Abstract
Purpose
This study aims to determine if the integrity of the sperm plasma membrane and acrosome vesicle could be limiting factors in sheep intracytoplasmic sperm injection (ICSI).
Methods
Prior to in vitro fertilization (IVF) or ICSI, the oocytes were subjected to in vitro maturation (IVM) for 24 h. First, to evaluate the need of artificial activation for ovine ICSI, 226 oocytes were injected with intact spermatozoa (IS), from which 125 were activated by incubation in ionomycin and 101 were cultured without activation. Next, spermatozoa were mechanically (by piezo-electrical pulses) and/or chemically (by ionomycin/Triton X-100) treated to break membranes and acrosomes and were injected into oocytes, grouped as follows: (i) piezo-pulsed spermatozoa (PPS), (ii) PPS pre-treated with ionomycin (PPS-I), (iii) PPS pre-treated with Triton X-100 (PPS-T), and (iv) intact and untreated spermatozoa as a control (CTR-IS).
Results
No differences were observed in the zygote/cleavage/blastocyst rate between chemically activated and non-activated oocytes (50 vs. 45 %, 11.6 vs. 10.1 %; 1.8 vs. 1.1 %, respectively), after ICSI with CTR-IS. Injection of PPS compared to CTR-IS increased the proportion of zygotes and blastocysts (84.6 vs. 45 %, p < 0.01; 15.5 vs. 1.1 %, p < 0.0001, respectively). Moreover, the percentage of PPS-derived blastocysts was not significantly different from that obtained by conventional IVF (15.5 vs. 20.2 %). The ICSI blastocysts’ development was also improved with PPS pre-treated with ionomycin (15.6 %), but was completely impeded with PPS pre-treated with Triton X-100 (0 %).
Conclusion
Our findings confirm that ICSI with spermatozoa whose plasma membrane and acrosome have been mechanically damaged substantially improves embryonic development until the blastocyst stage.
Keywords: ICSI, Ovine sperm, Plasma membrane, Acrosome
Introduction
The intracytoplasmic sperm injection (ICSI) bypasses fundamental physiological steps such as oocyte-sperm interaction and fusion. ICSI is well-established in human and mouse but, for unknown reasons, is less efficient and not yet standardized in other species, small ruminants in particular [5,6,16,35].
Oocyte/spermatozoon fusion at fertilization ensures the loss of the acrosomal vesicle and plasma membrane, whereas in ICSI, spermatozoon with an intact acrosome and membrane are injected into the oocyte. Several studies in mice have stressed the importance of the membrane and acrosome’s removal before ICSI [18,25]. Furthermore, the retention of spermatozoa membranes is known to delay oocyte activation in mouse and human model [19,38], and the acrosomal enzymes are harmful to hamster oocytes [25,37]. In this work, we investigated whether the presence of the plasma membrane and the sperm acrosomal vesicle could be the limiting factors in sheep ICSI. To address the issue, the plasma membranes and acrosomes of spermatozoa were damaged by chemical and/or mechanical treatment before ICSI. Our findings showed a significant increase in ICSI blastocyst development when the plasma membranes and acrosomes of spermatozoa were mechanically damaged by piezo pulses.
Materials and methods
All chemicals were purchased from Sigma (Milano, Italy), unless otherwise stated.
Oocyte recovery and in vitro maturation (IVM)
Sheep ovaries were collected from local slaughterhouses and transferred at 37 °C to the laboratory within 1–2 h. Oocytes were aspirated with 21 G needles in the presence of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered TCM-199 medium (Gibco, Life Technologies, Milan, Italy) and containing 0.005 % (w:v) heparin. Oocytes having at least 2–3 layers of compact cumulus cells were selected for IVM in bicarbonate-buffered TCM-199 (Gibco) containing 2 mM glutamine, 0.3 mM sodium pyruvate, 100 μM cysteamine, 10 % fetal bovine serum (FBS) (Gibco), 5 μg/ml follicle stimulating hormone FSH (Ovagen, ICP, Auckland, New Zealand), 5 μg/ml luteinizing hormone (LH), and 1 μg/ml estradiol. Maturation was conducted in 4-well culture plates (Nunclon, Roskilde, Denmark) containing 0.4 ml/well of IVM medium, incubated in a humidified atmosphere of 5 % CO2 in air at 38.5 °C for 24 h [24]. After IVM, only selected MII oocytes with expanded cumulus and normal morphology were used for ICSI.
