Abstract
Background
Diagnosis of children with attention-deficit/hyperactivity disorder (ADHD) is increasing. The present study sought to identify characteristics and medication treatment patterns of children with ADHD and compare them by relative age in class, sex, ethnicity, family size, sibling order, and other socioeconomic status, as well as find trends in disparity of pharmacotherapy.
Methods
This study was based on data from 1 013 149 Clalit Health Services members aged 6–17 years during 2006–2011. Centrally acting sympathomimetic drug purchases were compared according to children’s estimated relative age in class; youngest third (born August to November), middle third (born April to July), and oldest third (born December to March). Treatment trends were determined and compared according to sociodemographic and family-related factors.
Results
The overall prevalence of stimulant use in the population was 2.6% in 2006 and 4.9% in 2011. The annual incidence of stimulant use increased from 0.75% to 1.36%, rising more sharply among children in the older age groups (≥12) than among younger ones. Moreover, the youngest third of children in class was more likely to use medication than the oldest third (risk ratio (RR) 1.17, confidence interval (CI) 1.12–1.23) or the middle third (RR 1.06, CI 1.01–1.11). Of the different ethnic sectors, incidence of stimulant use was highest among general Jewish (1.8% in 2011) and lowest among Arabs (0.37% in 2011).
Conclusions
The use of stimulant medication is growing among children in Israel. Although the overall use does not exceed the estimated prevalence of ADHD among children, the appropriateness of prescribing to the Israeli pediatric population, especially to the youngest children in class, may be questionable.
Keywords: relative age, stimulant use, ADHD, children, ethnicity, Israel, pharmacoepidemiology
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a commonly diagnosed mental condition in children and may have lasting effects through adolescence and into adulthood.1–3 The pediatric diagnosis of ADHD typically involves a several-step process based on clinical evaluation, teacher ratings of behavior and school performance in school, and parental rating scales.4 Relative immaturity among peers may be a contributing factor in the evaluation of ADHD symptoms and medication prescribing; there is an excess risk of being identified with ADHD among children born shortly before school eligibility cutoff dates, compared with children born soon after the cutoff date. In North America and Iceland, countries where rates of medication use for ADHD are among the world’s highest,5 children youngest in the classroom are 1.5-fold to twofold more likely to be diagnosed or medicated for ADHD than their older classmates.6–8 Halldner et al. demonstrated an association of somewhat lower magnitude (odds ratio, 1.2–1.8) among children in Sweden, peaking at the age when they start school, at age 6 to 7 years.9 In Denmark,10,11 where prescribing of stimulants and medication for ADHD is still relatively conservative and school-entry age is flexible, there was a near absence of such a relative age effect. This suggests that such relative age effects are less likely to be artifacts in countries with more rigid school-entry cutoffs.
As in many Western countries,12–21 the medication of ADHD in Israel has risen substantially over the past decade and a half.22 The overall prescription of stimulants used to treat ADHD and covered by the national healthcare system doubled in 2005–2012, from 4.0 to 9.9 defined daily doses per 1000 inhabitants per day.20 Increases in the early to mid-2000s have been observed among both boys and girls and across geographically diverse areas.22,23 Yet, little is known about recent patterns of utilization by sociodemographic and family-related factors in the Israeli pediatric population.
In this study, we took advantage of being able to study the association between relative age in class and medication of ADHD among the Israeli children within a time frame when diagnostic trends were also changing. Further, we were able to assess the impact of sociodemographic and family factors, such as socioeconomic status (SES), ethnicity (general Jewish, Arab, and ultra-Orthodox Jewish), birth order among siblings, and number of siblings in the family, some of which have not been studied in relation to the relative age effect before.
Leveraging unique health data covering half the inhabitants of Israel in 2006–2011 (over a million children aged 6 to 17 years), we hypothesized that being young relative to one’s classmates increases the risk of being prescribed stimulant drugs for ADHD. We expected the relative age effect to be higher in younger children when maturity differences are more evident. Further, we hypothesized that this relative age effect increased over the past decade alongside rising stimulant use for ADHD in Israel.
METHODS
Setting and population
We obtained health insurance data from 1 January 2006 to 31 December 2011 from the Clalit Health Services’ dataset, which covers over 50% of the Israel population. The study population comprised all children aged 6 to 17 years (as psychostimulants are prescribed only from age 6 years) during the study period with complete information on date of birth and at least one full year of insurance coverage. In total, 1 013 149 children were followed for a sum of 4 169 264 person-years across the study period from 2006 to 2011. While members can freely move in or out of the Clalit health services membership, in fact such moves are rare, on average 1% a year. Thus, we had virtually a complete follow-up of the population.
