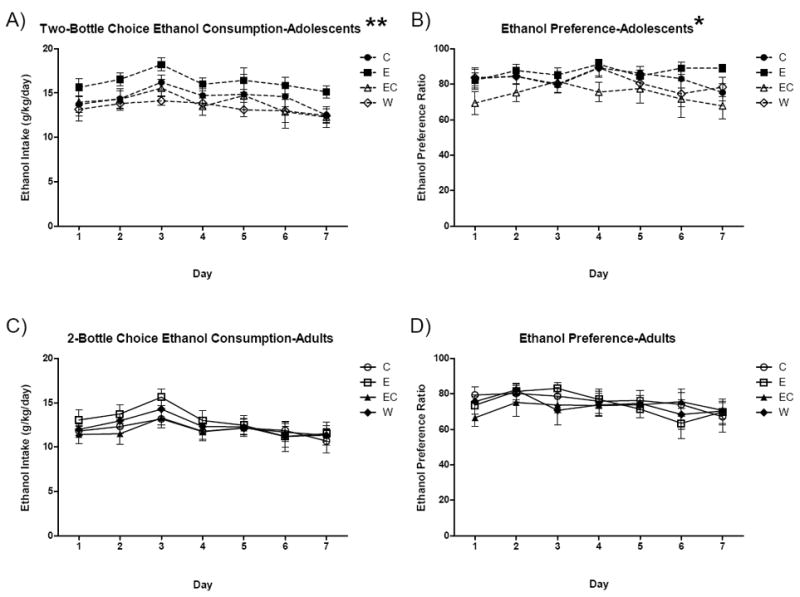
Ethanol intake and preference in A,B) mice that matured from adolescence to adulthood and C,D) mice previously tested in DID as adults. p* < 0.05, p** < 0.01 versus adults. n = 8-10.
DISCUSSION
The present study demonstrated that concomitant binge caffeine and ethanol consumption in DID can increase ethanol intake and resultant BECs in both adolescent and adult B6 mice. Furthermore, adolescent, but not adult mice exhibit additive stimulation produced by combined binge caffeine and ethanol co-consumption. Finally, the previous fluid consumption histories during DID consumption did not influence later continuous access two-bottle choice ethanol drinking or preference. Thus, adolescent mice may be particularly sensitive to the additive stimulant effect of combined caffeine and ethanol, although this response does not appear to be related to later ethanol consumption in adulthood (at least as assessed in a 2-bottle choice preference procedure).
Although water intake and home cage locomotion has been extensively analyzed in the DID model (Linsenbardt and Boehm, 2014), this is the first study, to our knowledge, to report concurrently assessed repeated water consumption in adolescent and adult B6 mice via DID. In our lab, we have observed that the volume of water consumed is either greater than (Linsenbardt and Boehm, 2012, 2014; Linsenbardt et al., 2011) or equivalent to that of ethanol consumed in DID (Fritz et al., 2014; current study) and the intake observed in the current study is very similar to that previously reported (~25-35 ml/kg). The observed main effect of age in the current study suggests that adolescents consumed more fluid in DID in general, however the fluid volume of water and ethanol consumed was quite similar within both adolescent and adult animals. Although adolescents consumed more fluid in DID, it appears that this is driven by particularly high consumption of caffeine, ethanol, and/or their combination in adolescent animals (Figure 1B-E). It should also be noted that a previous study in our lab found that adolescent and adult B6 mice did not differ in water intake during the circadian period at which DID was assessed (Moore et al., 2011), suggesting that the observed differences in caffeine and ethanol consumption between groups are likely not due to generally increased fluid intake.
The observation that adolescent mice generally consumed more ethanol is not surprising (Figures 2B,D). Numerous studies, including one conducted in our lab, have demonstrated increased ethanol intake in adolescent B6 mice (Holstein et al., 2011; Moore et al., 2010; Strong et al., 2010). In light of the generally greater fluid intake, higher caffeine intake in adolescent mice is also not particularly surprising. It should be noted, however, that this higher ethanol consumption in adolescent mice was not apparent in BEC measurements taken following DID on day 14 (Figure 2E). We have previously observed this in our lab (Moore et al., 2010) and we believe that the previously demonstrated increased metabolic rate of adolescent B6 mice (Linsenbardt et al., 2009) likely accounts for similar BECs between age groups. Given that ethanol consumption typically occurs rapidly in the DID paradigm (Linsenbardt and Boehm, 2014), it is still possible that the peak BECs of adolescent mice were indeed higher than those of adults.
