Abstract
Background
Platelet activation at sites of vascular injury is essential for hemostasis, but it is also a major pathomechanism underlying ischemic injury. As anti-inflammatory therapies limit thrombosis and anti-thrombotic therapies reduce vascular inflammation, we tested the therapeutic potential of two pro-resolving endogenous mediators: Annexin A1 N-terminal derived peptide (AnxA1Ac2–26) and aspirin (ASA) triggered lipoxin A4 (ATL, 15-epi-lipoxin A4), on the cerebral microcirculation following ischemia-reperfusion (I/R) injury. Furthermore, we tested whether the lipoxin A4 receptor, formyl-peptide receptor 2/3 (Fpr2/3, ortholog to human FPR2/ALX), evoked neuro-protective functions following cerebral I/R injury.
Methods and Results
Using intravital microscopy, we found that cerebral I/R injury was accompanied by neutrophil and platelet activation and neutrophil-platelet aggregate (NPA) formation within cerebral microvessels. Moreover, ATL activation of neutrophil Fpr2/3 regulated NPA formation in the brain, and inhibited the reactivity of the cerebral microvasculature. The same results were obtained with AnxA1Ac2–26 administration. Blocking Fpr2/ALX with the antagonist Boc2 reversed this effect, and treatments were ineffective in Fpr2/3 knockout mice, who displayed an exacerbated disease severity, evidenced by increased infarct area, blood brain barrier dysfunction, increased neurological score and elevated levels of cytokines. Furthermore, ASA treatment significantly reduced cerebral leukocyte recruitment, and increased endogenous levels of ATL, effects again mediated by Fpr2/3.
Conclusions
In summary, Fpr2/ALX is a therapeutic target for initiating endogenous pro-resolving, anti-inflammatory pathways following cerebral I/R injury.
Keywords: formyl peptide receptor, inflammation, ischemia reperfusion injury, stroke, ischemic
Introduction
Inflammation plays a crucial role in the pathophysiological cascade of ischemic stroke and related forms of brain injury.1 Although the exact mechanisms responsible for post-ischemic cerebral damage are not fully understood, there is increasing evidence suggesting the inflammatory state following ischemia reperfusion (I/R) as a crucial contributing factor to these pathogenic processes. It has been demonstrated that post I/R inflammation results in endothelial cell activation as well as consequent recruitment and activation of leukocytes and platelets leading to further cerebral microvascular dysfunction and subsequent tissue injury.1 Since the brain is highly susceptible to I/R injury, prevention of cerebral ischemic events has become a major part of modern health care. However, neuronal cells have a limited capacity for restoration following cerebral I/R; therefore, there is an unmet clinical need to explore novel therapeutic avenues to better resolve the aggressive inflammatory state after cerebral ischemic insults to limit the irrecoverable neuronal damage.
It is now appreciated that inflammatory mechanisms do not disperse spontaneously, but rather that resolution of inflammation involves a tightly orchestrated series of events, engaging specific endogenous mediators and protective pathways.2 Among these, Annexin A1 (AnxA1) and its N-terminal peptide AnxA1Ac2–26 (Ac-AMVSEFLKQAWFIENEEQEYVQTVK), the eicosanoid-derived lipoxin A4 (LXA4) and 15(R)-epi-lipoxin A4 (referred to as aspirin-triggered lipoxin [ATL], and produced after aspirin acetylation of inducible cyclooxygenase-2 [COX-2]) have been shown to play an active role in order to return local sites of inflammation to homeostasis.2–4 These pro-resolving mediators act at different phases of the inflammatory response and are involved in blockade of leukocyte recruitment, inhibition of cytokine release, promotion of apoptosis, stimulation of phagocytosis, and decreasing vascular permeability.3,4 Effectors of resolution ensure that these responses remain localized within the inflamed tissue and are extinguished within an adequate timeline. Of relevance, both AnxA1/AnxA1Ac2–26, and LXA4/ATL mediate their effects through a common G protein-coupled receptor: the formyl peptide receptor 2 (Fpr2/3), also called lipoxin A4 receptor (ALX).5 This receptor is expressed on a variety of cell types, such as endothelial cells and myeloid lineages,6 e.g. neutrophils, which are involved in the inflammation observed in early stages of reperfusion following ischemic insults.
Several lines of evidence, produced by our group and others, indicate protective effects of these pro-resolving mediators in a number of post-I/R events, including mesentery,7,8 heart,9 lung,10 and kidney.11 We have previously shown that AnxA1/AnxA1Ac2–26, as well as LXA4 and ATL mediate their effects through Fpr2/ALX in a global murine stroke model (a model which mimics human stroke following atherosclerotic degeneration of the common carotid arteries and respiratory or cardiac arrest).12 In a focal I/R model (the middle cerebral artery occlusion (MCAo) model), we reported that exogenous administration of AnxA1 markedly attenuated I/R injury, by acting on a member of the FPR family, which at the time, we speculated to be Fpr2/ALX,12 although this possibility was never confirmed, until now.
Here we demonstrate a novel role of AnxA1Ac2–26 and ATL in regulating leukocyte-platelet responses in the cerebral microvasculature following I/R as attained with pharmacological blockade, quantifying endogenous mediator levels, and using Fpr2/3−/− mice. We present novel evidence that specifically neutrophil Fpr2/3 regulates cerebral neutrophil-platelet aggregation (NPA) by rapid generation of ATL in an endogenous biosynthetic circuit to resolve inflammation and restore homeostasis. In summary, Fpr2/ALX is a therapeutic target for initiating endogenous pro-resolving, anti-inflammatory pathways following cerebral I/R injury.
Methods
A detailed Methods section is provided in the online-only Data Supplement.
Animals
Animal experiments complied with ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines and followed the European Union Directive (2010/63/EU) or LSUHSC-S IACUC. Wild-type (WT) C57BL/6 mice or Fpr2/3−/− mice were used.12
Receptor agonists and drug treatment
Vehicle (saline, or ethanol plus saline for ATL), AnxA1 mimetic peptide Ac2-26 (AnxA1Ac2–26, Ac-AMVSEFLKQAWFIENEEQEYVQTVK, Cambridge Research Biochemicals, Cleveland, UK) 100 μg/mouse,13 Boc2 (N-tert-butoxycarbonyl-L-Phe-D-Leu-L-Phe-D-Leu-L-Phe, MPBiomedicals, Cambridge, UK) 10 μg/mouse,13 and ATL (stable epimer of LXA4) 4.0 μg/mouse,12 were administered (100 μl) intravenously (i.v.) at the start of cerebral reperfusion. In a separate set of experiments, mice were intraperitoneally (i.p.) treated with Aspirin (ASA, 150 mg/kg Sigma-Aldrich, Dorset, UK) 60 minutes (min) prior to I/R.