Measures
From the Clalit dataset, we obtained information on children’s date and country of birth, sex, birth order among siblings (first, second to third, fourth, or subsequent), ethnic sector (general Jewish, Arab, and ultra-Orthodox Jewish), clinic-level SES (clinic-level SES, low, mid, and high), number of children in the family (one to two, three to five, six, or more), and centrally acting sympathomimetic drug purchases by the Anatomical Therapeutic Chemical classification system (codes starting with N06BA).
The ethnic sector and the SES of each Clalit member are determined by the clinic at which the member receives primary care medicine (clinic-level SES). The clinic is classified by the Clalit computer services unit according to geostatistical data from the Israeli Central Bureau of Statistics (CBS). Sector is attributed as general Jewish, ultra-Orthodox, or Arab by majority, and SES is stratified into three layers by the CBS and verified by the clinic manager. The general Jewish sector excludes the ultra-Orthodox sector, whose sociological, economical, and cultural behaviors are distinct. Given the Israeli social structure, the accuracy of ethnicity is well over 95%. SES at individual level during the study period was unavailable. Based on present-day data, the intraclass correlation for a single measure is 0.626, and the association between clinic and categorized individual-level values is fair (κ =0.44).
Ascertainment of exposure
We divided children into three relative age groups in class depending on their month of birth: oldest third (born December to March), middle third (born April to July), and youngest third (born August to November). Relative age in the Israeli classroom roughly coincides with the order of calendar months given the nationwide birthday cutoff, which is during December, dependent on the lunar calendar. Therefore, the oldest children in class are generally born in late December, and the youngest in November or early December. Normally, children start school in the calendar year during which they turn 6 years. But over the past decade, there has been an increasing tendency to leave children an additional year in preschool, and the estimated proportion has increased from 7.9% to 12.2%.24,25 Delayed start of school is most common among children born in November and early December. As an attempt to validate the consistency of our data and potential effect of delayed school start in the cohort, we computed the risks and crude risk ratios of starting treatment according to relative age in class, demographics, and family-related characteristics in 2011 where November-born children were removed from the analyses (Supporting Information Table S2). Further, to assess the sensitivity of the study exposure, we conducted analyses with relative age as a continuous linear variable, by month of birth beginning with December.
Ascertainment of outcome
The main study outcome measure was children’s start of stimulant treatment for ADHD. For each prescription to study children, we obtained information on the prescribed drug and calendar date. We defined the start of treatment to be the first prescription following a period of at least 1 year during which children were covered by Clalit insurance when no prescriptions for a stimulant drug were filled. After this period, we considered the start date of treatment for each child to be the date of the first prescription for a stimulant drug. Given our information on historical prescriptions pre-study, we ascertained that this was indeed the first prescription ever. Stimulant drugs for ADHD drugs were defined according to the World Health Organization Anatomic Therapeutic Chemical classification as drugs within the category of centrally acting sympathomimetics (N06BA).26 Chemical substances included were amphetamine (N06BA01), dexamfetamine (N06BA02), metamfetamine (N06BA03), methylphenidate (N06BA04), and the non-stimulant atomoxetine (N06BA09). Children diagnosed with ADHD and covered by Clalit insurance were almost exclusively prescribed methylphenidate (Ritalin, immediate and extended release, and Concerta). The Clalit data contain all diagnosed health conditions separate from the prescription data; thus, we lacked information on the indication for drug treatment. But as an ADHD diagnosis27,28 by a psychiatrist is a precondition for stimulant treatment among those covered by Clalit, we assumed children with filled stimulant prescriptions had an underlying diagnosis.
Data analysis
We first calculated incidence and prevalence proportions as the number of children starting stimulant treatment per 100 children in the population (incidence proportion), or filling a stimulant prescription at least once (prevalence proportion), during the relevant calendar year.29 We used the Cochran–Armitage test to assess time trends in incidence of stimulant use stratified by all available covariates.30,31 Incidence proportions were estimated for the whole sample by age within grade, as well as stratified by relevant sociodemographic and family factors.
We then used Kaplan–Meier curves to estimate the risk of ADHD stimulant treatment for each of the three relative age groups; individuals were considered cases based on the age at which they first received ADHD treatment; those who never received treatment were censored at age 17 years. The beginning of the information was at age 6 years, which served as the start date, and we could determine whether indeed children had received treatment before this date, in which case they were excluded from incidence and Kaplan–Meier analysis.
Finally, we estimated crude and sex-stratified risk ratios (incidence proportion ratios) and 95% confidence intervals (CI) comparing incidence (risk) of stimulant use according to children’s relative age in class using logistic regression models. We also built a multivariate Cox regression model for the age of ADHD treatment onset considering relative age in class, sex, ethnicity, and clinic-level SES as effect parameters30,31 and stratified analyses based on these characteristics too.
