Abstract
Membrane proteins mediate cell-cell interactions and adhesion, the transfer of ions and metabolites, and the transmission of signals from the extracellular environment to the cell interior. The extracellular domains of most cell membrane proteins are glycosylated, often at multiple sites. There is a growing awareness that glycosylation impacts the structure, interaction and function of membrane proteins. The application of glycoproteomics and glycomics methods to membrane proteins has great potential. However, challenges also arise from the unique physical properties of membrane proteins. Successful analytical workflows must be developed and disseminated to advance functional glycoproteomics and glycomics studies of membrane proteins. This review explores the opportunities and challenges related to glycomic and glycoproteomic analysis of membrane proteins, including discussion of sample preparation, enrichment, and tandem mass spectrometry (MS/MS) analyses, with a focus on recent successful workflows for analysis N- and O-linked glycosylation of mammalian membrane proteins.
Keywords: Glycoproteomics, Glycomics, Membrane Proteins, Receptors
I. Introduction
The cell (or plasma) membrane serves as a barrier between the cytoplasm and the external environment, and is populated by integral and lipid- and GPI-anchored proteins. Membrane proteins perform essential functions in multicellular organisms including mediation of cell-cell interactions and adhesion, transfer of ions and metabolites, and transmission of signals from the extracellular environment to the cell interior [1]. Peripheral membrane proteins, a subset of membrane proteins that lie outside the membrane and interact with integral membrane proteins or the cell membrane via non-covalent interactions, have quite different biophysical properties and will not be covered in this review. In eukaryotes, 20–30% of the genome encodes for integral membrane proteins [2, 3], providing evidence that these proteins fill important roles in cells. Prominent among these are cell surface receptors, including enzyme-linked, G-protein-linked, ligand-gated ion channel-linked, and cellular adhesion receptors that function as major transducers of cellular signaling. Integral membrane proteins contain an extracellular region and one or multiple transmembrane domains or lipid anchors, and may also contain a cytoplasmic region. Their extracellular domains (ectodomains) are frequently glycosylated. There is a growing awareness that interactions between integral membrane proteins and their ligands, cell-surface proteins of the same or neighboring cells, peripheral membrane proteins and/or secreted proteins are heavily influenced by protein glycosylation [4–8].
Most membrane proteins undergo folding and maturation in the ER and Golgi, where co-translational N-glycosylation aids protein folding and plays a role in protein quality control [9]. Many membrane proteins also undergo O-GalNAc (mucin-type) glycosylation, a process that occurs in the ER and Golgi after protein folding is complete. Glycosylation and glycan remodeling are non-template based processes, which result in the generation of a heterogeneous set of glycan modifications. Local steric and structural factors influence the access of glycosyltransferases to N- and O-glycosylation sites on a protein, and impact glycan structure; therefore, while glycosylation is influenced by cell-specific expression of glycosyltransferases, the supply of sugar nucleotides and the amount of time a protein spends passing though the ER/Golgi, local secondary and tertiary structure may also impact glycosylation type at specific sites on a protein [10, 11]. In eukaryotes, N-linked glycans are attached to amide side chains of asparagine (N) residues primarily within NXS/T (X≠P) sequons and share a common trimannosylchitobiose core (Man3GlcNAc2), while mucin-type O-linked glycans are linked to hydroxyl groups of serine (S) and threonine (T) residues. Mammalian N- and O-linked glycans vary considerably in topology and linkage but are all constructed from a small number of monosaccharide components including fucose, mannose, galactose, N-acetylglucosamine, N-acetylgalactosamine, N-acetylneuraminic acid and/or N-glycolylneuraminic acid. The heterogeneity of their glycan structures results in the existence of multiple protein glycoforms within a population of glycoprotein molecules. Notably, additional forms of glycosylation have been described [12–14]; however, for the purposes of this review we will restrict our discussion primarily to methods that facilitate analysis of N- and O-linked glycosylation in mammalian membrane proteins.
Opportunities and Challenges in Membrane Proteomics
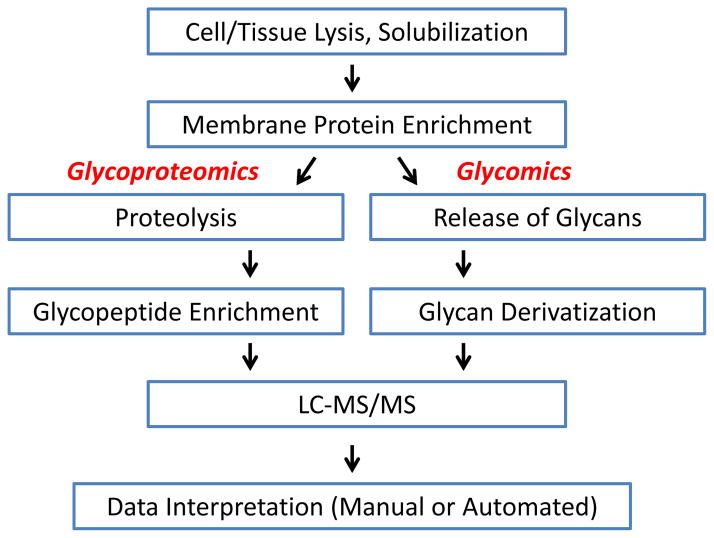
Glycosylation is a critical component of protein quality control and also serves important functional roles in mature membrane proteins, including involvement in adhesion and signaling. Recent publications that explore the role of glycosylation in signaling offer exciting clues regarding the potential rewards of applying glycomic and glycoproteomic approaches to the study of membrane proteins [4–8]. However, there are also challenges associated with the application of glycoproteomic methods to membrane proteins. Many of these challenges arise from the unique physical properties of membrane proteins, including the hydrophobicity of the transmembrane domain(s) of integral membrane proteins which frequently leads to aggregation and loss during isolation. Therefore, successful analytical workflows that include recovery of relatively hydrophobic species must be developed and disseminated to advance functional glycoproteomics and glycomics studies of membrane proteins. Figure 1 represents a generalized workflow for analysis of membrane protein glycosylation, including key steps such as solubilization and enrichment, parallel paths for glycomic and glycoproteomic analyses, and MS/MS analysis.
Figure 1.
Schema of Glycomics and Glycoproteomics Workflow for Membrane Proteins.