Sperm preparation for intracytoplasmic sperm injection (ICSI)
Frozen ram semen was purchased from commercial sources (Semenitaly; ca. 400 × 106 sptz/ml). The semen was fast-thawed by immersing the straw in 35 °C water for a few seconds. Then straw was opened directly into a 1.5-ml eppendorf, together with the freezing medium, and incubated in a humidified atmosphere at 38.5 °C and 5 % CO2 for 5 min. Next, 1 μl of semen was diluted in 500 μl of IVF medium (bicarbonate-buffered synthetic oviductal fluid (SOF) enriched with 20 % (v:v) heat-inactivated estrus sheep serum, 2.9 mM calcium lactate, and 16 μM isoproterenol) buffered with HEPES. The latter was diluted 1:1 with 12 % (w:v) polyvinylpyrrolidone (PVP), and 10 μl of this final solution was placed on the lid of a Petri dish on a warmed microscope stage covered by mineral oil.
We first sought to assess the need for artificial activation of sheep oocytes following ICSI. To do so, 226 oocytes injected with intact spermatozoa (IS) were divided into two groups: (i) group A (activated) was chemically activated by 5 min of incubation with 5 mg/ml ionomycin and then washed twice in H199 and cultured as described below (n = 125) and (ii) group NA (not-activated) did not undergo chemical activation (n = 101).
In the next experiments, spermatozoa were mechanically and/or chemically treated before ICSI, in order to remove sperm plasma membrane and to induce acrosomal reaction. Sperm plasma membrane was damaged by piezo pulses (mechanical removal) and by Triton X-100 (chemical dissolution). Acrosomal reaction in spermatozoa was induced by ionomycin (Ca2+ ionophore) as well as by piezo pulses (mechanical rupture). The spermatozoa were divided into the following four experimental groups: (i) piezo-pulsed spermatozoa (PPS), (ii) PPS pre-treated 5 min with 5 mg/ml ionomycin (in IVF medium) (PPS-I), (iii) PPS pre-treated 10 min with 0.2 % Triton X-100 (in IVF medium) (PPS-T), and (iv) intact and untreated spermatozoa, as control (CTR-IS).
The injection was performed under an inverted microscope (Nikon Eclipse E-800) connected to a micromanipulation system (Narishige, Tokyo, Japan), using a piezo micropipette-driving system (PiezoXpert, Eppendorf, Milan, Italy). The group of intact spermatozoa (CTR-IS) was injected without the piezo device, with a canonical beveled injection pipette, in order to avoid membrane damage. Oocytes were injected in groups of five to avoid prolonged light exposure, PVP/sperm suspension drops were renewed every ten injected oocytes.
Sperm plasma membrane and acrosomal integrity
To evaluate the membrane integrity/damage after mechanical treatment (piezo-electrical pulses) and chemical treatment (0.2 % Triton X-100 for 10 min), spermatozoa were incubated in a 5 μg/ml propidium iodide (PI) solution for 10 min and then observed under an epifluorescence microscope (Nikon Eclipse E-600). PI is a fluorescent dye able to permeate only damaged membranes. Intact spermatozoa were used as control.
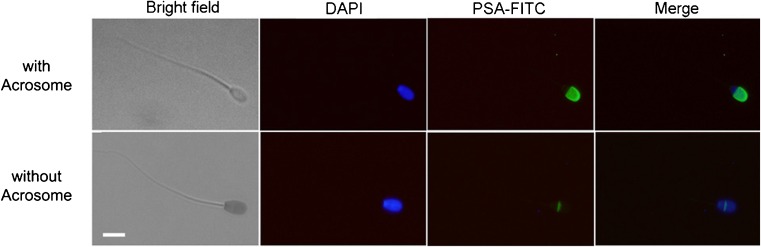
The acrosomal integrity/damage was evaluated by Pisum sativum agglutinin (PSA) assay, after mechanical (piezo-electrical pulses) and/or chemical (ionomycin/Triton X-100) treatments. The chemical treatments were performed by incubation of spermatozoa with 5 mg/ml ionomycin for 5 min or 0.2 % Triton for 10 min, to induce Ca2+-dependent acrosomal reaction and to extract the membranes, respectively. Briefly, treated semen was smeared on glass slides and fixed in ethanol for 5 min at room temperature (RT). After one wash in phosphate buffered saline (PBS), the slides were incubated with 40 μg/ml of PSA (PSA-FITC) for 12 min in a dark room to visualize the acrosome vesicle; nuclei were counterstained with 5 μg/ml DAPI (4′,6-diamidin-2-fenilindolo) for 5 min at RT. After a wash in bi-distilled water, the slides were mounted and images were captured by an epifluorescence microscope (Nikon Eclipse E-600). Spermatozoa with acrosomes showed intense fluorescence of the acrosomal cap, while those without showed no fluorescence or a faint signal around the equatorial area (Fig. 1).
