Abstract
Objective
Regulatory T cells (Tregs) contribute to HIV-1 disease progression by impairing antiviral immunity; however, the precise mechanisms responsible for the development of Tregs in the setting of HIV-1 infection are incompletely understood.
Design
In this study, we provide evidence that HIV-induced expansion of monocytic myeloid-derived suppressor cells (M-MDSCs) promote the differentiation of Foxp3+ Tregs.
Methods
We measured MDSC induction and cytokine expression by flow cytometry and analyzed their functions by co-culturing experiments.
Results
We observed a dramatic increase in M-MDSC frequencies in the peripheral blood of HIV-1 seropositive (HIV-1+) individuals, even in those on antiretroviral therapy (ART) with undetectable viremia, when compared to healthy subjects. We also observed increases in M-MDSCs after incubating healthy peripheral mononuclear cells (PBMCs) with HIV-1 proteins (gp120 or Tat) or Toll-like receptor (TLR) 4 ligand Lipopolysaccharides (LPS) in vitro, an effect that could be abrogated in the presence of the pSTAT-3 inhibitor, STA-21. Functional analyses indicated that M-MDSCs from HIV-1+ individuals express higher levels of IL-10, TGF-β, IL-4Rα, p47phex, PD-L1, and pSTAT-3 – all of which are known mediators of myelopoiesis and immunosuppression. Importantly, incubation of healthy CD4+ T cells with MDSCs derived from HIV-1+ individuals significantly increased differentiation of Foxp3+ Tregs. In addition, depletion of MDSCs from PBMCs of HIV-1+ individuals led to a significant reduction of Foxp3+ Tregs and increase of IFN-γ production by CD4+ T effector cells (Teffs).
Conclusions
These results suggest that HIV-induced MDSCs promote Treg cell development and inhibit T cell function - a hallmark of many chronic infectious diseases.
Keywords: HIV-1, gp120, MDSCs, pSTAT-3, Tregs
Introduction
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that are generated due to aberrant myelopoiesis under certain pathological conditions, such as cancer, inflammatory and infectious diseases [1-3]. MDSCs suppress immune responses via increased expression of inflammatory and immunosuppressive mediators, including inducible nitric oxide synthase (iNOS), arginase 1 (ARG1), reactive oxygen species (ROS), IL-4 receptor-α (IL-4Rα), programmed death-ligand 1 (PD-L1), interleukin-10 (IL-10), tumor growth factor-β (TGF-β), and phosphorylated signal transducer and activator of transcription 3 (pSTAT-3), all of which are important mediators of innate immune responses against pathogenic infections [4-10]. In mice, MDSCs are defined by the co-expression of the Gr-1 and CD11b surface antigens; whereas human MDSCs are less well-characterized due to the lack of uniform phenotypic markers. However, they usually express the common myeloid markers CD33 and CD11b, but lack the maturation marker HLA-DR [7-10]. Given the heterogeneous nature of MDSCs, human MDSCs are primarily dissected into two subsets: monocytic MDSCs (M-MDSCs; CD33+CD11b+CD14+HLA-DRlow/−) and granulocytic MDSCs (G-MDSCs; CD33+CD11b+CD14−HLA-DRlow/−). These 2 subtypes of MDSCs can have different biological functions and use different mechanisms for immune suppression [1-3].
While the immunosuppressive roles of MDSCs have been well-characterized in many tumor models, their roles during infectious diseases have been less well-studied, in particular during responses to retroviral infections that cause immunodeficiency. Although MDSCs can contribute to immune homeostasis after infection by limiting excessive inflammatory processes, their expansion may be at the expense of pathogen elimination and thus may lead to infection persistence or latency. Recent studies report MDSC expansion plays a role in suppressing T cell responses and disease progression of HIV-1 infection [11-18]. However, the phenotypic and functional features of MDSCs and their mechanisms in regulation of T cell responses in HIV-1+ individuals remain unclear. This is particularly true for those on antiretroviral therapy (ART) with undetectable viremia, as they exhibit persistent dampened immunity, such as lower expression of Th1 cytokines and poor response to vaccinations [19]. Thus, phenotypic and functional characterizations of MDSCs in ART-controlled HIV-infected individuals may delineate the mechanisms by which MDSCs mediate immunosuppression and promote viral persistence or latency.
In this study, we characterized MDSCs in HIV-1+ individuals on ART with undetectable viremia and determined whether MDSCs contribute to the induction or maintenance of regulatory T (Treg) cell development and suppression of T effector (Teff) cell function during latent HIV-1 infection. We provide evidence that HIV-induced MDSCs control T cell differentiation and functions, which represents a novel mechanism for viral persistence and a new strategy for immunotherapy against human viral diseases.
Methods
Subjects
The study protocol was approved by the joint institutional review board at East Tennessee State University and James H. Quillen VA Medical Center (ETSU/VA IRB, Johnson City, TN). The study subjects were composed of two populations: 65 chronically HIV-1 infected individuals and 72 healthy subjects (HS). In this study, we focused on HIV-1 patients who are on ART (truvada-based regimen) with undetectable viremia (aviremia, HIV RNA < 20 copies/ml) and CD4 count ranging from 220 ~ 1,250. Healthy subjects were negative for HBV, HCV, and HIV infection and blood buffy coats were obtained from Key Biologics, LLC, Memphis, TN or Physician's Plasma Alliance LLC, Gray, TN. Written informed consent was obtained from all participants.