For the purposes of this review, we have used the definition of glycoproteomics favored by Thaysen-Andersen and Packer: “the site-specific analysis of the glycoproteome at the systemwide level… [yielding] information about the protein carriers, the glycan attachment sites and the structure and occupancy of the glycan” [15]. Therefore, we will apply the term “glycoproteomics” to workflows and analyses that characterize intact glycopeptides, and “glycomics” to analyses of free or released glycans from unseparated glycoprotein pools or from an individual protein with multiple glycosylation sites. Glycoproteomics-focused analytical workflows that empower hypothesis-driven, site-specific study of membrane protein glycosylation are essential to understand the regulation of membrane proteins in signaling and adhesion. This review explores the opportunities and challenges related to glycoproteomic and glycomic analysis of membrane proteins. This will include discussion of sample preparation, enrichment, and tandem mass spectrometry (MS/MS) analyses, with a focus on recent successful workflows for membrane protein analysis.
II. Maintaining Membrane Protein Solubility
Preparation of membrane proteins is challenging due to their propensity to aggregate and/or precipitate. This ‘Achilles heel’ of integral membrane protein preparation arises from the specialized nature of these proteins, namely, the fact that they contain one or more hydrophobic transmembrane domains that are more at home in their native lipid environment than in aqueous solutions. By avoiding pitfalls in membrane protein fractionation and enrichment steps, innovations in sample preparation are expanding the reach of membrane protein enrichment workflows. One important aspect of membrane protein enrichment is the selection of the appropriate solvents, detergent(s), and/or organic acids for solubilization [16].
Solubilization using detergents is one of the best ways to remove membrane proteins from the surrounding hydrophobic lipid layer without causing protein aggregation. Most current strategies rely on either detergents or organic solvents to keep membrane proteins in solution once they are removed from their normal hydrophobic environment and are subjected to enrichment and purification steps. However, many detergents interfere with downstream mass spectrometry analyses by suppressing ionization of analytes. While anionic detergents including sodium dodecyl sulfate (SDS) and sodium deoxycholate (SDC) solubilize membrane proteins much more efficiently than other detergents, SDS unfortunately interferes with trypsin digestion and subsequent mass spectrometry analyses. SDC shows similar (though lower) ability to solubilize membrane proteins and has the advantage that it can be removed using ethyl acetate to prevent interference with trypsin digestion [17]. Acid-cleavable detergents such as RapiGest™ (an anionic detergent marketed by Waters Corp.) successfully solubilize some membrane proteins. RapiGest does not interfere with trypsin digestion and can be decomposed prior to MS analysis by acidifying the solution. Non-ionic detergents Triton X-114 and octyl-β-D-glucopyranoside (octyl glucoside) also show high efficiency in solubilization of membrane proteins; octyl glucoside has also been shown to improve selectivity of phosphotyrosine-modified membrane proteins in immunoprecipitation [18].
Solvents such as methanol and trifluoroethanol (TFE) have been used to solubilize membrane proteins prior to digestion [19–21]. However, methanol-aided digestion of membrane proteins requires repeated addition of trypsin because it decreases the efficiency of digestion. Of the two, TFE-aided digestion appears to be more advantageous for membrane protein studies, but it reportedly does not match the success of detergent-based approaches [22]. It may also cause some hydrolysis of peptide bonds.
III. Enrichment Strategies for Membrane Proteins
Enrichment methods for membrane proteins must be tailored to the desired goal(s) of the experiment, including whether the goal is to enrich a specific membrane protein or to enrich a broad set/subset of proteins. Differential centrifugation, two-phase separation, biotinylation, lectin affinity, cationic colloidal silica, antibody-mediated immunoaffinity enrichment, cell-capture and cell surface shaving have been applied successfully for membrane protein fractionation and enrichment [23–25]. A brief description of each of these methods and a summary of their enrichment properties can be found in Table 1.
Table 1.
Membrane Protein Enrichment Strategies.
| Method | Applications, Advantages, and Limitations | Reference(s) |
|---|---|---|
| Differential centrifugation | Broad enrichment of membrane proteins; also enriches mitochondrial proteins and proteins from other subcellular organelles | 19, 26–29 |
| Sodium carbonate treatment | Used in combination with differential centrifugation; removes interacting/non-specifically bound proteins, aids specificity of membrane protein enrichment | 30–31 |
| Two-phase separation | Aqueous/PEG to enrich membrane proteins from tissue lysate; aqueous/Triton X-114 to enrich membrane and GPI- anchored proteins; detergent removal may be challenging and result in sample loss | 32–35 |
| Biotinylation | Use of membrane-impermeable, cleavable reagents such as Sulfo-NHS-SS-Biotin followed by enrichment with immobilized avidin; selective enrichment of membrane proteins at the cell surface; limited to use in cell culture | 36 |
| Lectin affinity | Immobilized lectins bind glycosylated proteins; lectins may bias the subset of membrane glycoproteins that are enriched based on the subset of lectins used | 37–40 |
| Cationic colloidal silica | Colloidal silica is added to intact cells to coat the plasma membrane, followed by centrifugation; demonstrates low levels of contamination from cytoplasmic proteins | 46–49 |
| Antibody- mediated immunoaffinity enrichment | Immobilized antibodies are directed at cell membrane proteins; greatly increases enrichment of plasma membrane proteins and reduces the presence of mitochondrial proteins; this process is cell-type specific, so care must be taken to select the correct antigen target(s) | 44–45 |
| Cell-capture | Hydrazide chemistry, release of peptides mediated by PNGase F; formerly-N-glycosylated peptides can be analyzed via MS; can be used to enrich membrane proteins/peptides containing glycans; loss of information about the detailed glycan structures and their heterogeneity | 25,42–43 |
| Cell surface shaving | High-pH conditions, non-specific proteases; targets the soluble extracellular domains of membrane proteins | 41 |
Differential Centrifugation
Sucrose density gradient separation using ultracentrifugation has been effectively applied in a number of studies to enrich membrane proteins from cell and tissue lysates [19, 26–29]. A subsequent sodium carbonate enrichment step can aid in removal of proteins that interact with or are non-specifically bound to membrane proteins, increasing the level of membrane protein enrichment [30, 31]. Aqueous two-phase density-based separations using polyethylene glycol (PEG) and other molecules have been used to effectively enrich membrane proteins from tissue lysate [32, 33]. Finally, phase separation of membrane and GPI-anchored proteins can also be accomplished using Triton X-114™ and aqueous phases [34, 35], although subsequent detergent removal may be challenging and result in sample loss. While all of these methods can enrich broad subsets of membrane proteins from tissue and cell lysate, they suffer from the common drawback that the membrane protein fraction is often contaminated with mitochondrial proteins and proteins from other subcellular organelles. Even though this type of recovery may be adequate for certain applications, the limitation imposed by such contamination must be kept in mind when designing experiments that require higher purity.