Fig. 1.
Acrosomal integrity after PSA-FITC staining. Spermatozoa with acrosome show brilliant green signal on the acrosomal cap; spermatozoa without acrosome show green signal only in the equatorial region of the head. All heads (nuclei) were counterstained with DAPI (blue). Merge means DAPI + PSA-FITC. Scale bar = 5 μm
Transmission Electron Microscopy (TEM)
Sperm plasma membranes of intact (IS) and piezo-pulsed spermatozoa (PPS) were evaluated by TEM. For PPS, thawed semen was opportunely diluted in 12 % PVP to be piezo-pulsed, as previously described for ICSI. Then, IS and PPS were washed twice in PBS and fixed in glutaraldehyde (2.5 % in 0.1 M cacodylate buffer, pH 7.4) for 24 h. After washing in bi-distilled H2O (ddH2O), sperms were post-fixed in 2 % OsO4 in ddH2O for 4 h and washed three times in ddH2O. Next, spermatozoa were dehydrated through a graded series of ethanol solutions (30 % - 10 min, 50 % - 15 min, 70 % - 24 h, 80 % - 10 min, 96 % - 10 min, 100 % - 10 min, acetone - twice for 15 min), infiltrated with graded concentrations of EPON resin in 100 % acetone (1:3 - 20 min, 1:1 - 24 h, 3:1 - 2 h), infused twice for 1 h in pure EPON resin and polymerized at 65 °C for 24 h. Finally, 60 nm sections were prepared and examined using a LEO 912AB electron microscope. Images were captured using a Slow Scan CCD camera (Proscane) and EsiVision Pro 3.2 software (Soft Imaging Systems GmbH).
In vitro fertilization (IVF)
Oocytes in vitro fertilized by the conventional method were used as a control for embryo development until the blastocyst stage. Matured oocytes (MII) were fast pipetted in 300 U/ml hyaluronidase solution to separate well from each other and transferred into 50 μl drops of IVF medium covered by mineral oil. Ram semen was fast-thawed in 35 °C water and centrifuged in sperm wash medium (SOF containing 0.4 % (w/v) bovine serum albumin (BSA)), at 120 × g for 5 min. Fertilization was carried out at a final concentration of 5 × 106 spermatozoa/ml and left overnight in a humidified atmosphere at 38.5 °C, 5 % CO2, and 7 % O2 [24].
Embryo culture
All the ICSI groups, as well as oocytes after IVF, were incubated overnight in an IVF medium, in a humidified atmosphere at 38.5 °C, 5 % CO2, and 7 % O2.
After 22 h, presumptive zygotes (both ICSI and IVF) were washed in SOF medium and then transferred into 20 μl drops of SOF enriched with 2 % (v:v) basal medium Eagle (BME)-essential amino acids (EAA), 1 % (v:v) minimum essential medium (MEM)-nonessential amino acids (NEAA) (Gibco), 1 mM glutamine, and 8 mg/ml fatty acid-free BSA, covered by mineral oil. The medium was renewed on day 3 (SOF supplemented with 0.27 mg/ml glucose (SOF+), 2 % EAA, 1 % NEAA), on day 5 (SOF+ with 10 % of charcoal stripped FBS (csFBS), 2 % EAA, 1 % NEAA), and on day 6 (1:1 MEM/M199 enriched with 10 % csFBS, 2.5 μg/ml gentamicin, and 1 % sodium pyruvate) until day 8. The in vitro development was recorded on day 1 (cleavage stage) and 7/8th day (blastocyst stage) as previously described in Ptak et al. [24] and Iuso et al. [12].
Pronuclear staining
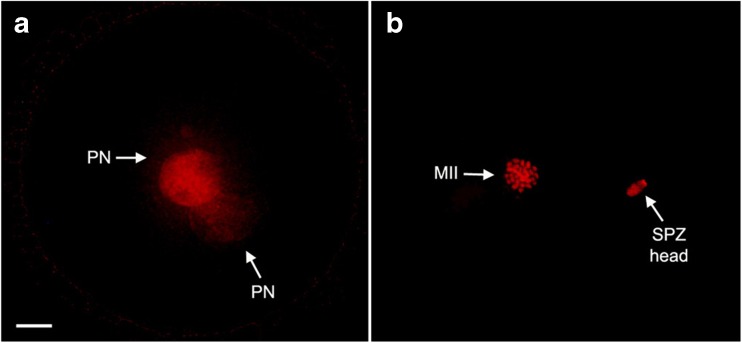
Fourteen to 16 h after spermatozoa injection, presumptive zygotes were fixed in 4 % paraformaldehyde (PFA) overnight at 4 °C and permeabilized for 15 min with 0.1 % Triton X-100 (in PBS). Then, the pronuclei (PN) were counterstained for 5 min with 5 μg/ml PI at RT. After two washes in 0.4 % PVP (in PBS), the slides were mounted and images were captured by an epifluorescence microscope (Nikon Eclipse E-600) (Fig. 2).
