Abstract
Purpose
Mature sperm cells can be found in testicular specimens extracted from azoospermic men with non-mosaic Klinefelter syndrome (KS). The present study evaluates the expression of various known molecular markers of spermatogenesis in a population of men with KS and assesses the ability of those markers to predict spermatogenesis.
Methods
Two groups of men with non-obstructive azoospermia who underwent testicular sperm-retrieval procedures were included in the study: 31 had non-mosaic KS (KS group) and 91 had normal karyotype (NK group). Each group was subdivided into mixed atrophy (containing some mature sperm cells) or Sertoli cell only syndrome according to testicular histology and cytology observations. Semi-quantitative histological morphometric analysis (interstitial hyperplasia and hyalinization, tubules with cells and abnormal thickness of the basement membrane) and expression of spermatogenetic markers (DAZ, RBM, BOLL, and CDY1) were evaluated and compared among those subgroups.
Results
Clear differences in the histological morphometry and spermatogenetic marker expression were noted between the KS and NK groups. There was a significant difference in the expression of spermatogenetic markers between the subgroups of the NK group (as expected), while no difference could be discerned between the two subgroups in the KS group.
Conclusion
We conclude that molecular spermatogenetic markers have a pattern of expression in men with KS that is distinctively different from that of men with NK, and that it precludes and limits their use for predicting spermatogenesis in the former. It is suggested that this difference might be due to the specific highly abnormal histological morphometric parameters in KS specimens.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0698-0) contains supplementary material, which is available to authorized users.
Keywords: Klinefelter syndrome, Azoospermia, Spermatogenesis, Infertility, Spermatogenic markers
Introduction
Although most men with non-mosaic Klinefelter syndrome (KS) have azoospermia [1, 2], it is well established that mature sperm cells can be found in testicular biopsies extracted from at least 40 % of them [3]. The mechanisms that enable sperm production in non-mosaic KS patients are still unknown, as is the genetic etiology for the presence or absence of sperm cells. Age has been proposed as one possible factor affecting the probability of sperm detection in men with KS [4, 5]. Occasional loss of one X-chromosome may allow the formation of clones of diploid spermatogonia capable of progressing and even achieving complete spermatogenesis [6]. The association between azoospermia factor (AZF) deletions and KS is controversial [7–11]. However, most studies showed no association between complete deletions of the Y-chromosome and KS [7–9].
Genes that are expressed during spermatogenesis encode proteins necessary for specific stages of sperm cell development as well as for maintaining the general functions of the cells involved. Of interest are the genes found in the azoospermic factor regions (AZF) at the Y-chromosome that tend to be deleted in infertile men. RNA binding motif (RBM) and deleted in azoospermia (DAZ) genes were isolated as candidate genes in controlling spermatogenesis from the AZF regions AZFb and AZFc, respectively [12, 13]. Both of them express exclusively in testicular germ cells and encode proteins that bind RNA, and both might be involved in its regulation and metabolism. ChromoDomain Y1 (CDY1) genes are of special interest because they are included in the AZFc region (the most frequently AZF-deleted region), and their expression in testicular tissue correlates with complete spermatogenesis [14, 15]. CDY1 genes belong to the CDY family of genes. CDY proteins were postulated to be involved in the spermiogenetic process and transcriptional co-repressor [16, 17]. An additional gene of interest that participates in the spermatogenesis process is the highly conserved autosomal BOULE gene. It belongs to the deleted in azoospermia (DAZ) gene family, and its quantitative expression in the human testis seems to be a good predictor of finding sperm cells in azoospermic men [18, 19]. BOULE protein expression in men with complete spermatogenesis was found to be restricted to different stages of spermatocytes [20] or round spermatids [21]. BOULE protein expression was completely lacking in spermatocytes of testicular biopsies with meiotic arrest [20]. It was suggested that BOULE may encode a key switch that regulates the progression of germ cells through meiosis and spermiogenesis [18, 22, 23].
Lack of expression of RBM, DAZ, and CDY genes was associated with spermatogenic impairments leading to the absence of testicular sperm cells in azoospermic men [14]. Expression in conjunction with both CDY1 and BOLL was recently shown to be a sensitive and feasible indicator for predicting the presence of sperm cells in the testicular tissue of men with non-obstructive azoospermia (NOA) [24]. Isolated focal spermatogenesis may exist in the testes of men with NOA, and tubules containing mature sperm cells can be missed by testicular sperm extraction (TESE) [25]. Therefore, genetic markers that predict spermatogenesis were proposed to assist in deciding whether to repeat testicular exploration for sperm cells when none were retrieved in the first trial [14, 26, 27]. Given that the etiology of azoospermia in KS men is different than the etiology in normal karyotype (NK) men, we questioned if the referred markers could be useful for predicting spermatogenesis in men with KS.
The present study evaluated, for the first time, the predictive efficiency of the previously referred molecular markers in spermatogenesis detection among men with KS compared with men with normal karyotype (NK), both groups comprised men with NOA. In addition to the assessment of the expression of pre-meiotic (RBM, DAZ, BOLL) and post-meiotic (CDY1) testicular markers, the study included meticulous histopathological analysis of testicular biopsies.