Enrichment of Targeted Subsets of Membrane Proteins
Biotinylation of cell-surface proteins followed by avidin-based enrichment, lectin affinity enrichment, cell shaving, cell-surface capture (CSC), immunoaffinity enrichment and cationic colloidal silica-based enrichment are all methods that allow enrichment of a targeted set/subset of membrane proteins. Biotinylation of cell-surface membrane proteins using membrane-impermeable, cleavable reagents such as amine-reactive sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (Sulfo-NHS-SS-Biotin) followed by enrichment with immobilized avidin is a powerful workflow for membrane protein enrichment, and enables efficient and selective enrichment of membrane proteins at the cell surface [36]. However, this method can only be applied to cells in culture. In addition, it is important to consider the impact of the covalent modification of primary-amine containing residues on subsequent proteomic analyses, including the impact on proteolysis and detection via mass spectrometry. Lectin-affinity enrichment typically involves the use of immobilized lectins (on beads) with an established affinity for one or more glycan classes. The immobilized lectins recognize and bind glycosylated membrane proteins [37–40]. The results must be interpreted within the context of the enrichment, as lectin specificity may be broader than expected; it may also bias the subset of membrane glycoproteins that are enriched [24]. Cell shaving involves use of high-pH conditions and high pH-tolerant, non-specific proteases to digest the soluble extracellular domains of membrane proteins; it is a helpful method if the extracellular/soluble domains of membrane proteins are the primary target of interest in an analysis [41]. Cell surface capturing (CSC) relies on periodate treatment followed by hydrazide chemistry in which proteins/peptides with oxidized carbohydrate moieties are reacted with a bifunctional compound containing (a) a hydrazide moiety and (b) a tag that can subsequently be used for affinity purification [25, 42, 43]. Following release mediated by the amidase/peptide glycosidase PNGase F, the released, deamidated, formerly-N-glycosylated peptides can be analyzed via MS. CSC can be used to enrich membrane proteins/peptides containing glycans; however, the serious drawback of this approach is the destruction of the glycan moiety and the resulting loss of information about the detailed glycan structures and their heterogeneity. Immunoaffinity-based enrichment and cationic colloidal silica beads have also been used to enrich plasma membrane proteins; however, they lack specificity compared to other membrane protein enrichment methods [44–49].
Fractionation of cell and tissue lysate is often essential for detection of membrane proteins due to the lower recovery of membrane proteins compared to soluble proteins. ‘Shotgun’ membrane proteomics strategies rely on global enrichment of membrane proteins or targeted enrichment of a subset of membrane proteins, while in-depth studies of post-translational modifications and glycosylation may require targeted enrichment of one or a few proteins. In general, the strategies discussed in this section provide poor specificity for enrichment of membrane proteins; however, these methods may still be of use as initial steps in a larger workflow for enrichment of membrane proteins. It is also important to consider at the outset of experimental design that some of these methods can only be used in cultured cells, while others can be used for cell and tissue lysates.
IV. Membrane Protein Digestion and Glycopeptide Enrichment
As membrane proteins have poor solubility due to the hydrophobic nature of their transmembrane domains, and have fewer potential (Lys and Arg) trypsin cleavage sites in the transmembrane domains, trypsin has limited overall proteolytic efficiency in membrane protein digestion, though it is suited for digestion of the more hydrophilic regions of these proteins, i.e., the extracellular domains. Furthermore, glycosylation may interfere with protein digestion due to steric hindrance that can diminish access to protease cleavage sites [50]. For some investigators, the path forward involves removal of the obstructive glycan moieties. However, since the focus of this review discussion of strategies that enable elucidation of the details of protein glycosylation, methods that rely on removal of glycosylation [42] will not be discussed in detail. Alternative digestion techniques may be employed if high/low pH conditions, the presence of detergents, or the lack of trypsin cleavage sites in membrane protein preparations lead to poor digestion using trypsin. Some of the techniques discussed in this section serve to improve sample preparation by enhancing trypsin digestion, while others forgo the use of trypsin and explore alternative methods for proteolysis to increase sequence coverage of membrane proteins. Enzymatic digestion using chymotrypsin, Lys-C, pepsin, and pronase, and/or chemical cleavage with cyanogen bromide have all been shown to improve membrane protein sequence coverage [51]. Parallel digestion techniques in which a sample is divided into multiple fractions and digested in parallel with multiple proteases can also be used to enhance coverage via assembly of overlapping and non-overlapping peptides from different digests.
The efficiency of enzymatic digestion of membrane proteins can also be improved by a number of ‘assisted digestion’ techniques [51]. One such method is microwave-assisted digestion, which increases membrane protein solubility and facilitates digestion [50, 52–54]. An important advantage of this method is that post-translational modifications (PTMs) are preserved. Alternatively, filter-aided separation enables experimentalists to take advantage of detergents for membrane solubility, and then achieve their removal via urea addition and filtration, followed by on-filter digestion, so that the presence of detergents does not inhibit enzymatic digestion [55, 56]. However, there are conflicting reports regarding protein loss and, therefore, the sensitivity achievable with this method [22]. TFE and methanol aid digestion of membrane proteins [57]. Guanidine hydrochloride has also been used to enhance the digestion efficiency of membrane proteins during in-gel digests [58]. Finally, use of high concentrations of urea for protein denaturation, Lys-C digestion, then dilution of urea followed by trypsin digestion has been shown to be the most reproducible method to aid in digestion of plasma proteins, and this approach also seems likely to benefit digestion of membrane proteins [59]. Thus, robust methods for digestion of membrane proteins that simultaneously preserve glycosylation and other PTMs and enhance protein sequence coverage are emerging, and promise to advance bottom-up membrane proteomics. Consideration of digestion efficiency and sensitivity are important for broad membrane proteome studies. If the primary goal of an analysis is the optimal digestion and coverage of a specific, enriched membrane protein, simultaneous maintenance of protein solubility and optimization of proteolysis/coverage must be built into the experimental design by proper selection of proteases and reagents that aid in solubility.