We used R version 3.1.1 for running the statistical analysis.32 Package doParallel33 was used to maximize efficiency given the large data size.34 The study was approved by the Clalit Health Services Ethics Committee.
RESULTS
Table 1 shows the sociodemographic and family-related characteristics of the entire study population in 2011. These characteristics did not vary significantly across children’s relative age in class. Overall, the population was composed of 56% general Jewish, 38.7% Arabs, and 5.2% ultra-Orthodox Jewish, an ethnic distribution that remained stable across children’s relative age group.
Table 1.
Demographic and family-related characteristics (in % of total) of the study population (6- to 17-year-old Clalit Health Service members) in 2011, according to relative age in class
| Children’s relative age in class | |||
|---|---|---|---|
|
| |||
| Oldest third N = 251 093 | Middle third N = 245 710 | Youngest third N = 270 974 | |
| Overall (%) | 32.7 | 32.0 | 35.3 |
| Sex (%) | |||
| Boy | 16.6 | 16.4 | 18.1 |
| Girl | 16.1 | 15.6 | 17.2 |
| Age, years (%) | |||
| 6 to 8 | 9.0 | 8.9 | 9.5 |
| 9 to 11 | 8.5 | 8.3 | 9.1 |
| 12 to 14 | 8.0 | 7.9 | 8.5 |
| 15 to 17 | 7.2 | 7.0 | 8.1 |
| Ethnic sector (%) | |||
| General Jewish | 18.4 | 17.8 | 19.8 |
| Arab | 12.5 | 12.6 | 13.6 |
| Ultra-Orthodox Jewish | 1.7 | 1.6 | 1.9 |
| Birth order among siblings (%) | |||
| First | 9.3 | 10.5 | 10.8 |
| Second to third | 18.4 | 17.2 | 19.4 |
| Fourth or subsequent | 5.0 | 4.3 | 5.0 |
| Number of children in family (%) | |||
| 1 to 2 | 5.7 | 5.7 | 6.4 |
| 3 to 5 | 19.6 | 19.6 | 21.4 |
| 6 or more | 7.5 | 6.7 | 7.5 |
| SES (%) | |||
| Low | 17.5 | 17.0 | 18.9 |
| Mid | 10.4 | 10.2 | 11.2 |
| High | 4.4 | 4.4 | 4.8 |
SES, socioeconomic status at the health clinic level.
The overall prevalence of stimulant use in the population was 2.6% in 2006 and 4.9% in 2011. Both incidence and prevalence of stimulant use varied considerably by children’s sociodemographic and family-related characteristics (Figure 1; Supporting Information Figs S1 and S2). Throughout the study period, boys were more likely than girls to start stimulant treatment (Supporting Information Fig. S1a), and use was most prevalent among children aged 9 to 11 years (6.0% in 2011, Figure 1). Of the different ethnic sectors, incidence of stimulant use was highest among general Jewish (1.8% in 2011) and lowest among Arabs (0.37% in 2011). First-born children and those with fewer than two siblings were more likely to start stimulant treatment than those subsequently born or with more siblings (Supporting Information Fig. S1d,e). Children from families categorized within the lowest clinic-level SES group were less likely to start stimulant treatment than those within the higher SES groups (Supporting Information Fig. S1f).
Figure 1.
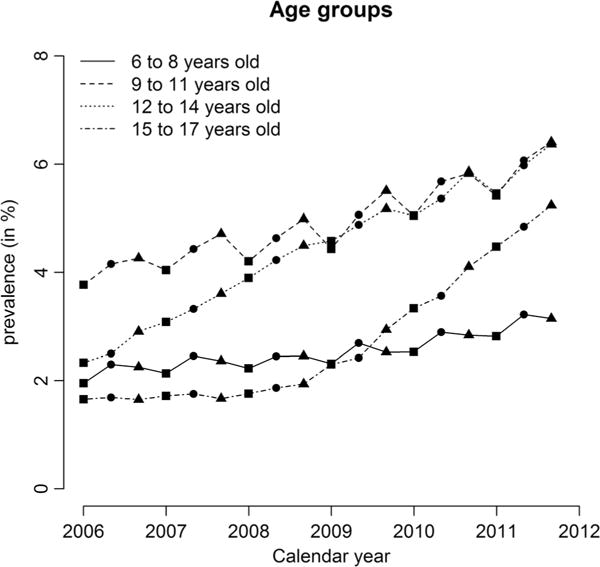
One-year prevalence for each relative age group* of stimulant use per 100 in the population 2006–2011, according to children’s age. *Three data points per calendar year, one for each relative age group (December to March, April to July, and August to November)
The annual incidence of stimulant use rose from 0.75% in 2006 to 1.36% in 2011, rising more among children in older age groups (12–14 and 15–17 years) than the younger age groups. The increasing incidence of stimulant use was evident in all ethnic sectors (Supporting Information Fig. S1c). A time trend of increasing incidence was significant for all population characteristics, according to Cochran–Armitage tests, except among children in families with greater than six children and children aged 15–17 years, for which the increase trend was not significant.