PBMC preparation and analysis by flow cytometry
PBMCs were isolated from whole blood by Ficoll-Percoll gradients centrifugation (GE Heathcare, Piscataway, NJ). For phenotypic analysis of MDSCs, PBMCs were immune-stained with CD14-PerCP710, CD11b-PE, CD33-APC (eBioscience, San Diego, CA), and HLA-DR-FITC (BD Bioscience, San Jose, CA) antibodies or isotype controls. The cells were collected on an Accuri C6™ flow cytometer (BD, Franklin Lakes, NJ) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). For intracellular cytokine staining, PBMCs were stimulated with 1 μg/ml LPS (Santa Cruz Biotechnology, Santa Cruz, CA) plus 2.5 μg/ml R848 (Santa Cruz) for 6 h, and 1 μg/ml Brefeldin A (BioLegend, San Diego, CA) was added 5 h prior to harvest. The cells were fixed and permeabilized using Inside Stain Kit (Miltenyi Biotec, Auburn, CA), and then stained with IL-10-PE, TGF-β-PE, PD-L1(CD274)-PE (BD Bioscience), IL-4Rα-PE (R&D, Minneapolis, MN), p47phox and rat anti-mouse IgG1-PE. For pSTAT3-PE (pY705) staining, PBMCs were processed with Fix Buffer I and Perm Buffer III (BD) following the BD Phosflow™ protocol. The cells were also stained with surface antigens CD14-PerCP710, CD33-APC (clone p67.6), and HLA-DR-FITC, then analyzed by flow cytometry. The fluorescence minus one (FMO) strategy and isotype controls were used to adjust multicolor compensation for cell gating and determine background levels.
Treatment of healthy PBMCs by HIV-1 proteins, LPS, and/or STA-21
PBMCs from HS were treated with HIV-1 gp120, Tat, β-gal (2 μg/ml, ViroGen), or LPS (1 μg/ml, Santa Cruz), in the presence or absence of specific pSTAT-3 inhibitor STA-21 or DMSO control (20 μM, Santa Cruz). The cells were harvested at different time points for staining with CD33-APC, HLA-DR-FITC, CD11b-PE, and CD14-PerCP710, or isotype controls, followed by flow cytometric analysis.
Treg cell induction
CD33+HLA-DR− and CD33−HLA-DR− were isolated from PBMCs of HIV-1+ individuals by first selection of HLA-DR− populations, followed by positive selection of CD33+ cells using their microbeads according to the manufacturer's instructions (Miltenyi Biotec., cell purity > 95%). The purified immature myeloid cells or non-myeloid cells from HIV-1+ individuals were co-cultured with healthy CD4+ T cells, or autologous HIV-1 CD4+ T cells, respectively, in a ratio of 1:2 in the presence of 1 μg/ml anti-CD3 and 2 μg/ml anti-CD28 (BD Bioscience) for 3 days. Treg cell differentiation were analyzed by flow cytometry after staining with CD4-PE, CD25-Alexa488, Foxp3-PE-Cy5 (eBioscience), or isotype controls, using Foxp3 Staining Buffer Set (Milenyi Biotec).
Teff cell cytokine assays
PBMCs from HIV+ individuals, with or without depletion of CD33+ myeloid cells using CD33 microbeads (Miltenyi Biotec), were stimulated with 1 μg/ml anti-CD3 and 2 μg/ml anti-CD28 for 72 hours. The cells were treated with 1 μg/ml Brefeldin A for 5 hours before harvest. Teff intracellular cytokine expressions were assessed by IFN-γ-PE (BD Bioscience) or isotype control staining, followed by flow cytometry analysis as described above.
Statistical analysis
The data were summarized as mean ± standard error of the mean (SEM) or median with interquartile range, depending on the characteristics of the data distribution. Comparisons among groups were made by one-way analysis of variance (ANOVA) or Kruskal-Wallis test, followed with Dunn's or Fisher's multiple comparisons test. Independent t test or paired t test was used to compare the difference of mean between each two groups. Mann-Whitney test or Wilcoxon signed rank test was used to compare the difference of median between each two groups. All the data were analyzed using Minitab 17.0 and Graphpad Prism v6.0. *P<0.05, or **P<0.01, and ***P<0.001 were considered significant or very significant, respectively.
