Abstract
Purpose
Testicular ischemia is the main consequence of testicular torsion, in both clinical and experimental aspects. Preservation and auto-transplantation of spermatogonial stem cells (SSCs) could be a new treatment for infertility in testicular ischemia following testicular torsion.
Methods
To apply the idea in this study, animals were randomly divided into four groups of control, sham, with torsion, and with torsion followed by transplantation (TT). Isolated SSCs from neonatal mice were cultured and identified by flow cytometry (C-KIT−, INTEGRIN β1+) and RT-PCR (Reverse transcription polymerase chain reaction) for specific spermatogonial cell markers (Oct4, Gfrα-1, Plzf, Vasa, Itgα6, and Itgβ1). SSCs were transplanted upon a 2-h testicular torsion in the TT group. Cultured cells were transplanted into ischemia reperfusion testicle 2 weeks post-testicular torsion. Eight weeks after SSCs transplantation, the SSCs-transplanted testes and epididymis were removed for sperm analysis, weight and histopathological evaluation, and pre- and post-meiotic gene expression assessment by qRT-PCR.
Results
Our findings indicated that all evaluated parameters (epididymal sperm profile, Johnsen score, Plzf, Gfrα-1, Scp-1, Tekt-1 expressions, and histopathological profile) were significantly decreased following testicular torsion (group 3) when compared to the control group (p ≤ 0.05). However, all abovementioned parameters showed a significant increase/improvement in torsion-transplantation group compared to torsion group. However, these parameters in the TT group were significantly lower in the sham and control groups (p ≤ 0.05).
Conclusion
SSCs transplantation could up-regulate the expression of pre- and post-meiotic genes in testicular ischemia, which resulted in improvement of both testicular function and structure after testicular torsion.
Keywords: SSCs transplantation, Pre- and post-meiotic genes, Testicular torsion
Introduction
Infertility is a common problem that affects approximately 15 % of couples trying to conceive a baby [1]. In more than 50 % of couples having difficulty getting pregnant, the problem is at least partly related to male reproductive issues [1, 2]. Different factors contribute to male infertility or sub fertility; including testicular torsion [3], which is one of the most common urological issues targeting young men. Annual incidence of testicular torsion among those less than 25 years old was reported to be 1 in 4000 [4]. The highest peak was observed around puberty (accounted for 65 % of all torsions) with another much smaller peak in the first year of life [4–6]. It is estimated that around 400 boys lose their testis because of testicular torsion in the UK annually [7]. Therefore, this high rate of testicular torsion makes it an important factor leading to infertility in men. It seems to be the second most common reason for emergency surgical in young men in Britain [8].
During testicular torsion, unilateral or bilateral testicles get twisted in the scrotum leading to an interruption of testicular blood flow and therefore ischemia dysfunction [9]. The injuries caused by testicular torsion are severe enough to lead to ipsilateral damage, which are the consequences of significant increase in blood flow after detorsion [10]. The success rate of treatment depends on the duration of torsion and early diagnosis which could be a routine management for preservation of spermatogenesis and fertility [11]. Although reperfusion is necessary for the survival of ischemic tissue, it can also trigger destructive pathophysiological cascades including generation of oxygen-derived free radicals [12]. Reactive oxygen species distort organ function owing to DNA damage, endothelial cell injury, and germinal cell necrosis [13, 14]. Research focusing on stem cells have generated great optimism in the treatment of many human diseases in the near future. Particularly, bone marrow stem cells (BMS) are well defined and have long been used therapeutically [15]. The merits of stem cell therapy for ischemia have been proved in several tissues, such as the muscle, heart, and even brain [16].
Germ cell transplantation has been widely used to investigate spermatogenesis recovery in various animal models of azoospermia [17–22]. For instance, it was found that transplanted spermatogonial stem cells (SSCs) lead to complete spermatogenesis in the treated testes of heat shock mice [22] and mice with genetic defects in spermatogenesis, such as, mutant mice [23]. Furthermore, efficient proliferation and colonization of SSCs were observed in irradiated, busulfan, and heat shock-treated mouse recipient testes [17, 19, 22]. The fate of SSCs is determined by factors associated with stem cell niche, which reside near the basement membrane of seminiferous tubules in the vicinity of Sertoli cells [24]. However, an assessment of the stem cell niche alterations remains elusive, so the only way in which stem cell activity and microenvironment can be determined is by using a transplantation assay [24, 25]. Although many efforts have been made to transplant SSCs into testis models, no report has yet been found regarding therapeutic effects of SSCs transplantation in ischemic testis. In the previous study, we introduced testicular torsion as an azoospermic mouse model for spermatogonial stem cell transplantation [26]. In this study, the effects of SSCs transplantation in ischemic testis were evaluated, and the alterations in stem cell niche following testicular torsion were assessed. In addition, a precise evaluation of pre- and post-meiotic-specific markers in transplanted testicular tissue with SSCs was carried out.
Material and methods
Animals
All animal experiments were approved by the Animal Ethics Committee at Iran University of Medical Sciences. Twenty-four adult male NMRI mice at 6–8 weeks of age were purchased from Razi Vaccine and Serum Research Institute (Karaj, Iran). The mice were fed with standard commercial laboratory chow ((pellet form), Javeneh Khorasan Co., Mashhad, Iran) and water and housed under standard laboratory conditions (12 h light to 12 h dark and 22 ± 2 °C) during the experimental period. All the animals used in this experiment were age matched.
Experimental design
Animals were randomly divided into four groups each containing six mice including (1) control (as the positive control), which was composed of mice that did not undergo any surgical procedure, and was just for determination of the basal values for all parameters; (2) sham, with all surgical procedures involving a midline scrotal incision and physical manipulation of the testis before placing it back into the scrotum; (3) torsion (as negative control), with a 2-h testicular torsion followed by vehicle (10 μl Dulbecco’s Modified Eagle Medium (DMEM) and trypan blue) injection to seminiferous tubules 2 weeks after reperfusion; and (4) torsion-transplantation (TT; as an experimental group); a treatment group consisting of animals treated by SSCs transplantation 2 weeks after a 2-h-long testicular torsion.
The mice were sacrificed by cervical dislocation 8 weeks after SSCs transplantation. Thereafter, the left testes and epididymis were harvested and weighed; histopathological changes and pre-/post-meiotic gene expressions were evaluated.
Spermatic cord torsion
All operations were performed under sterile condition. Surgery was done with the subject under 100 mg/kg of ketamine hydrochloride (Rotexmedica, Trittau, Germany) and 10 mg/kg xylazine (Alfasan, Woerden, Netherlands) anesthesia (single dose intraperitoneally).