V. Glycan and Glycopeptide Enrichment
Glycopeptide enrichment is a valuable, probably essential, step for site-specific glycosylation analysis using mass spectrometry because the hydrophilic glycan moieties on glycopeptides decrease the ionization efficiency of glycopeptides compared to non-glycosylated peptides. Enrichment is often required due to the presence of sub-stoichiometric levels of individual glycopeptides that result from glycosylation heterogeneity. To overcome these obstacles to detection, a number of methods have been developed to enrich glycopeptides prior to analysis, including differential solubility, hydrophilic-interaction chromatography (HILIC), porous graphitized carbon (PGC), and titanium dioxide enrichment [60–63]. HILIC enrichment is the most common strategy for glycopeptide enrichment; comparative studies have demonstrated non-biased, high-specificity glycopeptide enrichment using amide-(TSKgel Amide-80) and sulfobetaine-(ZIC-HILIC) bonded particles under high organic conditions [63]. HILIC phases have been used to enrich glycopeptides in a number of formats, including solid phase [64, 65], chromatographic [61, 66], and online enrichment followed by reversed phase separation [67]. Sialylated glycopeptides can be selectively enriched using titanium dioxide [68]; however, unless sialylated glycopeptides are specifically of interest, this method should not be employed as a profiling approach because it discriminates against asialo-glycoforms and also enriches for phosphopeptides. PGC chromatographic separation of glycopeptides from pronase digests have also shown utility for site-specific resolution of glycosylation in certain contexts [69, 70]. PGC has also been used to separate isomeric glycan structures. Recently, strategies involving metabolic labeling of glycopeptides to facilitate enrichment and detection have also been developed [71]. This method promises to enable enrichment of glycopeptides with smaller (less hydrophilic) glycan moieties, including small O-glycans that may be lost using other methods. For enrichment, differential solubility/acetone enrichment is also an option, though the specificity and losses that may be associated with this method have not been examined in detail [60]. Current workflows for enrichment of a broad range of glycoforms rely on HILIC resins, namely Amide-80 and ZIC-HILIC, which show high selectivity for glycopeptides and no apparent bias toward different glycoforms.
VI. Mass Spectrometry Analysis of Glycans and Intact Glycopeptides
In this section we will discuss MS and MS/MS analysis of glycans and intact glycopeptides. Recent review articles cover current methods for the analysis of glycans and glycopeptides in great detail [15, 72]. Here, we will briefly summarize relevant methods and techniques.
Analysis of Released Glycans
Native or derivatized N- and O-linked glycans can be analyzed after release via enzymatic or chemical methods [73]. Common ionization techniques for released glycans include both matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI). MALDI-MS of native glycans with acidic monosaccharides (such as sialic acids) and substituents (sulfate, phosphate) may lead to the loss of these labile groups, unless the pressure in the ion source is raised to provide vibrational cooling [74]. Permethylation or esterification of glycans prior to MALDI ionization stabilizes the glycans and prevents the loss of labile sialic acid residues [75–78]. ESI is a more gentle ionization method, and is recommended for ionization of underivatized glycans, but still results in loss of labile glycans, such as sialic acid, especially during operation in positive-ion mode [79]. Online HILIC or PGC separation for native and reductively aminated glycans, and reversed phase and PGC for permethylated glycans, can aid in separation of glycans prior to MS analysis [72].
‘Profiling’ intact glycans via MS can be a useful way to survey the compositions of glycans released from a protein or proteins of interest, while MS/MS analysis of glycans can lead to a greater understanding of glycan topology and linkage. Instruments with high mass accuracy should be used for glycan profiling experiments because glycan compositions can be assigned with higher confidence and isobaric species can be resolved. Isomeric mixtures provide a particular challenge for MS analysis of glycans. Tandem mass spectrometry can aid in characterization of glycan topology and linkage [80, 81]. Collision–induced dissociation (CID) and infrared multiphoton dissociation (IRMPD) are commonly used fragmentation techniques for intact glycan analysis. These methods enable fragmentation of glycosidic linkages and therefore enable determination of topology information of monosaccharides within a glycan, but give very limited information on branch positions. Rearrangement of monosaccharide residues has been observed using these fragmentation methods, so care should be taken in interpreting results [82]. Electron capture dissociation (ECD) and other electron activated dissociation (ExD) methods have shown great promise in determining glycan linkage and branching information [83, 84].
Analysis of Intact Glycopeptides
Tandem mass spectrometry of intact glycopeptides is sensitive and enables high-throughput analysis of glycosylation heterogeneity in complex samples. Online separation of glycopeptides using reversed phase chromatography followed by MS/MS analysis has proven to be an efficient and effective workflow for complex glycoproteomic analyses [85, 86]. Instruments used for glycopeptide analysis include Orbitrap hybrid (Thermo) and quadrupole time-of-flight (QTOF) mass spectrometers. While MALDI was used for ionization of glycopeptides in many early studies, ESI is used almost exclusively in current analytical workflows due to its compatibility with efficient LC-MS/MS workflows. Fragmentation of glycopeptides by CID and higher-energy collisional dissociation (HCD), ExD, and IRMPD has been widely documented [83]. Lower-energy CID of glycopeptides favors cleavage of glycosidic linkages in the carbohydrate moiety of a glycopeptide and enables determination of some glycan topological information. Higher-energy CID (in QTOFs) and HCD (in hybrid Orbitrap instruments) generate more peptide backbone fragmentation that enables characterization of the peptide sequence but lead to the loss (to varying degrees) of glycan information [85]. In contrast, ECD and ETD fragment the peptide backbone but not the glycan moiety, enabling determination of the site of glycosylation [87]. This is especially useful for the analysis of O-glycopeptides. Parallel analysis of samples using multiple fragmentation techniques can yield complementary information. Ultraviolet photodissociation (UVPD) of heparin-derived molecules and lipid A have also been demonstrated [88, 89]. Ion mobility separation (IMS) can be coupled with tandem mass spectrometry to afford separation of glycan and glycoconjugate isomers based on their cross-sections [90]. Our laboratory and others are actively pursuing the utilization of IMS for glycopeptide and glycoprotein separation and characterization.
VII. Automated Data Interpretation for Glycomics and Glycoproteomics
Several excellent reviews discuss the current state of automated glycomics and glycoproteomics analysis [91–93]. Here, we briefly describe notable developments in automated analysis of glycomics and glycoproteomics datasets. Tools including GlycoWorkbench [94], Cartoonist [95], and SimGlycan [96] have been developed to aid in the interpretation of glycomics data, including for analysis of intact glycan MS and glycan MS/MS data. GlycoWorkbench is publicly available and is useful for analysis of single spectra or small datasets, while commercially available SimGlycan is suited to analysis of large datasets with MS and MS/MS data. Automated analysis of intact glycopeptide LC-MS/MS datasets has also made strides in the past few years, and Hu et al. recently published a comprehensive review on automated glycoproteomics analysis that describes the capabilities of currently available software [92]. Byonic is one of the most widely distributed and commercially available software packages [97]. Others, including GlycoPep Evaluator [98], are publicly available. Notably, current tools differ in their ability to handle data generated using alternate fragmentation methods (CID, HCD, and ExD). One must always keep in mind that structural assignments based solely on molecular weight determinations rely on commonly reported structures and are not definitive. Glycoproteomics data interpretation is not as mature as proteomics data analysis, in part due to a lack of consensus regarding the best way(s) to estimate the false discovery rate and the existence of multiple formats for storage of glycan structure information. However, current tools accelerate data interpretation and offer a level of confidence unheard of a decade ago. Guidelines for the reporting of glycoproteomics and glycomics data are being developed (MIRAGE) and their utilization is strongly encouraged [99].