Results
M-MDSCs are expanded in HIV-1+ individuals on ART
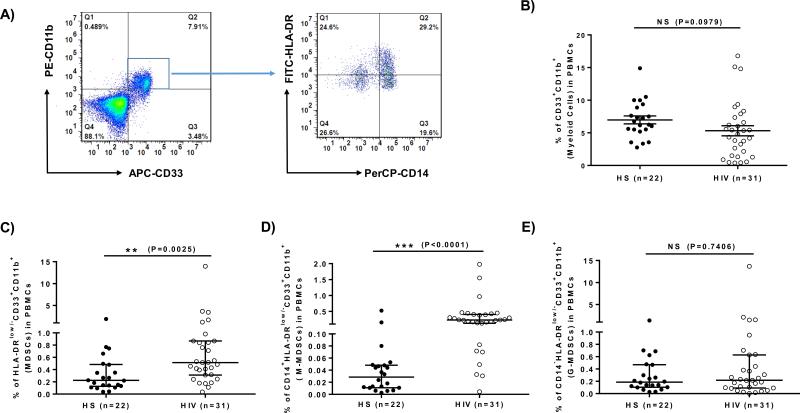
Phenotypically, human MDSCs express the common myeloid markers CD33 and CD11b, but lack the maturation marker HLA-DR [7-10]. As an initial approach to determine the role of MDSCs in the pathogenesis of HIV-1 persistence and latency, we first compared the frequency of MDSCs in the peripheral blood of 31 HIV-1+ individuals on ART with undetectable viremia and 22 HS. As shown in Fig.1, the representative dot plots of flow cytometry and summary data for the frequencies of different myeloid cell populations, PBMCs were first gated on CD11b+ and CD33+ myeloid cells, and then analyzed for the immature HLA-DRlow/− populations within the monocytic (CD14+) and granulocytic (CD14−) subsets (Fig.1A). Although the overall numbers of myeloid cells (CD33+CD11b+) in HIV-1+ individuals were slightly lower than those in HS (Fig.1B), the numbers of MDSCs (HLA-DRlow/−CD33+CD11b+) (Fig.1C), in particular the M-MDSC (CD14+HLA-DRlow/−CD33+CD11b+) subset (Fig.1D), but not the G-MDSC (CD14−HLA-DRlow/−CD3+CD11b+) subset (Fig.1E), were significantly higher in HIV-1+ individuals. These results indicate that latently HIV-infected patients on ART have elevated numbers of M-MDSCs in their peripheral blood, consistent with the reports of MDSC expansion in actively HIV-infected individuals [11-18]. While it has been reported that the elevated M-MDSC frequency in HIV-1 viremic patients correlated with prognostic disease markers, including the HIV-1 RNA level, CD4 T cell number, and activated T cells [13], we did not find such a correlation of MDSC and CD4 cell frequencies in these HIV-1 aviremic subjects on ART. These data are the first observation showing that HIV-1+ individuals on ART with undetectable viremia still exhibit expanded M-MDSCs.
Fig.1. Phenotypic analysis of myeloid cell frequencies in HIV-1 patients versus HS.
A) Representative dot plots for analysis of MDSCs by flow cytometry. CD33+CD11b+ myeloid cells were first gated in live cells of PBMCs isolated from HIV-1 patients and HS; monocytic MDSCs (M-MDSC, CD33+CD11b+CD14+HLA-DRlow/−) and granulocytic-MDSCs (G-MDSC, CD33+CD11b+CD14−HLA-DRlow/−) were further characterized by the expression level of CD14 and HLA-DR, respectively. B) Summary data of myeloid cell (CD33+CD11b+) frequencies in HS (filled circle, n=22) versus chronically HIV-infected patients (open circle, n=31). C) Pooled data of myeloid suppressor cell (CD33+CD11b+HLA-DRlow/−) frequencies in HS versus HCV. D) Pooled data of M-MDSC (CD14+CD33+CD11b+HLA-DRlow/−) frequencies in HS versus HCV. E) Pooled data of G-MDSC (CD14−CD33+CD11b+HLA-DRlow/−) frequencies in HS versus HCV. The frequencies of circulating MDSCs in the pooled data were calculated by the frequencies of CD33+CD11b+ cells in PBMCs multiplied by the frequencies of CD14+ HLA-DRlow/− or CD14− HLA-DRlow/− cells. Each symbol represents an individual subject, and the horizontal bars represent mean ± SEM (Fig.1B) or median with interquartile range (Fig.1C, 1D, 1E). **P<0.01; ***P<0.001; NS=no significance.
Immunosuppressive proteins are increased in M-MDSCs from HIV-1+ individuals
MDSCs are more accurately identified by their immunosuppressive functions rather than their phenotypic markers. MDSCs exert their suppressive activities via expression or secretion of copious amounts of immunosuppressive mediators [4-10]. For example, IL-4Rα, also known as CD124, is a 140kDa transmembrane glycoprotein that binds IL-4 and IL-13, which are primarily responsible for IL-4-dependent activation of transcription factors that induce Th2 cell expansion [20]. Programmed death-ligand 1 (PD-L1), also known as CD274 or B7-H1, is a 40kDa transmembrane protein that has been speculated to play a major role in suppressing the host immune system during inflammation or infection [21]. P47phox is a 47 kDa cytosolic subunit of the multi-protein complex NADPH oxidase that is responsible for respiratory burst of phagocytes during defense responses [22]. IL-10 and TGF-β are known to regulate immune responses via phosphorylation of STAT-3 (pSTAT-3) [23]. To functionally characterize MDSCs during HIV-1 infection, we measured these inhibitory proteins and regulatory cytokines that have been suggested to play a role in MDSC expansion or suppressive functions [4-10]. As shown in Fig.2A, the representative dot plots of flow cytometry, and Fig.2B-2G, summary data for the expression of different cell mediators in M-MDSCs, including IL-4Rα, PD-L1, p47phox, IL-10, TGF-β, and pSTAT-3, all these inhibitory mediators expressed in M-MDSCs were found to be significantly up-regulated in PBMCs from HIV-1+ individuals compared to HS, suggesting that these molecules are involved in the differentiation and/or suppressive functions of MDSCs during viral infection.
Fig.2. Analysis of IL-4Rα, PD-L1, p47phox, IL-10, TGF-β, and pSTAT-3 expressions in M-MDSCs from HIV-1 patients versus HS.