Materials and methods
Study population
The study included men with NOA who underwent testicular sperm-retrieval procedures. A total of 31 men diagnosed as having non-mosaic KS (47,XXY) were included in the KS group, while the second group included 91 men with NK. All 122 men presented with primary infertility of 1–9 years and consented to participate in the study. Repeated semen analyses (at least twice) with high-speed centrifugation established azoospermia. All the men with KS underwent genetic counseling.
The 31 men with KS, recruited between 1998 and 2012, underwent a complete andrological evaluation that included a physical examination and hormonal tests. Their mean ± SD age was 32 ± 3.4 years (range, 28–40), and their follicle-stimulating hormone (FSH) level was in the high abnormal range. A total of 25 men with KS consented to undergo Y-chromosome microdeletion assessment, and they were all found to have a complete Y-chromosome.
The control group (NK) included 91 men who underwent TESE in our institute and were found to have mixed atrophy or Sertoli cells only (SCO) [25]. Cases with complete meiotic arrest were excluded since none were detected among the men with KS. Their mean age was 34 ± 0.6 years (range, 23–57) and their FSH level was in the high abnormal range in 71 % of them. The entire group underwent karyotype and Y-chromosome microdeletion analysis, and all but one had normal karyotype and Y-chromosome: the exception was one man with NK who had complete AZFc microdeletion. All the men with NK underwent additional histological and expression evaluation and 16 of them underwent morphometric assessment as well.
The RNA expression of the spermatogenesis markers of 31 testicular specimens from men with KS were compared with 91 specimens of NK men who had similar testicular impairments (i.e., mixed atrophy or SCO). The expression of 53 of these 91 NK specimens was reported elsewhere [28]. The present study was approved by the local Institutional Review Board (IRB) committee in accordance with the Helsinki Declaration of 1975, and all the participants signed a written consent.
Testicular sperm extraction procedures
All the subjects enrolled in this study underwent a surgical procedure for testicular sperm extraction under general anesthesia. Between one and three samples (according to testis size) were taken from each testis, as described elsewhere [25]. Smears of each testicular biopsy were taken and immediately examined in our lab to be used as an additional means of sperm identification as well as for cytological evaluation. In the laboratory, the testicular tissue samples taken from each testis (1–3 samples) were treated and examined separately. Each sample was minced using 25-gauge sterile needles. The shredded tissue was collected, centrifuged at 300×g for 5 min and, after removing the supernatant, the pellet was suspended in human tubal fluid medium (Irvine Scientific) supplemented with 1 % human serum albumin (Kamapharm Human Albumin; Kamada, Kibbutz Beit Kama, Israel). The suspensions underwent a thorough search under high-power magnification using an inverted microscope as described elsewhere [25]. Testicular cell suspension containing sperm cells were then cryopreserved in a freezing medium (Irvine Scientific) in several tubes for future intracytoplasmic sperm injection cycles. A tiny part of a testicular biopsy (<10 mg) from one of the testis was dedicated to the molecular analysis.
Testicular tissue evaluation and semi-quantitative morphometric analysis
The extracted testicular biopsies underwent cytological and histological evaluations in both groups as described elsewhere [14, 15]. They were classified and included in the two subgroups according to the most advanced spermatogenic cells detected in the combined cytological and histological findings. The men with KS were classified into either mixed atrophy (when elongated spermatids/spermatozoa were detected in addition to the tubules with SCO) or SCO (when no spermatogenetic cells were detected by the combined histology and cytology). There was no evidence of arrest at the spermatocyte stage among the KS specimens.
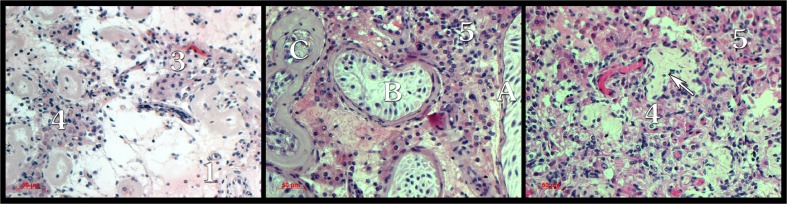
Paraffin-embedded sections were stained with hematoxylin/eosin (H&E) and histologically analyzed for the presence of germ cells. Additional histological morphometric parameters were evaluated in the paraffin-embedded sections of the 31 KS and 16 NK specimens which were chosen randomly. These included hyperplasia of the interstitial tissue, percent of hyalinized interstitium, number of tubules with cells (presence of at least Sertoli cells), and percent of tubules with normal basement membrane thickness. The values of the parameters calculated in each specimen were the result of the average of five fields assessed at ×200 magnification. Hyperplasia was measured on a scale of 1–5, where 1 represents normal concentration of interstitial cells and 5 represents severe hypertrophy. H&E-stained biopsies illustrating morphometric measurements are presented in Fig. 1.
Fig. 1.