Middle cerebral artery occlusion and reperfusion (MCAo)
MCAo was performed for 60 min followed by 4 h or 24 h of reperfusion according to standard operating procedure in our laboratory.13
Blood sampling and cell counts
Blood samples were taken before and after MCAo for measuring leukocytes (3% citric acid & 10% crystal violet) and platelet (1% buffered ammonium oxalate) counts with a hemocytometer.
Platelet and leukocyte labeling
Platelets were isolated from donor mice, labeled using carboxyfluorescein succinimidyl ester (CFSE, 90 μM, 10 min, Sigma-Aldrich) and injected into recipient mice, followed by 0.02 % rhodamine 6G (Sigma-Aldrich) to label circulating leukocytes.14
Cerebral intravital fluorescence microscopy (IVM)
IVM was performed using a Zeiss Axioskop microscope (Zeiss, New York, USA).12,13 (See online-only Data Supplement).
Infarct volume (IV)
After 24 h reperfusion, brains were removed and stained with 2 % 2,3,5-triphenyltetrazolium chloride (TTC, Sigma Aldrich). Sections were photographed and the digitized images of each brain section (and the infarcted area) were quantified using NIH 1.57 Image Software.13
Neurological score (NS)
A five-point neurological deficit score was used: 0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; and 4, unable to walk spontaneously.14
Blood–Brain Barrier (BBB) dysfunction
BBB permeability was assessed using Evans blue (EB) extravasation.15 BBB permeability was normalized by dividing tissue EB concentration (μg/g brain weight) by plasma concentration (μg/mL).
Myeloperoxidase (MPO) activity
Brain homogenates and MPO standards (Sigma-Aldrich) were placed onto a 96-well plate, and 200 μL of o-dianisidine (Sigma-Aldrich) solution and 10 μL of 0.1 % H2O2 (Sigma-Aldrich) were added. Absorbance was read after 5 min at 405 nm and expressed as units per mg of wet tissue.8
Confocal Microscopy
To visualize NPAs, mice were injected with specific antibodies to label: neutrophils (eFluor 660 (green)-labeled anti-Mouse Ly-6G, eBioscience, San Diego, CA. 2μg/mouse) and platelets (Dylight 649 (red)-labeled anti-mouse CD42, (Emfret Analytics, Eibelstadt, Germany. 1μg/mouse).16,17 (See online-only Data Supplement).
Cytokines in Plasma and Brain Tissue
After 24 h of reperfusion, plasma and brain hemisphere homogenates were obtained. Levels of pro- and anti-inflammatory cytokines, ATL and thromboxane B2 (TXB2) were measured using standard ELISAs, as described in the online-only Data Supplement.
Statistical Analysis
Results from IVM experiments were confirmed to follow a normal distribution using Kolmogorov-Smirnov test of normality with Dallal-Wilkinson-Lillie for corrected p value. Data that passed the normality assumption was analyzed using Student’s t-test (two groups) or ANOVA with Bonferroni post-tests (more than two groups), which were performed using GraphPad Prism5 software. Data that failed the normality assumption were analyzed using the non-parametric Mann-Whitney U test (two groups) or Kruskal-Wallis with Dunn’s test (more than two groups). Data are shown as mean values ± standard error of the mean (SEM), or median with interquartile range (neurological score only). Differences were considered statistically significant at a value of p < 0.05.
Results
Cellular responses are exacerbated in the cerebral microcirculation of Fpr2/3−/− mice
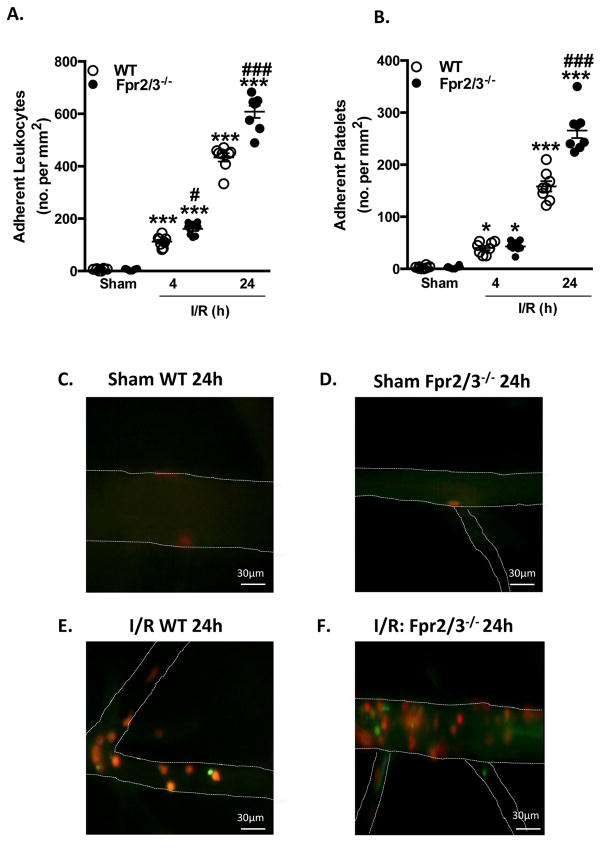
MCAo for 60 min, followed by 4 h or 24 h reperfusion induced heightened cellular trafficking within the cerebral microcirculation in both WT and Fpr2/3−/− mice vs. sham animals, with an enhanced inflammatory response in Fpr2/3−/− mice. At 4 h, Fpr2/3−/− mice displayed a significant increase in leukocyte (but not platelet) adhesion vs. WT mice at that time point. Furthermore, at 24 h, this inflammatory response was more pronounced, and Fpr2/3−/− demonstrated an increase in both leukocyte and platelet adhesion (p < 0.0001 for both), compared to WT mice (Figure 1A+B). Figure 1C+D illustrates representative images of cerebral venules from sham as well as I/R WT and Fpr2/3−/− mice at 24 h, displaying the marked accumulation of leukocytes and platelets (Figure 1E+F).
Figure 1.
Cellular responses are exacerbated in the cerebral microcirculation of Fpr2/3−/− mice following I/R. Wild type (WT, C57BL/6) and Fpr2/3−/− mice were subjected to MCAo for 60 min, followed by 4h or 24 h reperfusion. Leukocyte and platelet recruitment in the cerebral microcirculation was quantified in terms of: A) number (no.) of adherent leukocytes (cells stationary ≥ 30 sec) and B) number of adherent platelets (cells stationary for ≥ 2 sec). Representative intravital fluorescence microscopy video stills at 24 h showing few cellular interactions in the cerebral microcirculation of C) WT sham mice and D) Fpr2/3−/− sham mice, or increased leukocytes and platelets in E) WT mice and further heightened increase in F) Fpr2/3−/− mice. Images taken on an Olympus BW61WI microscope, magnification × 40. Data are mean ± SEM of 8 mice per group. *p < 0.05 & ***p < 0.001 vs. control. #p < 0.05 & ###p < 0.001 vs. genotype at same time point.