The skin of the scrotal area was shaved and disinfected with 10 % povidone iodine solution. Unilateral testicular torsion was created by scrotal approach through a midline incision, the left testis twisted 720° in a counter clockwise direction and by fixing the testis to the scrotum with a 6–0 nylon suture passing through the tunica albuginea and dartos [27]. Animals underwent 2 h of unilateral testicular ischemia. After 2 h, the suture was removed and the ischemic testis was untwisted and replaced in the scrotum and the incision was closed.
Spermatogonial cells isolation, purification, and culture
Testes from ten 3–6-day-old neonate NMRI mice were collected for the preparation of cell suspension following enzymatic digestions and purification steps. After removal of tunica, the testes were minced into small pieces and suspended in DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 1.37 g/L NaHCO3 (Sigma-Aldrich, St Louis, MO, USA), single-strength non-essential amino acids, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 40 μg/ml gentamycin (all from Life Technologies).
Single cell suspensions were obtained by two-step enzymatic dissociation procedure and used for culture utilizing the method previously described [28, 29] with some modifications. In brief, minced testis pieces were suspended in DMEM containing 1 mg/ml collagenase, 1 mg/ml trypsin, and 1 mg/ml hyaluronidase type II (Sigma-Aldrich, St Louis, MO, USA) and kept in shaking incubator at 37 °C for 15 min. After three washes in DMEM medium, interstitial cells were removed and seminiferous cord fragments were incubated in fresh enzymes for 30–45 min as described above. Cells were separated from the remaining tubule fragments by centrifugation at 1200 RPM for 5 min. After filtration through sterile mesh (40-μm opening, 5–6 cm2), the cells were pelleted and purified to eliminate the somatic cells (myoid and Sertoli cells). For this purpose, cells were incubated in 75 cm2 flasks at a density of 2 × 105 cells/cm2 (15 × 106 cells/flask) in DMEM with 2.5 % fetal bovine serum (FBS; Life Technologies) at 37 °C and 5 % CO2. After 16 h, the medium with unattached (suspended) cells was collected, washed as described, and cultured at 37 °C and 5 % CO2 in a humidified atmosphere in the presence of 5 % FBS and 40 ng/ml glial cell line-derived neurotropic factor (GDNF) for 2 weeks [30].
Identity confirmation of the spermatogonial cells
RT-PCR
Total RNA was extracted from the cultured cells by using the TRI reagent (Sigma, Pool, UK) according to the manufacturer’s recommendations. Complementary deoxyribonucleic acid (cDNA) synthesis was carried out with an RT kit (Fermentas, St. Leon-Rot, Germany). One microgram of total RNA was used as a template in order to generate cDNA. The PCR reaction was performed with Platinum Blue PCR Super Mix (Invitrogen, Pairsley, UK). Gene-specific primers designed using Gene Bank and Gene runner software (version 3.02; Hastings Software Inc., New York, NY, USA) and used for the RT reaction to assess Itgα6, Itgβ1, Plzf, Vasa, Oct-4, Gfr-α1, and Gapdh expression. Primer sequences and products size are listed in Table 1.
Table 1.
Primers used for qRT-PCR analysis of pre-and post-meiotic gene markers and RT-PCR analysis of the SSC-specific genes
| Gene | Primer sequence (5′–3′) | Annealing temperature (°C) | DNA size (bp) |
|---|---|---|---|
| Mvh(Vasa) | F: GATAATCATTTAGCACAGCCTC R: GTCAACAGATGCAAACACAG |
60 | 149 |
| Itgα-6 | F: CTCAGAATATCAAGCTCCCT R: AAACACTAATAGAGCCAGCA |
60 | 148 |
| Gfrα-1 | F: CTGTGGACTAGCTCGCTCTC R: GACCCGCTTTTAGGGGTTCA |
60 | 130 |
| Itgβ-1 | F: GACATTACTCAGATCCAACCA R: AGGTAGTAGAGATCAATAGGGT |
60 | 115 |
| Oct-4 | F:TGGATCCTCGAACCTGGCTA R: CTCAGGCTGCAAAGTCTCCA | 60 | 129 |
| Plzf | F: CCCGTTGGGGGTCAGCTAGAA R: CTGCAAGGTGGGGCGGTGTAG |
60 | 137 |
| Gapdh | F: CTGCTGGACAAGTGAGTCCC R: CCAAGTACCCTGGCCTCATC |
60 | 125 |
| Scp-1 | F: CGCAAGCTGACCTTGAAGTAG R: AGCCCTTCACACTGTTCGTC |
59 | 97 |
| Tekt-1 | F: GAAGGCCCTGTACCGCAGGC R: CCATGGTCGTCGCCGTCTCG |
61 | 132 |
The amplification was persistent for 40 cycles under the following setting after an initial denaturation step of 95 °C for 5 min: 95 °C for 30 s, specific annealing temperature for each primer pairs (Itgα6, 52 °C; Itgβ1, 55 °C; Plzf, 55 °C; Vasa, 62 °C; oct4, 60 °C; Gfr-α1 52 °C, and Gapdh 60 °C) for 30 s, and 72 °C for 30 s followed by a final step of 10 min at 72 °C. Electrophoresis was applied on 1.2 % agarose gel with Tris-Borate-EDTA (TBE) 1× loading buffer (Sigma- Aldrich) at a voltage of 95 for 45 min. The gels were stained with 0.1 μg/mL Gel Red™ (Biotium Inc, Hayward, CA, USA), the bands were visualized using Gel Logic (Carestream Health Inc., Rochester, NY, USA), and images were obtained.
Flow cytometry analysis
Flowcytometrical analysis was done on the obtained cell suspension before and after 2 weeks of culturing. Cultured SSCs trypsinated and resuspended in PBS containing 2 % FBS and incubated with conjugated antibodies for 20 min at 4 °C. C-Kit and INTEGRIN-α6 antibodies were conjugated to FITC and PE (Abcam, Cambridge, MA, USA), respectively. Stained cells were analyzed by flow cytometry (Partec AG, CH-4144 Arlesheim, and Switzerland) and cells without antibody staining served as negative controls.
Transplantation procedure and recipient testes assessment
The spermatogonial cell-derived colonies were labeled with DiI (Invitrogen, Cergy Pontoise, France) based on manufacturer’s protocol and injected into the seminiferous tubules of the recipient mice 2 weeks after ischemia reperfusion. The recipient mice (n = 10) were anesthetized as described, and then approximately 105 of cultured spermatogonial cells in 10 μl DMEM were injected into the seminiferous tubules in each affected testis. Germ cell transplantation was performed by retrograde microinjection through the efferent ducts into the seminiferous tubules [31]. Seminiferous tubules were tracked and visualized by adding trypan blue in the germ cell injection media. Approximately 80–90 % of the tubules were filled in each recipient testis.