Histological testicular illustrations of the morphometric measurements. Extent of interstitial hyperplasia: 1 low; 3–5 high. Arrow, interstitial hyalinization. Tubule with normal (A), mild (B), or thick and severely hyalinized membrane (C)
Evaluation of RNA spermatogenic markers
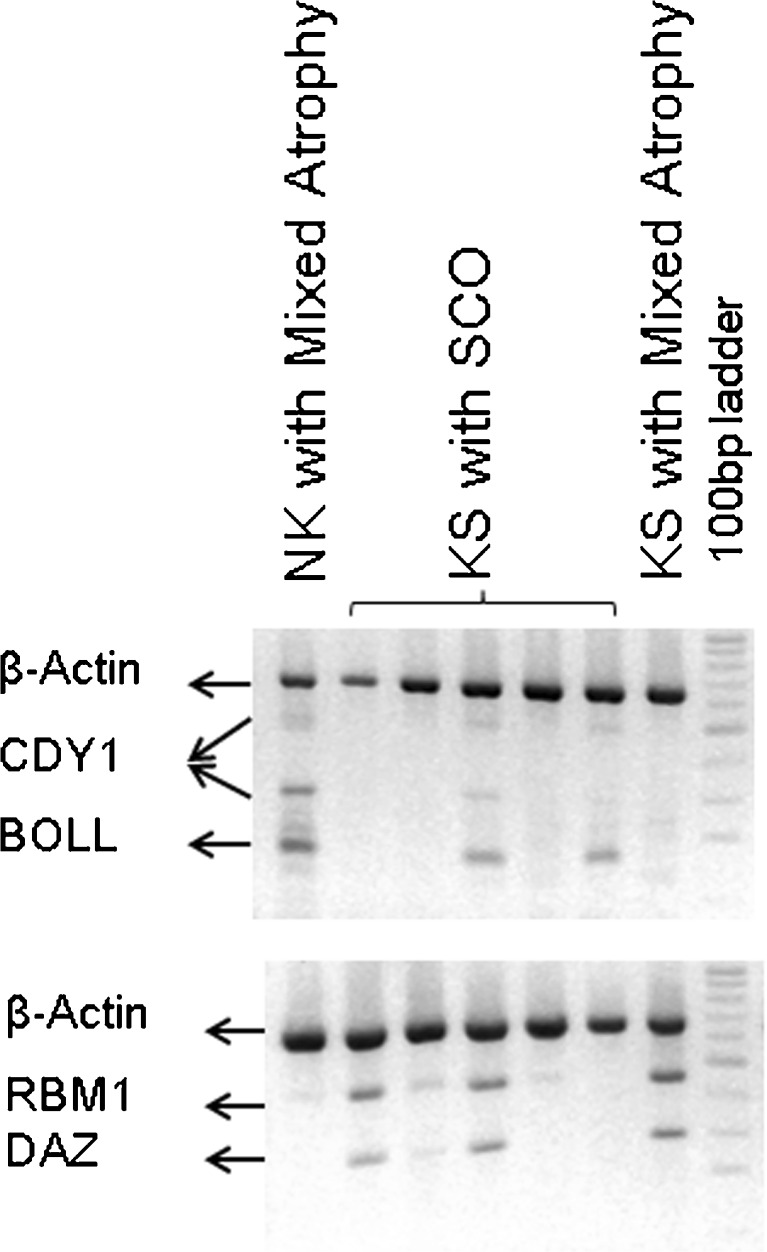
The expression of the transcripts RBM, DAZ, CDY, and β-ACTIN was assessed in the testicular tissue of 31 men with KS and 91 men with NK (Fig. 2) by reverse transcriptase with poly-dT oligonucleotides followed by multiplex gene amplification (RT-multiplex PCR) as previously described [24]. BOLL expression was analyzed in 24 KS and 41 NK specimens. The oligonucleotides primer sequences used for their amplification were reported elsewhere [14, 24]. The sample was further examined by single-gene PCR (RT-single PCR) whenever there was an absence of expression. The expression of β-ACTIN was evaluated in order to have an internal control for the RNA isolation and efficiency of the RT-PCR. DAZ, RBM, and BOLL expressions were indicative of the presence of pre-meiotic and meiotic germ cells, while CDY1 expression of post-meiotic cells was indicative of complete spermatogenesis [14, 24]. These germ cells transcripts were referred as “spermatogenetic markers.”
Fig. 2.
Detection of spermatogenetic markers expression by multiplex RT-PCR. NK NOA men with normal karyotype, KS men with Klinefelter syndrome, SCO Sertoli cell only
Statistics
Fisher’s exact test was used to assess the association between categorical parameters (RT-PCR gene expression and the testicular histological in conjunction with the cytological findings). The Mann-Whitney test was applied to assess the differences in numerical parameters (morphometric parameters) between the mixed atrophy and the SCO specimens in each group (KS and NK) and between the NK and KS groups. All statistics were performed at the Statistical Laboratory of our University.
Results
Sperm extraction in TESE procedures
All the men who underwent TESE were azoospermic. Three biopsies were obtained from the analyzed testis in the majority of the men with NK (80 %). Two or three testicular biopsies were taken from the men in the KS group (61 and 26 %, respectively), and mature sperm cells were found in nine of them (29 %). The rate of testicular sperm retrieval in KS was low (29 %) compared with the rate of retrieval in NK (62 %) [29].
