Abstract
Chronic and juvenile myelomonocytic leukemias (CMML and JMML) are myelodysplastic/myeloproliferative neoplasia (MDS/MPN) overlap syndromes that respond poorly to conventional treatments. Aberrant Ras activation due to NRAS, KRAS, PTPN11, CBL, and NF1 mutations is common in CMML and JMML. However, no mechanism-based treatments currently exist for cancers with any of these mutations. An alternative therapeutic strategy involves targeting Ras-regulated effector pathways that are aberrantly activated in CMML and JMML, which include the Raf/MEK/ERK and phosphoinositide-3´-OH kinase (PI3K)/Akt cascades. Mx1-Cre, KrasD12 and Mx1-Cre, Nf1flox/− mice accurately model many aspects of CMML and JMML. Treating Mx1-Cre, KrasD12 mice with GDC-0941 (also referred to as pictilisib), an orally bioavailable inhibitor of class I PI3K isoforms, reduced leukocytosis, anemia, and splenomegaly while extending survival. However, GDC-0941 treatment attenuated activation of both PI3K/Akt and Raf/MEK/ERK pathways in primary hematopoietic cells, suggesting it could be acting through suppression of Raf/MEK/ERK signals. To interrogate the importance of the PI3K/Akt pathway specifically, we treated mice with the allosteric Akt inhibitor MK-2206. This compound had no effect on Raf/MEK/ERK signaling, yet it also induced robust hematologic responses in Kras and Nf1 mice with MPN. These data support investigating PI3K/Akt pathway inhibitors as a therapeutic strategy in JMML and CMML patients.
Introduction
Juvenile and chronic myelomonocytic leukemias (JMML and CMML) are aggressive myeloid malignancies characterized by monocytosis, splenomegaly, anemia, and thrombocytopenia.1 They are categorized as myelodysplastic syndromes/myeloproliferative neoplasms (MDS/MPN), a designation that reflects both expansion of myelomonocytic populations and aberrant multi-lineage differentiation, especially dyserythropoiesis and anemia. Chemotherapy has little benefit for MDS/MPN patients, and most die from their disease within 3 years.2, 3 Hematopoietic growth and differentiation is deregulated in JMML and CMML as a result of mutations in KRAS, NRAS and in three genes that modulate the levels of active Ras-GTP (NF1, PTPN11, and CBL).4–6 A promising therapeutic strategy for Ras-driven malignancies is to target the two downstream kinase effector pathways most frequently activated in cancer: Raf/MEK/ERK (or mitogen activated protein kinase; MAPK) and phosphotidylinositol-3 kinase (PI3K)/Akt. Traditionally, Raf and PI3K have been viewed as parallel outputs of Ras.7 However, PI3K can also be activated directly by growth factor receptors, and functions upstream of Ras in some cell types.8, 9
Mouse models of JMML and CMML are valuable for characterizing disease mechanisms and testing new therapies.6 Mx1-Cre, KrasLSL-D12 (hereafter designated KrasD12) mice harbor a conditional oncogenic Kras allele that is activated when Cre recombinase is expressed from the Mx1 promoter, which is itself induced by polyinosinic-polycytidylic acid (pIpC).10–12 These mice develop an aggressive and fully penetrant MDS/MPN that causes leukocytosis, splenomegaly, anemia, and death by 10–16 weeks of age. Mx1-Cre, Nf1flox/− mice (hereafter Nf1Δ/−) undergo conditional loss of Nf1. These mice also develop MDS/MPN, but the disease is much more indolent and anemia is less prominent than in KrasD12 mice.
We previously showed that the MEK inhibitor PD0325901 induced dramatic hematologic improvement in both KrasD12 and Nf1Δ/− mice.13, 14 These encouraging preclinical data provide evidence that signal transduction therapy might be efficacious in JMML and CMML. However, the activity of PD0325901 in both models had some limitations. Importantly, treatment fails to reduce the proportion of Kras or Nf1 mutant bone marrow cells, and T-lineage acute lymphoblastic leukemia (T-ALL) emerged in some mice despite ongoing treatment. These data support investigating other Ras effector pathways as potential therapeutic targets. Recent data implicate aberrant PI3K signaling in JMML.15–17 When activated by growth factor receptors or Ras-GTP, PI3K phosphorylates phosphatidylinositol (3,4)-bis-phosphate (PIP2) in the plasma membrane to create phosphatidylinositol (3,4,5)-tris-phosphate (PIP3), which recruits and thereby activates a variety of downstream effectors. Akt is a serine/threonine kinase that becomes active when bound to PIP3 via its PH domain and phosphorylated by the kinases PDK and mTORC2. Akt also has multiple effectors that regulate cell survival, growth, metabolism and differentiation.
Here, we test the importance of PI3K/Akt signaling in MDS/MPN using a pharmacologic approach in KrasD12 and Nf1 mouse models. We show that the class I PI3K inhibitor GDC-0941 improves hematologic function and prolongs survival in KrasD12 mice with MDS/MPN. Further corroborating the relevance of this pathway, the allosteric Akt inhibitor MK-2206 also induces substantial hematologic improvement in both KrasD12 and Nf1 mice.18 Collectively, these studies support the idea that inhibitors targeting the PI3K/Akt pathway may have a role in treatment of JMML or CMML.
Materials and Methods
Mice
Mice were maintained in the specific pathogen free UCSF animal facility, and the institutional animal research committee approved all experimental procedures. Four-week-old F1 (129Sv/Jae × C57BL/6) Mx1-Cre, KrasLSL-D12 mice and control littermates were injected intraperitoneally once with 250 µg of pIpC (Sigma-Aldrich) at weaning.13 F1 (129X1/SvJ x C57BL/6) Mx1-Cre, Nf1flox/− mice were injected intraperitoneally once with 500 µg of pIpC 2 or 3 days after birth. KrasLSL-D12 allele burden was determined by quantitative PCR for the LSL cassette (see supplemental methods).
In Vivo Inhibitor studies
GDC-0941 (Genentech) was administered at the maximally tolerated dose (MTD) of 125 mg/kg/day or 0.5% hydroxypropylmethylcellulose (Sigma-Aldrich H9262)/0.2% Tween-80 vehicle by gavage once daily. MK-2206 (Proactive) was given at 240 mg/kg in Captisol vehicle (Ligand Pharmaceuticals) by gavage every Monday, Wednesday and Friday. Treatment of KrasD12 mice began at 8–10 weeks of age, and treatment of Nf1Δ/− mice began at 6 months of age; in both cases, MDS/MPN was well established prior to therapy. Mice were removed from trial if moribund or severely anemic (Hb < 6 g/dL). Blood counts were measured by Hemavet analyzer (Drew Scientific). Mice were randomly assigned to treatment groups without respect to sex; all experiments were unblinded.