PBMCs isolated from HIV-1-infected patients (n=12) and HS (n=9) were stimulated with TLR ligand LPS/R848 for 6 h, immune stained for IL-4Rα, PD-L1, p47phox, IL-10, TGF-β, and pSTAT-3 in M-MDSCs, followed by flow cytometric analysis. A) Representative dot plots for analysis of IL-10 expression in MDSCs by flow cytometry. Monocytic myeloid cells (CD14+CD33+) were first gated in PBMCs, followed by analysis of IL-10 expression in HLA-DRlow/− populations. B) - G) Summary data of IL-4Rα+ M-MDSCs, PD-L1+ M-MDSCs, p47phox + M-MDSCs, IL-10+ M-MDSCs, TGF-β+ M-MDSCs, and pSTAT-3+ M-MDSCs in PBMCs from HS and HCV patients. Each symbol represents an individual subject, and the horizontal bars represent mean ± SEM (Fig.2B, 2C, 2F) or median with interquartile range (Fig.2D, 2E, 2G). *P<0.05.
HIV-1 proteins (gp120 and Tat) prevent myeloid cell maturation and drive MDSC expansion
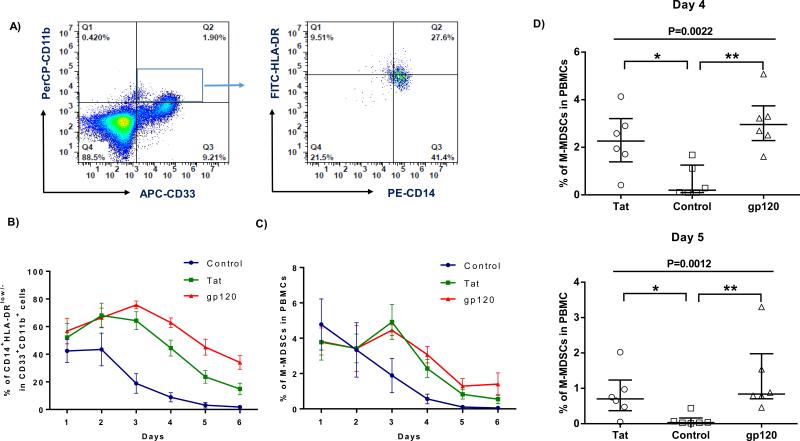
Despite several reports of increases in the numbers of MDSCs in HIV-1+ individuals, factors responsible for MDSC expansion during HIV-1 infection are unclear. Since patients with chronic HIV-1 infection often suffer from confounding factors that may drive MDSC expansion, we determined whether the increased numbers of MDSCs during HIV-1 infection were directly caused by HIV-1 or other factors. To this end, purified PBMCs from HS were incubated with recombinant HIV-1 gp120 or Tat, two important proteins that have been shown to exert immunomodulatory effects [14, 24-26], or the control protein β-gal for different times, followed by the analysis of myeloid cell differentiation and maturation with flow cytometry. As shown in Fig.3A, the representative dot plots of flow cytometry, and Fig.3B-3D, summary data for the frequencies of different myeloid cell populations by days after the treatment, healthy PBMCs treated with HIV-1 gp120 or Tat protein exhibited a slightly lower number of CD33+CD11b+ myeloid cells (data not shown), but a relatively higher number of CD14+ cells and HLA-DRlow/− cells, when compared to those treated with the control protein, leading to an increased CD14+HLA-DRlow/− subsets in the CD11b+CD33+ myeloid cell populations after the treatment (Fig.3B). Overall, an increased M-MDSCs in PBMCs was observed (Fig.3C), significantly higher at day 4-5 after gp120 or Tat treatment (3D), when compared to those exposed to the control protein. Notably, the frequencies of M-MDSCs decreased following PBMC incubation with the control protein, which is contributed by the decreased expression of CD14 but increased expression of HLA-DR maturation marker, indicating myeloid cell differentiation into a mature status when cultured in vitro over a period of time; whereas the CD14+HLA-DRlow/− population of myeloid cells remain in an immature state after exposure to HIV-1 proteins. These findings, which are in line with the observations in HIV-infected patients vs HS in vivo (Fig.1B and 1E), suggest that HIV-1 derived proteins prevent myeloid cell maturation and drive them toward differentiation into M-MDSCs.
Fig.3. HIV-1 proteins (gp120 and Tat) prevent myeloid cell maturation and drive MDSC differentiation.
PBMCs isolated from HS (n=6) were treated with HIV-1 gp120, Tat or control protein for different days as indicated, followed by flow cytometric analysis. A) Representative dot plots for analysis of myeloid cells by flow cytometry. CD33+CD11b+ myeloid cells were first gated in PBMCs treated with HIV-1 protein or control protein; monocytic MDSCs (M-MDSC, CD33+CD11b+CD14+HLA-DRlow/−) were further characterized by the expression level of CD14 and HLA-DR. B) Summary data for the percentage of CD14+HLA-DRlow/− cell frequencies in CD11b+CD33+ myeloid cells by days following various treatments. C) Summary data for the percentage of M-MDSC (CD33+CD11b+CD14+HLA-DRlow/−) induction in PBMCs by days after treatments as described above. D) Summary data for M-MDSC (CD33+CD11b+CD14+HLA-DRlow/−) induction with PBMCs treated by HIV-1 gp120 (triangle), Tat (circle), or control (square) protein for 4-5 days. Each symbol represent one particular donor, and the horizontal bars represent mean ± SEM (Fig.3B, 3C) or median with interquartile range (Fig.3D). *P<0.05, **P<0.01.