Mice lacking Fpr2/3 have increased cerebral damage following I/R
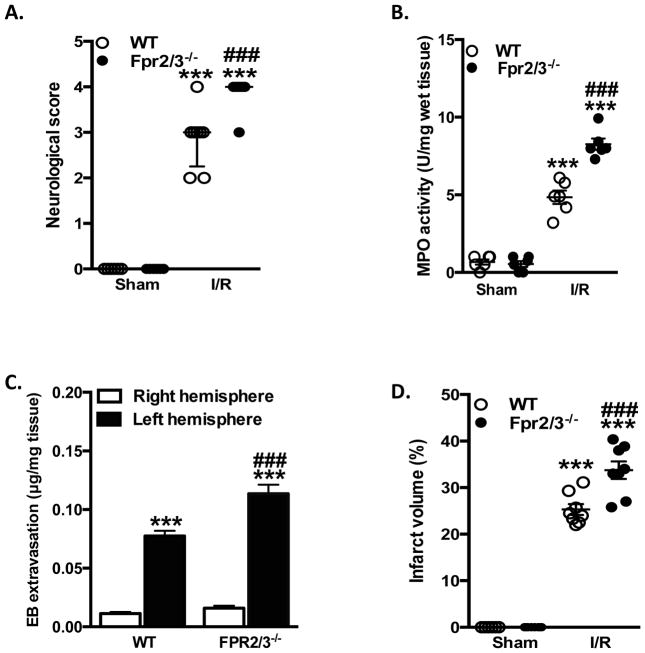
NS and IV in WT and Fpr2/3−/− mice subjected to 60 min of MCAo 24 h reperfusion or sham are presented in Figure 2A+B. While after 4h of reperfusion differences were noted between sham and I/R, no changes were noted between the genotypes (data not shown). However, neurological scores assessed after 24 h of reperfusion were significantly worse in Fpr2/3−/− than in WT mice (Figure 2A), a response that correlated with elevated IVs (Figure 2B). No significant differences among mouse genotypes were detected with respect to the hemodynamic parameters under assessment (Supplemental Table 1).
Figure 2.
Lack of Fpr2/3 leads to increased tissue damage in the brain following I/R. Wild type (WT, C57BL/6) and Fpr2/3−/− mice were subjected to MCAo for 60 min, followed by 24 h reperfusion. A) Neurological score was assessed and brains removed to quantify B) infarct volume. In a separate cohort of animals, brain homogenate samples were taken. C) Blood brain barrier dysfunction was measured in right and left hemispheres from mice using Evan’s blue (EB) dye extraction. D) Myeloperoxidase (MPO) was measured in whole brain homogenates. **p < 0.01 & ***p < 0.001 vs. control. ###p < 0.001 vs. genotype at same time point.
Fpr2/3−/− mice have augmented cerebral and systemic inflammatory responses following I/R
We further investigated the involvement of Fpr2/3−/− in local and systemic inflammatory responses. BBB integrity was monitored in WT and Fpr2/3−/− mice at 24 h reperfusion using the EB extravasation method. Figure 2C shows that BBB permeability was significantly elevated in the left (injured) hemisphere of WT and Fpr2/3−/− mice after I/R compared to their respective contralateral side. Sham animals had no BBB dysfunction (data not shown). Most importantly, when compared to WT mice, Fpr2/3−/− mice showed a significant higher EB extravasation, indicating an exacerbated BBB dysfunction (Figure 2C). Furthermore, we also found a marked increase in MPO (a marker of neutrophil activation) after I/R, which again was significantly higher in Fpr2/3−/− mice (Figure 2D). All mice undergoing MCAo/R had decreased white blood cell (WBC) counts, which was reflected by a decrease in lymphocyte count. The neutrophil count was increased in MCAo/R mice, and further exacerbated in MCAo/R Fpr2/3−/− mice vs. WT MCAo/R mice (Table 1). No differences were noted in: platelet count (Table 1); the relative proportions of immature vs. mature platelets (Supplemental Figure 1A+B);18,19 nor in the level of activation within these populations (Supplemental Figure 1C+D),21 in any groups or genotypes.
Table 1.
Cell counts of WT and Fpr2/3−/− mice.
| Genotype | Treatment | Total WBC count(x 109/L) |
 Neutrophils (per μl blood) |
 Monocytes (per μl blood) |
 Lymphocytes (per μl blood) |
 Platelets(per μl blood) |
|---|---|---|---|---|---|---|

|
Sham | 6.7 ± 0.2 | 1697 ± 47.0 | 875.5 ± 55.1 | 8322 ± 563.2 | 993.8 ± 17.3 |
| I/R | 4.3 ± 0.3* | 3728 ± 538.4** | 1001 ± 210.4 | 1508 ± 85.93*** | 980.0 ± 35.2 | |

|
Sham | 5.7 ± 0.4 | 1365 ± 250.3 | 953.0 ± 87.7 | 7967 ± 468.0 | 861.3 ± 29.4 |
| I/R | 3.6 ± 0.2* | 5467 ± 452.2**# | 1295 ± 102 | 2133 ± 283.6*** | 855.8 ± 34.7 |
I/R = 60 min ischemia + 24 h reperfusion.
p < 0.05 &
p < 0.001 vs. sham of same genotype.
p < 0.05 vs. WT I/R
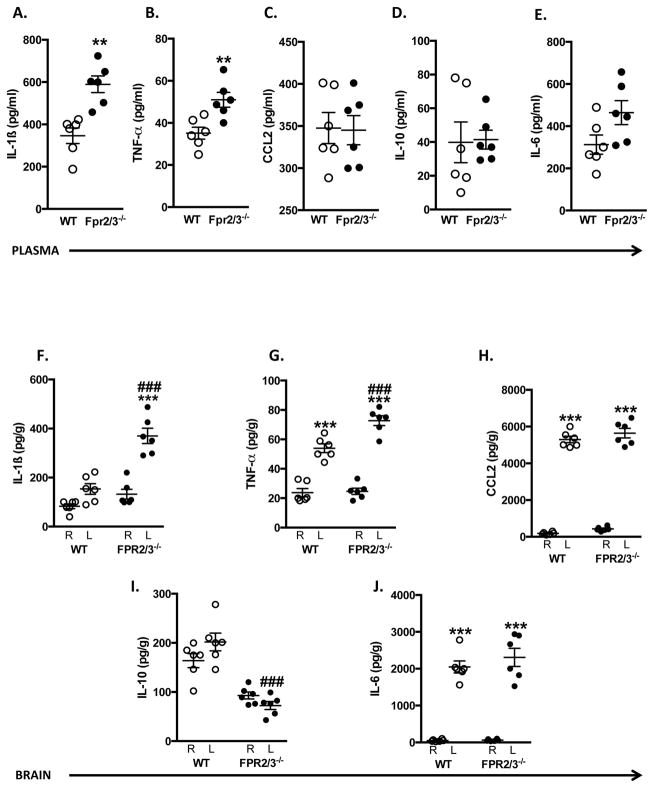
Finally, we determined serum and tissue cytokine levels. All cytokines (with the exception of IL-10) measured in plasma of WT mice were elevated post-I/R compared to sham mice (data not shown), whereas in the brain homogenates, MCAo/R elicited discrete increases in TNFα, CCL2 and IL-6 compared to sham mice (data not shown). When comparing WT and Fpr2/3−/− mice subjected to MCAo for 60 min, followed by 24 h reperfusion, we found that absence of Fpr2/3−/− resulted in heightened serum levels of IL-1β and TNFα (Figure 3A+B), with no significant difference in CCL2, IL-10 and IL-6 levels vs. WT mice (Figure 3C–E).