VIII. Proteomic Workflows for Membrane Proteins
The low relative recoveries and hydrophobic natures of membrane proteins largely explain the under-representation of membrane proteins in large ‘shotgun’ proteomics datasets. Membrane proteins bear extensive post-translational modifications and many of the modified peptides are not detected in large-scale proteomics datasets. Multidimensional protein identification technology (MudPIT), which relies on separation of peptides based on charge and hydrophobicity, has increased the number of membrane proteins identified as well as the sequence coverage [100, 101]. One strategy for increasing the detection rate for membrane proteins in complex digests is the application of workflows that remove N-linked glycans from proteins prior to proteolysis to prevent missed cleavage resulting from N-glycan steric hindrance of proteases [6]. This approach also expands the number of identified peptides based on detection of deglycosylated (and deamidated) peptides. This strategy may be beneficial if protein identification/sequence coverage is the primary goal; however, removal of N-glycans is not helpful in the many instances where biological insight may be gained from the knowledge of site-specific glycosylation. A more informative alternative is to boost the temperature of chromatographic separations, which increases the resolution and recovery of peptides from the hydrophobic stationary phase, and leads to detection of greater numbers of peptides derived from lower-abundance peptides, including those of membrane proteins [102]. As discussed earlier in section III, the use of MS-compatible detergents can also boost identification of membrane proteins [103, 104]. Hydrazide capture of glycopeptides after oxidation of glycans, followed by release with PNGase F, is a strategy for proteomic analysis of formerly glycosylated peptides that can be applied to membrane proteomics that is successful for recovering a representative set of glycoproteins [42, 105], but it destroys the glycan structures and thus sacrifices important biological information. Quantitative proteomics approaches have also been applied to peptides (and formerly-glycosylated peptides) derived from membrane proteins and glycoprotein. Such approaches include stable isotope incorporation via chemical means (iTRAQ, TMT) [106, 107], metabolic incorporation (SILAC) [108, 109], and enzymatic incorporation of heavy isotopes into formerly-glycosylated sites (TOSIL) [110]. However, as noted above, methods that rely on removal of N-glycans from peptides are not compatible with glycoproteomic studies that seek to characterize glycan heterogeneity, although such strategies are helpful in the identification (and quantitative analysis) of occupied glycosylation sites, and therefore can play some role in studying glycosylation. Membrane proteomics methods can inform membrane protein glycomics and glycoproteomics workflows; the use of MS-compatible detergents, higher temperature chromatographic separation of peptides, and multidimensional separation of digests can improve glycoproteomics workflows.
IX. Glycomics of Membrane Proteins
The release of glycans from protein pools or purified proteins, by chemical or enzymatic means, followed by either direct analysis via MS or derivatization followed by MS analysis, is a powerful means of exploring glycan structure and heterogeneity. Common workflows rely on PNGase F for (enzymatic) release of N-linked glycans and reductive β-elimination for (chemical) release of O-linked glycans; hydrazinolysis can be used for both N- and O-linked glycans [72]. The released glycans are often derivatized to make them more amenable to MS analyses and to increase the information content of the mass spectra. The primary goal of derivatization is to make these hydrophilic molecules more hydrophobic, which enhances glycan signal strength in MALDI and ESI ionization. Permethylation of glycans introduces methyl groups onto free hydroxyl, amine, and carboxyl groups [75, 111]. The free reducing end of N-glycans released via PNGase F or O-glycans released by chemical cleavage can also be reduced or labeled via reductive amination reactions, enabling the attachment of fluorescent or UV-absorbing chemical groups that can be used for detection of glycans or to enhance ionization for MS analysis [112].
Glycomics of Complex Mixtures
The majority of glycomics studies of membrane proteins to date focus on N-linked glycans released from enriched membrane protein fractions. Generally, membrane proteins are enriched from tissue or cells, followed by release of N-linked glycans with PNGase F, permethylation, and analysis via MALDI-TOF MS or ESI-MS/MS. Alternatively, released glycans are labeled on the reducing end and separated via PGC chromatography followed by MS analysis [113, 114]. Comparative membrane protein glycome studies of diseases including galactosemia, liver cancer, and colorectal cancer offer models for study design [113–116]. Comparative glycomics studies have also been applied to analyze glycosylation of membrane proteins derived from different cell types, including comparisons of embryonic stem cells versus somatic cells, and differentiated versus undifferentiated epithelial cells [117, 118]. A successful (though not universal) workflow for membrane protein enrichment in these studies is phase partitioning of membrane proteins with Triton-X114 and an aqueous phase, followed by immobilization of proteins on a PVDF membrane and PNGase F treatment (on membrane), followed by PGC-LC separation and MS analysis [119]. These glycomics studies offer interesting insight into global changes in glycosylation of membrane proteins depending on the degree of cell differentiation or based on disease state, as well as serving as models for future membrane protein glycomics studies. Recently, a method for direct release of N-linked glycans from the cell surface, followed by MS analysis, has also been employed [120]. One advantage of global analyses of the membrane protein glycome is that they can detect major shifts in glycoforms, which are likely to have a major impact on cell adhesion and signaling. However, in addition to global studies, some mechanistic studies of protein signaling require more targeted analysis of protein glycosylation. In such cases, it may be helpful to analyze the glycosylation of individual membrane proteins; therefore, we will discuss this topic in the next section.