Fig. 2.
Pronuclear formation after ICSI. The presence of pronuclei (PN) was analyzed 14–16 h post sperm injection. a Activated oocytes showed two clearly distinguishable pronuclei; b non-activated oocytes showed chromosomal metaphase (MII) and a non-decondensed sperm head (SPZ head) closer to it. All nuclei were counterstained with propidium iodide. Scale bar = 10 μm
Statistical analyses
Fisher’s exact and Chi-square tests were used to compare in vitro development. Statistical significance was considered when probability values (p) were lower than 0.05. Statistical analyses were performed using GraphPad Prism 5.0 software.
Results
Sperm plasma membrane and acrosome integrity
All piezo-pulsed spermatozoa’s nuclei (100 % of PPS), as well as all the Triton X-100-treated ones (100 %), were reached by PI, indicating membrane breakages, while only 29 % (58/197) of CTR-IS showed damaged membranes.
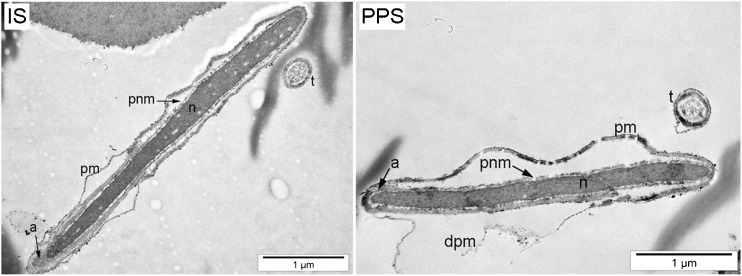
TEM analysis confirmed our previous observations on the integrity of the sperm plasma membrane. Images clearly show broken membrane in PPS and intact one in IS (Fig. 3).
Fig. 3.
Integrity of sperm plasma membrane evaluation. The integrity of sperm plasma membrane was evaluated by Transmission Electron Microscopy (TEM) in intact (IS) and piezo-pulsed spermatozoa (PPS). Abbreviations: pm plasma membrane, dpm damaged plasma membrane, a acrosome, n nucleus, t tail, pnm perinuclear material
Staining for the presence of acrosome vesicle showed a higher percentage of acrosomal loss in PPS and ionomycin-treated spermatozoa, compared to intact and untreated spermatozoa (CTR-IS): 46.2 % (48/104), 42.7 % (47/110), and 20 % (24/120), respectively (PPS vs. CTR-IS and ionomycin treated vs. CTR-IS, p < 0.0001). Moreover, we found that also Triton X-100 treatment rapidly denatured also the acrosomal cap in all observed spermatozoa (100 %) (Fig. 2, Table 1).
Table 1.
Acrosome loss in the different spermatozoa groups
| Spermatozoa treatments | Without acrosome (%) |
|---|---|
| Mechanical (piezo pulses) | 48/104 (46.2)a |
| Chemical (ionomycin) | 47/110 (42.7)b |
| Chemical (Triton X-100) | 100/100 (100)c |
| Intact, untreated (CTR-IS) | 24/120 (20) |
Mechanical treatment indicates PPS; chemical treatments indicate spermatozoa treated with ionomycin for 5 min (ionomycin) or with Triton X-100 for 10 min (Triton X-100). Fisher’s exact test
aPPS vs. CTR-IS, p < 0.0001
bChemical ionomycin vs. CTR-IS, p < 0.0001
cChemical Triton X-100 vs. CTR-IS, p < 0.0002
The results indicate that piezo pulses not only damaged the membrane but also induced acrosomal loss (Table 1), as ionomycin does. No statistical differences were found between the reacted spermatozoa of the two groups.
ICSI outcome
Pronuclear formation (2 PN), cleavage, and blastocyst rate did not differ between activated and non-activated oocytes after injection with intact spermatozoa (CTR-IS) (20/40 (50 %) and 18/40 (45 %); 13/112 (11.6 %) and 9/89 (10.1 %); 2/112 (1.8 %) vs. 1/89 (1.1 %), respectively, Table 2). Given that our findings indicated that activation is not necessary for sheep embryo development, we decided to skip this step in the following experiments.
Table 2.