The existence and proliferation of injected cells were evaluated 8 weeks post-transplantation by fluorescent microscope (Olympus, type CH2, ×40 magnifications). Both tissue and cultured cells were stained utilizing DAPI (Invitrogen, Cergy Pontoise, France) in order to confirm the results.
Assessment of morphological changes
For histological study, the testes of mice in all four groups of our study (control, sham, with torsion, with torsion followed by transplantation/TT) were removed, weighed, and fixed in a Bouin’s solution for 48 h, embedded in paraffin, sectioned at 5-μm thickness, and finally stained with hematoxylin-eosin (H&E). One hundred round or nearly round seminiferous tubules were assessed for each testis randomly. The mean diameter and epithelium thickness of the seminiferous tubules were measured in each testes using Image J (Version 1.240; National Institutes of Health, USA). The seminiferous tubules in each section were also graded and scored according to the Johnsen score system to fulfill a systematic evaluation [32].
Epididymal sperm parameters
Cauda epididymis and vas deferens were isolated in all four groups of our study (control, sham, with torsion, with torsion followed by transplantation/TT) and placed in 1 ml of PBS (pH = 7.4). The tissue of cauda epididymis at the beginning of vas deferens was minced using sharp scissors. The spermatozoa were allowed to swim out for 15 min in an atmosphere of 5 % CO2 at 37 °C, prior to determination of sperm quality [33]. Sperm suspension was analyzed under light microscope (Olympus, type CH2, ×400 magnifications). The suspension was used to determine the percentage of motile spermatozoa (light microscope, room temperature). The sperm suspensions were counted using Neubauer counting chamber (Thoma, assistant Sondheim/Rhon, Germany) for determination of sperm concentration (×106/ml). Eosin B (0.5 % in saline) (Sigma-Aldrich, USA) was applied to determine the percentage of viable sperm (sperm with pink head was counted as dead sperm).
Gene expression evaluation by qRT-PCR
Pre-meiotic (Plzf, Gfr-α1) and post-meiotic (Tekt-1, Scp-1) specific markers were evaluated by real-time PCR. Total RNA was prepared from experimental testis tissue using the TRI reagent (Sigma, Pool, UK) according to the manufacturer’s protocol and treated three times with DNase I (Fermentas, St. Leon-Rot, Germany) to remove genomic DNA contaminations from the samples. RNA was then reverse-transcribed to cDNA using the oligo dT primers and later purified by RT kit (Fermentas, St. Leon-Rot, Germany), subjected to real-time PCR. Q-PCR reactions assessment were carried out using the ABI PRISM 7300 sequence detection system (Applied Biosystems, USA) with SYBR Green reagent (Applied Biosystems, USA) according to the manufacturer’s instructions. The PCR conditions were 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s, and 60 °C for 1 min. Each PCR was repeated at least three times using specific primers (Table 1). Expression assay of stage-specific markers for pre- and post-meiotic genes were analyzed using the comparative ΔΔCT method [34].
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey’s test using the SPSS software was used (SPSS Inc, Chicago, IL, USA) to analyze the data, and p ≤ 0.05 is considered as statistically significant.
Results
Culture and identification of spermatogonial stem cells
Cell viability was assessed after isolation of testicular cells by the dye exclusion test (0.04 % trypan blue solution). The viability of the cells after isolation was more than 90 %, and the clusters appeared in 2–3 days after primary culture. Upon enzymatically disperse and re-plate of these clusters, their SSC contents start new clusters by 2 weeks of culture.
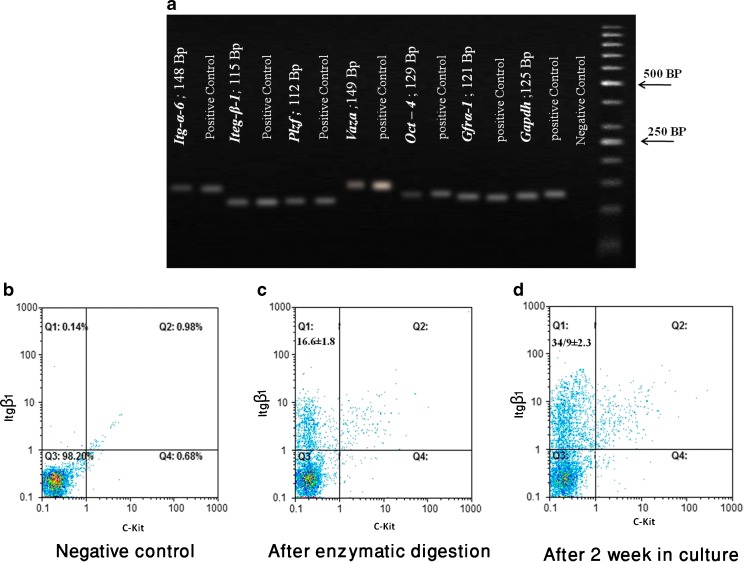
The expression of spermatogonial cell markers (Itg-α6, Itg-β1, Plzf, Vasa, Gfr-α1, and Oct-4) were detected by RT-PCR, which provided additional evidence for cultured cell identification (Fig. 1a). Flow cytometric analysis of spermatogonial cells (Fig. 1b–d) indicates 16.06 ± 1.8 % cells were positive for INTEGRIN β1 and negative for C-KIT after two-step enzymatic digestion (Fig. 1c) whereas the 2-week cultivation significantly enhanced the purity of these cells to 34.9 ± 2.3 % (Fig. 1d).
Fig. 1.
RT-PCR and flow cytometric analysis. RT-PCR was used to determine the expression of specific spermatogonia and germ cell markers. a Expression of the specific spermatogonia and germ cell-specific markers was detected by RT-PCR of Itgα6 (148 bp), Itgβ1 (3115 bp), Plzf (137 bp), VASA (149 bp), Oct4 (129 bp), and Gfrα-1 (130 bp); genes were expressed in testis tissue (positive control) and spermatogonial cells after culture. Flow cytometric analysis of spermatogonial cells. b The cells without antibody staining considered as negative control, c spermatogonial cells after two-step enzymatic digestion, and d after 2 week in culture. The population of cultured cells were double stained with antibodies against β1-integrin and c-kit. In three experiments, the average percent of the spermatogonial cells (C-KIT−, INTEGRIN β1 +) after two-step enzymatic digestion was 16.06 ± 1.8 %, whereas cultivation enhanced the purity of these cells to 34.9 ± 2.3 %
Epididymal sperm parameters following testicular torsion
Analysis of sperm parameters showed no significant discrepancy between the sham and control groups. However, epididymal sperm count had vanished following testicular torsion compared to the control (0.10 ± 0.00 vs. 5.72 ± 0.48; p ≤ 0.05). A significant increase in sperm count was also observed in the TT group compared to the torsion group (0.10 ± 0.00 vs. 2.92 ± 0.77; p ≤ 0.05). Furthermore, the sperm count in the TT group was not the same as that in the control group. However, there was a significant difference in this group compared to the sham and control groups (2.92 ± 0.77 vs. 5.64 ± 0.41/5.72 ± 0.48, respectively) (p ≤ 0.05) (Table 2).