Pharmacokinetics
Proteins were precipitated by the addition of acetonitrile (250 µL) containing losartan (0.5 µM) as internal standard, followed by vortexing and centrifugation. Supernatants (200 µL) were transferred to new low retention micro-centrifuge tubes and dried under vacuum at 45°C. The dried samples were resuspended in 50 µL of 70/30 water/acetonitrile with vortexing and subsequently centrifuged for 5 minutes at 16,000 × g at 4°C to remove insoluble material. The supernatants were transferred to a 96 well plate and samples were injected (10 µL) for PD0325901 and MK 2206 analysis. Both analytes were separated on an Agilent 1290 UPLC system with a c18 column using a gradient run of 20 – 95% acetonitrile in water with 0.1 % formic acid over 2 minutes at a flow rate of 0.4 mL/min and detected on an Agilent 6520 QTOF mass spectrometer. Standard curves were generated from the area under the curve for standards within the quantifiable range. The lower limit of quantification was 30 nM for MK2206 and 10 nM for PD0325901 in plasma.
Flow Cytometry
Antibodies (eBioscience, BioLegend, and BD Biosciences) used: PE-Cy7 lineage markers (“Lin”: CD3, CD4, CD5, CD8, B220, TER119, CD11b, Gr1), PacificBlue CD105, BV510 Sca1, FITC CD34, PE CD150, PerCP-Cy5.5 c-kit, APC CD48, and Alexa700 CD16/32. Intracellular phosphoproteins were analyzed as described.13, 19 Data were collected on a LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo (TreeStar).
Progenitor Assays
Bone marrow was incubated for 7 days in M3231 methylcellulose (STEMCELL Technologies) with recombinant murine GM-CSF (granulocyte-macrophage colony stimulating factor; PeproTech) and/or inhibitors (GDC-0941 or MK-2206) in DMSO 0.2%.
Statistics
Data were analyzed in Prism 6 (GraphPad) and R20; specific tests appear in figure legends. Linear mixed-effects modeling of blood counts over time is described in supplementary methods. Sample sizes were based on prior preclinical studies.13, 14
Results
PI3K inhibitor GDC-0941 suppresses MDS/MPN in KrasD12 mice
GDC-0941 is a selective, ATP-competitive inhibitor of class I PI3K isoforms with favorable pharmacokinetic and pharmacodynamic properties that showed activity in preclinical models of breast and lung cancer.21–25 We treated KrasD12 and control littermates with GDC-0941 or vehicle from age 6–8 weeks, when MDS/MPN is well established. The maximum daily dose of GDC-0941 tolerated in this background strain produces inhibitory concentrations in the bloodstream for 8 hr.26 As expected, this dose of GDC-0941 was generally well tolerated in control mice, which only showed modest weight loss (data not shown).
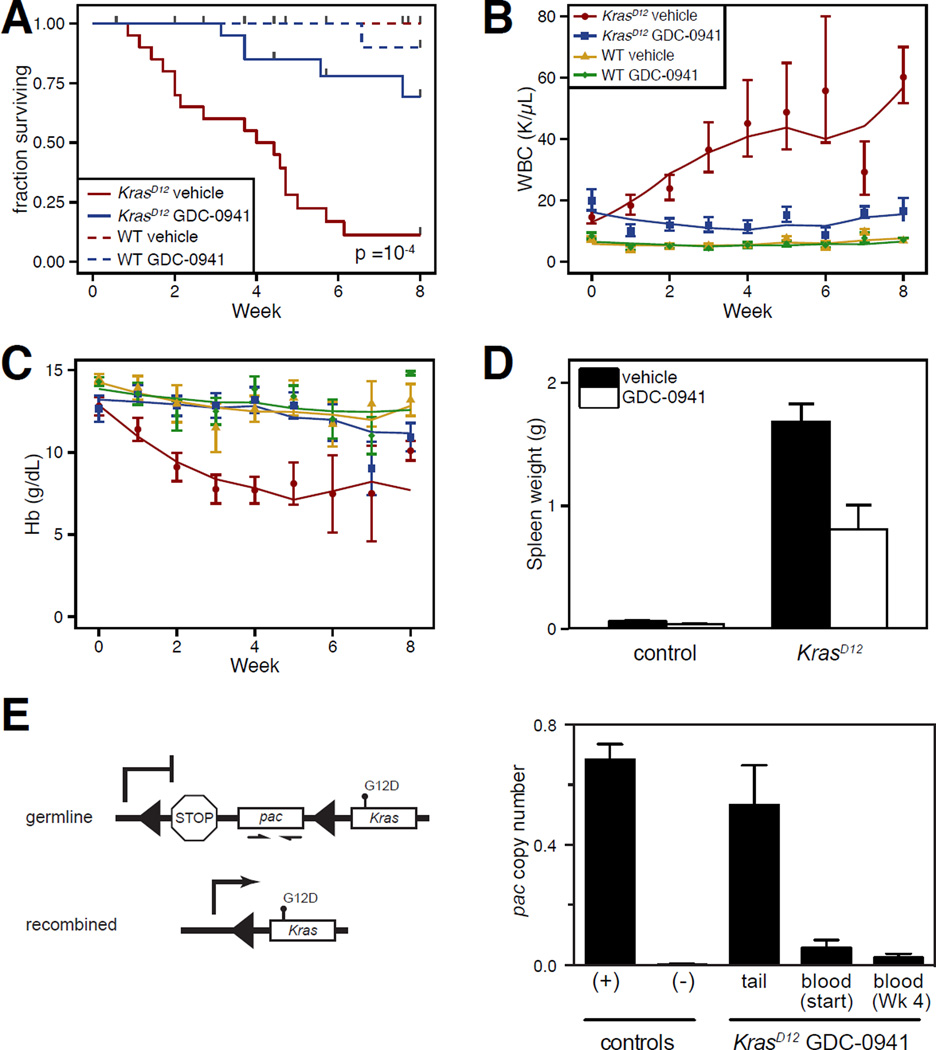
GDC-0941 treatment significantly extended the survival of KrasD12 mice and also improved peripheral blood cell counts (Figure 1A–C). Anemia was largely prevented by GDC-0941 treatment, and leukocyte counts were reduced, though they remained above control values. Treatment also partially corrected splenomegaly in KrasD12 mice (Figure 1D). Several KrasD12 mice treated with GDC-0941 died from T-ALL. This, along with incomplete correction of peripheral blood counts, suggested that GDC-0941 reduced the severity of MDS/MPN without eradicating Kras mutant cells. This was confirmed by demonstrating persistence of the KrasD12 allele in the active, recombined configuration in the blood of mice with a hematologic response to treatment (Figure 1E).
Figure 1. Efficacy of PI3K inhibition in KrasD12 mice.
KrasD12 and control mice were randomly assigned to receive 125 mg/kg of GDC-0941 or vehicle daily for up to 8 weeks. All error bars indicate standard error of the mean. Cohort sizes were: KrasD12 GDC-0941: n=20; KrasD12 vehicle: n=20; control GDC-0941: n=18; control vehicle: n=10.