Figure 3.
Inflammatory responses are augmented in Fpr2/3−/− mice. Wild type (WT, C57Bl/6) and Fpr2/3−/− mice were subjected to MCAo for 60 min, followed by a 24 h reperfusion, after which plasma and brain homogenate samples were taken. Cytokines were measured in plasma (A–E) and in homogenates from the left (L: ipsilateral) and right (R: contralateral) sides of the brain (F–J). Data are mean ± SEM of 8 mice per group. **p < 0.01 & ***p < 0.001 vs. same genotype right side. ###p < 0.001 vs. WT left side.
Local cytokine levels (TNFα, CCL2 and IL-6) were significantly elevated in the left (injured) hemisphere of WT and Fpr2/3−/− mice after I/R (Figure 3G,H +J). Moreover, both TNFα, and IL-1β levels were elevated in Fpr2/3−/− mice above WT mice. IL-10 levels were decreased in Fpr2/3−/− mice vs. WT mice. No cytokine was above control values in the contralateral hemisphere of either WT or Fpr2/3−/− mice (with the exception of IL-10), suggesting these inflammatory mechanisms are confined to the ischemic region (i.e. ipsilateral side).
Fpr2/3−/− mice have reduced cerebral NPAs
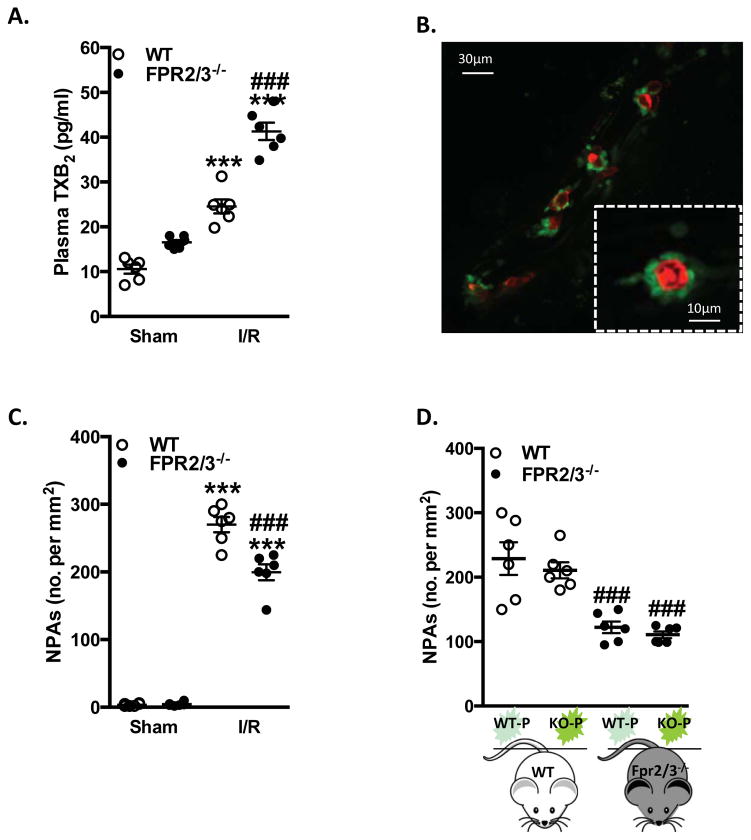
Once stimulated at sites of inflammation, activated platelets are prone to adhering to neutrophils, forming circulating NPAs, a mechanism which has been demonstrated in ischemic stroke.20 Since the IVM data suggested an augmented platelet activation in Fpr2/3−/− mice (Figure 1B), we further investigated the state of activation in platelets following cerebral I/R injury by measuring the thromboxane (TX) A2 metabolite TXB2, which is a soluble marker for platelet activation. Plasma levels of stable TXB2 were increased in both WT and Fpr2/3−/− mice after I/R, and further elevated in absence of Fpr2/3 when comparing I/R groups to each other (Figure 4A). We then assessed the potential of these activated platelets to form aggregates. By using fluorescence IVM, we revealed a dynamic formation of NPAs within the brain microcirculation, showing intravascular neutrophils interacting with platelets (Figure 4B). Surprisingly, we found that Fpr2/3−/− mice subjected to MCAo for 60 min, followed by 24 h reperfusion, had significantly fewer NPAs than WT mice (Figure 2C). While the IVM data had shown an increase in total adherent leukocyte counts in Fpr2/3−/− mice compared with WT mice, we found that approximately 33% of these were platelet-bearing in Fpr2/3−/− mice compared to ~60% platelet-bearing leukocytes in WT mice (no significant differences were measured between WT and Fpr2/3−/− mice in sham conditions).
Figure 4.
Neutrophil Fpr2/3−/− is key for NPAs in I/R injury. Wild type (WT, C57Bl/6) and Fpr2/3−/− mice were subjected to MCAo for 60 min, followed by 24 h reperfusion. A) Plasma TXB2 levels were measured. B) representative image of NPAs in cerebral I/R as seen with confocal intravital microscopy (Neutrophil: red; platelets: green). C) neutrophil/platelet aggregates (NPAs) were quantified in sham and I/R WT and Fpr2/3−/− mice. D) Adoptive transfer experiments were performed by injecting CFSE-labeled platelets from donor mice (P) into recipient mice (i.e. KO-P = platelets isolated from Fpr2/3−/− donor mice), and assessed via intravital microscopy. Data are mean ± SEM of 6–8 mice per group. ***p < 0.001 vs. sham. ###p < 0.001 vs. control
Next it was important to ascertain whether platelet or neutrophil Fpr2/3 was functional in these settings. Data in Figure 4D show that, following I/R, CSFE-labeled platelets from Fpr2/3−/− mice injected into WT animals led to similar numbers of NPAs to those quantified with WT platelets transferred into WT recipients. Thus, NPA formation in WT animals was independent of the presence of Fpr2/3 on the infused platelets. In addition, lower aggregate numbers were found when platelets from WT or Fpr2/3−/− mice were injected into Fpr2/3−/− mice subjected to I/R (Figure 4D), demonstrating that NPA formation in Fpr2/3−/− mice was also independent from the type of transferred platelets. Thus these findings reveal that it is not the platelet Fpr2/3, which is important in NPA formation, but rather a functional neutrophil Fpr2/3.