In vitro development of ICSI embryos
| Injected spermatozoa | Oocytes | 2 PN (%) | 2 cells (%) | Blastocysts (%) | |
|---|---|---|---|---|---|
| CTR-IS | Group A | 125 | 10/20 (50) | 13/112 (11.6) | 2/112 (1.8) |
| Group NA | 101 | 9/20 (45) | 9/89 (10.1) | 1/89 (1.1) | |
| PPS | 110 | 22/26 (84.6)a,b | 44/84 (52.3)c | 13/84 (15.5)d | |
| PPS-I | 64 | / | / | 10/64 (15.6) | |
| PPS-T | 70 | / | / | 0/70e | |
Fisher’s exact test
Abbreviations: PPS piezo-pulsed spermatozoa, PPS-I piezo-pulsed spermatozoa pre-treated with ionomycin, PPS-T piezo-pulsed spermatozoa pre-treated with Triton X-100, CTR-IS intact and untreated spermatozoa, Group A activated oocytes, Group NA non-activated oocytes
aPPS vs. Group NA, p = 0.0099
bPPS vs. Group A, p = 0.0221
cPPS vs. Group A, p < 0.0001
dPPS vs. Group A, p = 0.0005
ePPS-T vs. PPS, p = 0.0003
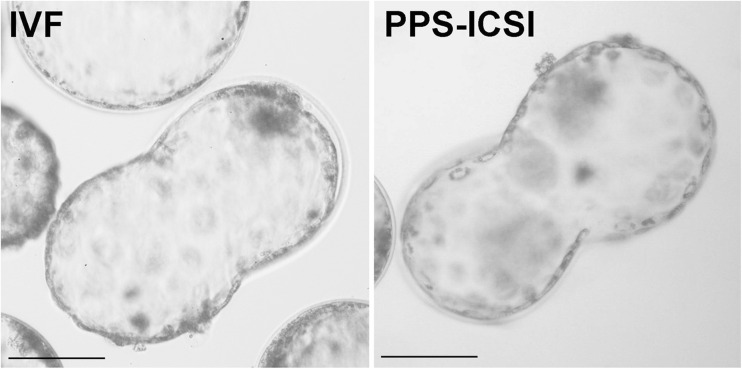
Injection of piezo-pulsed spermatozoa (PPS) improved embryo development. 2PN were visible in 84.6 % of the oocytes (CTR-IS: 9/20 (45 %) vs. PPS: 22/26 (84.6 %); p < 0.01) (Table 2). Moreover, the injection of PPS significantly improved the number of embryos reaching the blastocyst stage (CTR-IS: 2/112 (1.8 %) vs. PPS: 13/84 (15.5 %); p = 0.0005), which approached the frequency of development and quality of the IVF-derived blastocysts (IVF: 25/124 (20.2 %)) (Fig. 4).
Fig. 4.
Sheep blastocysts at 8th day of culture. Image compares blastocysts derived from IVF (left) and ICSI with PPS (right). IVF in vitro fertilized blastocyst, PPS-ICSI blastocysts obtained from ICSI with piezo-pulsed spermatozoa. Scale bar = 100 μm
We then verified the effects of chemical treatment of spermatozoa (ionomycin and Triton X-100) on ICSI outcomes. Injection of PPS pre-treated with ionomycin (PPS-I) resulted in extremely similar blastocyst rates as PPS injected oocytes (10/64 (15.6 %) vs. 13/84 (15.5 %), respectively), while none of the oocytes injected with PPS pre-treated with Triton X-100 (PPS-T) entered the first mitosis stage (Table 2).
Discussion
Here, we show that the mechanically induced membrane and acrosome damage in ram spermatozoa before ICSI significantly improved the embryonic development.
Setting up the experimental design, we opted to use the in vitro matured sheep oocytes. Although their developmental competence is lower than in vivo recovered oocytes, they could be successfully used in ruminant assisted reproduction [7]. We believe that in vitro matured oocytes shall give us great and easily repeatable results in every laboratory of ruminant reproduction.
The low success of ICSI in sheep has generally been ascribed to inadequate oocyte activation by ram spermatozoon; therefore, an external stimulus has usually been provided [3,4,11,30].
Our findings demonstrate that chemical activation is not fundamental to ovine ICSI (Table 2), because the spermatozoon by itself activates the oocyte, provided that the spermatozoa’s inner content becomes accessible to the ooplasm, as occurs following breakage of its membrane.