(A) Kaplan-Meier survival analysis including deaths from all causes (p ≤ 0.0001 for KrasD12 GDC-0941 vs. KrasD12 vehicle by Mantel-Cox Log-Rank test). Among 20 KrasD12 mice treated with GDC-0941, 3 died from progressive MPN and 4 died from T-ALL.
(B,C) Hemoglobin (Hb) concentrations and white blood cell (WBC) counts in peripheral blood sampled weekly during treatment. Blood counts from mice with T-ALL are excluded. Data were analyzed statistically using a linear mixed-effects model as described in the supplemental methods. Bootstrap 95% confidence intervals demonstrated a significant difference of treated vs. untreated KrasD12 mice for WBC by both time terms and intercept, and for Hb by the second-order time term and intercept.
(D) Spleen weights after treatment for 10 weeks or removal from trial for progressive MPN or T-ALL (p ≤ 0.01 for KrasD12 GDC-0941 vs. KrasD12 vehicle by two-tailed Mann-Whitney test).
(E) The abundance of the silent, unrecombined KrasLSL-D12 allele was determined by quantitative PCR for puromycin N-acetyl-transferase (pac) sequences in the LSL cassette. Excision of the LSL by Cre therefore prevents amplification. Controls are DNA from tails of wild type (−) or Cre-negative KrasLSL-D12 (+) mice. As expected, LSL sequences are rare in the blood of untreated KrasD12 mice with MPN. Treatment with GDC-0941 for 4 weeks did not result in increased abundance of the unrecombined allele despite improved blood counts.
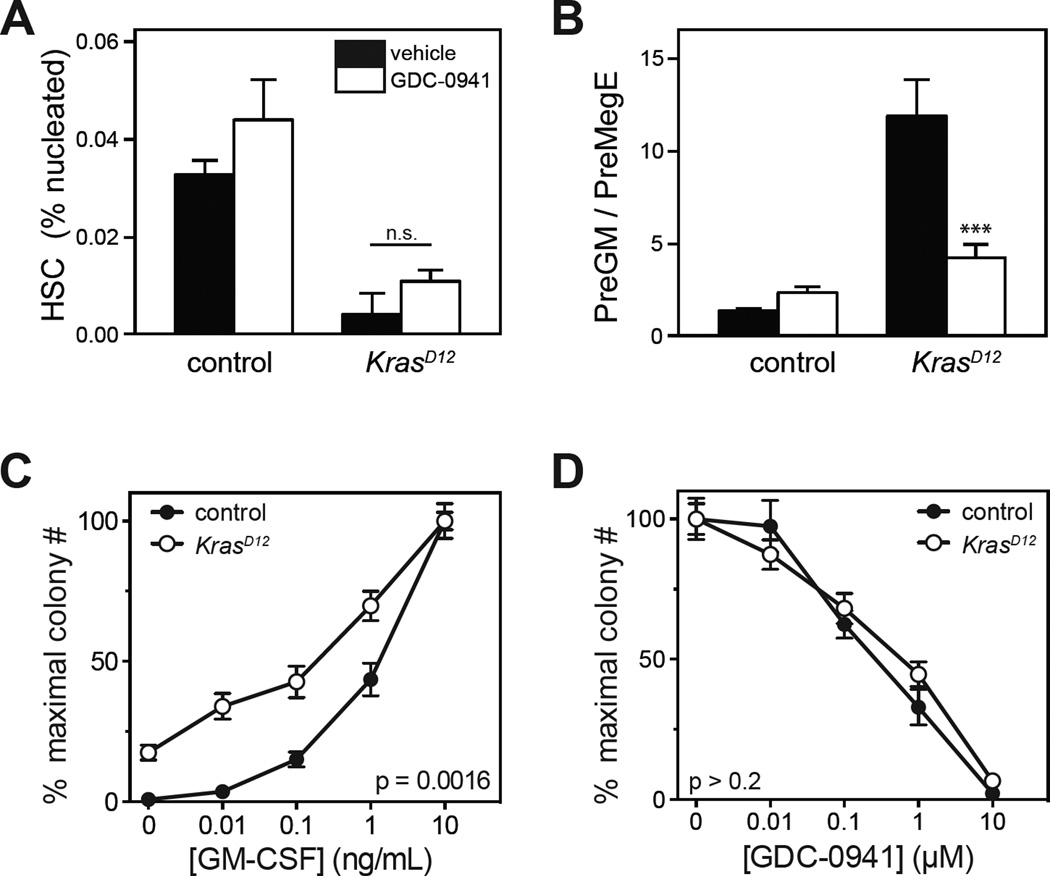
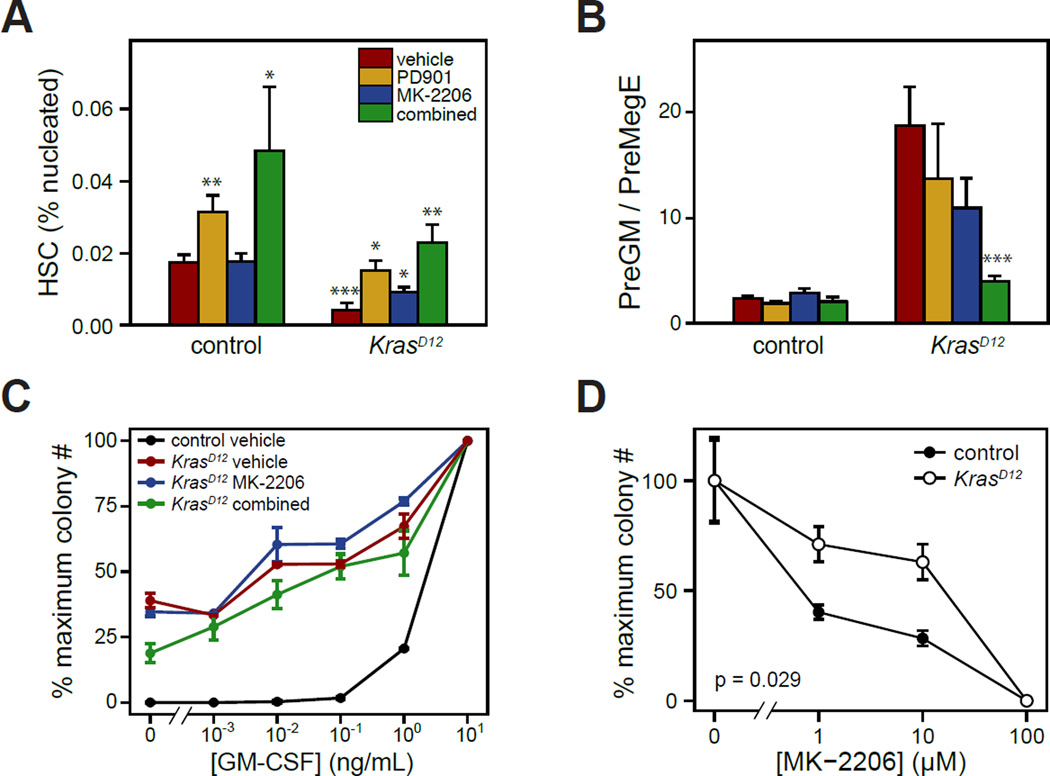
We performed flow cytometry to assess the effects of GDC-0941 on early hematopoietic populations in bone marrow (Figure 2). As expected from prior studies12, 13, KrasD12 mice had reduced numbers of phenotypic bone marrow HSC, which persisted with GDC-0941 treatment despite improvement in peripheral blood cell counts (Figure 2A). Hematopoiesis in KrasD12 mice is biased toward the granulocyte/monocyte lineage and away from megakaryocyte/erythroid differentiation.10, 13, 19, 27 This lineage output bias is most apparent in skewing of the ratio of PreGM to PreMegE populations, which was partly corrected by GDC-0941 (Figure 2B).28 We also examined the effects of GDC-0941 on the growth of myeloid progenitor colonies. Bone marrow harvested from KrasD12 mice after treatment with GDC-0941 for 5 weeks remained abnormal and demonstrated cytokine independence and GM-CSF hypersensitivity, which is a cellular hallmark of human JMML and murine KrasD12 progenitors (Figure 2C). GDC-0941 suppressed myeloid colony formation in a dose-dependent fashion. However, KrasD12 and WT granulocyte-macrophage colony forming unit (CFU-GM) progenitors were equally sensitive to GDC-0941 in the presence of saturating levels of GM-CSF (Figure 2D).