HIV-1 protein (gp120) induces MDSC development via the STAT-3 pathway
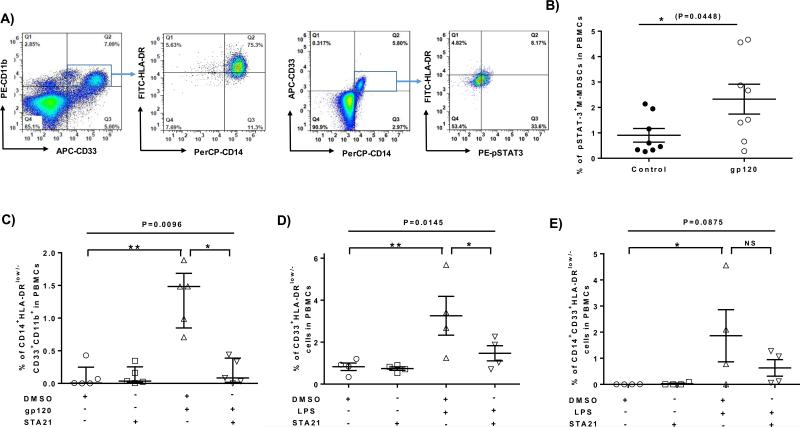
STAT-3 phosphorylation and activation has been shown to play a pivotal role in myelopoiesis [27-29]. To further study the role of STAT-3 signaling in HIV-1-induced expansion of M-MDSCs, we incubated healthy PBMCs with HIV-1 gp120 or control β-gal protein with or without the pSTAT-3 specific inhibitor STA-21 [30] for 5 days, followed by flow cytometric analysis. As shown in Fig.4A, the representative dot plots of flow cytometry and Fig.4B-4C, summary data for the frequencies of pSTAT-3 expression and M-MDSC development following the treatment, healthy PBMCs treated with HIV-1 gp120 exhibited a significantly higher number of pSTAT-3+ M-MDSCs compared to those exposed to the control protein. This in vitro result is in line with the findings in vivo that HIV-1+ individuals exhibit significantly higher numbers of pSTAT-3+ M-MDSCs compared to the HS (Fig.2G). In addition, the HIV-1 gp120-induced M-MDSC expansion could be abrogated by pSTAT-3 inhibitor when compared to those cells treated with DMSO control. Of note, STA-21 treatment alone did not affect the M-MDSC development. Overall, these data suggest that HIV-1 gp120 induces M-MDSC differentiation via the STAT-3 pathway.
Fig.4. Induction of M-MDSC development by HIV-1 gp120 via the STAT-3 pathway.
A) Representative dot plots for induction of M-MDSCs by HIV-1 gp120 via the STAT-3 pathway, analyzed by flow cytometry. PBMCs were first gated for CD33+CD11b+ or CD14+CD33+ myeloid cells, followed by analysis of CD14+HLA-DRlow/− or pSTAT-3+HLA-DRlow/− subsets. B) Summary data for the percentages of pSTAT-3+ M-MDSC frequencies in healthy PBMCs treated with HIV-1 gp120 or control protein for 3 days. C) Summary data for the percentages of M-MDSCs in PBMCs with different treatments for 5 days. D) Summary data for the percentages of CD33+HLA-DRlow/− cells in PBMCs treated with or without LPS in the presence of STA-21 or DMSO for 5 days. E) Summary data for the percentages of CD14+CD33+HLA-DRlow/− cells in PBMCs treated without or with LPS in the presence of DMSO or STA-21 for 5 days. Each symbol represents one subject with relevant treatment, and the horizontal bars represent mean ± SEM (Fig.4B, 4D, 4E) or median with interquartile range (Fig.4C). *P<0.05, **P<0.01, NS = no significance.
HIV-1 or its proteins may induce MDSC differentiation in the active or early phase of viral infection, but it remains unclear what factors can induce or maintain MDSC generation in the latent phase of viral infection, in particular for patients on ART with undetectable viremia (Fig.1). Recent reports suggest that ART-controlled, HIV-1+ individuals exhibit a chronic inflammatory state with over-activation of the immune system and T cell exhaustion during latent viral infection, which can be promoted by multiple inflammatory mediators, including TLR ligands such as LPS [31-34]. In addition, we have recently observed, in a gene array analysis, that TLR4 was upregulated on CD33+ myeloid cells derived from HIV-1+ individuals as well as HCV-infected individuals versus HS (data not show), suggesting that TLR4 pathway may be involved in MDSC expansion during viral infections. To determine whether TLR4 stimulation can cause MDSC development through the STAT-3 pathway, we incubated healthy PBMCs with or without LPS in the presence of pSTAT-3 inhibitor STA-21 or DMSO for 5 days, followed by flow cytometric analysis for the MDSC development. As shown in Fig.4D, PBMCs treated with LPS in vitro resulted in a significant increase in CD33+HLA-DRlow/− MDSCs, and these LPS-induced MDSC increases could be abrogated by the presence of pSTAT-3 inhibitor STA-21 when compared to the DMSO control. Of note, monocytic marker CD14 expression was almost lost on the surface of myeloid cells when they were cultured in vitro for 5 days, whereas LPS treatment prevented CD14 loss and HLA-DR expression, resulting in an inhibition of myeloid cell maturation and increase in M-MDSC numbers (Fig.4E). Again, these LPS-induced M-MDSCs were diminished by the presence of STAT-3 inhibitor. Taken together, these results indicate that, in addition to HIV-1 proteins, LPS/TLR4-mediated inflammatory response can also induce MDSC development via the STAT-3 pathway.