Prior studies evaluating whether caffeine influences ethanol intake in rodents, however, have observed very different results with two showing increases in ethanol intake (Franklin et al., 2013; Kunin et al., 2000; current study), one showing a decrease in intake (Rezvani et al., 2013), and two others showing no effect (Fritz et al., 2014; Hughes, 2011). These studies have many procedural differences that likely influence these discordant findings including species (rat or mouse), route of caffeine administration (injection versus oral co-consumption with ethanol), and the ethanol drinking paradigm (two-bottle choice drinking, forced continuous access, or binge-like drinking). As we had previously observed no effect of caffeine on ethanol intake in a similar study with adult B6 mice when both compounds were co-consumed (Fritz et al., 2014), it is not clear why caffeine increased ethanol intake in adult mice in the current study. One potential reason is that mice were moved into the behavioral testing room prior to DID drinking in the previous study, whereas mice remained undisturbed in their colony room in the present study. Perhaps moving cages interfered with these group differences in adult mice in the former study. Additionally, it is possible that the addition of the extended 4-hr DID session on day 7 increased the degree of caffeine and ethanol co-exposure which later contributed to the increase in later 2hr DID intake. Nevertheless, both adolescent and adult mice increased their ethanol intake when the solution contained caffeine and BECs were similarly increased. As previously mentioned, the lack of BEC differences between adolescent and adult animals does not necessarily negate the possibility that peak BECs were indeed elevated and adolescent EC mice could have reached very high peak BECs. To our knowledge this is the first published demonstration of increased ethanol consumption via voluntary co-consumption with caffeine in mice as well as the first demonstration in adolescent animals. Collectively, these results demonstrate that binge co-consumption of caffeine with ethanol may further increase the binge-like ethanol intake of already high-consuming adolescent mice.
Perhaps more interestingly, co-consumption produced a uniquely robust motor response in adolescent mice (Figures 3A,B). As expected on the basis of our prior research (Fritz et al., 2014), caffeine consumption elicited locomotor stimulation in all mice. In adult mice, the ethanol and caffeine combination was no more effective in stimulating locomotion than caffeine alone. In adolescents however, the addition of caffeine to the ethanol solution augmented stimulation above caffeine consumption alone. What is most striking about this is that adolescent C and EC mice consumed statistically equivalent doses of caffeine. In addition, even though caffeine-consumption increased ethanol intake in both age groups, the increase in stimulation was only seen in adolescent mice. Therefore, this particularly robust stimulant response in adolescent EC mice likely cannot simply be explained by greater ethanol intake/BECs. Together, these observations indicate that adolescent mice may be uniquely sensitive to additive motor stimulation produced by binge caffeine and ethanol co-consumption. The findings with adult mice are consistent with our previous work, demonstrating that caffeine and caffeinated ethanol consumption produce equivalent stimulation (Fritz et al., 2014). A number of prior studies also evaluating the locomotor stimulant properties of caffeine and ethanol combinations have also observed additive stimulation (Hilbert et al., 2013; Kuribara et al., 1992; May et al., 2015), however these studies were conducted with adult animals and the route of administration was either intraperitoneal injection or intragastric infusion. As stressors have been shown to sensitize the stimulant response to ethanol and psychostimulants (Phillips et al., 1997; Robinson, 1988), it is possible that these modes of drug administration contributed to these observations in adult mice. To our knowledge, this is the first demonstration of a unique adolescent response to ethanol and caffeine co-exposure and the first demonstration of additive caffeine and ethanol stimulation induced by voluntary consumption in animals.
Finally, we assessed whether caffeine and ethanol drinking histories via DID during adolescence influenced later ethanol consumption and/or preference. Previous reports have demonstrated that adolescent ethanol consumption can increase ethanol consumption in adulthood in the DID model as well as 2-bottle choice drinking (Holstein et al., 2011; Moore et al., 2010; Strong et al., 2010). We observed that the specific fluid that mice were assigned to in DID had no bearing on their later 2-bottle choice intake or preference in the current study (Figure 4). It is unclear, however, whether later binge drinking via DID could have been influenced by age and/or fluid type of DID pre-exposure. Two-bottle choice drinking does not reliably produce ethanol consumption resulting in pharmacologically-relevant BECs in B6 mice (Dole and Gentry, 1984; Matson and Grahame, 2013) and binge drinking via DID may be a more relevant assessment of later intake. We did, however, observe that mice that initiated testing in adolescence, in general, consumed more ethanol and demonstrated significantly higher ethanol preference in adulthood. The reason for this is not clear. Speculating about why this may have been observed, the initial exposure to the modified drinking tubes used in both paradigms during adolescence could have produced greater habituation to the procedures, resulting in greater consumption. Alternatively, these mice were singly housed upon arrival and spent their entire adolescent period in isolated housing. A number of studies have previously shown that isolated housing conditions in adolescence can increase alcohol intake (Hall et al., 1998; Parker and Radow, 1974; Schenk et al., 1990). However, this has not always been observed (Doremus et al., 2005). These observations heavily depend on when an animal is isolated during development and whether the housing condition is acute or chronic. For a review on the effects of stressors on alcohol consumption in preclinical studies, the interested reader is referred to Becker et al. (2011). As adolescence is a crucial developmental period, chronic isolated housing during this time may have increased the ethanol intake of these mice in adulthood which is in line with this body of previous work.