Figure 2. Effects of GDC-0941 in primary hematopoietic progenitors.
(A,B) Frequencies of hematopoietic stem and progenitor cell populations in the bone marrows of KrasD12 and control mice were determined by flow cytometry after treatment with 125 mg/kg GDC-0941 or vehicle daily for up to 8 weeks. For each genotype, 10 mice were treated with GDC-0941 and 7 control mice were treated with vehicle. Surface markers for indicated populations are: HSC (hematopoietic stem cells): Lin− Kit+ Sca1+ CD48− CD150+; PreGM: Lin− Kit+ Sca1− CD34+ CD16/32− CD150−; PreMegE: Lin− Kit+ Sca1− CD34+ CD16/32− CD150+. Data were analyzed by Wilcoxon rank sum test; p=0.0001 for PreGM/PreMegE ratio in KrasD12 GDC-0941 vs. KrasD12 vehicle.
(C) Bone marrow cells were harvested from 6 KrasD12 and 6 control mice after treatment with GDC-0941 or vehicle for 5 weeks, and plated in methylcellulose (5×104 cells per culture) containing 0.01–10 ng/mL GM-CSF. Myeloid colony numbers were normalized to the saturating GM-CSF concentration to determine the dose-response curve. Mean ± s.e.m. are displayed for each data point; p = 0.0016 for KrasD12 GDC-0941 vs. KrasD12 vehicle by permutation significance testing.40, 41
(D) CFU-GM from untreated KrasD12 or control mice were grown in the presence of saturating GM-CSF (10 ng/mL) and 0.01–10 µM GDC-0941 or DMSO 0.2% (n=3). KrasD12 does not alter the GDC-0941 dose response curve in CFU-GM (analyzed by permutation significance testing).
Akt inhibitor MK-2206 suppresses MDS/MPN in KrasD12 mice
PI3K inhibition attenuates MAPK signaling in primary hematopoietic cells due to complex “upstream” effects on lipid signaling networks.9 These data raised the possibility that hematologic improvement in response to GDC-0941 might be due to partial MAPK pathway inhibition. Furthermore, PI3K signals through effectors in addition to Akt.29 Therefore, we asked if suppression of Akt signaling is sufficient to alleviate MDS/MPN initiated by endogenous KrasD12 expression. MK-2206 is an allosteric inhibitor that binds to the interface of the PH and kinase domains of Akt and does not inhibit any of 250 other kinases at 1 µM.18, 30, 31
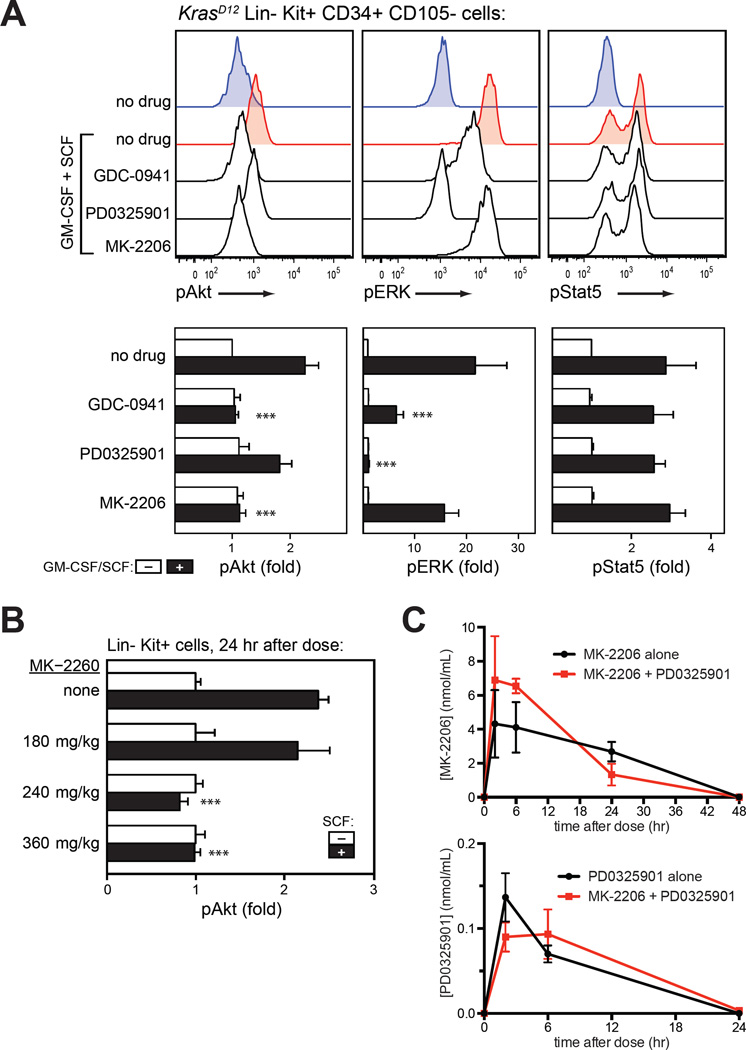
First, we verified that MK-2206 would inhibit Akt but not Raf/MEK/ERK signaling in myeloid progenitors. We used phospho-specific flow cytometry to analyze signal transduction in immature populations of bone marrow mononuclear cells that were exposed to signal transduction inhibitors in vitro. Bone marrow was harvested from control and KrasD12 mice, briefly starved in the presence of inhibitors, and then stimulated with a combination of stem cell factor (SCF) and GM-CSF. As expected, GDC-0941 strongly inhibited Akt phosphorylation (Figure 3A) and caused a modest but reproducible decrease in ERK phosphorylation. By contrast, MK-2206 inhibited Akt phosphorylation without affecting ERK. These data agree with our prior observations using structurally distinct PI3K inhibitors, and they are consistent with a model in which lipid second messengers downstream of PI3K regulate Raf/MEK/ERK signaling in myeloid progenitors.9, 19 As expected, the potent and selective MEK inhibitor PD0325901 abrogated ERK phosphorylation, but did not alter Akt phosphorylation.