Testicular tissue evaluation and semi-quantitative morphometric analysis
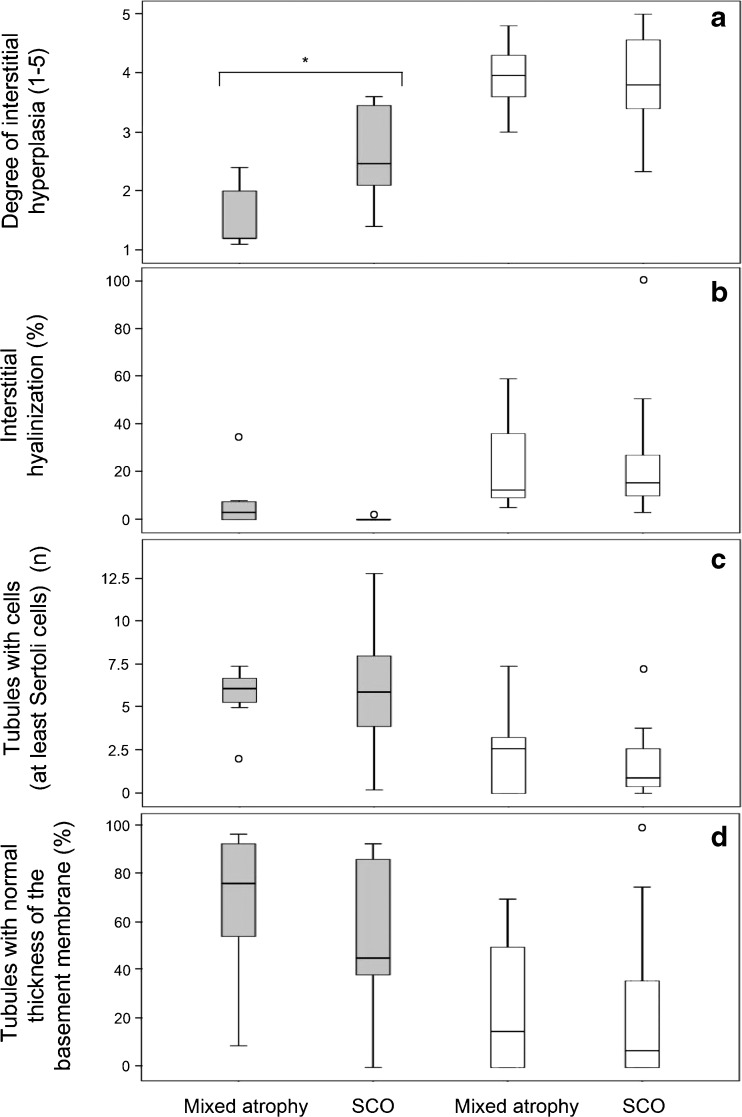
The KS and NK groups were classified into mixed atrophy and SCO according to the combined histology and cytology assessment. The histological features of the KS and NK subgroups were further assessed by a quantitative morphometric analysis for a better estimate of their pathological differences. There was no difference between the two KS subgroups (n = 31 in total) with regard to the degree of interstitial hyperplasia, the percent of hyalinized interstitial surface, the number of tubules with cells (at least Sertoli cells), and the percent of tubules with a normal thickness of the basal membrane (Mann-Whitney test, P > 0.5, Fig. 3). One specimen was excluded from the calculations of average hyperplasia because it had complete hyalinization of the interstitium.
Fig. 3.
Morphometric measurements of 31 men with Klinefelter syndrome (KS) and 16 men with normal karyotype (NK) are presented by “box and whisker” plots. The men were classified according to overall histological and cytological testicular findings into mixed atrophy and Sertoli cells only (SCO). The KS group (white boxes) included nine mixed atrophy specimens and 22 SCO specimens. The NK group (gray boxes) included eight specimens in each subgroup. Each box encloses the first and third quartiles and the median of the data (the band inside the box). The vertical lines at the top and bottom of the box indicate the range of “typical” data values. Extreme values are displayed as empty circles. Significant differences (P < 0.005) between KS and NK in both mixed atrophy and SCO were observed in a–d. There was a significant difference (P < 0.015) between the NK subgroups in the degree of interstitial hyperplasia (asterisk)
Significant differences between the KS and NK groups with regard to all the morphometric parameters that were assessed were demonstrated in both the mixed atrophy and SCO subgroups (Mann-Whitney test, P < 0.05) (Fig. 3). The interstitium parameters that were measured by the degree of hyperplasia and hyalinization showed greater damage in the KS specimens than in the NK specimens. The average number of tubules with at least Sertoli cells observed per field in the KS specimens was lower than in the NK ones, and the majority of them had a thicker basal membrane.
Unlike the KS group, there was a higher degree of interstitial hyperplasia in the SCO subgroup compared with the mixed atrophy subgroup of the NK group (P = 0.015, Fig. 3a).