Glycomic Analyses of Individual Membrane Proteins
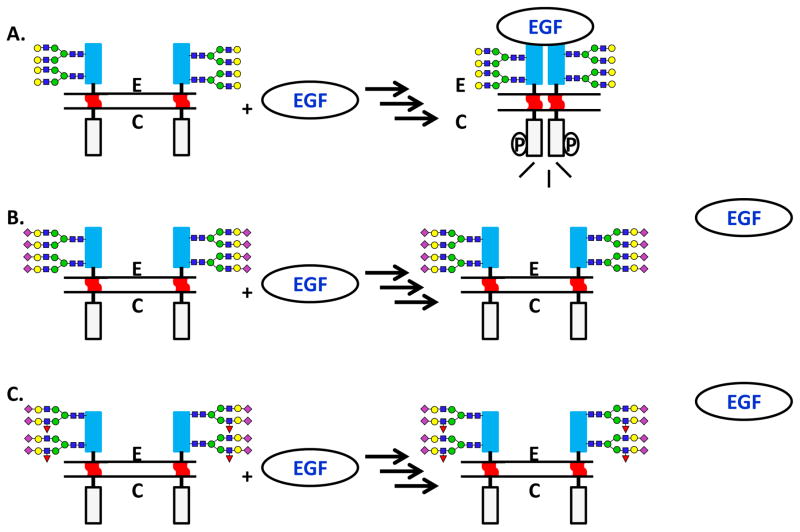
Glycomic analysis of individual membrane proteins is a laborious process that requires purification of sufficient quantities of membrane proteins prior to release of N- and O-glycans. Parallel proteomic analyses are essential to confirm the identity of the protein and confirm that other glycosylated protein contaminants are not present. An early membrane protein glycomics study involved the GPI-linked membrane protein CD59 glycoprotein (CD59) [121]. In this study, N- and O-linked glycans were released from erythrocyte- and platelet-derived CD59 by PNGase F treatment and hydrazinolysis, followed by glycosidase treatment and MALDI-MS analysis. Based on the N- and O-linked glycans present on membrane-bound CD59, the authors of this study concluded that the glycans limit the interaction of the protein with the membrane surface and may also restrict the movement of the extracellular domain and orient the active side of the protein outward. This model study reflects the importance of studying membrane protein glycosylation to understand how proteins perform biological functions. Several additional exemplar glycomics studies have characterized N- and O-linked glycosylation in Mucin 1 (MUC1), an important mucin that is overexpressed in many tumors; CD98 heavy chain (CD98hc) (derived from a tumor cell line) containing heavily fucosylated termini; and Scavenger receptor expressed by endothelial cells 1 (SREC-I), an endothelial cell protein that mediates endocytosis, in which N-glycans modulate ligand binding, proteolysis and subcellular localization [122–124]. Molecular biology studies targeting glycosylated membrane proteins including Proteinase-activated receptor 1 (PAR1) (a G-protein coupled receptor) [125], β4 Integrin [126], and Platelet endothelial cell adhesion molecule (PECAM) [127] offer clues to the important role of glycosylation in cellular signaling and adhesion, as well as enticing targets for future glycomics studies. Several tantalizing studies have demonstrated the impact of N-glycosylation on Epidermal growth factor receptor (EGFR) dimerization and downstream signaling [4–7], indicating a role for glycosylation in fine-tuning receptor signaling (Figure 2). This important receptor tyrosine kinase serves as a model to study the impact of protein glycosylation on modulation of cell signaling. It is clear that further study of membrane protein glycosylation will lead to a greatly expanded understanding of how glycosylation impacts biological processes in normal and disease states. Prominent tools in glycomics, including the use of glycosidases and the expansion of mass spectrometry fragmentation techniques, enable structural characterization of glycans which will ultimately lead to greater understanding of glycans in membrane protein function.
Figure 2. The Impact of N-Glycosylation on Epidermal Growth Factor Receptor (EGFR) Dimerization, Activation, and Signaling.
A. The extracellular (E) domain of EGFR (dark gray box) is N-glycosylated at multiple sites, whereas the transmembrane region (gray wavy line) and cytosolic (C) (light gray box) domains are not glycosylated. B. Increased sialylation of glycans on the receptor suppresses ligand-induced dimerization and activation. C. Increases in the number of alpha 1,3-fucosylated N-glycans also suppress ligand-induced dimerization and activation. Dark squares: N-acetylglucosamine; gray circles: mannose; light circles: galactose; diamonds: sialic acid. ‘E’ indicates extracellular, ‘C’ indicates cytoplasmic. Circled ‘P’ indicates phosphorylation. Circled ‘EGF’ represents the ligand. Sources: Fernandes et al., 2001 [4], Liu et al., 2011 [6], Yen et al., 2015 [7], Kaszuba et al., 2015 [5].
X. Glycoproteomics of Membrane Proteins
Glycoproteomics is best defined as the site-specific characterization of glycosylation in which information about the peptide (or protein), glycosylation site, and the glycan moiety are retained [15]. Advances in glycopeptide analysis using mass spectrometry have empowered the field of glycoproteomics. Collision-induced dissociation (CID) and electron activated dissociation (ExD) of glycopeptides provide complementary information about both the peptide and glycan components of glycopeptides [72, 128, 129]. However, using current LC-MS/MS based workflows experimentalists are usually forced to choose between depth and breadth of analysis. Interlaboratory studies of glycoproteins have shown that results may vary from one laboratory to another [79, 130, 131], emphasizing the importance of experimental design in minimizing sources of error.
Current ‘shotgun’ glycoproteomics workflows can successfully analyze glycopeptides from hundreds of proteins in one LC-MS run [132]. In this study, glycopeptides were enriched using lectin affinity, and over 2500 unique N- and O-linked glycopeptides from 453 proteins were identified. This feat sets the bar for future analytical workflows. Using a similar approach, membrane protein-enriched brain and liver fractions were subjected to glycopeptide analysis to determine tissue-specific glycosylation of secreted and membrane proteins [133]. Other studies of membrane protein glycosylation have used similar workflows [134–136]. These studies serve to emphasize the fact that current technologies for enrichment, separation, and analysis of glycopeptides on a proteome-wide scale can sacrifice some information, as the heterogeneity of glycosylation at individual glycosylation sites is rarely captured at this scale.
To date, studies that focus on glycopeptide characterization from a single membrane protein (or a simple mixture) are able to characterize site-specific glycosylation heterogeneity with greater depth compared to strategies that seek to characterize a broad set of glycoproteins [137–139]. In the first MALDI-MS study of site-specific glycopeptide heterogeneity, characterization of N-linked glycosylation contributed to an enhanced understanding of the assembly of (Na, K)-ATPase subunits into a functional oligomer [137]. Rhodopsin is one of the first receptors studied using glycomics methods, and was a successful target for analysis, in part due to its abundance and relative ease of purification [140]. This study generated glycopeptides, purified them and then released the glycans from the two individual sites. Unambiguous determination of mucin-type O-linked glycosylation on mucins is important for understanding the conformation, resistance to proteases, and function of these unique, abundant proteins [138, 141]. A recent publication offers evidence of differential site-specific glycosylation between soluble (secreted) and membrane-bound forms of Epidermal Growth Factor Receptor (EGFR) which should be further investigated [142]. Quantitative analysis of protein glycosylation by application of multiple reaction monitoring (MRM) to glycopeptides is a promising approach that has been applied to secreted N- and O-glycoproteins enriched from human plasma [143–145]. Glycopeptide fragment ions, including oxonium ions and peptide-HexNAc ions were selected as glycopeptide-specific fragments for MRM analysis. While this approach has not been used to quantitatively study membrane protein N-glycosylation, it can clearly be applied to any enriched glycoprotein. Targeted analysis of purified proteins is well suited to expand on studies such as these, which seek to investigate the functional role of glycosylation in membrane proteins and receptors.