Fpr2/3 agonists attenuate I/R-induced cerebrovascular injury in WT mice
Having demonstrated an exacerbated cerebral inflammatory response in mice lacking Fpr2/3, coupled with the importance for a functional neutrophil Fpr2/3, we next tested the protective potential of targeting FPR2/ALX in stroke. Treatment of WT mice with either AnxA1Ac2–26 or ATL markedly attenuated the inflammatory response, as assessed by NS, IVM, and IV (Supplemental Figure 2). No significant differences in parameters measured were observed between either vehicle (saline, or saline + ethanol), so only saline vehicle is presented. Intravenous administration of AnxA1Ac2–26 to I/R WT mice reduced the number of adherent leukocytes (303.0±23.5 cells/mm2. Figure 5A) and platelets (92.1±10.1 cells/mm2), compared to saline-injected animals (433.8±15.8 cells/mm2 and 158.5±10.0 cells/mm2 respectively). These protective effects translated in reduced IV (17.2±1.3% vs. 25.3±1.2% in saline treated mice) and NS (1.5±0.2 vs. 2.9±0.2). Similar effects were produced with ATL, decreasing leukocyte adhesion by 35%, platelet adhesion by 45%, IV by 42% and NS by 58%, all compared to WT mice. When either compound was co-administered with the Fpr2/3 antagonist, Boc2, there was a loss of effect (Supplemental Figure 2), whilst the antagonist was ineffective when given alone.
Figure 5.
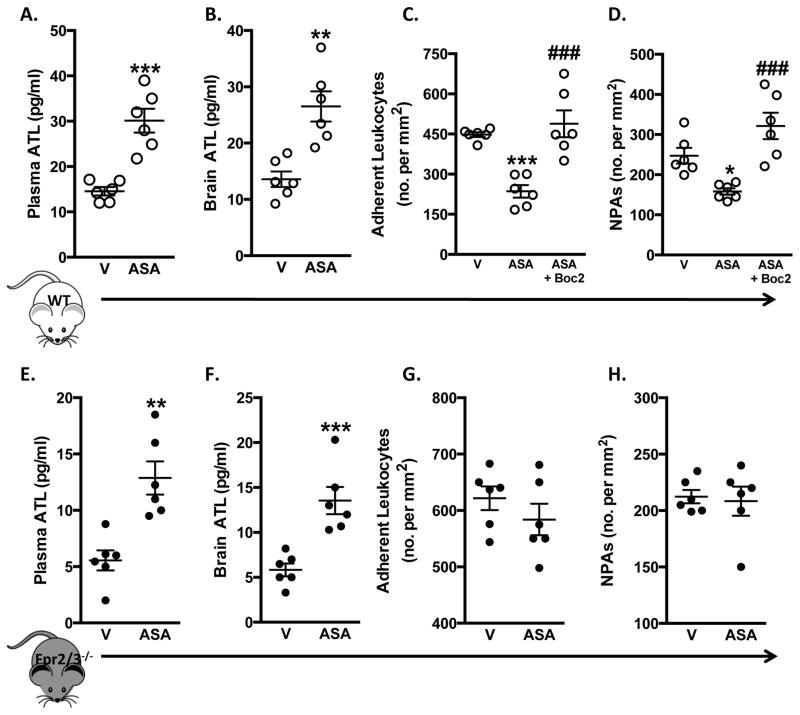
Protective effects of ASA are inhibited in Fpr2/3−/− mice. Wild type (WT, C57Bl/6) and Fpr2/3−/− mice were subjected to MCAo for 60 min, followed by 24 h reperfusion. Vehicle (V. saline) or ASA (150 mg/kg) were administered 60 min prior to I/R. A+E) Plasma ATL levels; B+F) ATL levels in whole brain homogenates; C+G) adherent leukocytes and D+H) NPAs were measured in WT and Fpr2/3−/− mice. Data are mean ± SEM of 6–8 mice per group. **p < 0.01 & ***p < 0.001 vs. vehicle. ###p < 0.001 vs. ASA.
Harnessing endogenous Fpr2/3 activation as a therapeutic strategy
Finally, we assessed whether a pharmacological dose of ASA (150mg/kg) changed ATL levels in our stroke model. Plasma and brain homogenate ATL concentrations were measured following cerebral I/R, with and without ASA treatment. ASA induced a significant increase in both plasma and brain homogenate ATL levels vs. vehicle treated WT mice (Figure 5A+B). Furthermore, ASA treatment reduced leukocyte adherence and NPAs in WT mice (Figure 5C+D). Next we determined whether increased levels of endogenous ATL produced by ASA treatment contributed to the cerebro-protective effects that we observed. Administration of the Fpr2/3 antagonist Boc215 alone had no effect on leukocyte adhesion and NPA formation, however when administered in combination with ASA, it reversed the effects of ASA i.e. it increased leukocyte adhesion and NPA formation (Figure 5C+D). These pharmacological results were complemented by analysis in Fpr2/3−/− mice. In spite of the fact that administration of ASA to Fpr2/3−/− and WT mice resulted in increased plasma and brain homogenate ATL concentrations (Figure 5E+F), ASA was unable to reduce leukocyte adherence or NPAs in Fpr2/3−/− mice (Figure 5G+H). Furthermore, we found that ASA was unable to reduce NS and IV in Fpr2/3−/− mice, unlike it was able to do in WT counterparts (data not shown).
Discussion
There are several novel key findings from this study: 1) absence of Fpr2/3 heightens the inflammatory response following cerebral I/R; 2) agonistic targeting of Fpr2/3 is protective in stroke; 3) ATL is required for ASA protection in cerebral I/R injury, and furthermore, 4) functional neutrophil Fpr2/3 is needed for formation of NPAs, through which ATL exerts its effects within the cerebral microvasculature (Figure 6).
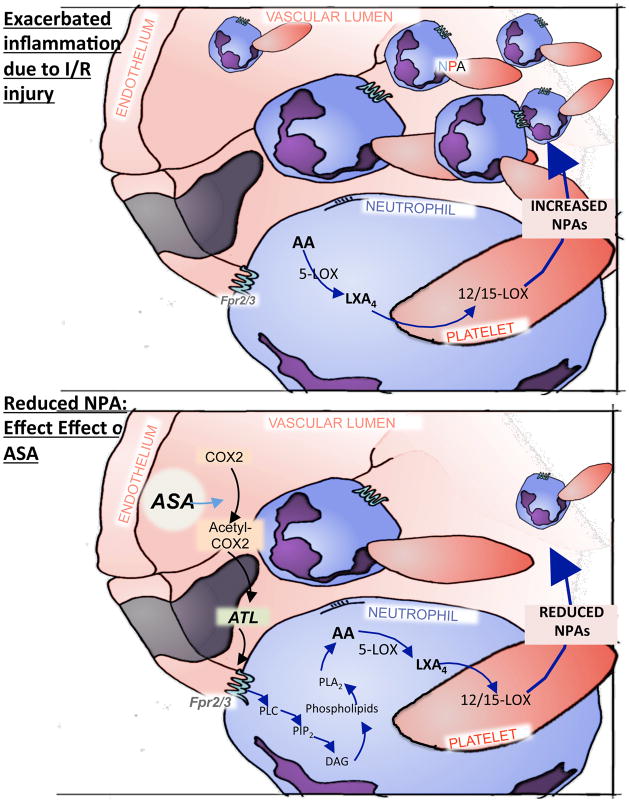
Figure 6.