We decided to faithfully follow what happens during normal fertilization: the sperm enters the oocyte after plasma membrane fusion, releasing its cytoplasmic content into the oocyte, activating the oocyte, and triggering embryo development [15]. Disorganization of the spermatozoon’s plasma membrane after the fusion step has been associated with the release of sperm-borne oocyte-activating factor (SOAF) [20,28,31]. It has been shown that in human, disruption of sperm plasma membrane induces activation earlier than membrane-intact ones, when injected into oocytes [8], and that, conversely, Ca2+ oscillations—the first signal of activation—are delayed when the intact spermatozoon is injected [19,38].
The rupture of the sperm membrane as coadjuvant in ICSI fertilization has already been highlighted in murine and human models, where ICSI efficiency is higher than in large animals. In the mouse ICSI, the isolated head or piezo-pulsed demembraned spermatozoa are routinely injected into the oocytes [14,39]. On the other hand, in human ICSI, the spermatozoa are subjected to membrane injuries when hit with the pipette to immobilize them [9,17]. This mechanical stress seems to be associated with an increased proportion of oocyte activation [13,21].
However, despite the fact that the damaging of the plasma membrane positively influences the success of ICSI, its complete extraction by Triton X-100 incubation turned out to be deleterious for embryonic development, in contrast to what has been described in mouse [18]. The dissolution of the plasma membrane by detergent could induce the loss of the sperm-activating factor, abolishing its activation ability, or perhaps it might denature the centriole (an issue not explored here). On the other hand, it is likely that piezo pulses cause mechanical damage to the sperm membrane without compromising the sperm’s activation activity, in agreement with previously published papers [33,35,36].
We believe that mechanical damaging of the spermatozoon’s membrane, immediately before injection, triggers the oocyte activation as physiologically occurs. Likely, as for a normal fertilization, sperm phospholipase Cζ spreads into the oocyte, gives rise to the phosphoinositide pathway that results in the leak of calcium (Ca2+) from the endoplasmic reticulum [1,2,27,34].
The second biological question addressed in our study regarded the role of the acrosome in ICSI efficiency. Several reports showed higher efficiency in mouse and human ICSI following removal of the acrosomal vesicle [18,29,32], which rested on the assumption that its hydrolytic enzymes could harm the oocytes [25,37]. Notwithstanding that ionomycin induces acrosomal reaction (as suggested by Rotem et al. [26] and confirmed in our experiments), we reached the same blastocyst rate by injecting piezo-pulsed spermatozoa pre-treated both with and without ionomycin (PPS: 15.6 % vs. PPS-I: 15.5 %). Our conclusion is that the mechanical force exerted by the piezo pulses on the sperm membrane, as well as on the acrosome, ensures efficient oocyte activation and improves embryo development, representing the best way to increase the success of ICSI in sheep.
Our findings represent a significant improvement to ICSI in sheep, and our data could have implications for other species, like cattle. The number of good quality blastocysts produced following ICSI in our experiments approached those produced by normal IVF. Although further work might push the technology further, the number and quality of embryos obtained with our approach could boost the use of ICSI in several fields of reproduction biotechnologies, such as the production of large transgenic animals as disease models [22,23], as well as the replacement/expansion of animals threatened with extinction [10].
Acknowledgments
This work was supported by Program FIRB GA B81J12002520001 (GenHome) to PL, Program OVI-TE-CA 2011 (financing-Fondazione Tercas) to GP, and Program P.O. FSE Abruzzo “007-2013” to DAA. The authors are participating in the COST action FA 1201 “Epiconcept” Epigenetic and Peri-conception Environment. A warm acknowledgement goes to Dr. Nicola Ferri, Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale” Campo Boario (IZSAM), Teramo, Italy, for his technical and scientific support.
Authors’ contributions
DAA, DI, and PL conceived the experiments. DAA and DI performed all experiments. DAA, DI, and PL wrote the manuscript. MC discussed the results and edited the manuscript. GP supervised the embryological work.
Footnotes
Capsule Intracytoplasmic sperm injection (ICSI) with spermatozoa whose plasma membrane and acrosome have been mechanically damaged substantially improves sheep embryonic development until the blastocyst stage.
Contributor Information
Debora A. Anzalone, Email: debora.anzalone@gmail.com
Domenico Iuso, Email: dome.iuso@gmail.com.
Marta Czernik, Email: czernik.ma@gmail.com.
Grazyna Ptak, Email: gptak@unite.it.
Pasqualino Loi, Phone: +39-0861-266856, Email: ploi@unite.it.