Current methods enable glycopeptides from hundreds of glycoproteins to be identified (with assignment of the structures of the top 1–2 glycoforms at each glycosylation site) or a single protein or simple mixture of proteins can be analyzed to reveal dozens of site-specific glycoforms [132, 137]. The decision to pursue either depth or breadth is highly dependent on the research question and goals of the experiment.
XI. The Promising Future of Membrane Glycoproteomics
The study of membrane proteins, including their structure and function, can benefit from glycomic and glycoproteomic approaches. Nearly all membrane proteins and receptors are N- and/or O-glycosylated, and these modifications play a role in membrane protein function including in adhesion and signaling [4–8]. Applying glycomics and glycoproteomics techniques to study the role of glycosylation in membrane proteins is enticing, as it will further our understanding of the role glycosylation plays in modulating signaling and adhesion. Glycomics, the study of released and free glycans including their structure and heterogeneity, enables a view into glycan structure that can be applied in functional studies of membrane proteins. The “site-specific analysis of the glycoproteome at the system-wide level… [yielding] information about the protein carriers, the glycan attachment sites and the structure and occupancy of the glycan” [15] is approaching for membrane proteins. Barriers to the study of membrane protein glycosylation include (a) limitations in MS-compatible enrichment of membrane proteins, (b) the challenges of achieving quantitative analyses of intact glycopeptides, and (c) time-consuming manual and automated analysis of glycopeptide datasets. We envision a future in which tools for analysis of glycosylation can be applied to study membrane protein function and signaling with glycoform-level specificity to expand on current knowledge of membrane protein biology. However, based on current technology, this ideal vision of glycoproteomics has not yet been met, as studies of membrane protein glycosylation still require some tradeoffs in regard to breadth and depth of glycoprotein heterogeneity at specific glycosylation sites.
The application of glycomics and glycoproteomics methods to the study of membrane proteins requires the careful tailoring of membrane protein purification and enrichment techniques to glycoproteomics and glycomics workflows. Lessons can be learned from current methods that focus on (1) proteomic characterization of membrane proteins and (2) glycomics and glycoproteomics. However, in merging these two goals, several challenges arise that are unique to “membrane glycoproteomics”, among them the conflicting goals of maintaining the solubility of membrane proteins (commonly through the use of detergents) and removal/avoidance of detergents in sample preparation steps prior to MS/MS analyses. A second balancing act involves the desire to achieve both breadth (in terms of the number of membrane proteins analyzed) and depth (in terms of the number of glycosylation sites and even individual glycoforms at each site) of glycoproteomic analyses. Currently, one must still choose between depth and breadth, though the gap that limits achieving these two goals is closing.
Clearly, global studies of glycosylation that cover hundreds of proteins may reveal global trends in signaling and adhesion, while targeted studies of a single protein or a simple mixture are appropriate to answer mechanistic questions regarding the role of glycosylation in protein function. These strategies may also benefit by supplementation with parallel glycomic analysis, which may enable greater structural characterization of glycan moieties. Site-specific analysis of glycosylation, in combination with glycomic studies to aid in structural characterization of glycans, should play a larger role in the study of signaling and adhesion. The prospect of performing quantitative analysis of glycopeptides in membrane proteins is tantalizing. Glycopeptide MRM approaches are currently limited to enriched glycoproteins because the specificity of MRM transitions suffers when complex samples are considered. Advances in quantitative analysis of glycopeptides are promising [145] but must overcome current limitations of glycopeptide MRM-based methods. The application of new glycopeptide fragmentation methods including ExD, maturation of glycopeptide enrichment tools (including HILIC-RP), expansion of separation capabilities and informatics tools that accelerate the interpretation of glycopeptide MS/MS datasets are expanding and transforming the reach of membrane protein glycosylation studies. Glycomics and glycoproteomics have advanced rapidly in recent years due to advances in instrumentation, enrichment, and chromatography. These advances will benefit the study of membrane proteins if the scientific community embraces these changes to advance membrane protein research.
Acknowledgments
Preparation of this manuscript was supported by grants from the National Institutes of Health, including F32 CA196157 (to KBC) and P41 GM104603 (to CEC).
Abbreviations
- CSC
cell-surface capture
- ECD
electron capture dissociation
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- ETD
electron transfer dissociation
- ExD
electron activated dissociation
- GPI
glycophosphatidylinositol
- HCD
higher-energy collisional dissociation
- IRMPD
infrared multiphoton dissociation
- MudPIT
multidimensional protein identification technology
- PGC
porous graphitized carbon
- SDC
sodium deoxycholate
- TFE
trifluoroethanol
- UVPD
Ultraviolet photodissociation
- ZIC-HILIC
zwitterionic hydrophilic interaction chromatography
Footnotes
The contents are solely the responsibility of the authors and do not represent the official views of the awarding offices.