Figure 3. Pharmacologic properties of MK-2206.
(A) In vitro activity. Phosphorylation of Akt (S473), ERK (T202/Y204) and STAT5 (Y694) measured by intracellular flow cytometry in primary KrasD12 bone marrow cells. After harvest, cells were incubated with GDC-0941 (10 µM), the MEK inhibitor PD0325901 (10 µM; PD901), the Akt inhibitor MK-2206 (10 µM), or DMSO (0.001%) for 20 minutes prior to a 10-minute stimulation with GM-CSF (10 ng/mL) and stem cell factor (100 ng/mL). Lin− Kit+ CD34+ CD105− myeloid progenitor cells are shown. Data from replicate experiments appear in the lower panels. Basal signal levels (white) and stimulated levels (black) are shown for each drug treatment. Fold change in median fluoresence intensity (MFI) is displayed. Data from 5 indepdendent replicates on different days were analyzed using a linear model for MFI as predicted by stimulus, drug, replicate, and the drug-stimulus interaction, with log-transformation of MFI to correct heteroscedasticity. Asterisks indicate conditions in which the drug had a significant effect on the stimulated MFI, as indicated by the drug-stimulus interaction term (***, p<0.001); this was not statistically significant (p>0.05) where asterisks are not shown.
(B) In vivo activity. Bone marrow was harvested from mice 24 h after MK-2206 administration, immediately stimulated with SCF, fixed, and analyzed for Akt phosphorylation (S473) by intracellular flow cytometry. Data are displayed and analyzed as in panel A.
(C) Pharmacokinetic profile of MK-2206 and PD0325901 in plasma on the eighth day of treatment with either agent alone or the combination. Drug exposure was not affected by combination therapy.
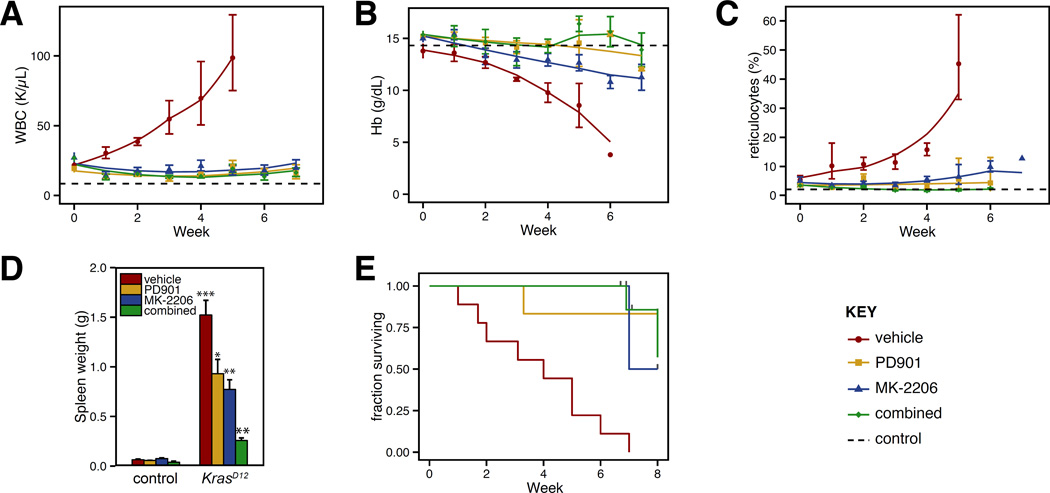
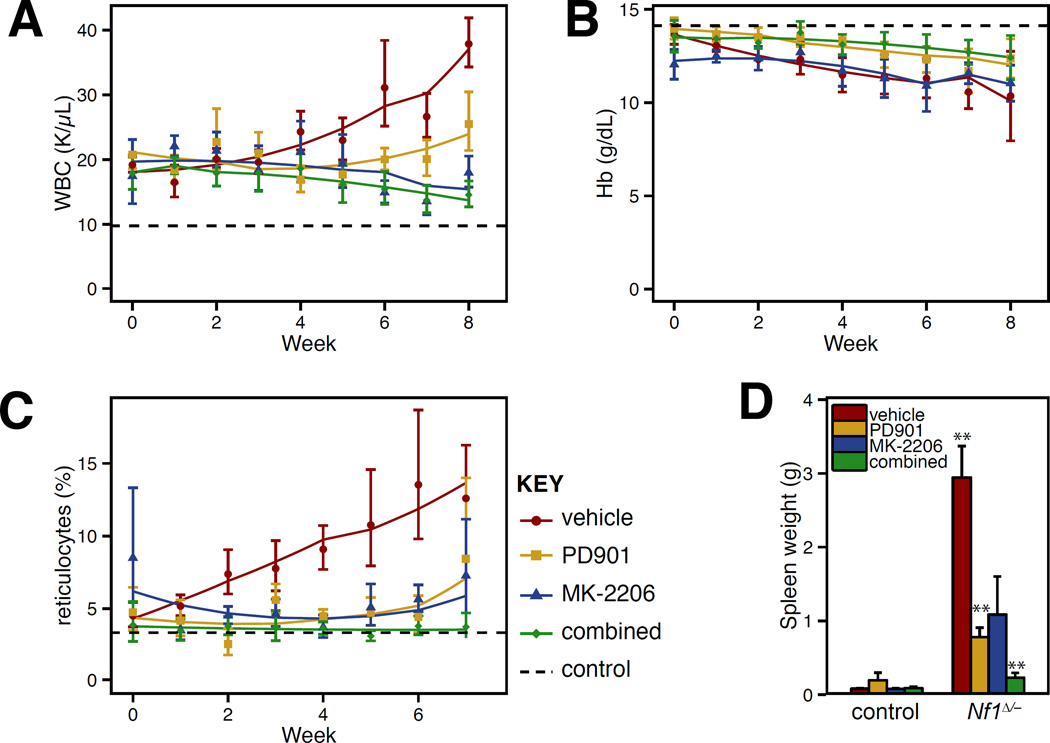
We next performed pharmacodynamic studies of MK-2206 by harvesting bone marrow from mice 24 h after drug administration, and immediately stimulating the marrow with SCF. Flow cytometry was used to assess Akt phosphorylation in progenitor cells, identified by absence of mature lineage markers and expression of c-kit, the receptor for SCF. While we did not observe target inhibition at a dose of 180 mg/kg, Akt phosphorylation was suppressed 24 hr after doses of 240 mg/kg and 360 mg/kg (Figure 3B). Pharmacokinetic studies confirmed that MK-2206 persists in the blood for >24 hr (Figure 3C). Based on this long duration of action, mice were given MK-2206 three times each week. At this schedule, mice receiving 360 mg/kg had excessive weight loss, but those receiving 240 mg/kg demonstrated no overt toxicity. KrasD12 mice with MPN that received 240 mg/kg of MK-2206 had significantly increased survival, markedly improved blood cell counts, and reduced splenomegaly (Figure 4). Control mice remained well throughout treatment and maintained normal blood counts (Supplementary Figure 1).