Table 2.
Spermatogenesis evaluation. Spermatogenesis evaluation between different groups of the present study by assessment of histopathological factors (testicular weight, seminiferous tubule diameter, and seminiferous epithelium thickness Johnsen score) and sperm parameters (mean epididymis sperm count, percentage of sperm motility, and percentage of viable sperm)
| Groups | Control ± SD | Sham ± SD | Torsion ± SD | Transplantation ± SD |
|---|---|---|---|---|
| Testicular weight | 0. 09 ± 0.05 | 0. 08 ± 0.05 | 0. 07 ± 0. 01 | 0. 08 ± 0. 01 |
| Seminiferous diameter | 199. 98 ± 2. 48 | 198. 32 ± 1. 98 | 135. 64 ± 11. 41a | 165. 44 ± 2. 36ab |
| Epithelium thickness | 55. 52 ± 2. 02 | 55. 86 ± 3. 94 | 12. 46 ± 2. 23a | 47. 22 ± 0. 90ab |
| Johnson grade | 9. 2 ± 0. 51 | 9. 16 ± 0. 52 | 2. 90 ± 0. 37a | 8. 03 ± 0. 43ab |
| Sperm count (×106) | 5. 72 ± 0. 48 | 5. 64 ± 0. 41 | 0. 10 ± 0. 00a | 2. 92 ± 0. 77ab |
| Sperm morphology | 74. 60 ± 1. 14 | 69. 20 ± 5. 17 | 0. 60 ± 1. 34 a | 78. 40 ± 2. 88b |
| Sperm motility | 52. 80 ± 2. 77 | 53. 20 ± 4. 82 | 1. 00 ± 2. 24 a | 33. 40 ± 12. 22 ab |
| Sperm viability | 60. 00 ± 1. 58 | 60. 80 ± 8. 23 | 1. 40 ± 3. 13 a | 57. 20 ± 8. 93b |
aSignificant difference vs. control and sham groups(p ≤ 0.05)
bSignificant difference vs. torsion group (p ≤ 0.05)
Moreover, a remarkable decline was noticed in the percentages of sperm motility following testicular torsion in comparison with that of control and sham groups (1.00 ± 2.24 vs. 52.80 ± 2.77/53.20 ± 4.82, respectively) (p ≤ 0.05). This parameter was augmented in the TT group compared to the torsion group (33.40 ± 12.22 vs. 1.00 ± 2.24) (p ≤ 0.05). In addition, sperm motility in the TT group was remarkably found lower than that in the control and sham groups (33.40 ± 12.22 vs. 52.80 ± 2.77/53.20 ± 4.82, respectively) (p ≤ 0.05) (Table 2).
Furthermore, a reduction in sperm viability was significantly perceived after testicular torsion compared to the control and sham (1.40 ± 3.13 vs. 60.00 ± 1.58/60.80 ± 8.23, respectively) (p ≤ 0.05). However no significant difference was seen in sperm viability between the TT and control groups (57.20 ± 8.93 vs. 60.80 ± 8.23/60.00 ± 1.58) (Table 2).
A statistically significant decline in the sperm with normal morphology was also observed following a testicular torsion. The mean percentage of morphologically normal sperm in the testicular torsion group showed a decrease when compared with control and sham groups (0. 60 ± 1. 34 vs. 74. 60 ± 1. 14/69. 20 ± 5. 17), respectively (p ≤ 0.05). However, no significant difference was seen in sperm morphology between the TT and control groups (78. 40 ± 2. 88 vs. 60.80 ± 8.23/74. 60 ± 1. 14) (Table 2).
Assessment of morphological changes
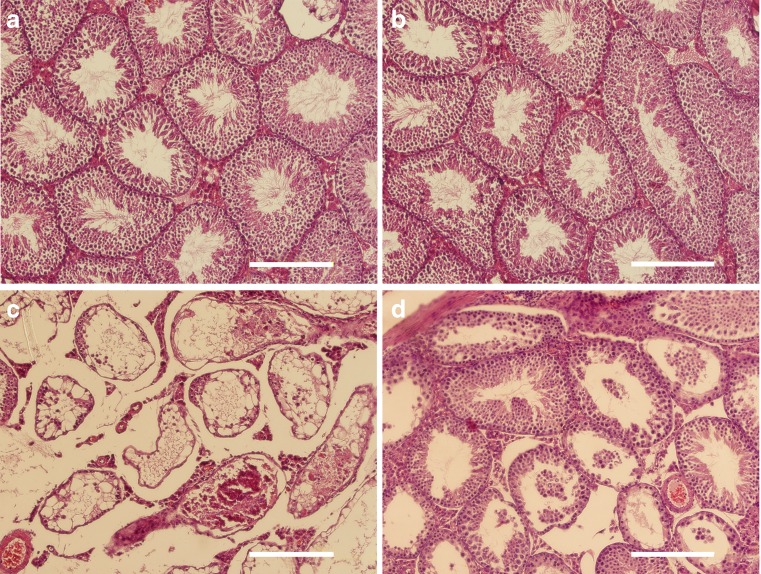
The histopathological findings in the sections were illustrated in Fig. 2 (control, sham, and experimental groups). Histological examinations of the control and sham testes revealed normal seminiferous tubules (Fig. 2a, b). The epithelium of all seminiferous tubules in ischemic testis was severely disrupted. Most of tubules depleted while just the basement membrane was observed in some of them (Fig. 2c). Despite the improvement in seminiferous tubule structure following SSCs transplantation, some sclerotic and depleted seminiferous tubules were still observed in transplanted testes 10 weeks post-testicular torsion (Fig. 2d).
Fig. 2.