Table 2 demonstrates the incidence (risk) of starting stimulant treatment across children’s relative age in class. Throughout the study period, the youngest third (born August to November) in class was more likely than the oldest third (born December to March) and mid-third (born April to July) of children to start stimulant treatment (see age and sex-adjusted incidence, Supporting Information Fig. S3). In 2011, the hazard ratio for the youngest third was 1.19 (95%CI 1.18–1.21) and for the middle third 1.08 (95%CI 1.06–1.10), compared with the oldest third and mid-third in class, respectively (Table 3). This excess risk appeared stable across most sociodemographic and family-related characteristics, but as children were older, the risk ratio decreased (Table 3). Adjusting for the available sociodemographic and family-related factors in multivariable models (Table 3; Supporting Information Table S1) did not alter the observed associations.
Table 2.
Children’s risks (incidence proportions) of starting stimulant treatment in 2011, according to relative age in class, sociodemographic and family-related characteristics
| Children’s relative age in class | |||
|---|---|---|---|
|
| |||
| Oldest third risk % (n/N) | Mid-third risk % (n/N) | Youngest third risk % (n/N) | |
| Overall | 1.20 (2867/238 763) | 1.33 (3087/232 446) | 1.41 (3602/255 559) |
| Sex | |||
| Boy | 1.63 (1939/118 698) | 1.77 (2058/116 315) | 1.87 (2387/127 398) |
| Girl | 0.77 (928/120 065) | 0.89 (1029/116 131) | 0.95 (1215/128 161) |
| Sex and age (years) | |||
| Boys 6 to 8 | 2.26 (772/34 128) | 2.58 (871/33 825) | 2.68 (980/36 540) |
| Boys 9 to 11 | 1.83 (559/30 577) | 2.07 (620/29 884) | 2.32 (763/32 852) |
| Boys 12 to 14 | 1.32 (375/28 437) | 1.36 (382/28 072) | 1.35 (400/29 651) |
| Boys 15 to 17 | 0.91 (233/25 556) | 0.75 (185/24 534) | 0.86 (244/28 355) |
| Girls 6 to 8 | 0.92 (307/33 492) | 1.09 (352/32 436) | 1.20 (424/35 398) |
| Girls 9 to 11 | 0.83 (259/31 054) | 0.90 (267/29 563) | 1.04 (342/32 794) |
| Girls 12 to 14 | 0.62 (181/29 178) | 0.76 (216/28 390) | 0.73 (222/30 425) |
| Girls 15 to 17 | 0.69 (181/26 341) | 0.75 (194/25 742) | 0.77 (227/29 544) |
| Ethnic sector | |||
| General | 1.80 (2355/130 553) | 1.98 (2487/125 357) | 2.06 (2867/138 939) |
| Arab | 0.37 (352/95 686) | 0.42 (402/95 646) | 0.52 (535/103 267) |
| Ultra-Orthodox Jewish | 1.28 (160/12 524) | 1.73 (198/11 443) | 1.50 (200/13 353) |
| Birth order among siblings | |||
| First | 1.46 (973/66 457) | 1.53 (1149/75 196) | 1.68 (1297/77 261) |
| Third to third | 1.26 (1692/134 525) | 1.35 (1689/124 906) | 1.43 (2017/140 677) |
| Fourth or subsequent | 0.53 (202/37 781) | 0.77 (249/32 344) | 0.77 (288/37 621) |
| Number of children in family | |||
| 1 to 2 | 1.8 (716/39 830) | 2.02 (804/39 747) | 2.04 (916/44 865) |
| 3 to 5 | 1.30 (1848/142 657) | 1.38 (1973/142 653) | 1.52 (2351/154 586) |
| 6 or more | 0.54 (303/56 276) | 0.62 (310/50 046) | 0.60 (335/56 108) |
| SES | |||
| Low | 0.78 (1022/131 309) | 0.85 (1076/127 185) | 0.96 (1353/140 901) |
| Mid | 1.63 (1230/75 264) | 1.80 (1325/73 561) | 1.90 (1521/80 221) |
| High | 1.93 (614/31 842) | 2.18 (683/31 387) | 2.14 (728/34 047) |
SES, socioeconomic status at the health clinic level.