Molecular markers for predicting spermatogenesis
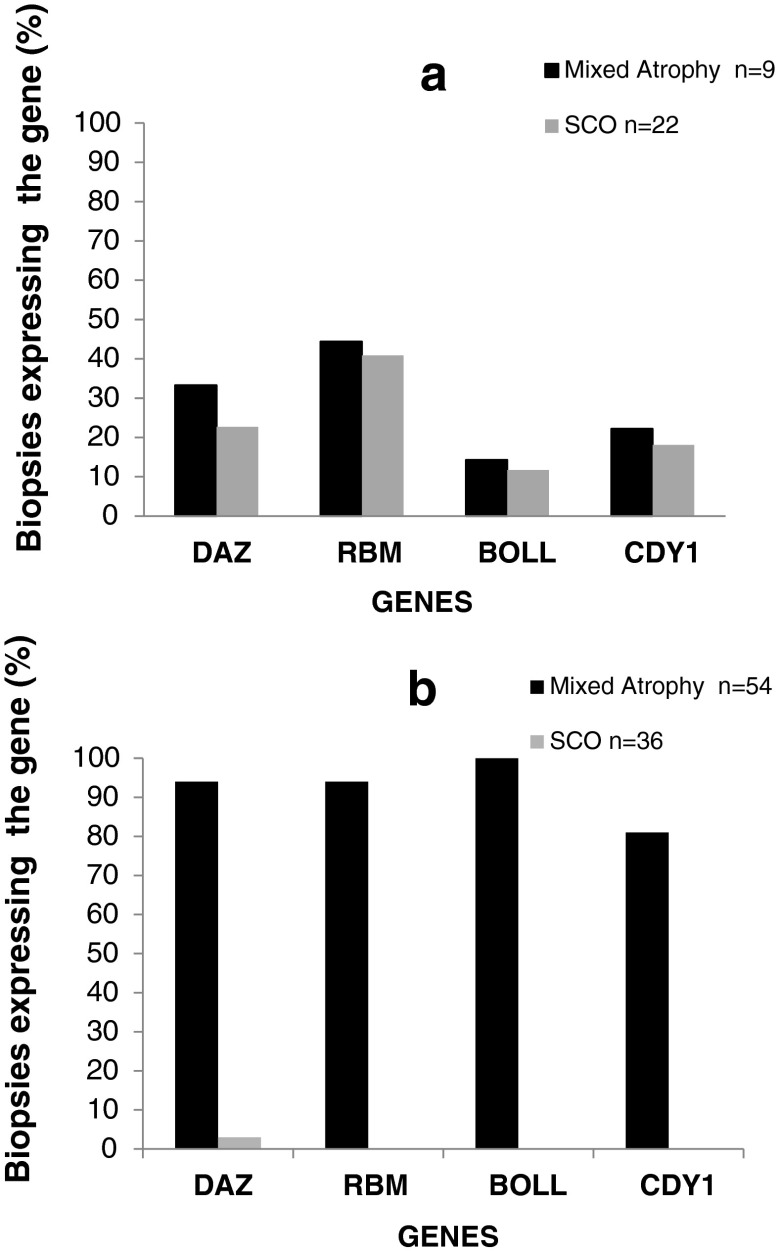
Testicular expression of the spermatogenetic markers is illustrated in Fig. 2. As expected, the expression of all spermatogenic markers was found in the NK group, it was positive in the mixed atrophy subgroup and it was negative in the SCO subgroup (Fig. 4). Expression of at least one out of the three spermatogenetic markers (DAZ, RBM, and BOLL) was observed in 14 of the 31 (45 %) biopsies of the KS group. The post-meiotic spermatogenetic marker (CDY1) was detected in six of the 31 (19 %) KS samples, always in addition to the other spermatogenetic markers. Unexpectedly, spermatogenic markers in the two KS subgroups (mixed atrophy and SCO) revealed that the frequency of the markers’ expression was very similar among the biopsies that contained sperm cells (mixed atrophy subgroup) and those in the SCO subgroup, with no difference between them (Fig. 4). The itemized clinical and molecular measures of each KS man that had positive spermatogenic markers were presented in Supplementary Table 1.
Fig. 4.
Percent of specimens with mixed atrophy and Sertoli cells only (SCO) subgroups expressing the spermatogenetic genes tested. a Men with Klinefelter syndrome; b NOA men with normal karyotype
Findings on testicular expression of the spermatogenetic markers in the KS group were entirely different from those observed in biopsies of the azoospermic control group (NK) in which spermatogenetic markers were almost exclusively observed in biopsies with mixed atrophy and absent in those with SCO (Fig. 4). (Fisher exact test, P = 0.001 and P < 0.000 between the mixed atrophy and SCO subgroups, respectively). Similar findings were observed in the NK group when the samples were limited to those with high FSH levels (Supplementary Fig. 1).
Discussion
The results of the present study showed no correlation between the expression of known spermatogenic markers and the success of sperm cell retrieval in the KS group. This was in contrast to the high correlation between the expression of spermatogenic markers and both spermatogenesis and sperm cell retrieval in the NK group [14, 28]. These findings suggest the presence of sperm cells that express the same markers in both KS subgroups (mixed atrophy with sperm cells and SCO group). When sperm cells were found in the testis (mixed atrophy), the frequency of expression of the spermatogenic markers was significantly lower in KS than in NK. This might be partially explained by the more severe pathology observed in KS biopsies than in NK ones (e.g., tubules with cell, interstitial hyalinization). The reason for a higher frequency of expression of the spermatogenic markers in KS with SCO compared with NK with SCO remains unexplained. It can be hypothesized that a process of progressive degeneration [30] might have left sporadic cells expressing spermatogenetic markers along with a histology that resembles SCO. In support of our finding, a recent testicular transcriptome study of KS men with SCO reported down-regulation of only 24 transcripts being specifically expressed in germ cells [31].