References
- 1.Albertini DF. What case reports can and cannot reveal. J Assist Reprod Genet. 2015;32(9):1297–8. doi: 10.1007/s10815-015-0580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anifandis G, Messini CI, Dafopoulos K, Daponte A, Messinis IE. Sperm contributions to oocyte activation: more that meets the eye. J Assist Reprod Genet, Jan 2016 [DOI] [PMC free article] [PubMed]
- 3.Beaujean N, Taylor JE, McGarry M, Gardner JO, Wilmut I, Loi P, Ptak G, Galli C, Lazzari G, Bird A, Young LE, Meehan RR. The effect of interspecific oocytes on demethylation of sperm DNA. Proc Natl Acad Sci U S A. 2004;101(20):7636–40. doi: 10.1073/pnas.0400730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalá MG, Izquierdo D, Rodríguez-Prado M, Hammami S, Paramio MT. Effect of oocyte quality on blastocyst development after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a sheep model. Fertil Steril. 2012;97(4):1004–8. doi: 10.1016/j.fertnstert.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 5.Catt JW, Gomez MC, Maxwell WM, Evans G. Birth of a male lamb derived from an in vitro matured oocyte fertilised by intracytoplasmic injection of a single presumptive male sperm. Vet Rec. 1996;139(20):494–5. doi: 10.1136/vr.139.20.494. [DOI] [PubMed] [Google Scholar]
- 6.Catt JW, Rhodes SL. Comparative intracytoplasmic sperm injection (ICSI) in human and domestic species. Reprod Fertil Dev. 1995;7:161–7. doi: 10.1071/RD9950161. [DOI] [PubMed] [Google Scholar]
- 7.Dattena M, Ptak G, Loi P, Cappai P. Survival and viability of vitrified in vitro and in vivo produced ovine blastocysts. Theriogenology. 2000;53(8):1511–9. doi: 10.1016/S0093-691X(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 8.Dozortsev D, Rybouchkin A, De Sutter P, Dhont M. Sperm plasma membrane damage prior to intracytoplasmic injection: a necessary condition for sperm nucleus decondensation. Hum Reprod. 1995;10:2960–4. doi: 10.1093/oxfordjournals.humrep.a135829. [DOI] [PubMed] [Google Scholar]
- 9.Fishel S, Lisi F, Rimaldi L, Green S, Hunter A, Dowell K, Thornton S. Systemic examination of immobilizing spermatozoa before intracytoplasmic sperm injection in the human. Hum Reprod. 1995;10:497–500. doi: 10.1093/oxfordjournals.humrep.a135974. [DOI] [PubMed] [Google Scholar]
- 10.Garcìa-Rosellò E, Garcìa-Mengual E, Coy P, Alfonso J, Silvestre MA. Intracytoplasmic sperm injection in livestock species: an update. Reprod Domest Anim. 2009;44:143–51. doi: 10.1111/j.1439-0531.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 11.Gómez MC, Catt JW, Evans G, Maxwell WM. Sheep oocyte activation after intracytoplasmic sperm injection (ICSI) Reprod Fertil Dev. 1998;10(2):197–205. doi: 10.1071/R98002. [DOI] [PubMed] [Google Scholar]
- 12.Iuso D, Czernik M, Zacchini F, Ptak G, Loi P. A simplified approach for oocyte enucleation in mammalian cloning. Cell Reprogram. 2013;15(6):490–4. doi: 10.1089/cell.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai T, Hoshi K, Yanagimachi R. Effect of sperm immobilisation and demembranation on the oocyte activation rate in the mouse. Zygote. 1999;7(3):187–93. doi: 10.1017/S0967199499000568. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–20. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–41. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 16.López-Saucedo J, Paramio-Nieto MT, Fierro R, Piña-Aguilar RE. Intracytoplasmic sperm injection (ICSI) in small ruminants. Anim Reprod Sci. 2012;133(3–4):129-38. Review [DOI] [PubMed]
- 17.Mansour R. Intracytoplasmic sperm injection: a state of the art technique. Hum Reprod Update. 1998;4(1):43–56 ESHRE [DOI] [PubMed]
- 18.Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci U S A. 2006;103(47):17661–6. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod. 1997;3(12):1087–93. doi: 10.1093/molehr/3.12.1087. [DOI] [PubMed] [Google Scholar]
- 20.Ogonuki N, Sankai T, Yagami K, Shikano T, Oda S, Miyazaki S, Ogura A. Activity of a sperm-borne oocyte-activating factor in spermatozoa and spermatogenic cells from cynomolgus monkeys and its localization after oocyte activation. Biol Reprod. 2001;65:351–7. doi: 10.1095/biolreprod65.2.351. [DOI] [PubMed] [Google Scholar]