Table 3.
Hazard ratio for onset of methylphenidate treatment by covariates, univariable (raw), and multivariable analysis (adjusted)
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
|
|
|
||||
| HR | 95% CI | HR | 95%CI | ||
| Sex | Boy | 1 | — | 1 | — |
| Girl | 0.41 | 0.41–0.42 | 0.41 | 0.40–0.41 | |
| Relative age in class | Oldest third | 1 | — | 1 | — |
| Mid-third | 1.08 | 1.06–1.10 | 1.08 | 1.06–1.10 | |
| Youngest third | 1.19 | 1.18–1.21 | 1.19 | 1.17–1.21 | |
| Ethnic sector | Arab | 1 | — | 1 | — |
| Ultra-Orthodox Jews | 2.17 | 2.09–2.23 | 2.24 | 2.16–2.32 | |
| General | 3.18 | 3.11–3.24 | 3.18 | 3.11–3.25 | |
| Birth order among siblings | First | 1 | — | 1 | — |
| Second or third | 1.19 | 1.17–1.21 | 1.19 | 1.17–1.21 | |
| Fourth or subsequent | 1.24 | 1.22–1.26 | 1.23 | 1.21–2.26 | |
| Number of children in family | 1 to 2 | 1 | — | 1 | — |
| 3 to 5 | 1.48 | 1.45–1.50 | 1.64 | 1.61–1.67 | |
| 6 and more | 0.78 | 0.76–0.80 | 1.12 | 1.09–1.16 | |
| SES | Low | 1 | — | 1 | — |
| Medium | 1.76 | 1.73–1.79 | 1.18 | 1.16–1.20 | |
| High | 1.78 | 1.74–1.81 | 1.09 | 1.07–1.11 | |
SES, socioeconomic status at the health clinic level; RR, risk ratio; CI, confidence interval.
Figure 2 depicts the cumulative hazard of starting stimulant treatment by children’s age and relative age in class based on Kaplan–Meier analysis of the difference of the age of ADHD treatment with the relative age in class. Those in the youngest third within grade had the highest cumulative hazard (4.3%), followed by those in the middle third (3.9%); those in the oldest third had the lowest cumulative hazard (3.6%).
Figure 2.
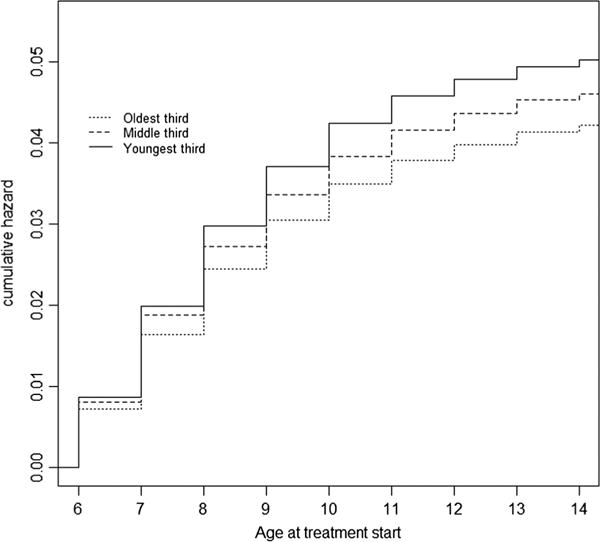
Cumulative hazard of starting stimulant treatment by children’s age and relative age in class
A Cox proportional hazards multivariate regression modeling the age of stimulant treatment on relative age in class was estimated, stratified by sex, ethnicity, and SES as effect parameters. Comparing the youngest third with the oldest third within class, there was a significantly higher risk of stimulant use among both boys and girls, among general Jewish and Arab ethnic groups, and among those at any birth order. Risk was also heightened among those at younger absolute age, those with less than six children in the family, and those in low and middle income.
To assess the effect of delayed school start on the observed associations of stimulant treatment according to relative age, we removed children born in November, resulting in risks and risk ratios similar to those of the main analysis (Supporting Information Table S2). The sensitivity analysis using relative age as a continuous linear variable demonstrated that children’s month of birth was strongly correlated with the incidence of stimulant treatment (Supporting Information Table S3).
DISCUSSION
In this study covering roughly half the pediatric population in Israel, we demonstrated the rising use of stimulants for ADHD over a 5-year period. The annual incidence per 1000 children increased from 7.5 in 2006 to 13.6 in 2011, mainly among Jewish children, older age groups, and within smaller families of higher clinic-level SES. Furthermore, we found that in the Israeli classroom, the youngest third of children were at 17% increased risk of being treated with stimulants for ADHD, compared with the oldest third. The excess risk among the youngest in class remained stable across the 5-year study period and fairly similar across most sociodemographic and family-related factors.