The histological morphometric findings in men with KS were much more abnormal than those observed in men with NK as judged by the semi-quantitative morphometric assessment (Fig. 3), and they reflected the severe pathology of the testicular tissue relative to the NK group. Moreover, the abnormal morphometric findings of extensive hyalinization, interstitial hyperplasia, and scarcity of tubules with at least Sertoli cells as well as tubules with normal thickness of the basement membrane in KS was similar in both the mixed atrophy and SCO subgroups. These pathological features of KS testis are probably an end result of the transcriptional deregulation of Sertoli and Leydig cells leading to increased apoptosis [31].
An atypical process in the testicular tissue, such as cell neoplasia, may lead to an abnormal expression of spermatogenetic markers in men with KS. Indeed, an abnormal expression of fertility-related genes has been reported in seminomas and melanomas [32, 33]. In addition, a stem cell marker, such as OCT, was proposed to be useful as a specific marker for the detection of intratubular germ cell neoplasia [34]. Genome-wide differential methylation was recently observed in the blood of men with KS. Nonetheless, the presence of this phenomenon in testis and its functional significance remain essentially unexplored [35]. Interestingly, an increased incidence of breast cancer and mediastinal cancer, but not of testicular cancer, was recently reported in men with KS [36, 37]. The increased hyperplasia only among KS specimens and the expression of germ cells markers in the subgroup of KS SCO specimens (classified according to the findings of all the biopsies taken from the same testis) observed in our study might support an atypical process in the KS pathology.
Age has been proposed as a predictive factor for the success of sperm retrieval in men with KS [30], although a recently published prospective study reported no increase in the sperm-retrieval rate among young versus adult non-mosaic KS men [38]. The current study findings support that report, since there was no difference in the mean age of our subgroups in either classification.
Although at least two biopsies were extracted per KS testis, we cannot completely rule out the possible presence of germ cells. Successful retrieval of sperm cells in repeated micro-TESE was reported in three of 18 men with KS previously classified as having SCO and failure of sperm retrieval [39]. It seems that micro-TESE provides a feasible assessment of the entire small testis found in men with KS and the prospect of a higher sperm retrieval [30, 39].
When repeated surgery is needed to collect testicular tissue, it is necessary to very carefully consider whether or not to perform it in men with KS. In the NK group, the TESE procedure may have negative effects on testicular functions, with a temporary or long-term decline in serum testosterone [40–42]. The group of men with KS willing to undergo a repeated TESE would therefore be at a higher risk of experiencing an additional decrease in their already low serum testosterone level due to their reduced testicular volume. Based on the present results, the option of repeat TESE for KS men whose first attempt at sperm extraction had been unsuccessful should be thoroughly discussed with the patient. Additional research is recommended in order to identify new markers for a greater selection of spermatogenic predictors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 17 kb)
Percent of specimens with mixed atrophy and Sertoli cells only (SCO) subgroups expressing the spermatogenetic genes tested. a NOA men with normal karyotype; b NOA men with normal karyotype and high FSH levels (PPTX 41.9 kb)
Acknowledgments
The authors thank Esther Eshkol for editorial assistance and Ilana Gelenter (Statistical Department, Tel Aviv University) for expert statistical analysis.
Compliance with ethical standards
The present study was approved by the local Institutional Review Board (IRB) committee in accordance with the Helsinki Declaration of 1975, and all the participants signed a written consent.
Conflict of interest
All authors declare no competing financial interests.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the local Institutional Review Board (IRB) committee in accordance with the Helsinki Declaration of 1975.
Footnotes
Capsule Molecular spermatogenetic markers have a distinct pattern of expression in non-mosaic Klinefelter syndrome that precludes their use for the prediction of spermatogenesis in affected men.