It is also important to acknowledge that isolation, or even shipping stress, may have influenced the observed differences in ethanol and/or caffeine motor effects. As previously mentioned, stressors have been demonstrated to sensitize the stimulant response to drugs of abuse in rodents (Robinson, 1988). Adolescence is a developmental period that is particularly sensitive to the effects of stressors (Burke and Miczek, 2013) and therefore, shipping or isolation stress may have influenced the sensitivity of these mice to ethanol and/or caffeine. These data should therefore be interpreted with this caveat in mind.
Together with findings from the human literature, our results suggest that caffeine co-consumption with ethanol may positively influence binge alcohol consumption in adolescents/young adults. Importantly, this age group may be particularly sensitive to the additive stimulant effects of caffeinated alcohol consumption, an effect which may be related to the high incidence of associated negative outcomes in this population. These observations are particularly concerning considering the heightened plasticity of the adolescent brain (Spear, 2000; Spear and Varlinskaya, 2005). As this form of binge drinking is popular and unregulated, more research into its protracted consequences is needed.
Acknowledgments
This work was supported by NIAAA grants AA016789 (S. B.) and AA07462 (B.F. & C.R.K).
Footnotes
The authors have no conflicts of interest to declare.
References
- Attwood A, Rogers P, Ataya A, Adams S, Munafò M. Effects of caffeine on alcohol-related changes in behavioural control and perceived intoxication in light caffeine consumers. Psychopharmacology. 2012;221:551–560. doi: 10.1007/s00213-011-2601-0. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models:Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2013;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS. The biphasic effects of centrally and peripherally administered caffeine on ethanol-induced motor incoordination in mice. Journal of Pharmacy and Pharmacology. 1988;40:482–487. doi: 10.1111/j.2042-7158.1988.tb05282.x. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proceedings of the National Academy of Sciences. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Turner L, Scofield HM, Roth T. Caffeine reversal of ethanol effects on the multiple sleep latency test, memory, and psychomotor performance. Neuropsychopharmacology. 2003;28:371–378. doi: 10.1038/sj.npp.1300026. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;45:977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, Abrahao KP, Souza-Formigoni MLO. Expression of behavioral sensitization to ethanol is increased by energy drink administration. Pharmacology Biochemistry and Behavior. 2013;110:245–248. doi: 10.1016/j.pbb.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Formigoni ML. Can energy drinks affect the effects of alcoholic beverages? A study with users. Rec Assoc Med Bras. 2004a;50:48–51. doi: 10.1590/s0104-42302004000100034. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, Hartmann Quadros IM, Trindade ÁA, Takahashi S, Koyama RG, Souza-Formigoni MLO. Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiology & Behavior. 2004b;82:841–847. doi: 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Bell RL, Engleman EA. Caffeinated Alcoholic Beverages – An Emerging Trend in Alcohol Abuse. Journal of addiction research & therapy. 2013;(Suppl 4):S4–012. doi: 10.4172/2155-6105.S4-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Companion M, Boehm SL. “Wired,” Yet Intoxicated: Modeling Binge Caffeine and Alcohol Co-Consumption in the Mouse. Alcoholism: Clinical and Experimental Research. 2014;38:2269–2278. doi: 10.1111/acer.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles GE, Mahoney CR, Brunyé TT, Gardony A, Taylor HA, Kanarek RB. Differential cognitive effects of energy drink ingredients: caffeine, taurine, and glucose. Pharmacology Biochemistry and Behavior. 2012;102:569–577. doi: 10.1016/j.pbb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Hall SF, Huang S, Fong WG, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology. 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, de Wit H, Lilje TC, Kassel JD. The combined effects of alcohol, caffeine, and expectancies on subjective experience, impulsivity, and risk-taking. Experimental and Clinical Psychopharmacology. 2013;21:222. doi: 10.1037/a0032337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert ML, May CE, Griffin WC., III Conditioned reinforcement and locomotor activating effects of caffeine and ethanol combinations in mice. Pharmacology Biochemistry and Behavior. 2013;110:168–173. doi: 10.1016/j.pbb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J Mice Show Elevated Alcohol Intake, but Reduced Taste Aversion, as Compared to Adult Mice: A Potential Behavioral Mechanism for Binge Drinking. Alcoholism: Clinical and Experimental Research. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Jones SC, Barrie L, Berry N. Why (not) alcohol energy drinks? A qualitative study with Australian university students. Drug and Alcohol Review. 2012;31:281–287. doi: 10.1111/j.1465-3362.2011.00319.x. [DOI] [PubMed] [Google Scholar]
- Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z. Caffeine promotes ethanol drinking in rats: Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–277. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Asahi T, Tadokoro S. Ethanol enhances, but diazepam and pentobarbital reduce the ambulation-increasing effect of caffeine in mice. Arukoru Kenkyuto Yakubutsu Ison. 1992;27:528–539. [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Role of novelty and ethanol history in locomotor stimulation induced by binge-like ethanol intake. Alcoholism: Clinical and Experimental Research. 2012;36:887–894. doi: 10.1111/j.1530-0277.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Alterations in the rate of binge ethanol consumption:implications for preclinical studies in mice. Addiction Biology. 2014;19:812–825. doi: 10.1111/adb.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2 Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcoholism: Clinical and Experimental Research. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., Ii Sensitivity and Tolerance to the Hypnotic and Ataxic Effects of Ethanol in Adolescent and Adult C57BL/6J and DBA/2J Mice. Alcoholism: Clinical and Experimental Research. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cruz L, Salamone JD, Correa M. The impact of caffeine on the behavioral effects of ethanol related to abuse and addiction: A review of animal studies. Journal of Caffeine Research. 2013;3:9–21. doi: 10.1089/jcr.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Clubgoers and their trendy cocktails: Implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Experimental and Clinical Psychopharmacology. 2006;14:450–458. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR. Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Experimental and Clinical Psychopharmacology. 2012;20:129–138. doi: 10.1037/a0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology. 2013;18:921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CE, Haun HL, Griffin WC. Sensitization and Tolerance Following Repeated Exposure to Caffeine and Alcohol in Mice. Alcoholism: Clinical and Experimental Research. 2015;39:1443–1452. doi: 10.1111/acer.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Linsenbardt DN, Melón LC, Boehm SL. Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: Anxiety-like, locomotor, and consummatory behaviors. Developmental Psychobiology. 2011;53:141–156. doi: 10.1002/dev.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL. Adolescent C57BL/6J (but not DBA/2J) Mice Consume Greater Amounts of Limited-Access Ethanol Compared to Adults and Display Continued Elevated Ethanol Intake into Adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MC, McCoy TP, Egan KL, Goldin S, Rhodes SD, Wolfson M. Caffeinated Alcohol, Sensation Seeking, and Injury Risk. Journal of Caffeine Research. 2013;3:59–66. doi: 10.1089/jcr.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MC, McCoy TP, Rhodes SD, Wagoner A, Wolfson M. Caffeinated cocktails: Energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Academic Emergency Medicine. 2008;15:453–460. doi: 10.1111/j.1553-2712.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiology & behavior. 1974;12:1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Martin FH, Carr A. The Impact of Alcohol and Energy Drink Consumption on Intoxication and Risk-Taking Behavior. Alcoholism: Clinical and Experimental Research. 2013a;37:1234–1242. doi: 10.1111/acer.12086. [DOI] [PubMed] [Google Scholar]
- Peacock A, Martin FH, Carr A. Energy drink ingredients. Contribution of caffeine and taurine to performance outcomes Appetite. 2013b;64:1–4. doi: 10.1016/j.appet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacology Biochemistry and Behavior. 1997;57:487–493. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K, Levin ED. Effects of Caffeine on Alcohol Consumption and Nicotine Self-Administration in Rats. Alcoholism: Clinical and Experimental Research. 2013;37:1609–1617. doi: 10.1111/acer.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Stimulant drugs and stress: factors influencing individual differences in the susceptibility to sensitization. Sensitization of the nervous system. 1988:145–173. [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–326. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Springer; 2005. Recent developments in alcoholism; pp. 143–159. [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones and Behavior. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL. “Drinking in the Dark”(DID): A Simple Mouse Model of Binge-Like Alcohol Intake. Current Protocols in Neuroscience. 2014:9.49 41–49.49 12. doi: 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs DL, O’Mara RJ, Tsukamoto M, Rossheim ME, Weiler RM, Merves ML, Goldberger BA. Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addictive Behaviors. 2010;35:325–330. doi: 10.1016/j.addbeh.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Verster JC, Aufricht C, Alford C. Energy drinks mixed with alcohol: misconceptions, myths, and facts. International journal of general medicine. 2012;5:187. doi: 10.2147/IJGM.S29313. [DOI] [PMC free article] [PubMed] [Google Scholar]