Schematic overview of the important anti-inflammatory role that Fpr2/3 plays in abrogating I/R-induced cerebrovascular injury. I/R leads to the production of Fpr2/-mediated formation of neutrophil/platelet aggregates (NPAs), with neutrophil Fpr2/3 mediating the signal. Aggregates allow the biosynthesis of LXA4 via neutrophil 5-lipoxygenase (5-LOX) and platelet 12/15-LOX. High levels of aspirin-triggered lipoxin (ATL) are also generated during I/R. The effect of ATL on the cerebral microcirculation was absent in mice that lack the Fpr2/3, but pharmacological targeting of the Fpr2/3 (with AnxA1Ac2–26 or ATL) can resolve these inflammatory effects. The administration of aspirin (ASA) is able to produce ATL via COX2 acetylation, which in turn acts on Fpr2/3 and monitors the vascular permeability in the brain. In summary, Fpr2/3 (and we propose the human counterpart ALX/FPR2) promotes the biosynthesis and production of lipoxins and ATL, which in turn regulates the inflammatory process and restores homeostasis in the brain.
Despite the positive effects of blocking various aspects of the inflammatory cascade, which ameliorates injury in experimental stroke,21 there are currently few effective treatment options for patients affected by ischemic stroke. The second phase of inflammation is the resolution one, where active engagement of molecular and cellular circuits ensures termination.5 Thus, endogenous anti-inflammatory mediators like AnxA1Ac2-26 and ATL could be exploited for guiding innovative drug discovery programs to develop compounds likely to be devoid of the major side effects associated with current anti-inflammatory and vasculo-protective therapies (such as vomiting, nausea, constipation, diarrhea, ulcers, bleeding, organ-failure (e.g. liver and kidney)), as their application would be mimicking a way the body naturally disposes of inflammation.22 These mediators target Fpr2/3, hence we began here by assessing whether Fpr2/3 played functional roles in cerebral I/R.
Fpr2/3 belongs to a receptor family of the haematopoietic/myeloid cell population. Within this family, FPR1 is one of the best-studied G-protein coupled receptors, dating back to the early 1970s. Three functional receptors (FPR1, FPR2/ALX, and FPR3) exist in humans (as well as mice), with a wide tissue distribution; they interact with a number of structurally diverse naturally-occurring ligands that can promote opposing biological actions.23 We demonstrate herein that Fpr2/3−/− mice (lacking the orthologue of FPR2/ALX) display an exacerbated cerebral inflammatory response following I/R injury. This was observed by increased adherent leukocytes and platelets, which is in-keeping with the cerebral effects observed in other models (e.g. systemic administration of LPS),24 along with increased IV (which is inextricably linked to levels of inflammation during cerebral reperfusion) and NS. We also found that whilst there was a reduced circulating-lymphocyte count in MCAo/R mice (which may account for the decrease in WBC, and confirms findings from other group)25, there was no difference between WT vs. Fpr2/3−/− mice. However, the exacerbation detected in peripheral neutrophil counts in MCAo/R Fpr2/3−/− vs. MCAo/R WT mice may account for the increased adherent neutrophils quantified in the brain microvasculature of these mice. These findings are inline with the experimental and clinical notion that supports the importance of neutrophil infiltration in ischemic stroke. Previous focal I/R models have shown that the recruitment of neutrophils in the ischemic brain occurs within 30 min to a few hours and peaks in the first 3 days.26 Furthermore, in the clinic, neutrophil counts are increased in the first 3 days after symptom onset in stroke patients, and this is associated with larger final infarct volumes (on computed tomography (CT) and magnetic resonance imaging (MRI)) and increased stroke severity.27
As stroke is associated with platelet activation, which we monitored by the heightened platelet adhesion in the brain, we determined whether Fpr2/3 deficiency changes the number of total, immature and mature platelets, and the level of activation within these platelet populations pre and post stroke. Whilst we noted no change in platelet count due to stroke, or between genotypes, we investigated this further using thiazole orange (TO. A nucleic acid intercalating dye which has previously been used to detect and quantify immature platelets [which contain more RNA than their mature counterparts] in both humans28 and mice29 to assess the number of both mature and immature circulating platelets, and the level of activation within these populations (by using the antibody [JONA] that recognizes the activated conformation of the murine GPIIb/IIIa integrin on the platelet surface.19 These data confirmed that the relative proportions of immature versus mature platelets did not change, and the level of activation within these populations was also unchanged. These observations demonstrate that the effects of our MCAo/R on platelet activation are local, whilst levels of circulating platelets and their maturity are not affected.
The BBB is a critical guardian of communication between the periphery and the brain,30 and was also compromised to a greater degree in the ipsilateral side compared to the WT mice, suggesting that Fpr2/3, either directly or indirectly, preserve BBB permeability. This concurs with previous observations in which this receptor has been shown to be involved in the maintenance of BBB structure and function, by down-regulating RhoA GTPase activity following binding of AnxA1, and in so doing enabled the organization of the inter-endothelial cell tight and adherens junctions.30
Overexpression of pro-inflammatory cytokines is another important component of the neuroinflammatory response. The precise concoction of pro-inflammatory cytokines deployed during I/R is variable, especially when assessed in the brain and not the periphery. We found an increase in IL-6 in I/R animals, which is in-keeping with clinical data (over 10pg/ml in patients), and can correlate with poor clinical outcomes.31 We measured higher levels of TNFα and IL-1β following cerebral I/R (heightened in the Fpr2/3−/− mice). These cytokines not only attract leukocytes into ischemic sites, promoting tissue necrosis, but they can also damage the microvascular endothelium, disrupt the BBB, and accelerate the formation of cerebral edema. We also found low IL-10 levels in both plasma and brain homogenates in the Fpr2/3−/− mice. This is fitting with the clinical situation, in which stroke patients with lower plasma concentrations (< 6pg/ml) of IL-10 deteriorated within 48 h of stroke.32 Having assessed that Fpr2/3−/− mice are more susceptible to the macroscopic and microscopic effects of cerebral I/R, we proposed that targeting of Fpr2/3 with pharmacological interventions might prove beneficial. Indeed treatment of mice with AnxA1Ac2–26 or ATL administered at the start of reperfusion corroborated this hypothesis, as many of the parameters under analysis were reduced. Co-administration of AnxA1Ac2–26 or ATL with the Fpr pan antagonist Boc2, elicited an abrogation of their protective responses. Interestingly, responses observed following pharmacological blockade of the Fpr2/3 receptor (i.e. administration of Boc2 alone) were not as pronounced as those observed with the genetic ablation of Fpr2/3 (i.e. using Fpr2/3−/− mice). This type of response is not unusual and has been observed in other studies,33 however, whilst the dose of Boc2 used in our study has been used previously, 11,21 the differences in response could be related to the dose of Boc2 administered (i.e. sufficient to block the receptors, but not sufficient to initiate an exaggerated inflammatory response). Furthermore, the results from the Fpr2/3−/− mice suggests that Fpr2/3 plays a more dominant role in controlling the vascular reactivity to ensure adequate counter-regulation of the inflammatory response during MCAo/R, and as such, genetic ablation of Fpr2/3 results in a heightened inflammatory response, and one which is exacerbated in comparison to exogenous administration of Boc2.