Assessment of morphological changes: normal seminiferous was observed in a control and b sham animal testis. c Depleted tubule was perceived by 2-h testicular torsion; d restoration of seminiferous tubule following SSCs transplantation is illustrated (bar = 100 μm)
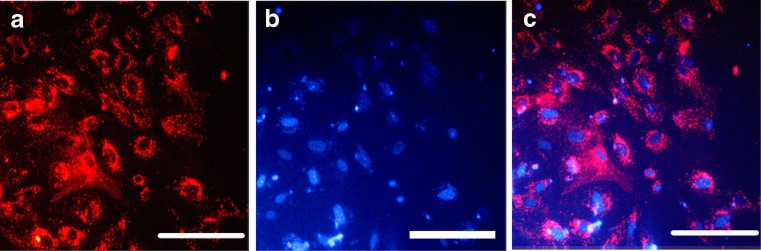
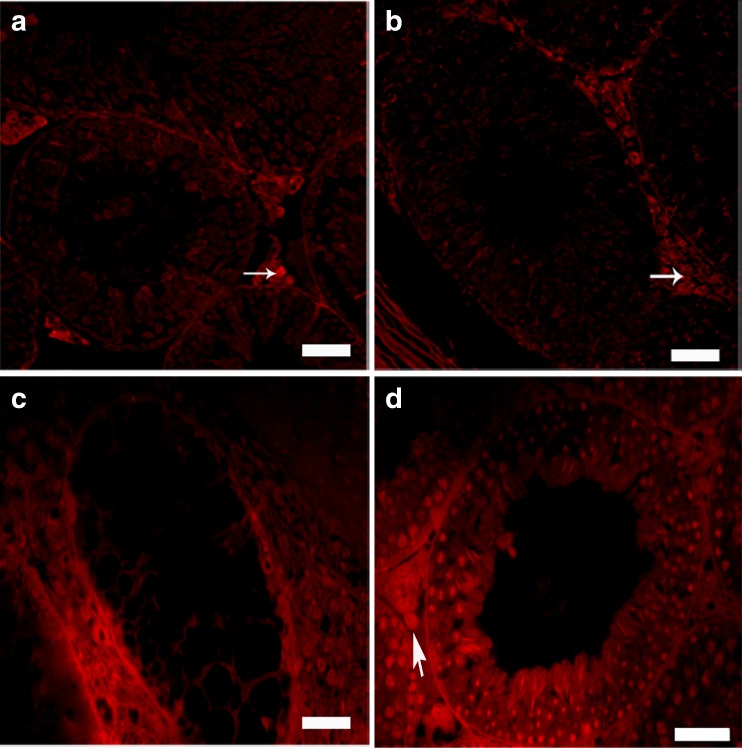
Spermatogonial cells were labeled with DiI before transplantation. Fluorescence indicated that before transplantation, approximately all of the cells had been labeled with DiI (Fig. 3a–c). Then, labeled cell were transplanted in order to confirm the presence of SSCs in cell suspension as well as to assess SSCs colonization in the testis. Eight weeks after transplantation, fluorescent-labeled cells were considered transplanted cells. The labeled cells were localized in the seminiferous tubules of the recipient testes. In addition, colonization and proliferation of transplanted cells were observed 8 weeks after cells transplantation (Fig. 4).
Fig. 3.
SSC labeling before transplantation. a DiI-labeled SSCs was observed under fluorescence; b same cells were stained with DAPI; c panels a and b were merged
Fig. 4.
Colonization of spermatogonial stem cells into recipient mouse testis. a Control and b sham groups without any positive signals in the tubules. Auto-fluorescent signals in interstitial tissue were considered as an artifact (arrow). c The tubules were still without germ cells (depleted) 10 weeks after testicular torsion. d Proliferation and differentiation of transplanted SSCs were detected by tracing labeled cell in the recipient testes 8 weeks after transplantation; seminiferous tubule were stained with DAPI and observed under UV microscope (bar = 50 μm)
As shown in Table 2, there were no significant morphological changes between the sham and control groups. However, in ischemic testis compared to the sham and control, a reduction in tubule diameter (135.64 ± 11.41 μm, vs. 198.32 ± 1.98 μm and 199.98 ± 2.48 μm, respectively; p ≤ 0.05) and epithelial thickness (12.46 ± 2.23 μm, vs. 55.86 ± 3.94 μm and 55.52 ± 2.02 μm, respectively, p ≤ 0.05) was discovered. Although in the TT group, the diameter of seminiferous and epithelial tubules increased compared to the torsion group, they also showed a difference between the sham and control groups. The alterations in testicular weighs and seminiferous lumen diameters were not statistically significant in all the groups of our study (Table 2).
Johnsen score assessment
Following torsion, the Johnsen score was declined in comparison with the sham and control groups (2.90 ± 0.37 vs. 9.16 ± 0.52 and 9.2 ± 0.51, respectively; p ≤ 0.05). Seminiferous tubule score for SSCs-transplanted testis was considerably lower than that in the control group, while it was higher when compared to the ischemic testis group (8.03 ± 0.43 vs. 9.2 ± 0.51, 2.90 ± 0.37, respectively; p ≤ 0.05) (Table 1).
Gene expression evaluation by qRT-PCR
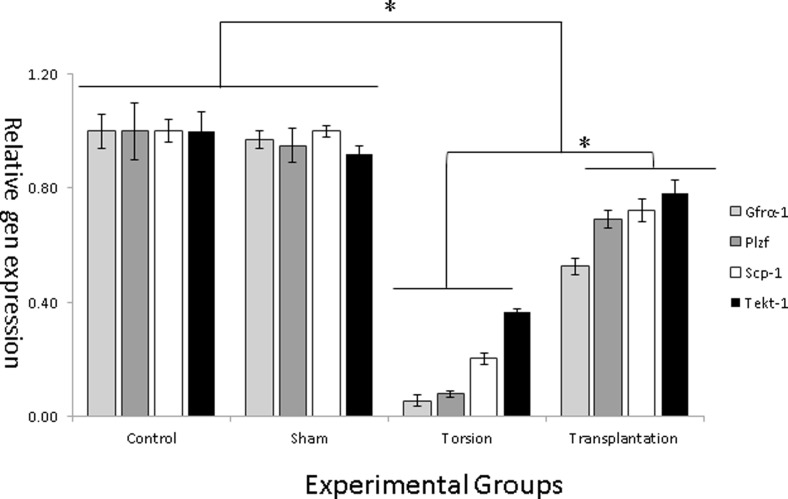
To determinate the increase in SSC number and differentiation, we transplanted the cultured cells into seminiferous tubules of the torsion testes. In these experiments, at least three recipients received donor cell transplantation. In order to evaluate proliferation and differentiation of transplanted cells, we assessed and analyzed the expression levels of the SSC-specific pre-mitotic (Plzf, Gfrα-1) and post-mitotic (Scp-1, Tekt-1) genes by quantitative RT-PCR (qRT-PCR). Figure 5 shows the results of qRT-PCR analysis in testicular tissue of experimental groups.