Figure 4. Efficacy of Akt inhibition in KrasD12 mice.
(A) White blood cell (WBC) counts, (B) hemoglobin (Hb) concentrations, and (C) reticulocyte counts in peripheral blood of KrasD12 and control mice sampled weekly during MK-2206 treatment. Dashed horizontal lines indicate average values for control mice (presented in greater detail in Supplemental Figure 1). Cohort sizes: KrasD12 vehicle: 9; KrasD12 MK-2206: 9; KrasD12 PD0325901: 6; KrasD12 combination: 7; control vehicle: 12; control MK-2206: 8; control PD0325901: 5; control combination: 3. All deaths in drug treated mice were attributable to T-ALL except for one mouse treated with MK-2206 with advanced MPN after 7 weeks. Data were analyzed statistically as described in Figure 1; among KrasD12 mice, each drug treated group in panels A–C demonstrated at least one signficiant difference in mixed linear model coefficients fom the vehicle treated cohort. (D) Spleen weights in mice measured at time of death or trial end. Data were analyzed using the Wilcoxon rank sum test. Samples from vehicle treated KrasD12 mice were compared to those from vehicle treated control mice. Samples from drug treated mice were compared to vehicle treated mice of the same genotype. Indicators for p values: * <0.05; ** < 0.01; *** < 0.001.
(E) Kaplan-Meier analysis of overall survival. Log-rank p values are 0.003, 0.0001 and 0.0002 for comparison of vehicle treated mice with PD0325901, MK-2206, or the combination, respectively.
Combined inhibition of Akt and MEK
In contrast to GDC-0941, MK-2206 does not reduce Raf/MEK/ERK pathway activation. Therefore MK-2206 and PD0325901 are likely to alleviate MDS/MPN through distinct biochemical mechanisms of action, and combining these agents might be more effective than using either alone. We developed a combined therapy regimen to test this hypothesis. While our single agent studies of PD0325901 in KrasD12 and Nf1Δ/− mice administered 5 mg/kg/day, reducing the dose to 1.5 mg/kg/day inhibited MEK activity for at least 12 hours and was efficacious in Nf1 and Kras mutant mice with MDS/MPN.14 Importantly, because this lower dose of PD0325901 is better tolerated in combination with MK-2206 than 5 mg/kg (data not shown), we tested the efficacy of MK-2206 240 mg/kg thrice weekly with PD0325901 1.5 mg/kg daily in KrasD12 mice with MPN. We found no pharmacokinetic interaction between these two agents (Figure 3C). Control mice exhibited no hematologic changes or any other overt toxicity from combined therapy. Combined MEK + Akt inhibition did not further improve in most hematologic parameters in KrasD12 mice, which were already largely corrected by either agent alone (Figure 4). However, spleen size was smallest in mice receiving combination therapy (Figures 4E).
Effects of MK-2206 on KrasD12 hematopoietic progenitors
The frequency of HSC in the marrow was increased in KrasD12 mice receiving the combination of MK-2206 and PD0325901, and to a lesser extent in mice receiving either single agent (Figure 5A). Furthermore, the myeloid to erythroid progenitor bias was corrected more by the combination than by either compound alone (Figure 5B). Despite this improvement in bone marrow progenitor distributions, however, myeloid progenitors continued to demonstrate an abnormal growth phenotype when cultured in vitro (Figure 5C). CFU-GM grown from KrasD12 mice after 5 weeks of therapy with either MK-2206 or MK-2206 + PD0325901 retained the abnormal, hypersensitive dose response to GM-CSF characterisitic of MDS/MPN. This suggests that continuous drug treatment would be needed to maintain the clinical response to Akt and/or MEK inhibition. Finally, we asked whether expression of KrasD12 would influence the sensitivity of myeloid progenitors to MK-2206 in a colony assay. Bone marrow from untreated KrasD12 or control mice was cultured in methylcellulose containing MK-2206 over a range of 1–100 µM and a saturating concentration of GM-CSF (10 ng/mL). KrasD12 CFU-GM demonstrated a modestly increased sensitivity to MK-2206 as compared to controls.
Figure 5. Effects of MK-2206 in KrasD12 hematopoietic progenitors.
(A,B) Frequencies of hematopoietic stem and progenitor cell populations in the bone marrows of KrasD12 and control mice were determined by flow cytometry after treatment with vehicle, PD0325901, MK-2206, or both agents for up to 8 weeks. 5 to 13 mice were analyzed in each group, and data were analyzed as described for Figure 4D, comparing vehicle treated KrasD12 mice to vehicle treated control mice, and drug treated mice to respective vehicle treated mice.
(C) Bone marrow cells were harvested from KrasD12 and control mice after treatment with MK-2206, MK-2206+PD0325901, or vehicle for 5 weeks, and plated in methylcellulose (5×104 cells per culture) containing 0.01–10 ng/mL GM-CSF. Myeloid colony numbers were normalized to the saturating GM-CSF concentration to determine the dose-response curve. Mean ± s.e.m. are displayed for each data point.
(D) CFU-GM from untreated KrasD12 or control mice were grown in the presence of saturating GM-CSF (10 ng/mL) and 1–100 µM MK-2206 or DMSO 0.2% (n=4). Colony numbers were normalized to DMSO control; mean ± s.e.m. are displayed. CFU-GM from KrasD12 mice were more sensitive to MK-2206 (p = 0.029 by permutation significance testing).
MK-2206 suppresses MDS/MPN in Nf1 mutant mice
Conditional inactivation of Nf1 in mice provides an alternative preclinical model of JMML.14, 32 Homozygous Nf1 inactivation causes a subacute MDS/MPN with gradual onset of leukocytosis, splenomegaly, reticulocytosis and mild anemia beginning around 6 months of age, and death at 8–12 months. We treated Nf1Δ/− mice with MK-2206 to test whether Akt inhibition would also be effective in a less fulminant disease initiated by more subtle biochemical activation of Ras. Treatment with MK-2206 improved all aspects of the MDS/MPN phenotype in Nf1Δ/− mice, and the combination of PD0325901 and MK-2206 again yielded the biggest reduction in spleen size (Figure 6). These data are consistent with our observations in the Kras model.
Figure 6. Efficacy of Akt inhibition in Nf1Δ/− mice.
(A) White blood cell (WBC) counts, (B) hemoglobin (Hb) concentrations, and (C) reticulocyte counts in peripheral blood of Nf1Δ/− and control mice sampled weekly during MK-2206 treatment. Statistical analysis was performed as described in Figure 4. Cohort sizes: Nf1 vehicle: 5; Nf1 MK-2206: 4; Nf1 PD0325901: 7; Nf1 combination: 7; control vehicle: 6; control MK-2206: 5; control PD0325901: 7; control combination: 3. (D) Spleen weights in mice measured at time of death or trial end, analyzed as in Figure 4D.