It has long been known that neutrophil, endothelial cell and platelet interactions all facilitate neutrophil recruitment and extravasation into brain parenchyma after stroke,34 with post-ischemic neutrophil infiltration being a major component of the neuroinflammatory response.35 Once in the brain, neutrophils damage tissue by releasing a number of detrimental substances, such as oxygen free radicals, proteases, phospholipases and neutrophil extracellular traps (decondensed DNA and proteases).16,34 MPO activity is a marker of neutrophil activity, and in this study we found it to be upregulated in the brain of WT mice and further exacerbated in brain homogenates from Fpr2/3−/− mice, suggesting that absence of this GPCR removes an endogenous tonic control on neutrophil influx, hence a sharp increase was observed following I/R injury. This is in-keeping with studies in which Fpr2/3 agonists (especially LXA4 and its derivatives) are able to decrease MPO levels, This could be due to the fact that LXs are able to inhibit neutrophil migration,35 enhance neutrophil apoptosis,36 and in some cases suppress ROS production.37
Growing evidence suggests that platelet-derived microparticles38 amplify leukocyte activation and transmigration. Augmented plasma levels of TXB4 following I/R suggest amplified platelet activation, which was markedly increased in FPR2/3−/− mice. Previous reports of NPA formation in inflammation have shown that neutrophils bound to the endothelium secondarily capture platelets and that NPA formation, and platelet activation are increased in ischemic stroke and are involved in the pathogenesis of stroke.39 However, interestingly, our findings demonstrated for the first time that NPAs formed in the cerebral microcirculation in animals following I/R were significantly less in Fpr2/3−/− vs. WT animals. It is known that neutrophils can use platelet-derived arachidonate to generate lipoxgenase products,33 and that NPA interactions utilize bidirectional transcellular routes to contribute to LXA4 and ATL formation. Our results correlate with these findings, and those found by Brancaleone et al.,33 in the mesenteric microcirculation, that NP interactions allow for the production of LXA4. Herein, as we also found there to be increased levels of ATL in both plasma and brain homogenates. That being said, we have not ruled out the possibility that generation of other Fpr2/3 agonists could be contributing to the lipid mediator release, examples being: resolvins,40 or humanin.23 Further experiments will shed light on this.
Neutrophil accumulation is fundamental to acute inflammation. In response to tissue injury, circulating NPAs form for secondary capture. Counter regulation of acute inflammatory processes by specialized pro-resolving mediators is essential to restore homeostasis.41 In peripheral organs, such as the mesentery33 and the lung,41 endogenous biosynthetic routes have been shown to be amplified during NPA formation to restrain these aggregates using counter regulatory circuits to regulate the extent of the inflammation and protect the tissue from further damage. Whilst this has been shown in systemic organs, the effect in the brain microcirculation was unknown. Using adoptive transfer of donor platelets into recipient mice and similar strategies, we concluded that in the cerebral microvasculature, neutrophil Fpr2/3 is important informing aggregates with platelets. Whilst these findings are in line with previous findings in the systemic circulation (i.e. mesentery)33 following I/R injury, it would appear that in an LPS-induced lung injury model, neither neutrophil nor platelet Fpr2/3 alone is required for the formation of aggregates, but rather that Fpr2/3 on both cell types is necessary to inhibit inflammation.40 Additionally, it cannot be ruled out that that engagement of Fpr2/3 on other cell types such as endothelial cells6 or microglia42 could play a role in these findings, however, Fpr2/3 levels of expression are very low in endothelial cells,23 and as such are unlikely to have an impact on the NPA response observed here.
Finally, to establish the clinical relevance of Fpr2/3 as a therapeutic target for stroke treatment and other neurovascular diseases like: multiple sclerosis43 and Alzheimer’s disease,44 we determined its impact in the pharmacological actions of ASA. This NSAID has the ability to activate a number of mechanisms, such as interfering with transcription factor of adenosine signaling,45 and the acetylation of COX-2 to block prostanoid synthesis.45 Contact between platelets and neutrophils gives rise to the transcellular production of lipoxins and also leukotrienes,45 and even low-dose ASA administered to healthy volunteers is capable of producing bioactive levels of ATL.33 Here we showed that ASA, administrated to stroked mice, could produce ATL, which triggers pro-resolving responses through the engagement of Fpr2/3.
In summary, our results demonstrate that an absence of Fpr2/3 leads to an aberrant inflammatory response to stroke, and that compounds targeting Fpr2/3 e.g. AnxA1Ac2-26 and ATL are powerful inhibitors of these inflammatory events. Our findings shed light on an endogenous biosynthetic circuit in neutrophils, in which neutrophil Fpr2/3 controls NPA formation by rapid generation of ATL, which is engaged to restrain NPA formation and acute inflammation and restore homeostasis in the brain. Furthermore, this study shows that Fpr2/3 is an attractive therapeutic target and ATL, as well as other potentially other pro-resolving lipid mediators, are attractive treatment option for patients with stroke, a disease that currently lacks any effective pharmacotherapies.
Supplementary Material
Clinical Perspectives.
The brain is highly susceptible to ischemia reperfusion (I/R) injury, thus prevention of cerebral ischemic events has become a major part of modern health care. However, neuronal cells have a limited capacity for restoration following cerebral I/R; therefore, there is an unmet clinical need to explore novel therapeutic avenues to better resolve the aggressive inflammatory state after cerebral ischemic insults to limit the irrecoverable neuronal damage. Our paper shows for the first time the novel role of AnxA1Ac2–26 and ATL in regulating leukocyte-platelet responses in the cerebral microvasculature following I/R. These results were attained by pharmacological blockade, quantification of endogenous mediator levels, and the use of Fpr2/3−/− mice. Furthermore, we demonstrate that specifically neutrophil Fpr2/3 regulates cerebral neutrophil-platelet aggregation by rapid generation of ATL in an endogenous biosynthetic circuit. This pathway helps to resolve inflammation and restore homeostasis. For the first time this has been shown in the brain, and we feel that these data are exciting because they confirm that Fpr2/ALX is a therapeutic target for initiating endogenous pro-resolving, anti-inflammatory pathways following cerebral I/R injury, which has clear clinical implications in helping to limit neuronal damage following ischemic insults.