Fig. 5.
Gene expression evaluation by quantitative RT-PCR (qRT-PCR). Relative gene expression of pre-meiotic (Plzf, Gfrα-1) and post-meiotic (Scp-1, Tekt-1) by qRT-PCR. Asterisk shows significant difference
All amplified products had the expected size for that particular gene. Amplified products in control samples were not observed, which suggests lack of genomic DNA contamination.
The expression levels of specific pre- (Plzf, Gfrα-1) and post- (Tekt-1, Scp-1) meiotic markers between the sham and control groups were not significantly different. The abovementioned markers decreased in transplanted group following testicular torsion compared to the sham and control groups (p ≤ 0.05). SSC transplantation in mice significantly increased the expression of pre- and post-meiotic markers compared to ischemia reperfusion testicles (torsion group). However, the expression levels of these markers in SSCs-transplanted testis were considerably and significantly lower than the sham and control groups (Fig. 5).
Discussion
In this study, we propagated neonatal SSCs in vitro and then transplanted them into testicular ischemia reperfusion model. We demonstrated that SSCs transplantation could increase relative expression of pre-/post-meiotic genes and improve sperm parameters and testis structure in the ischemic (torsion) condition. The main and immediate outcome of testicular torsion is ischemia [35, 36]; therefore, a delay in presentation or failure of diagnosis and/or mismanagement of the condition leads to the loss of the testicle on the affected side. In our study, severe seminiferous epithelium disruption occurred during the ischemia or upon reperfusion.
SSCs transplantation was developed in mice approximately a decade ago and opened new approaches for biological investigations [18, 37]. There are various applications of spermatogonial stem cell transplantation beyond basic research [38]. For instance, cancer treatment can cause infertility; thus, obtaining and storing of spermatogonial stem cells from patients could be a promising way to restore and preserve fertility [38–40]. Also in testicular torsion, testicular biopsy is likely to provide the spermatogonial stem cells for cryopreservation, storage, and transplantation.
SSC transplantation has been examined in different azoospermic animal models [17, 20, 41, 42]. SSCs may be proliferated via in vitro culture [43], which in turn may increase the likelihood of successful transplantation [44, 45]. In addition, it provides a large number of stem cells for genetic manipulation, as well as biochemical and molecular analysis [46]. Perhaps, a recommended way to reach these goals is culture of stem cells in the presence of growth factors. Among the growth factors, GDNF (Glial-Cell-Line-Derived Neurotrophic Factor) is the most crucial factor to promote SSC self-renewal leading to a balance set between SSCs self-renewal and differentiation [47–49]. Without GDNF, spermatogonial aggregation cannot occur or SSCs may be perished [50]. In the cell culture, we experienced increased number of SSCs via self-renewal in presence of GDNF and somatic cells. Based on our flow cytometric results, SSCs were proliferated by twofolds after 2-weeks of culture. After isolation, the cell suspension contained 16 % SSCs while differential plating and in vitro culture enhanced the purity of these cells to 35 %. Our finding was consistent with previous in vitro studies reporting that culture increases the number of SSCs [51, 52].
On the basis of previously studies on SSCs isolation and purification, the availability of markers, which can confirm the exact identity of these cells, is essential. One of these markers is c-kit, the receptor for stem cell factor (SCF). c-kit is mostly more expressed by differentiated spermatogonia than by spermatogonial stem cells; thus, it could be used as a negative marker for SSCs identification [53, 54]. In addition, presence of integrin α-6 on mouse SSCs provided a positive marker for SSCs selection or identification [55]. Proper criteria for the recipients of SSCs transplantation include the removal of germ cell populations, existence of empty cavities, and presence of Sertoli cells within the seminiferous tubules and these might simplify might simplify the transplanted SSCs accommodation and colonization [56]. Hence, in this study, the best time for successful SSCs transplantation following testicular torsion (i.e., 14 days after 2 h of unilateral testicular torsion reperfusion) was selected according to the findings from our earlier study [26].
According to the results obtained from the evaluation of epididymal sperm parameters in this study, there was a significant improvement in sperm concentration, motility, and viability in the SSCs-transplanted group compared to the torsion group. However, this value in SSCs-treated animals was still significantly less compared to the sham and control groups 8 weeks after transplantation.
Seminiferous tubular diameter and seminiferous tubules epithelium thickness indicate the testis function and spermatogenesis [57]; however, the Johnsen score describes a new and rapid method for registration of spermatogenesis in testes [32]. In this study, a high correlation was found between testicular biopsy score and sperm count. Based on Johnsen scoring, most seminiferous tubules were restored 8 weeks following SSCs transplantation and were consistent with the other findings of our study. Our pervious study suggested that SSCs transplantation can produce sperm in the recipient’s testes after autologous transplantation. Although improvement of sperm parameters, testicular structure, and testicular weight were observed, no improvement in live birth has been detected. Moreover, assessed parameters in experimental group were less than those in the sham and control groups 2 months after transplantation [17, 58]. Honaramooz et al. showed that SSCs homologous transplantation increases the epididymal sperm concentration in busulfan-treated mice [20]. Izadyar et al. demonstrated that autologous transplantation of bovine spermatogonial stem cells resulted in an increase of the germ cells within the tubules and therefore a complete regeneration of spermatogenesis [19]. Our finding agreed with the reports by the aforementioned investigators.
So far, SSC-specific markers have been used to recognize the SSCs enrichment via in vivo and in vitro methods [53, 59, 60]. In addition, selected testicular cells by SSC markers have been employed to improve the restoration of fertility following SSC transplantation [61]. Therefore, to determine the efficiency of transplantation and to confirm the morphology, in this study, relative expressions of pre- and post-meiotic genes were evaluated by real-time PCR. GFRα-1 is expressed in SSCs as one of the two receptors for GDNF, to regulate the ratio of self-renewal and differentiation of SSCs. PLZF is a transcription factor that contributes to the maintenance of the male germ line stem cell [62]. These markers are well-known spermatogonial-specific markers in many species and are expressed in spermatogonial stem and progenitor cells (As-Aal) [63, 64] but not expressed in post-meiotic spermatogonial cells [65, 66]. Based on these studies, we conclude that assessment and comparison of pre-meiotic (Plzf, Gfrα-1) gene expressions among the groups can identify proliferation of transplanted SSCs in ischemic testicular tissue. As noted in the results, expression of pre-meiotic genes were significantly declined in ischemic testis compared to control and sham groups. The relative expressions of these genes were increased after SSCs transplantation, although it was still less than the control and the sham. These results are consistent with other findings of this study, indicating an increase in the number of spermatogonial cells in ischemic testis after SSCs transplantation.