Our results from Clalit are consistent with findings of the several large studies conducted in North America and Europe demonstrating associations between children’s relative age among classmates and the risk of being diagnosed with ADHD or receiving stimulant treatment. The estimated overall 17% relative age effect in this study is lower than that estimated in Canada (33–77%),21 the USA (50–100%),6,35 Iceland (50%),8 and Sweden (20–60%),9 but higher than in Denmark,10,11 where virtually no effect was reported. In England, being among the youngest in a school cohort has similarly been positively associated with being referred to mental health services.36 In the Israeli school system, the parents of younger children often opt, or are recommended by kindergarten teacher, to delay commencement of grade school. A specialist panel in consultation with the family performs the final decision in such cases.
Mirroring the overall global trends of stimulant use among higher-income countries,21 the use of stimulants in the study population rose in near all sociodemographic and family-related categories. An estimated 5–10% of school aged children worldwide are diagnosed with ADHD.37 Our results indicated an overall 4.9% prevalence of stimulant use among children in Israel in 2011, and age and gender distribution coinciding rather well with the epidemiologic patterns of ADHD.3,38
A relative maturity disadvantage within school grade in childhood has been associated with long-lasting negative effects on personal achievements and health outcomes.39–41 Nevertheless, in our data, the excess risk of stimulant treatment among the youngest children in class in our data seemed diminished as children were older in absolute age. This matches previous results from Sweden9 and supports the hypothesis that the relative age effect on stimulant prescribing is related to maturity differences between the oldest and youngest children in class, yielding elevated risks for the least mature children in the classroom. As children grow older, the maturity difference becomes less distinct and hence the difference in de novo prescribing to these relative age groups. The diminishing effect with increasing age may also be consequent to the majority of children being diagnosed with ADHD before age 12 years. The diagnostic criteria used during the study period (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, 10th revision of the International Statistical Classification of Diseases and Related Health Problems) required that inattentive or hyperactive–impulsive symptoms causing impairment be present by age 6–7 years but have since been revised to several symptoms being present before age 12 years (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition).27,28,42
We set out to examine whether the hypothesized relative age effect varied by other sociodemographic and family-related factors, but did not find any substantial differences between categories of clinic-level SES, family size, nor birth order among siblings. As many previous studies,8,9,11 but not all,7 we did not find a substantial gender difference in the relative age effect on stimulant treatment for ADHD. The elevated relative age effect observed among children of Arab ethnicity could reflect random variability from small numbers within this ethnic category, but it also might be consequent to real differences in stimulant prescription to the youngest and oldest Arab children in class. In any case, this finding warrants further scrutiny.
Contrary to our hypothesis, the relative age effect on stimulant use for ADHD had no secular trend, that is, it did not increase with increasing stimulant use in the population. Thus, the rise in stimulant use in Israel was not limited to children with a maturity disadvantage but rather a global increase across all children and with stability in the magnitude of the association with maturity within grade level. There is concern in the literature about “diagnostic creep,” that is, diagnosing and treating children with less severe manifestations of the disorder.43,44 We had hypothesized that such a diagnostic creep for ADHD would manifest in children with maturity disadvantage, but this was not supported by the study.
Limitations
This is the first study to examine the relative age effect on ADHD treatment among children in Israel. Based on Clalit data covering over one million children, it illustrates the recent patterns of stimulant utilization among children in Israel. While the Clalit population is in many respects similar to the general population (Supporting Information Table S4), the populations are not identical. The Clalit population is somewhat poorer than the general population, with a mean income 95% of that of the general population, with 49.9% as opposed to 48.1% making up to the national mean income.45 Moreover, overall ethnic distribution of the study population, 56% general Jewish, 5.2% ultra-Orthodox Jewish, and 38.7% Arab, does not fully reflect the total Israeli demographic composition. Overall, Clalit members include a higher proportion Arabs than the general population, yielding a slightly higher proportion of children within the lowest clinic-level SES than the general population and larger families. Thus, our estimates of incidence and prevalence of stimulant use may slightly underestimate utilization in the overall Israeli pediatric population, given the observed heightened use among children with higher clinic-level SES and lower use among Arabs. As almost routinely all children 0–17 years in a family are enrolled in the same health maintenance organization, the sibling order distribution relative to the general population will reflect this same preponderance of larger family size and hence higher sibling order. As children’s relative age in class is unaffected by the demographic composition of the study population, the estimated relative age should remain unbiased in this respect. A second important limitation is that we estimated children’s grade level from their age and school-entry guidelines. We did not have individual-level information on whether children were indeed on grade level, that is, whether they had been accelerated or delayed from the expected grade level. Thus, exposure misclassification is a possibility. To assess the magnitude of such bias, we conducted a sensitivity analysis excluding November-born children, that is, those with birthdays shortly before the school eligibility cutoff date, which resulted in effect estimates of similar magnitude as in the main analysis. Finally, we lacked concrete information on underlying diagnosis, or validity thereof, for medicated children. Likewise, we could not study children diagnosed with ADHD but not treated with stimulants. Thus, the results of the relative age effect are only generalizable for the use of stimulants, not the diagnosis of ADHD.