MDSCs promote Treg cell development during HIV-1 infection
In addition to producing inhibitory proteins, it has been suggested that MDSCs exert their immunosuppressive functions by inducing CD4+CD25+Foxp3+ Treg cell differentiation in cancer patients and organ transplant recipients [35-37]. Therefore, we next sought to determine whether HIV-induced MDSCs can induce Foxp3+ Treg development. To this end, we first incubated healthy CD4+ T cells with or without MDSCs derived from HIV+ individuals for 3 days, followed by flow cytometric analysis for the differentiation of CD25+Foxp3+ Treg cells. As shown in Fig.5A, the representative dot plots of flow cytometry and summary data for the co-culture experiments, healthy CD4+ T cells co-cultured with CD33+ myeloid cells isolated from HIV+ individuals induced a significant increase in Foxp3+ Tregs when compared to healthy CD4+ T cells incubated alone without the presence of HIV CD33+ myeloid cells. Given the heterogeneous populations of MDSCs, we further tested the role of MDSCs in induction of Tregs by co-culturing healthy CD4+ T cells with or without HIV immature myeloid cells (CD33+HLA-DR−). In this case, we use HIV immature non-myeloid cells (CD33−HLA-DR−), in addition to the HS CD4+ T cells cultured alone, as controls. As shown in Fig.5B, healthy CD4+ T cells co-cultured with CD33+HLA-DR− cells derived from HIV+ individuals induced a significant increase in Foxp3+ Tregs when compared to those co-cultured with HIV immature non-myeloid control cells (CD33−HLA-DR−), or healthy CD4+ T cells incubated alone (baseline). Since accumulation of Tregs is a characteristic of many chronic infectious diseases, including latent HIV-1 infection on ART [38-39], we also examined whether MDSCs are essential for maintaining the Foxp3+ Treg cells in the cultured conditions. To this end, we incubated HIV CD4+ T cells with or without autologous MDSCs ex vivo for 3 days, followed by flow cytometric analysis for the frequencies of Foxp3+ Tregs. As shown in Fig.5C, purified HIV CD4+ T cells incubated alone without the presence of HIV CD33+HLR-DR− MDSCs resulted in a significant decrease in Foxp3+ Treg cells when compared to those cultured with HIV CD33+HLA-DR− MDSCs. In addition, HIV CD4+ T cells co-cultured with HIV immature non-myeloid cells (CD33−HLA-DR−, MDSC depleted) also resulted in a significant decrease in the Foxp3+ Treg cells when compared to those co-cultured with HIV immature myeloid cells (CD33+HLA-DR−). Taken together, these results indicate that MDSCs promote Treg cell development during HIV-1 infection.
Fig.5. MDSCs from HIV-1 patients induce Foxp3+ Treg cell differentiation.
A) Representative dot plots and summary data of flow cytometric analysis for the induction of CD25+Foxp3+ Treg cells by co-culture of healthy CD4+ T cells with or without CD33+ myeloid cells derived from HIV+ individuals. B) Induction of Treg cells by co-culture of healthy CD4+ T cells with or without immature myeloid cells (CD33+HLA-DR−) or immature non-myeloid cells (CD33−HLA-DR−) derived from HIV+ individuals. C) Depletion of immature myeloid cells from HIV patients reduces Treg cells. CD4+ T cells isolated from HIV+ individuals were co-cultured with or without autologous CD33−HLA-DR− or CD33+HLA-DR− cells for 3 days, followed by immune staining and flow cytometric analysis for CD25+Foxp3+ Treg cells. Each symbol represents one subject with relevant treatment, and the horizontal bars represent mean ± SEM (Fig.5B, 5E) or median with interquartile range (Fig.5C, 5D). D) Depletion of MDSCs from HIV patients boosts IFN-γ production by CD4+ Teff cells. PBMCs derived from HIV-1 patients, with or without depletion of CD33+ myeloid cells, were cultured ex vivo in the presence of anti-CD3/CD28 stimulation, followed by intracellular staining for IFN-γ and flow cytometric analysis. Representative overlaid histogram of flow cytometry and cumulative results for the percentages as well as the MFI of IFN-γ expression by CD4+ T cells from 8 HIV-1+ patients are shown. Each symbol represents one individual subject, and the horizontal bars represent mean ± SEM (MFI) or median with interquartile range (Percentage). *P<0.05, **P<0.01, ***P<0.001.
MDSCs inhibit IFN-γ production by Teff cells during HIV-1 infection
In addition to inducing Treg cell development, we also studied whether MDSCs from HIV+ individuals can directly or indirectly inhibit Teff cell functions by depletion experiments. To this end, PBMCs from HIV-1+ individuals were depleted for CD33+ myeloid cells (enrich in MDSCs), stimulated by anti-CD3 and anti-CD28 for 3 days, followed by flow cytometric analysis for IFN-γ production by CD4+ T cells. As shown in Fig.5D, the representative overlaid histogram of flow cytometry and summary data for the IFN-γ production by CD4+ T cells, compared to the cultures of bulk PBMCs, depletion of CD33+ myeloid cells from PBMCs derived from HIV+ individuals resulted in a significant increase of IFN-γ production by CD4+ T cells. This holds true by measuring both the percentage of IFN-γ expressing CD4+ T cell frequencies and the mean florescence intensity (MFI) of the IFN-γ expression levels in CD4+ T cells. Overall, these results indicate that HIV-mediated MDSCs can inhibit Teff cell functions.