References
- 1.Selice R, Di Mambro A, Garolla A, Ficarra V, Lafrate M, et al. Spermatogenesis in Klinefelter syndrome. J Endocrinol Investig. 2010;33:789–93. doi: 10.1007/BF03350343. [DOI] [PubMed] [Google Scholar]
- 2.Foresta C, Galeazzi C, Betella A, Marin P, Rossato M, et al. Analysis of meiosis in intratesticular germ cells from subjects affected by classic Klinefelter’s syndrome. J Clin Endocrinol Metab. 1999;84:3807–10. doi: 10.1210/jcem.84.10.6029. [DOI] [PubMed] [Google Scholar]
- 3.Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 4.Wikström AM, Raivio T, Hadziselimovic F, Wikström S, Tuuri T, et al. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89:2263–70. doi: 10.1210/jc.2003-031725. [DOI] [PubMed] [Google Scholar]
- 5.Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, et al. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol. 2014;191:175–8. doi: 10.1016/j.juro.2013.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciurano RB, Luna Hisano CV, Rahn MI, Brugo Olmedo S, Rey Valzacchi G, et al. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod. 2009;24:2353–60. doi: 10.1093/humrep/dep180. [DOI] [PubMed] [Google Scholar]
- 7.Choe JH, Kim JW, Lee JS, Seo JT. Routine screening for classical azoospermia factor deletions of the Y chromosome in azoospermic patients with Klinefelter syndrome. Asian J Androl. 2007;9:815–20. doi: 10.1111/j.1745-7262.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 8.Simoni M, Tüttelmann F, Gromoll J, Nieschlag E. Clinical consequences of microdeletions of the Y chromosome: the extended Münster experience. Reprod Biomed Online. 2008;16:289–303. doi: 10.1016/S1472-6483(10)60588-3. [DOI] [PubMed] [Google Scholar]
- 9.Rajpert-De Meyts E, Ottesen AM, Garn ID, Aksglaede L, Juul A. Deletions of the Y chromosome are associated with sex chromosome aneuploidy but not with Klinefelter syndrome. Acta Paediatr. 2011;100:900–2. doi: 10.1111/j.1651-2227.2011.02169.x. [DOI] [PubMed] [Google Scholar]
- 10.Hadjkacem-Loukil L, Ghorbel M, Bahloul A, Ayadi H, Ammar-Keskes L. Genetic association between AZF region polymorphism and Klinefelter syndrome. Reprod Biomed Online. 2009;19:547–51. doi: 10.1016/j.rbmo.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ceylan C, Ceylan GG, Serel TA. The azoospermia factor locus-c region was found to be related to Klinefelter syndrome in Turkish patients. Genet Mol Res. 2010;9:1229–33. doi: 10.4238/vol9-2gmr826. [DOI] [PubMed] [Google Scholar]
- 12.Ma K, Inglis JD, Sharkey A, Bickmore WA, Hill RE, et al. A Y chromosome gene family with RNA-binding protein homology: candidates for the azoospermia factor AZF controlling human spermatogenesis. Cell. 1993;75:1287–95. doi: 10.1016/0092-8674(93)90616-X. [DOI] [PubMed] [Google Scholar]
- 13.Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–92. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 14.Kleiman SE, Lagziel A, Yogev L, Botchan A, Paz G, et al. Expression of CDY1 may identify complete spermatogenesis. Fertil Steril. 2001;75:166–73. doi: 10.1016/S0015-0282(00)01639-3. [DOI] [PubMed] [Google Scholar]
- 15.Kleiman SE, Yogev L, Hauser R, Botchan A, Bar-Shira Maymon B, et al. Members of the CDY family have different expression patterns: CDY1 transcripts have the best correlation with complete spermatogenesis. Hum Genet. 2003;113:486–92. doi: 10.1007/s00439-003-0990-9. [DOI] [PubMed] [Google Scholar]
- 16.Lahn BT, Tang ZL, Zhou J, Barndt RJ, Parvinen M, et al. Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc Natl Acad Sci U S A. 2002;99:8707–12. doi: 10.1073/pnas.082248899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caron C, Pivot-Pajot C, van Grunsven LA, Col E, Lestrat C, et al. Cdyl: a new transcriptional co-repressor. EMBO Rep. 2003;4:877–82. doi: 10.1038/sj.embor.embor917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maines JZ, Wasserman SA. Post-transcriptional regulation of the meiotic Cdc25 protein Twine by the Dazl orthologue Boule. Nat Cell Biol. 1999;1:171–4. doi: 10.1038/11091. [DOI] [PubMed] [Google Scholar]
- 19.Lin YM, Kuo PL, Lin YH, Teng YN, Nan Lin JS. Messenger RNA transcripts of the meiotic regulator BOULE in the testis of azoospermic men and their application in predicting the success of sperm retrieval. Hum Reprod. 2005;20:782–8. doi: 10.1093/humrep/deh647. [DOI] [PubMed] [Google Scholar]
- 20.Luetjens CM, Xu EY, Rejo Pera RA, Kamischke A, Nieschlag E, et al. Association of meiotic arrest with lack of BOULE protein expression in infertile men. J Clin Endocrinol Metab. 2004;89:1926–33. doi: 10.1210/jc.2003-031178. [DOI] [PubMed] [Google Scholar]
- 21.Lin YM, Chung CL, Cheng YS. Posttranscriptional regulation of CDC25A by BOLL is a conserved fertility mechanism essential for human spermatogenesis. J Clin Endocrinol Metab. 2009;94:2650–7. doi: 10.1210/jc.2009-0108. [DOI] [PubMed] [Google Scholar]