Acknowledgments
The authors thank Roderick J. Flower at the William Harvey Research Institute, Queen Mary University, London (UK) for providing the Fpr2/3−/− mice; Helen K. Smith (LSUHSC-S) for the schematic and Karen Y. Stokes for consultancy regarding platelets.
Funding Sources: This work was supported by the NIH/NHLBI (HL125572-01A1) and the German Research Foundation (DFG, BE 5619/1-1). MP acknowledges the financial support of the Wellcome Trust (086867/Z/08/Z).
Footnotes
Disclosures: None.
References
- 1.Rodrigues SF, Granger DN. Cerebral microvascular inflammation in DOCA salt-induced hypertension: role of angiotensin II and mitochondrial superoxide. J Cereb Blood Flow Metab. 2012;32:368–375. doi: 10.1038/jcbfm.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan C, Savill NJ. Resolution of inflammation: the beginning programms the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 3.Trentin PG, Ferreira TP, Arantes AC, Ciambarella BT, Cordeiro RS, Flower RJ, Perretti M, Martins MA, Silva PM. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol. 2015;172:3058–3071. doi: 10.1111/bph.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 5.Gavins FN. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia-reperfusion injury? Trends Pharmacol Sci. 2010;31:266–276. doi: 10.1016/j.tips.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 7.Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- 9.Gavins FN, Kamal AM, D’Amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19:100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- 10.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith HK, Gil CD, Oliani SM, Gavins FN. Targeting formyl peptide receptor 2 reduces leukocyte-endothelial interactions in a murine model of stroke. FASEB J. 2015;29:2161–2171. doi: 10.1096/fj.14-263160. [DOI] [PubMed] [Google Scholar]
- 13.Gavins FNE, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:2306–2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 15.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 16.Yipp BG, Kubes P. Antibodies against neutrophil LY6G do not inhibit leukocyte recruitment in mice in vivo. Blood. 2013;121:241–242. doi: 10.1182/blood-2012-09-454348. [DOI] [PubMed] [Google Scholar]
- 17.Lu SJ, Li F, Yin H, Feng Q, Kimbrel EA, Hahm E, Thon JN, Wang W, Italiano JE, Cho J, Lanza R. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21:530–545. doi: 10.1038/cr.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildirim A, Russell J, Yan S, Senchenkova EY, Granger DN. Leukocyte-dependent responses of the microvasculature to chronic angiotensin II exposure. Hypertension. 2012;60:1503–1509. doi: 10.1161/HYPERTENSIONAHA.112.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan SL, Russell J, Harris NR, Senchenkova EY, Yildirim A, Granger DN. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis. 2013;19:1245–1253. doi: 10.1097/MIB.0b013e318281f3df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Htun P, Fateh-Moghadam S, Tomandl B, Handschu R, Klinger K, Stellos K, Garlichs C, Daniel W, Gawaz M. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke. 2006;37:2283–2287. doi: 10.1161/01.STR.0000236638.75591.61. [DOI] [PubMed] [Google Scholar]
- 21.Smith HK, Gavins FN. The potential of stem cell therapy for stroke: is PISCES the sign? FASEB J. 2012;26:2239–2252. doi: 10.1096/fj.11-195719. [DOI] [PubMed] [Google Scholar]
- 22.Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavins FN, Hughes EL, Buss NA, Holloway PM, Getting SJ, Buckingham JC. Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB J. 2012;26:4977–4989. doi: 10.1096/fj.12-205971. [DOI] [PubMed] [Google Scholar]
- 25.Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs. 2011;13:154–163. doi: 10.1177/1099800410384500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793: Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 27.Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, Ali LK, Kim D, Villablanca JP, Salamon N, Razinia T, Ovbiagele B. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39:355–360. doi: 10.1161/STROKEAHA.107.490128. [DOI] [PubMed] [Google Scholar]
- 28.Robinson M, MacHin S, Mackie I, Harrison P. In vivo biotinylation studies: specificity of labelling of reticulated platelets by thiazole orange and mepacrine. Br J Haematol. 2000;108:859–864. doi: 10.1046/j.1365-2141.2000.01939.x. [DOI] [PubMed] [Google Scholar]
- 29.Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23:996–1001. [PubMed] [Google Scholar]
- 30.Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J, Christian HC, Weksler BB, Malaspina A, Solito E. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci U S A. 2013;110:832–841. doi: 10.1073/pnas.1209362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waje-Andreassen U, Kråkenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, Vedeler CA. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 32.Vila N, Castillo J, Dávalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 33.Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, Vergnolle N, Nourshargh S, Perretti M. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuartero MI, Ballesteros I, Lizasoain I, Moro MA. Complexity of the cell-cell interactions in the innate immune response after cerebral ischemia. Brain Res. 2015;1623:53–62. doi: 10.1016/j.brainres.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 36.El Kebir D, Filep JG. Modulation of Neutrophil Apoptosis and the Resolution of Inflammation through β2 Integrins. Front Immunol. 2013;4:60. doi: 10.3389/fimmu.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou XY, Wu P, Zhang L, Xiong W, Li YS, Feng YM, Ye DY. Effects of lipoxin A(4) on lipopolysaccharide induced proliferation and reactive oxygen species production in RAW264.7 macrophages through modulation of G-CSF secretion. Inflamm Res. 2007;56:324–33. doi: 10.1007/s00011-007-7012-7. [DOI] [PubMed] [Google Scholar]
- 38.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmalbach B, Stepanow O, Jochens A, Riedel C, Deuschl G, Kuhlenbäumer G. Determinants of platelet-leukocyte aggregation and platelet activation in stroke. Cerebrovasc Dis. 2015;39:176–180. doi: 10.1159/000375396. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124:2625–2634. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet–neutrophil interactions is organ-protective. PNAS. 2014;111:16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K, Iribarren P, Huang J, Zhang L, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Induction of the formyl peptide receptor 2 in microglia by IFN-gamma and synergy with CD40 ligand. J Immunol. 2007;178:1759–1766. doi: 10.4049/jimmunol.178.3.1759. [DOI] [PubMed] [Google Scholar]
- 43.Kostic M, Stojanovic I, Marjanovic G, Zivkovic N, Cvetanovic A. Deleterious versus protective autoimmunity in multiple sclerosis. Cell Immunol. 2015;296:122–132. doi: 10.1016/j.cellimm.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 45.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.