- 21.Palermo GD, Schlegel PN, Colombero LT, Zaninovic N, Moy F, Rosenwaks Z. Aggressive sperm immobilization prior to intracytoplasmic sperm injection with immature spermatozoa improves fertilization and pregnancy rates. Hum Reprod. 1996;11(5):1023–9. doi: 10.1093/oxfordjournals.humrep.a019290. [DOI] [PubMed] [Google Scholar]
- 22.Pereyra-Bonnet F, Fernàndez-Martìn R, Olivera R, Jarazo J, Vichera G, Gibbons A, Salamone D. A unique method to produce transgenic embryos in ovine, porcine, feline, bovine and equine species. Reprod Fertil Dev. 2008;20:741–9. doi: 10.1071/RD07172. [DOI] [PubMed] [Google Scholar]
- 23.Pereyra-Bonnet F, Gibbons A, Cueto M, Sipowicz P, Fernàndez-Martìn R, Salamone D. Efficiency of sperm-mediated gene transfer in the ovine by laparoscopic insemination, in vitro fertilization and ICSI. J Reprod Dev. 2011;57:188–96. doi: 10.1262/jrd.10-063A. [DOI] [PubMed] [Google Scholar]
- 24.Ptak G, Clinton M, Tischner M, Barboni B, Mattioli M, Loi P. Improving delivery and offspring viability of in vitro produced and cloned sheep embryos. Biol Reprod. 2002;67:1719–25. doi: 10.1095/biolreprod.102.006171. [DOI] [PubMed] [Google Scholar]
- 25.Roldan ER. Better intracytoplasmic sperm injection without sperm membranes and acrosome. Proc Natl Acad Sci U S A. 2006;103(47):17585–6. doi: 10.1073/pnas.0608752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotem R, Paz GF, Homonnai ZT, Kalina M, Lax J, Breitbart H, Naor Z. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992;131(5):2235–43. doi: 10.1210/endo.131.5.1425422. [DOI] [PubMed] [Google Scholar]
- 27.Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol Hum Reprod. 2015;21(10):783–91. doi: 10.1093/molehr/gav042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 29.Seita Y, Ito J, Kashiwazaki N. Removal of acrosomal membrane from sperm head improves development of rat zygotes derived from intracytoplasmic sperm injection. J Reprod Dev. 2009;55(5):475–9. doi: 10.1262/jrd.20216. [DOI] [PubMed] [Google Scholar]
- 30.Shirazi A, Ostad-Hosseini S, Ahmadi E, Heidari B, Shams-Esfandabadi N. In vitro developmental competence of ICSI-derived activated ovine embryos. Theriogenology. 2009;71:342–8. doi: 10.1016/j.theriogenology.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Swann K. Soluble sperm factors and Ca2+ release in eggs at fertilization. Rev Reprod. 1996;1:33–9. doi: 10.1530/ror.0.0010033. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi T, Colombero LT, Neri QV, Rosenwaks Z, Palermo GD. Does ICSI require acrosomal disruption? An ultrastructural study. Hum Reprod. 2004;19(1):114–7. doi: 10.1093/humrep/deg511. [DOI] [PubMed] [Google Scholar]
- 33.Van den Bergh M, Bertrand E, Biramane J, Englert Y. Importance of breaking a spermatozoon’s tail before intracytoplasmic injection: a prospective randomized trial. Hum Reprod. 1995;10(11):2819–20. doi: 10.1093/oxfordjournals.humrep.a135798. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online. 2014;28(5):560–71. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Baldassarre H, Pierson J, Cote F, Rao KM, Karatzas CN. The in vitro and in vivo development of goat embryos produced by intracytoplasmic sperm injection using tail-cut spermatozoa. Zygote. 2003;11(3):219–27. doi: 10.1017/S0967199403002260. [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Fukui Y. Births of calves derived from embryos produced by intracytoplasmic sperm injection without exogenous oocyte activation. Zygote. 2002;10(2):149–53. doi: 10.1017/S0967199402002204. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi Y, Yanagimachi R, Horiuchi T. Full-term development of golden hamster oocytes following intracytoplasmic sperm head injection. Biol Reprod. 2002;67(2):534–9. doi: 10.1095/biolreprod67.2.534. [DOI] [PubMed] [Google Scholar]
- 38.Yanagida K, Katayose H, Hirata S, Yazawa H, Hayashi S, Sato A. Influence of sperm immobilization on onset of Ca(2+) oscillations after ICSI. Hum Reprod. 2001;16(1):148–52. doi: 10.1093/humrep/16.1.148. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida N, Perry ACF. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI) Nat Protoc. 2007;2:296–304. doi: 10.1038/nprot.2007.7. [DOI] [PubMed] [Google Scholar]