SCP1 and TEKT1 are post-meiotic markers [67, 68]. SCP1 is involved in synaptonemal complex during meiosis, and TEKT1 is the specific marker of more mature male haploid gametes implicating in sperm flagellum formation [67, 69, 70]. Spermatogonial cell differentiation, spermatogenesis, and the overall testis function might be evaluated via analysis of post-meiotic genes expressions in testicular tissue. Evaluation of post-meiotic genes expressions in SSCs-transplanted ischemic testis may also indicate the transplantation and its efficiency.
In most studies, donor SSC colonization was evaluated via fluorescent microscopy; perception of high fluorescence intensity in seminiferous tubules shows donor SSCs proliferation and differentiation from multi-layered, undifferentiated germ cells to haploid spermatid stage. However, a moderate and a low fluorescence intensity observations reveal the presence and absence of differentiating cells to the spermatocyte stage, respectively [69, 71]. Although a real-time observation of the proliferation and differentiation of transplanted SSCs via the described method was not performed in this study, it was speculated and perceived by assessment of pre- and post-meiotic markers.
Conclusion
This study showed that SSCs transplantation was effective for the treatment of testicular ischemia due to testicular torsion in a mouse model. Isolation, proliferation, and SSCs transplantation may be a new approach for fertility preservation in testicular torsion.
Acknowledgments
This study was funded by a grant from Iran University of Medical Sciences (IUMS) (Number: 90-04-30-14879) for PhD student thesis, and all experiments have been performed at Cellular and Molecular Research Center, IUMS, Tehran, Iran.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule SSCs transplantation increase relative expression of pre-/post-meiotic genes and improve sperm parameters and testis structure in testicular torsion-detorsion mice.
Summary sentence
SSCs transplantation increase relative expression of pre-/post-meiotic genes and improve sperm parameters and testis structure in the ischemic condition.
References
- 1.Schilling K, Toth B, Rösner S, Strowitzki T, Wischmann T. Prevalence of behaviour-related fertility disorders in a clinical sample: results of a pilot study. Archives of Gynecology and Obstetrics. 2012:1–8. [DOI] [PubMed]
- 2.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G et al. European Association of Urology Guidelines on Male Infertility: the 2012 update. European urology. 2012. [DOI] [PubMed]
- 3.Ahmed A, Bello A, Mbibu NH, Maitama HY, Kalayi GD. Epidemiological and aetiological factors of male infertility in northern Nigeria. Niger J Clin Pract. 2010;13(2):205–9. [PubMed] [Google Scholar]
- 4.Anderson J, Williamson R. Testicular torsion in Bristol: a 25‐year review. Br J Surg. 1988;75(10):988–92. doi: 10.1002/bjs.1800751015. [DOI] [PubMed] [Google Scholar]
- 5.Melekos M, Asbach H, Markou S. Etiology of acute scrotum in 100 boys with regard to age distribution. J Urol. 1988;139(5):1023–5. doi: 10.1016/s0022-5347(17)42756-x. [DOI] [PubMed] [Google Scholar]
- 6.Cuckow P, Frank J. Torsion of the testis. BJU Int. 2000;86(3):349–53. doi: 10.1046/j.1464-410x.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 7.Bennett S, Nicholson MS, Little TM. Torsion of the testis: why is the prognosis so poor? Br Med J. 1987;294(6575):824. doi: 10.1136/bmj.294.6575.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson J, Williamson R. Fertility after torsion of the spermatic cord. Br J Urol. 2008;65(3):225–30. doi: 10.1111/j.1464-410X.1990.tb14715.x. [DOI] [PubMed] [Google Scholar]
- 9.Cox AM, Patel H, Gelister J. Testicular torsion. Br J Hosp Med. 2012;73(3):C34–6. doi: 10.12968/hmed.2012.73.sup3.C34. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen L, Lievano G, Ghosh L, Radhakrishnan J, Fornell L, John E. Effect of unilateral testicular torsion on blood flow and histology of contralateral testes. J Pediatr Surg. 1999;34(5):680–3. doi: 10.1016/S0022-3468(99)90355-X. [DOI] [PubMed] [Google Scholar]
- 11.Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613–26. doi: 10.1016/j.pop.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Torres MA, Bordin ALB, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Asp Med. 2004;25(1–2):199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Aitken RJ, Baker MA. Oxidative stress and male reproductive biology. Reprod Fertil Dev. 2004;16(5):581–8. doi: 10.1071/RD03089. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Prabakaran SA. Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43(11):963. [PubMed] [Google Scholar]
- 15.Abdallah B, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2007;15(2):109–16. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 16.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, et al. Derivation of male germ cells from bone marrow stem cells. Lab Investig. 2006;86(7):654–63. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 17.Koruji M, Movahedin M, Mowla SJ, Gourabi H, Pour-Beiranvand S, Arfaee AJ. Autologous transplantation of adult mice spermatogonial stem cells into gamma irradiated testes. Cell J(Yakhteh) 2012;14(2):82–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126(6):765–74. doi: 10.1530/rep.0.1260765. [DOI] [PubMed] [Google Scholar]
- 20.Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64(4):422–8. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 21.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17(1):55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Ma W, An L, Wu Z, Wang X, Guo M, Miao K, et al. Efficient and safe recipient preparation for transplantation of mouse spermatogonial stem cells: pretreating testes with heat shock. Biol Reprod. 2011;85(4):670–7. doi: 10.1095/biolreprod.110.089623. [DOI] [PubMed] [Google Scholar]
- 23.Ohta H, Tohda A, Nishimune Y. Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biol Reprod. 2003;69(6):1815–21. doi: 10.1095/biolreprod.103.019323. [DOI] [PubMed] [Google Scholar]
- 24.McLean DJ, Friel PJ, Johnston DS, Griswold MD. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol Reprod. 2003;69(6):2085–91. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- 25.McLean DJ. Spermatogonial stem cell transplantation, testicular function, and restoration of male fertility in mice. Methods Mol Biol. 2008;450:149–62. doi: 10.1007/978-1-60327-214-8_11. [DOI] [PubMed] [Google Scholar]
- 26.Azizollahi S, Aflatoonian R, Sedigi-Gilani MA, Asghari Jafarabadi M, Behnam B, Azizollahi G, et al. Recruiting testicular torsion introduces an azoospermic mouse model for spermatogonial stem cell transplantation. Urol J. 2014;11(3):1648–55. [PubMed] [Google Scholar]
- 27.Lysiak JJ, Turner SD, Nguyen QAT, Singbartl K, Ley K, Turner TT. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biol Reprod. 2001;65(3):718–25. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- 28.van Pelt AM, Morena AR, van Dissel-Emiliani FM, Boitani C, Gaemers IC, de Rooij DG, et al. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55(2):439–44. doi: 10.1095/biolreprod55.2.439. [DOI] [PubMed] [Google Scholar]