Discussion
This series of preclinical trials demonstrated that MDS/MPN driven by hyperactive Ras responds to inhibition of PI3K/Akt signaling. Together, these studies implicate aberrant signaling through PI3K and Akt in the pathogenesis of CMML and JMML. The improvement in Nf1Δ/− mice treated with MK-2206 demonstrates that this pathway remains important even when the disease is more indolent. Together with prior studies demonstrating efficacy of MEK inhibitors, MDS/MPN appears to follow a long-standing model whereby multiple effectors cooperate to mediate oncogenic signals downstream of Ras-GTP.13, 14, 33, 34 Consistent with this idea, combined inhibition of MAPK and PI3K/Akt signaling yielded the best improvement in splenomegaly in both MDS/MPN models, and the most significant correction of bone marrow progenitor frequencies in KrasD12 mice.
Our data complement recent studies by Gritsman et al., who used a genetic strategy to ablate the p110α isoform of PI3K in the hematopoietic compartment.17 This delayed lethality but did not prevent the development of a JMML-like disease in KrasD12 mice. In contrast to this early, complete, and permanent loss of a single PI3K isoform, treating mice with GDC-0941 at the maximally tolerated daily dose (MTD) inhibits multiple PI3K isoforms for only ~8 hours, and we initiated treatment after MDS/MPN was well established. The robust response to GDC-0941 suggests that isoforms other than p110α are important in MDS/MPN, and no mice succumbed from MPN. This idea is consistent with studies showing that p110δ activity is required for MPN initiated by oncogenic SHP-2, a pleiotropic signal relay protein encoded by PTPN11 that functions upstream of Ras-GTP.16 Furthermore, our studies of GDC-0941 demonstrate that a therapeutic index in MPN requires neither continuous nor isoform-selective inhibition of PI3K.
A recent report from Staffas et al. demonstrated that anemia and mortality in KrasD12 mice are influenced by non-hematopoietic tissues and identified blood loss from gastrointestinal neoplasia as a potential mechanism.35 However, in vitro and in vivo studies also show that oncogenic Ras has direct cell-intrinsic effects on erythropoiesis.36–38 Therefore, both cell-intrinsic and cell-extrinsic factors underlie the anemia in KrasD12 mice, and PI3K/Akt inhibition in either hematopoietic or non-hematopoietic cells could explain improvement in anemia and mortality. Importantly, however, leukocytosis in KrasD12 mice is intrinsic to the hematopoietic system, and this is also corrected by PI3K/Akt pathway inhibition. Thus, the activity of these inhibitors cannot be mediated fully by effects on KrasD12 phenotypes outside the hematopoietic system. This idea is consistent with the concordant responses in Nf1Δ/− mice, which have milder extra-hematopoietic phenotypes. Finally, the differential effect of MK-2206 on KrasD12 myeloid progenitors grown in vitro also suggests some of the activity of MK-2206 acts in a cell-intrinsic manner (Fig. 5).
Akt is one of many PI3K effectors that are activated by recruitment to PIP3 in the plasma membrane. The efficacy of MK-2206 in the MDS/MPN models shows that Akt mediates an essential component of the PI3K signal in this disease. Akt, in turn, has >100 substrates, including many with known relevance to hematopoiesis. Effectors such as mTOR, FOXO transcription factors, GSK3β, MDM2, NF-κB, and BAD collectively regulate growth, metabolism, survival, and fate decisions in hematopoietic cells.39 Interestingly, our data suggest that beneficial and adverse effects of PI3K/Akt inhibition are mediated by distinct biochemical pathways. The maximally tolerated dose of GDC-0941 inhibits PI3K for only ~8 hours, whereas deep inhibition of Akt for >24h is well tolerated. This suggests that toxicity may partly reflect loss of PI3K signals that are not dependent on Akt. The lipid second messengers that link PI3K to MEK in hematopoietic cells represent one example of an Akt-independent pathway that may contribute to efficacy and/or toxicity of PI3K inhibition.9
Many CMML patients suffer from chronic anemia, splenomegaly, transfusion dependence and iron overload, but are currently not eligible for hematopoietic stem cell transplantation due to risk of morbidity.2 A therapy that reduces symptoms with minimal toxicity may have a role in the clinical management of this aggressive MDS/MPN. While HSCT is the standard of care for JMML, these vulnerable young patients might also benefit from treatment with MAPK and/or PI3K/Akt inhibitors to enhance hematopoiesis and improve their overall clinical condition before undergoing transplantation. Our preclinical data suggest that this can be achieved by targeting either Raf/MEK/ERK or PI3K/Akt signaling. The experiments presented here also implicate Akt as a potential effector of PI3K that could be targeted in MDS/MPN. This is significant, since having multiple targets for therapy would allow risks and benefits to be more closely tailored to individual patients, and may also enable combination therapy.
Importantly, however, T-ALL emerges during treatment with any signal transduction inhibitor used to treat KrasD12 mice: PD0325901, GDC-0941, MK-2206 or PD325901 + MK-2206. These data suggest there is a high probability that mutant HSC will ultimately acquire cooperating mutations that enable them to overcome these targeted agents. Given this, developing drugs or drug combinations capable of eradicating mutant HSC remains a fundamental challenge in JMML and CMML.
Supplementary Material
Acknowledgments
This work was supported by the NF Preclinical Consortium and NF Therapeutic Consortium of the Children's Tumor Foundation, and by the National Cancer Institute Cancer Center Support Grant P30CA082103. B.S.B was a St. Baldrick’s Foundation Scholar and received support for this work from NIH awards U54CA143874 and R01CA173085, an ASH Bridge Grant from the American Society of Hematology, and the Frank A. Campini Foundation. T.Q.H was supported by NIH training grants T32CA128583 and T32HD044331 and C.L.C was supported by T32CA108462. T.C. is a St. Baldrick’s Foundation Fellow. M.D. was supported by grants from the William Lawrence and Blanche Hughes Foundation and NIH award K99CA157950.
Footnotes
Conflict of interest:
D. Sampath and L. Friedman are employed by Genentech, Inc.; M. Dail and T. Chang are currently employed by Genentech, Inc., but were not when contributing to this work. GDC-0941 was provided by Genentech, Inc. There are no other relevant disclosures.