Discussion
Since the discovery of MDSCs, there has been rapid progress in the understanding of this particular type of suppressor cells in tumor progression and antitumor response. However, the role of MDSCs in viral persistence or latency remains controversial and far from clear. For example, while some studies have shown that monocytic MDSCs are remarkably elevated and play a role in the pathogenesis of HIV or HCV infection [13-15, 40-42], Bowers et al [16] reported an immune suppression by neutrophils during HIV-1 infection; whereas Nonnenmann et al [43] found no significant increases in MDSCs and lack of their suppressive functions in peripheral blood of chronically HCV-infected individuals. We postulated that these discrepancies in MDSC frequency and suppressive capability might partially result from measuring different MDSC subsets, using different methodologies, investigating different patient populations and/or focusing on different disease stages with specific cytokine milieus that may induce distinct MDSC phenotypes. In this study, we observed substantial increases in the monocytic (CD14+) immature (HLA-DRlow/−) myeloid cells (CD33+CD11b+) in latently HIV-1-infected patients on ART with undetectable viremia. These M-MDSCs, expressing significantly high levels of IL-4Rα, PD-L1, P47phox, IL-10, TGF-β, and pSTAT-3, could induce Foxp3+ Treg cell expansion and inhibit IFN-γ production by CD4+ Teff cells, which may play a pivotal role in the chronicity of HIV-1 infection.
The mechanism underlying MDSC expansion in virus-infected individuals remains unclear. It has been suggested that the generation of MDSCs can be induced by the virus itself or by its coding proteins, including HCV, HIV, and lymphocytic choriomeningitis virus [13-15, 40-42, 44]. For example, Garg and Spector [14] reported that HIV gp120-induced MDSC expansion is dependent upon IL-6 signaling. Here, we demonstrated that HIV-1 gp120 can induce M-MDSC expansion via the STAT-3 pathway, which is closely related to IL-6 signaling. While HIV-1 or its proteins may induce MDSC development in the active or early phase of viral infection, the mediators involved in induction or maintenance of MDSC development in the latent phase of viral infection have not been identified, in particular for patients well-controlled by effective ART, which is our targeted population in this study. Notably, MDSCs expand in nearly all conditions where inflammation exists [1-3], and compelling data have shown that latently HIV-infected individuals, despite being well-controlled by ART and exhibiting unmeasurable viral RNA or proteins, still exhibit a dampened immunity, as evidenced by low expression of Th1 cytokines and poor response to vaccinations [19]. These latently HIV-infected patients exhibit a chronic inflammatory state with immune cells over-activation followed by exhaustion or anergy and/or premature T cell aging or senescence; a series of detrimental effects that appear to be a major factor in HIV-1 disease progression [31-34, 45]. Therefore, chronic inflammation or TLR stimulation, as we show LPS-mediated MDSC expansion via the STAT3 pathway in this study, may be the continuing impetus underlying the expansion of MDSCs during the latent phase of HIV-1 infection. These findings underscore the importance of transcription factor STAT-3 in the regulation of MDSC development and suppressive functions, and call for the use of STAT-3 as a potential therapeutic target for infection-associated expansion of MDSCs, during which pSTAT-3 is often induced [26-28]. Indeed, inhibition of STAT-3 signaling has been shown to directly regulate the production of immunosuppressive cytokines, such as IL-6 and IL-10 [29, 46].
In summary, this study demonstrates that HIV proteins or inflammatory mediators such as LPS-induced, STAT-3-mediated MDSC expansion contribute to Treg cell development and Teff cell suppression during HIV infection, shedding new light on the features of MDSCs and providing a novel mechanism for T cell regulation in chronic viral infection. Therefore, targeting MDSCs and/or STAT-3 signaling pathway may be a promising strategy for immunotherapy to treat human viral diseases.
Acknowledgements
This work was supported by an NIH NIDDK grant to ZQY/JPM (R01DK093526), an NIH NIAID grant to ZQY/JPM (R01AI114748). The role of each of the authors in this study: Ling Wang, Juan Zhao, and Jun P. Ren performed the experiments and analyzed the data; Xiao Y. Wu and Zheng D. Morrison provided technical supports; Mohamed A Elgazzar, Shun B. Ning, and Jonathan P. Moorman participated the data discussion and manuscript revision; Zhi Q. Yao designed the experiments and wrote the manuscript. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-Derived Suppressor Cells: Paradoxical Roles in Infection and Immunity. J. Innate Immun. 2015;7:116–26. doi: 10.1159/000368233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 7.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 8.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 10.Vasquez-Dunddel D1, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vollbrechi T, Striner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. 2012;26:F31–37. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 12.Macatangay BJ, Landay AL, Rinaldo CR. MDSC: a new player in HIV immunopathogenesis. AIDS. 2012;26:1567–1569. doi: 10.1097/QAD.0b013e328355e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J. Virol. 2013;87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg A, Spector SA. HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J. Infect. Dis. 2014;209:441–451. doi: 10.1093/infdis/jit469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gama L, Shirk EN, Russell JN, Carvalho KI, Li M, Queen SE, Kalil J, Zink MC, Clements JE, Kallas EG. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J Leukoc Biol. 2012;91:803–16. doi: 10.1189/jlb.1111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: Role of PD-L1/PD-1 pathway. PLoS Pathog. 2014;10:e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony DD, Umbleja T, Aberg JA, Kang M, Medvik K, Lederman MM, Peters MG, Koziel MJ, Overton ET. Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals. Vaccine. 2011;29:3558–63. doi: 10.1016/j.vaccine.2011.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehraj V, Jenabian MA, Vyboh K, Routy JP. Immune Suppression by Myeloid Cells in HIV Infection: New Targets for Immunotherapy. Open AIDS J. 2014;8:66–78. doi: 10.2174/1874613601408010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao ZQ, Moorman JP. Immune exhaustion and immune senescence – two distinct pathways for HBV vaccine failure during HCV and/or HIV infection. AITE. 2013;61:193–201. doi: 10.1007/s00005-013-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 21.Said Elias A., et al. PD-1 Induced IL10 Production by Monocytes Impairs T-cell Activation in a Reversible Fashion. Nature Medicine. 2009;2010;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamel El-Benna Pham My-Chan Dang, Marie-Anne Gougerot-Pocidalo Jean-Claude Marie, Braut-Boucher Françoise. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Experimental & Molecular Medicine. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchins AP, Takahashi Y, Miranda-Saavedra D. Genomic analysis of LPS-stimulated myeloid cells identifies a common pro-inflammatory response but divergent IL-10 anti-inflammatory responses. Sci Rep. 2015;5:9100. doi: 10.1038/srep09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Pauza CD. Critical roles for Akt kinase in controlling HIV envelope-mediated depletion of CD4 T cells. Retrovirology. 2013;10:60. doi: 10.1186/1742-4690-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovav AH, Santosuosso M, Bivas-Benita M, Plair A, Cheng A, Elnekave M, Righi E, Chen T, Kashiwagi S, Panas MW, Xiang SH, Furmanov K, Letvin NL, Poznansky MC. X4 human immunodeficiency virus type 1 gp120 down-modulates expression and immunogenicity of codelivered antigens. J Virol. 2009;83:10941–50. doi: 10.1128/JVI.00394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Boppana R, Mishra GC, Saha B, Mitra D. HIV-1 Tat suppresses gp120-specific T cell response in IL-10-dependent manner. J Immunol. 2008;180:79–88. doi: 10.4049/jimmunol.180.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Sido JM, Yang X, Nagarkatti PS, Nagarkatti M. Δ9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J Leukoc Biol. 2015;97:677–88. doi: 10.1189/jlb.1A1014-479R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, Garrett WS. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015;12:244–57. doi: 10.1016/j.celrep.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S, Kortylewski M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin Cancer Res. 2015;21:3771–82. doi: 10.1158/1078-0432.CCR-14-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song H, Wang RX, Wang SM, Lin JY. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. PNAS. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H, Gao J, Taxman DJ, Ting JP, Su L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem. 2014;289:21716–26. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Routy JP, Angel JB, Patel M, Kanagaratham C, Radzioch D, Kema I, Gilmore N, Ancuta P, Singer J, Jenabian MA. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015;16:48–56. doi: 10.1111/hiv.12171. [DOI] [PubMed] [Google Scholar]
- 33.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, 3rd, Blankson JN, Pardoll D, Cox AL. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang JJ, Altfeld M. Immune activation and the role of TLRs and TLR agonists in the pathogenesis of HIV-1 infection in the humanized mouse model. J Infect Dis. 2013;208(Suppl 2):S145–9. doi: 10.1093/infdis/jit402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J, Murphy B. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant. 2013;13:3123–31. doi: 10.1111/ajt.12461. [DOI] [PubMed] [Google Scholar]
- 37.Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, Eckart MJ, Krause SW, Oefner PJ, Le Blanc K, Mackensen A, Mougiakakos D. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124:750–60. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 38.Saison J, Maucort Boulch D, Chidiac C, Demaret J, Malcus C, Cotte L, Poitevin-Later F, Miailhes P, Venet F, Trabaud MA, Monneret G, Ferry T. Increased Regulatory T-Cell Percentage Contributes to Poor CD4(+) Lymphocytes Recovery: A 2-Year Prospective Study After Introduction of Antiretroviral Therapy. Open Forum Infect Dis. 2015;2:ofv063. doi: 10.1093/ofid/ofv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao YM, Liu CE, Luo LJ, Zhu WJ, Zhang T, Zhang LG, Su LS, Li HJ, Wu H. CD4+CD25+CD127 regulatory cells play multiple roles in maintaining HIV-1 p24 production in patients on long-term treatment: HIV-1 p24-producing cells and suppression of anti-HIV immunity. Int J Infect Dis. 2015;37:42–9. doi: 10.1016/j.ijid.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng QL, Yang B, Sun HQ, Feng GH, Jin L, Zou ZS, Zhang Z, Zhang JY, Wang FS. Myeloid-derived suppressor cells are associated with viral persistence and downregulation of TCR ζ chain expression on CD8+ T cells in chronic hepatitis C patients. Mol. Cells. 2014;37:66–73. doi: 10.14348/molcells.2014.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, Xu M, Fu Y, Zhou J, Tang X. Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J. Clin. Immunol. 2013;33:798–808. doi: 10.1007/s10875-012-9861-2. [DOI] [PubMed] [Google Scholar]
- 43.Nonnenmann J, Stirner R, Roider J, Jung MC, Schrudl K, Bogner JR, Draenert R. Lack of significant elevation of myeloid-derived suppressor cells in peripheral blood of chronically hepatitis C virus-infected individuals. J Virol. 2014;88:7678–82. doi: 10.1128/JVI.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity. 2013;38:309–21. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandberg JK, Falconer K, Gonzalez VD. Chronic immune activation in the T cell compartment of HCV/HIV-1 co-infected patients. Virulence. 2010;1:177–9. doi: 10.4161/viru.1.3.11206. [DOI] [PubMed] [Google Scholar]
- 46.Korrylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]