In sum, we found that the use of stimulant medication is growing among children in Israel. Although the overall use does not exceed the estimated prevalence of ADHD among children, the appropriateness of prescribing to the Clalit pediatric population, especially to the youngest children in class, may be questionable.
Supplementary Material
Key Points.
Diagnosis of children with attention-deficit/hyperactivity disorder (ADHD) is increasing.
ADHD stimulant medication is common in the primary and secondary school populations in Israel.
The increased risk of stimulant use among children, who are among the youngest in class, compared with children older in class, warrants questions of the appropriateness of the ADHD medication prescription in Israel.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Clalit Health Services Ethics Committee.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web site.
References
- 1.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 2.Steinhausen HC. The heterogeneity of causes and courses of attention-deficit/hyperactivity disorder. Acta Psychiatr Scand. 2009;120(5):392–399. doi: 10.1111/j.1600-0447.2009.01446.x. [DOI] [PubMed] [Google Scholar]
- 3.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA. Attention-deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. Guilford Press; New York: 2005. [Google Scholar]
- 5.Board INC. Report of the International Narcotics Control Board on the Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes. UN; New York: 2011. [Google Scholar]
- 6.Evans WN, Morrill MS, Parente ST. Measuring inappropriate medical diagnosis and treatment in survey data: the case of ADHD among school-age children. J Health Econ. 2010;29(5):657–673. doi: 10.1016/j.jhealeco.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Morrow RL, Garland EJ, Wright JM, Maclure M, Taylor S, Dormuth CR. Influence of relative age on diagnosis and treatment of attention-deficit/hyperactivity disorder in children. Can Med Assoc J. 2012;184(7):755–762. doi: 10.1503/cmaj.111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoëga H, Valdimarsdóttir UA, Hernández-Díaz S. Age, academic performance, and stimulant prescribing for ADHD: a nationwide cohort study. Pediatrics. 2012;130(6):1012–1018. doi: 10.1542/peds.2012-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halldner L, Tillander A, Lundholm C, et al. Relative immaturity and ADHD: findings from nationwide registers, parent- and self-reports. J Child Psychol Psychiatry. 2014;55(8):897–904. doi: 10.1111/jcpp.12229. [DOI] [PubMed] [Google Scholar]
- 10.Dalsgaard S, Humlum MK, Nielsen HS, Simonsen M. Relative standards in ADHD diagnoses: the role of specialist behavior. Econ Lett. 2012;117(3):663–665. [Google Scholar]
- 11.Pottegård A, Hallas J, Zoëga H. Children’s relative age in class and use of medication for ADHD: a Danish Nationwide Study. J Child Psychol Psychiatry. 2014;55(11):1244–1250. doi: 10.1111/jcpp.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giacobini M, Medin E, Ahnemark E, Russo LJ, Carlqvist P. Prevalence, patient characteristics, and pharmacological treatment of children, adolescents, and adults diagnosed with ADHD in Sweden. J Atten Disord. 2014 doi: 10.1177/1087054714554617. 1087054714554617. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry. 2009;194(3):273–277. doi: 10.1192/bjp.bp.107.045245. [DOI] [PubMed] [Google Scholar]
- 14.Asheim H, Nilsen KB, Johansen K, Furu K. Prescribing of stimulants for ADHD in Nordland County. Tidsskr Nor Laegeforen. 2007;127(18):2360–2362. [PubMed] [Google Scholar]
- 15.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J Atten Disord. 2007;10(4):335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 16.Zoega H, Baldursson G, Halldorsson M. Use of methylphenidate among children in Iceland 1989–2006. Laeknabladid. 2007;93(12):825–832. [PubMed] [Google Scholar]
- 17.Zuvekas SH, Vitiello B, Norquist GS. Recent trends in stimulant medication use among U.S. children Am J Psychiatry. 2006;163(4):579–585. doi: 10.1176/ajp.2006.163.4.579. [DOI] [PubMed] [Google Scholar]
- 18.Scheffler RM, Hinshaw SP, Modrek S, Levine P. The global market for ADHD medications. Health Aff (Millwood) 2007;26(2):450–457. doi: 10.1377/hlthaff.26.2.450. [DOI] [PubMed] [Google Scholar]