- 22.Mikhaylova LM, Boutanaev AM, Nurminsky DI. Transcriptional regulation by Modulo integrates meiosis and spermatid differentiation in male germ line. Proc Natl Acad Sci U S A. 2006;103:11975–80. doi: 10.1073/pnas.0605087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanGompel MJ, Xu EY. A novel requirement in mammalian spermatid differentiation for the DAZ-family protein Boule. Hum Mol Genet. 2010;19:2360–9. doi: 10.1093/hmg/ddq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleiman SE, Lehavi O, Hauser R, Botchan A, Paz G, et al. CDY1 and BOULE transcripts assessed in the same biopsy as predictive markers for successful testicular sperm retrieval. Fertil Steril. 2011;95:2297–302. doi: 10.1016/j.fertnstert.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Hauser R, Botchan A, Amit A, Ben Yosef D, Gamzu R, et al. Multiple testicular sampling in non-obstructive azoospermia—is it necessary? Hum Reprod. 1998;13:3081–5. doi: 10.1093/humrep/13.11.3081. [DOI] [PubMed] [Google Scholar]
- 26.Malcher A, Rozwadowska N, Stokowy T, Kolanowski T, Jedrzejczak P, et al. Potential biomarkers of nonobstructive azoospermia identified in microarray gene expression analysis. Fertil Steril. 2013;100:1686–94. doi: 10.1016/j.fertnstert.2013.07.1999. [DOI] [PubMed] [Google Scholar]
- 27.Dorosh A, Tepla O, Zatecka E, Ded L, Koci K, Peknicova J. Expression analysis of MND1/GAJ, SPATA22, GAPDHS and ACR genes in testicular biopsies from non-obstructive azoospermia (NOA) patients. Reprod Biol Endocrinol. 2013;15:11–42. doi: 10.1186/1477-7827-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiman SE, Yogev L, Hauser R, Botchan A, Maymon BB, et al. Expression profile of AZF genes in testicular biopsies of azoospermic men. Hum Reprod. 2007;22:151–8. doi: 10.1093/humrep/del341. [DOI] [PubMed] [Google Scholar]
- 29.Hauser R, Yogev L, Paz G, Yavetz H, Azem F, et al. Comparison of efficacy of two techniques for testicular sperm retrieval in nonobstructive azoospermia: multifocal testicular sperm extraction versus multifocal testicular sperm aspiration. J Androl. 2006;27:28–33. doi: 10.2164/jandrol.05055. [DOI] [PubMed] [Google Scholar]
- 30.Fullerton G, Hamilton M, Maheshwari A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum Reprod. 2010;25:588–97. doi: 10.1093/humrep/dep431. [DOI] [PubMed] [Google Scholar]
- 31.D’Aurora M, Ferlin A, Di Nicola M, Garolla A, De Toni L, et al. Deregulation of sertoli and leydig cells function in patients with Klinefelter syndrome as evidenced by testis transcriptome analysis. BMC Genomics. 2015;16:156. doi: 10.1186/s12864-015-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–99. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 33.dos Santos NR, Torensma R, de Vries TJ, Schreurs MW, de Bruijn DR, et al. Heterogeneous expression of the SSX cancer/testis antigens in human melanoma lesions and cell lines. Cancer Res. 2000;60:1654–62. [PubMed] [Google Scholar]
- 34.Hessel M, Ramos L, Hulsbergen AF, D'Hauwers KW, Braat DD, et al. A novel cell-processing method ‘AgarCytos’ in conjunction with OCT3/4 and PLAP to detect intratubular germ cell neoplasia in non-obstructive azoospermia using remnants of testicular sperm extraction specimens. Hum Reprod. 2013;28:2608–20. doi: 10.1093/humrep/det311. [DOI] [PubMed] [Google Scholar]
- 35.Wan ES, Qiu W, Morrow J, Beaty TH, Hetmanski J, et al. Genome-wide site-specific differential methylation in the blood of individuals with Klinefelter syndrome. Mol Reprod Dev. 2015;82:377–86. doi: 10.1002/mrd.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokol RZ. It's not all about the testes: medical issues in Klinefelter patients. Fertil Steril. 2012;98:261–5. doi: 10.1016/j.fertnstert.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome—a clinical update. J Clin Endocrinol Metab. 2013;98:20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]
- 38.Plotton I, Giscard d’Estaing S, Cuzin B, Brosse A, Benchaib M, Fertipreserve group et al. A prospective study of testicular sperm extraction in young versus adult patients with non-mosaic 47, XXY Klinefelter syndrome. Preliminary results. J Clin Endocrinol Metab. 2015;100:961–7. doi: 10.1210/jc.2014-3083. [DOI] [PubMed] [Google Scholar]
- 39.Haliloglu AH, Tangal S, Gulpinar O, Onal K, Pabuccu R. Should repeated TESE be performed following a failed TESE in men with Klinefelter Syndrome? Andrology. 2014;2:42–4. doi: 10.1111/j.2047-2927.2013.00157.x. [DOI] [PubMed] [Google Scholar]
- 40.Okada H, Shirakawa T, Ishikawa T, Goda K, Fujisava M, et al. Serum testosterone levels in patients with nonmosaic Klinefelter syndrome after testicular sperm extraction for intracytoplasmic sperm injection. Fertil Steril. 2004;82:237–8. doi: 10.1016/j.fertnstert.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 41.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 42.Takada S, Tsujimura A, Ueda T, Matsuoka Y, Takao T, et al. Androgen decline in patients with nonobstructive azoospermia after microdissection testicular sperm extraction. Urology. 2008;72:114–8. doi: 10.1016/j.urology.2008.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb)
Percent of specimens with mixed atrophy and Sertoli cells only (SCO) subgroups expressing the spermatogenetic genes tested. a NOA men with normal karyotype; b NOA men with normal karyotype and high FSH levels (PPTX 41.9 kb)