- 29.Eslahi N, Hadjighassem MR, Joghataei MT, Mirzapour T, Bakhtiyari M, Shakeri M, et al. The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int J Nanomedicine. 2013;8:4563–76. doi: 10.2147/IJN.S45535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61(1):225–30. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41(1):111–22. [PubMed] [Google Scholar]
- 32.Johnsen SG. Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormone Research in Paediatrics. 1970;1(1):2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 33.Tajaddini S, Ebrahimi S, Behnam B, Bakhtiyari M, Joghataei MT, Abbasi M, et al. Antioxidant effect of manganese on the testis structure and sperm parameters of formalin-treated mice. Andrologia. 2014;46(3):246–53. doi: 10.1111/and.12069. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.van Pelt AMM, Roepers-Gajadien HL, Gademan IS, Creemers LB, de Rooij DG, van Dissel-Emiliani FMF. Establishment of cell lines with rat spermatogonial stem cell characteristics. Endocrinology. 2002;143(5):1845–50. doi: 10.1210/endo.143.5.8806. [DOI] [PubMed] [Google Scholar]
- 36.Turner TT. Acute experimental testicular torsion. No effect on the contralateral testis. J Androl. 1985;6(1):65–72. doi: 10.1002/j.1939-4640.1985.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 37.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota H, Brinster RL. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Rev Endocrinol. 2006;2(2):99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Liu B, Xipeng G, Xie G, Liao S, Quan D, et al. Microstructure and properties of nano-fibrous PCL-b-PLLA scaffolds for cartilage tissue engineering. Eur Cell Mater. 2009;18:63–74. doi: 10.22203/ecm.v018a06. [DOI] [PubMed] [Google Scholar]
- 40.Brinster RL. Male germline stem cells: from mice to men. Science (New York) NY. 2007;316(5823):404–5. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oatley JM, Reeves JJ, McLean DJ. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. 2004;71(3):942–7. doi: 10.1095/biolreprod.104.028894. [DOI] [PubMed] [Google Scholar]
- 42.Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66(1):21–8. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 44.Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115(7):1855–61. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston DS, Russell LD, Griswold MD. Advances in spermatogonial stem cell transplantation. Rev Reprod. 2000;5(3):183–8. doi: 10.1530/ror.0.0050183. [DOI] [PubMed] [Google Scholar]
- 46.Kanatsu-Shinohara M, Inoue K, Lee J, Miki H, Ogonuki N, Toyokuni S, et al. Anchorage-independent growth of mouse male germline stem cells in vitro. Biol Reprod. 2006;74(3):522–9. doi: 10.1095/biolreprod.105.046441. [DOI] [PubMed] [Google Scholar]
- 47.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–86. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288(1–2):95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato T, Aiyama Y, Ishii-Inagaki M, Hara K, Tsunekawa N, Harikae K, et al. Cyclical and patch-like GDNF distribution along the basal surface of Sertoli cells in mouse and hamster testes. PLoS One. 2011;6(12):e28367. doi: 10.1371/journal.pone.0028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huleihel M, Abuelhija M, Lunenfeld E. In vitro culture of testicular germ cells: regulatory factors and limitations. Growth Factors. 2007;25(4):236–52. doi: 10.1080/08977190701783400. [DOI] [PubMed] [Google Scholar]
- 51.Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124(1):85–94. doi: 10.1530/rep.0.1240085. [DOI] [PubMed] [Google Scholar]
- 52.Smith LA, Liu X, Ma PX. Tissue engineering with nano-fibrous scaffolds. Soft Matter. 2008;4(11):2144–9. doi: 10.1039/b807088c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97(15):8346–51. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140(12):5894–900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 55.Shinohara T, Avarbock MR, Brinster RL. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96(10):5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa T, Dobrinski I, Brinster R. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31(5):461–72. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- 57.Russell L, Ettlin R, Sinha Hikim A, Clegg E. Mammalian spermatogenesis. Histological and histopathological evaluation of the testis. 1990;1:1–40. [Google Scholar]
- 58.Koruji M. Autograft of fresh and freezed spermatogonial cells of adult mouse after coculture with Sertoli cells and treatment with GDNF, SCF and GM-CSF cytokines to the azoospermic mice with gamma-ray. Presented for the Ph D, Tehran Tarbiat Modares University. 2007.
- 59.Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in steel and cryptorchid infertile mouse models. Dev Biol. 2000;220(2):401–11. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 60.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100(11):6487–92. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLean DJ. Spermatogonial stem cell transplantation and testicular function. Cell Tissue Res. 2005;322(1):21–31. doi: 10.1007/s00441-005-0009-z. [DOI] [PubMed] [Google Scholar]
- 62.Von Schönfeldt V, Wistuba J, Schlatt S. Notch-1, c-kit and GFRα-1 are developmentally regulated markers for premeiotic germ cells. Cytogenet Genome Res. 2004;105(2–4):235–9. doi: 10.1159/000078194. [DOI] [PubMed] [Google Scholar]
- 63.Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73(5):1011–6. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 64.Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27(12):3043–52. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- 65.Hofmann M-C, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–24. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36(6):647–52. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 67.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28(36):5401–6. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirzapour T, Movahedin M, Tengku Ibrahim TA, Haron AW, Nowroozi MR. Evaluation of the effects of cryopreservation on viability, proliferation and colony formation of human spermatogonial stem cells in vitro culture. Andrologia. 2013;45(1):26–34. doi: 10.1111/j.1439-0272.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- 69.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60(6):1429–36. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amos LA. The tektin family of microtubule-stabilizing proteins. Genome Biol. 2008;9(229):231–42. doi: 10.1186/gb-2008-9-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohta H, Yomogida K, Yamada S, Okabe M, Nishimune Y. Real‐time observation of transplanted ‘green germ cells’: proliferation and differentiation of stem cells. Develop Growth Differ. 2000;42(2):105–12. doi: 10.1046/j.1440-169x.2000.00495.x. [DOI] [PubMed] [Google Scholar]