- 19.Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24–31. doi: 10.1345/aph.1K143. [DOI] [PubMed] [Google Scholar]
- 20.Ponizovsky AM, Marom E, Fitoussi I. Trends in attention deficit hyperactivity disorder drugs consumption, Israel, 2005–2012. Pharmacoepidemiol Drug Saf. 2014;23(5):534–538. doi: 10.1002/pds.3604. [DOI] [PubMed] [Google Scholar]
- 21.Scheffler RM, Hinshaw SP, Modrek S, Levine P. The global market for ADHD medications. Health Aff. 2007;26(2):450–457. doi: 10.1377/hlthaff.26.2.450. [DOI] [PubMed] [Google Scholar]
- 22.Vinker S, Vinker R, Elhayany A. Prevalence of methylphenidate use among Israeli children 1998–2004. Clin Drug Investig. 2006;26(3):161–167. doi: 10.2165/00044011-200626030-00006. [DOI] [PubMed] [Google Scholar]
- 23.Fogelman Y, Vinker S, Guy N, Kahan E. Prevalence of and change in the prescription of methylphenidate in Israel over a 2-year period. CNS Drugs. 2003;17(12):915–919. doi: 10.2165/00023210-200317120-00005. [DOI] [PubMed] [Google Scholar]
- 24.State of Israel, statistical abstract of Israel, Table 8.10. Central Bureau of Statistics; Jerusalem: 2009. [Google Scholar]
- 25.State of Israel, statistical abstract of Israel 2013, Table 8.10. Central Bureau of Statistics; Jerusalem: 2013. [Google Scholar]
- 26.WHO. WHO Collaborating Centre for Drug Statistics Methodology: ATC/DDD Index. World Health Organization; Oslo: 2008. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington D.C.: 2000. [Google Scholar]
- 28.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. World Health Organization; Geneva: 2004. [Google Scholar]
- 29.Stevenson M, Nunes T, Heuer C, et al. epiR: An R package for the analysis of epidemiological data. R package version 0.9-59. 2014 http://CRAN.R-project.org/package=epiR (accessed 2 January 2015)
- 30.Therneau T. A package for survival analysis in S. R package version 237-7. 2012 Available at: http://CRANR-projectorg/package=survival. (accessed 2 January 2015)
- 31.Therneau T, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- 32.R Core Team. R: a language and environment for statistical computing. 2014 http://www.R-project.org/ (accessed 2 January 2015)
- 33.Analytics R. Weston S. doParallel: foreach parallel adaptor for the parallel package. R Package Version. 2014;1(8) [Google Scholar]
- 34.Bourne PE. What Big Data means to me. J Am Med Inform Assoc. 2014;21(2):194. doi: 10.1136/amiajnl-2014-002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elder TE. The importance of relative standards in ADHD diagnoses: evidence based on exact birth dates. J Health Econ. 2010;29(5):641–656. doi: 10.1016/j.jhealeco.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg S, Berg E. The youngest children in each school cohort are overrepresented in referrals to mental health services. J Clin Psychiatry. 2014;75(5):530–534. doi: 10.4088/JCP.13m08594. [DOI] [PubMed] [Google Scholar]
- 37.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 39.Bedard K, Dhuey E. The persistence of early childhood maturity: international evidence of long-run age effects. Q J Econ. 2006;121(4):1437–1472. [Google Scholar]
- 40.Helsen WF, Van Winckel J, Williams M. The relative age effect in youth soccer across Europe. J Sports Sci. 2005;23(6):629–636. doi: 10.1080/02640410400021310. [DOI] [PubMed] [Google Scholar]
- 41.Goodman R, Gledhill J, Ford T. Child psychiatric disorder and relative age within school year: cross sectional survey of large population sample. BMJ. 2003;327(7413):472. doi: 10.1136/bmj.327.7413.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Association; Washington DC: 2013. [Google Scholar]
- 43.Slomski A. Chronic mental health issues in children now loom larger than physical problems. JAMA. 2012;308(3):223–225. doi: 10.1001/jama.2012.6951. [DOI] [PubMed] [Google Scholar]
- 44.Graf WD, Miller G, Nagel SK. Addressing the problem of ADHD medication as neuroenhancements. Expert Rev Neurother. 2014;14(5):569–581. doi: 10.1586/14737175.2014.908707. [DOI] [PubMed] [Google Scholar]
- 45.State of Israel Ministry of Health. The Health Insurance Law: 20 years from the enactment of the law (Hebrew) Available at: http://www.health.gov.il/PublicationsFiles/HealthInsuranceLaw_20Years.pdf (accessed 2 January 2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