References
- 1.Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008 Jul;22(7):1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- 2.Bacher U, Haferlach T, Schnittger S, Kreipe H, Kroger N. Recent advances in diagnosis, molecular pathology and therapy of chronic myelomonocytic leukaemia. Br J Haematol. 2011 Mar 9; doi: 10.1111/j.1365-2141.2011.08631.x. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Nollke P, Zecca M, Korthof E, Lanino E, Peters C, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005 Jan 1;105(1):410–419. doi: 10.1182/blood-2004-05-1944. [DOI] [PubMed] [Google Scholar]
- 4.Loh ML. Recent advances in the pathogenesis and treatment of juvenile myelomonocytic leukaemia. Br J Haematol. 2011 Mar;152(6):677–687. doi: 10.1111/j.1365-2141.2010.08525.x. [DOI] [PubMed] [Google Scholar]
- 5.Kohlmann A, Grossmann V, Klein HU, Schindela S, Weiss T, Kazak B, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol. 2010 Aug 20;28(24):3858–3865. doi: 10.1200/JCO.2009.27.1361. [DOI] [PubMed] [Google Scholar]
- 6.Ward AF, Braun BS, Shannon KM. Targeting oncogenic Ras signaling in hematologic malignancies. Blood. 2012 Oct;120(17):3397–3406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 8.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002 Jul;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Flores E, Goldschmidt H, Depeille P, Ng V, Akutagawa J, Krisman K, et al. PLC-γ and PI3K Link Cytokines to ERK Activation in Hematopoietic Cells with Normal and Oncogenic Kras. Sci Signal. 2013;6(304):ra105. doi: 10.1126/scisignal.2004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J, et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004 Feb;113(4):528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabnis AJ, Cheung LS, Dail M, Kang HC, Santaguida M, Hermiston ML, et al. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol. 2009 Mar 17;7(3):e59. doi: 10.1371/journal.pbio.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyubynska N, Gorman MF, Lauchle JO, Hong WX, Akutagawa JK, Shannon K, et al. A MEK inhibitor abrogates myeloproliferative disease in Kras mutant mice. Sci Transl Med. 2011 Mar;3(76):76ra27. doi: 10.1126/scitranslmed.3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO, et al. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest. 2013 Jan;123(1):335–339. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YL, Castleberry RP, Emanuel PD. PTEN deficiency is a common defect in juvenile myelomonocytic leukemia. Leuk Res. 2009 May;33(5):671–677. doi: 10.1016/j.leukres.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin CB, Li XJ, Mali RS, Chan G, Kang M, Liu Z, et al. PI3K p110δ uniquely promotes gain-of-function Shp2-induced GM-CSF hypersensitivity in a model of JMML. Blood. 2014 May;123(18):2838–2842. doi: 10.1182/blood-2013-10-535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gritsman K, Yuzugullu H, Von T, Yan H, Clayton L, Fritsch C, et al. Hematopoiesis and RAS-driven myeloid leukemia differentially require PI3K isoform p110α. J Clin Invest. 2014 Apr;124(4):1794–1809. doi: 10.1172/JCI69927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan L. Abstract #DDT01-1: MK-2206: A potent oral allosteric AKT inhibitor. Cancer Research. 2009;69(9 Supplement):DDT01-01. [Google Scholar]
- 19.Van Meter ME, Diaz-Flores E, Archard JA, Passegue E, Irish JM, Kotecha N, et al. K-RasG12D expression induces hyperproliferation and aberrant signaling in primary hematopoietic stem/progenitor cells. Blood. 2007 May 1;109(9):3945–3952. doi: 10.1182/blood-2006-09-047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2014 [Google Scholar]
- 21.Folkes A, Ahmadi K, Alderton W, Alix S, Baker S, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008 Sep;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 22.Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009 Jul;8(7):1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3' kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010 Jul;16(14):3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 24.Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD, et al. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin Cancer Res. 2012 Jul;18(14):3901–3911. doi: 10.1158/1078-0432.CCR-11-2088. [DOI] [PubMed] [Google Scholar]
- 25.Spoerke JM, O'Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, et al. Phosphoinositide 3-Kinase (PI3K) Pathway Alterations Are Associated with Histologic Subtypes and Are Predictive of Sensitivity to PI3K Inhibitors in Lung Cancer Preclinical Models. Clin Cancer Res. 2012 Nov; doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 26.Dail M, Wong J, Lawrence J, O'Connor D, Nakitandwe J, Chen SC, et al. Loss of oncogenic Notch1 with resistance to a PI3K inhibitor in T-cell leukaemia. Nature. 2014 Sep;513(7519):512–516. doi: 10.1038/nature13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun BS, Archard JA, Van Ziffle JA, Tuveson DA, Jacks TE, Shannon K. Somatic activation of a conditional KrasG12D allele causes ineffective erythropoiesis in vivo. Blood. 2006 Sep 15;108(6):2041–2044. doi: 10.1182/blood-2006-01-013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007 Oct 11;1(4):428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010 May;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 30.Wu WI, Voegtli WC, Sturgis HL, Dizon FP, Vigers GP, Brandhuber BJ. Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS One. 2010;5(9):e12913. doi: 10.1371/journal.pone.0012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehan M, Beg MA, Parveen S, Damanhouri GA, Zaher GF. Computational insights into the inhibitory mechanism of human AKT1 by an orally active inhibitor, MK-2206. PLoS One. 2014;9(10):e109705. doi: 10.1371/journal.pone.0109705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Kong N, Zhu Y, Lauchle JO, Aiyigari A, Braun BS, et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004 Jun 1;103(11):4243–4250. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 33.Matsuguchi T, Kraft AS. Regulation of myeloid cell growth by distinct effectors of Ras. Oncogene. 1998 Nov;17(21):2701–2709. doi: 10.1038/sj.onc.1202201. [DOI] [PubMed] [Google Scholar]
- 34.Shieh A, Ward AF, Donlan KL, Harding-Theobald ER, Xu J, Mullighan CG, et al. Defective K-Ras oncoproteins overcome impaired effector activation to initiate leukemia in vivo. Blood. 2013 Jun;121(24):4884–4893. doi: 10.1182/blood-2012-05-432252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staffas A, Karlsson C, Persson M, Palmqvist L, Bergo MO. Wild-type KRAS inhibits oncogenic KRAS-induced T-ALL in mice. Leukemia. 2015 May;29(5):1032–1040. doi: 10.1038/leu.2014.315. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003 Dec 1;102(12):3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Lodish HF. Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood. 2004 Sep 15;104(6):1679–1687. doi: 10.1182/blood-2004-04-1362. [DOI] [PubMed] [Google Scholar]
- 38.Tang P, Gao C, Li A, Aster J, Sun L, Chai L. Differential roles of Kras and Pten in murine leukemogenesis. Leukemia. 2013 Apr;27(5):1210–1214. doi: 10.1038/leu.2012.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, et al. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis. Biochim Biophys Acta. 2010 Sep;1803(9):991–1002. doi: 10.1016/j.bbamcr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Elso CM, Roberts LJ, Smyth GK, Thomson RJ, Baldwin TM, Foote SJ, et al. Leishmaniasis host response loci (lmr1–3) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 2004 Mar;5(2):93–100. doi: 10.1038/sj.gene.6364042. [DOI] [PubMed] [Google Scholar]
- 41.Smyth G, Thompson R, Satterley K. http://bioinf.wehi.edu.au/software/compareCurves/ [Accessed on December 17, 2013]; [cited; Available from: http://bioinf.wehi.edu.au/software/compareCurves/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.