References
- 1.Zhao YY, et al. Cancer Sci. 2008;99(7):1304–10. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallin E, von Heijne G. Protein Sci. 1998;7(4):1029–38. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieper U, et al. Nat Struct Mol Biol. 2013;20(2):135–8. doi: 10.1038/nsmb.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes H, Cohen S, Bishayee S. J Biol Chem. 2001;276(7):5375–83. doi: 10.1074/jbc.M005599200. [DOI] [PubMed] [Google Scholar]
- 5.Kaszuba K, et al. Proc Natl Acad Sci U S A. 2015;112(14):4334–9. doi: 10.1073/pnas.1503262112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YC, et al. Proc Natl Acad Sci U S A. 2011;108(28):11332–7. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen HY, et al. Proc Natl Acad Sci U S A. 2015;112(22):6955–60. doi: 10.1073/pnas.1507329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty P, et al. Mol Cell Biochem. 2015 [Google Scholar]
- 9.Xu C, DTNg Nat Rev Mol Cell Biol. 2015 doi: 10.1038/nrm4073. [DOI] [PubMed] [Google Scholar]
- 10.Zielinska DF, et al. Cell. 2010;141(5):897–907. doi: 10.1016/j.cell.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Thaysen-Andersen M, Packer NH. Glycobiology. 2012;22(11):1440–52. doi: 10.1093/glycob/cws110. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Hart GW. Expert Rev Proteomics. 2013;10(4):365–80. doi: 10.1586/14789450.2013.820536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells L. J Biol Chem. 2013;288(10):6930–5. doi: 10.1074/jbc.R112.438978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moremen KW, Tiemeyer M, Nairn AV. Nat Rev Mol Cell Biol. 2012;13(7):448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaysen-Andersen M, Packer NH. Biochim Biophys Acta. 2014;1844(9):1437–52. doi: 10.1016/j.bbapap.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Seddon AM, Curnow P, Booth PJ. Biochim Biophys Acta. 2004;1666(1–2):105–17. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1002:144–51. doi: 10.1016/j.jchromb.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Masuda T, Tomita M, Ishihama Y. J Proteome Res. 2008;7(2):731–40. doi: 10.1021/pr700658q. [DOI] [PubMed] [Google Scholar]
- 19.Blonder J, et al. J Invest Dermatol. 2004;123(4):691–9. doi: 10.1111/j.0022-202X.2004.23421.x. [DOI] [PubMed] [Google Scholar]
- 20.Mbeunkui F, Goshe MB. Proteomics. 2011;11(5):898–911. doi: 10.1002/pmic.200900698. [DOI] [PubMed] [Google Scholar]
- 21.Liebler DC, Ham AJ. Nat Methods. 2009;6(11):785. doi: 10.1038/nmeth1109-785a. author reply 785–6. [DOI] [PubMed] [Google Scholar]
- 22.Vuckovic D, et al. Proteomics. 2013;13(3–4):404–23. doi: 10.1002/pmic.201200340. [DOI] [PubMed] [Google Scholar]
- 23.Cordwell SJ, Thingholm TE. Proteomics. 2010;10(4):611–27. doi: 10.1002/pmic.200900521. [DOI] [PubMed] [Google Scholar]
- 24.Mechref Y, Madera M, Novotny MV. Methods Mol Biol. 2008;424:373–96. doi: 10.1007/978-1-60327-064-9_29. [DOI] [PubMed] [Google Scholar]
- 25.Wollscheid B, et al. Nat Biotechnol. 2009;27(4):378–86. doi: 10.1038/nbt.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam PJ, et al. J Biol Chem. 2003;278(8):6482–9. doi: 10.1074/jbc.M210184200. [DOI] [PubMed] [Google Scholar]
- 27.Foster LJ, et al. Stem Cells. 2005;23(9):1367–77. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 28.Navarre C, et al. Proteomics. 2002;2(12):1706–14. doi: 10.1002/1615-9861(200212)2:12<1706::AID-PROT1706>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, et al. Proteomics. 2005;5(17):4510–24. doi: 10.1002/pmic.200401318. [DOI] [PubMed] [Google Scholar]
- 30.Fujiki Y, et al. J Cell Biol. 1982;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molloy MP. Methods Mol Biol. 2008;424:397–401. doi: 10.1007/978-1-60327-064-9_30. [DOI] [PubMed] [Google Scholar]
- 32.Cao R, et al. J Proteome Res. 2006;5(3):634–42. doi: 10.1021/pr050387a. [DOI] [PubMed] [Google Scholar]
- 33.Schindler J, Nothwang HG. Proteomics. 2006;6(20):5409–17. doi: 10.1002/pmic.200600243. [DOI] [PubMed] [Google Scholar]
- 34.Bordier C. J Biol Chem. 1981;256(4):1604–7. [PubMed] [Google Scholar]
- 35.Elortza F, et al. Mol Cell Proteomics. 2003;2(12):1261–70. doi: 10.1074/mcp.M300079-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Shin BK, et al. J Biol Chem. 2003;278(9):7607–16. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh D, Beavis RC, Wilkins JA. J Proteome Res. 2008;7(4):1572–83. doi: 10.1021/pr070509u. [DOI] [PubMed] [Google Scholar]
- 38.Kullolli M, Hancock WS, Hincapie M. J Sep Sci. 2008;31(14):2733–9. doi: 10.1002/jssc.200800233. [DOI] [PubMed] [Google Scholar]
- 39.Vercoutter-Edouart AS, et al. Proteomics. 2008;8(16):3236–56. doi: 10.1002/pmic.200800151. [DOI] [PubMed] [Google Scholar]
- 40.Ueda K, et al. Proteomics. 2009;9(8):2182–92. doi: 10.1002/pmic.200800374. [DOI] [PubMed] [Google Scholar]
- 41.Wu CC, et al. Nat Biotechnol. 2003;21(5):532–8. doi: 10.1038/nbt819. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, et al. Nat Biotechnol. 2003;21(6):660–6. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 43.Mi W, et al. Glycoconj J. 2012;29(5–6):411–24. doi: 10.1007/s10719-012-9420-3. [DOI] [PubMed] [Google Scholar]
- 44.Watarai H, et al. Proteomics. 2005;5(15):4001–11. doi: 10.1002/pmic.200401258. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, et al. J Proteome Res. 2007;6(1):34–43. doi: 10.1021/pr060069r. [DOI] [PubMed] [Google Scholar]
- 46.Chaney LK, Jacobson BS. J Biol Chem. 1983;258(16):10062–72. [PubMed] [Google Scholar]
- 47.Durr E, et al. Nat Biotechnol. 2004;22(8):985–92. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 48.Oh P, et al. Nature. 2004;429(6992):629–35. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 49.Schnitzer JE, et al. Science. 1995;269(5229):1435–9. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 50.Segu ZM, et al. J Proteome Res. 2010;9(7):3598–607. doi: 10.1021/pr100129n. [DOI] [PubMed] [Google Scholar]
- 51.Switzar L, Giera M, Niessen WM. J Proteome Res. 2013;12(3):1067–77. doi: 10.1021/pr301201x. [DOI] [PubMed] [Google Scholar]
- 52.Ye X, Li L. Anal Chem. 2012;84(14):6181–91. doi: 10.1021/ac301169q. [DOI] [PubMed] [Google Scholar]
- 53.Zhong H, Marcus SL, Li L. J Am Soc Mass Spectrom. 2005;16(4):471–81. doi: 10.1016/j.jasms.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Vaezzadeh AR, et al. J Microbiol Methods. 2010;80(1):56–62. doi: 10.1016/j.mimet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Wisniewski JR, Zielinska DF, Mann M. Anal Biochem. 2011;410(2):307–9. doi: 10.1016/j.ab.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Erde J, Loo RR, Loo JA. J Proteome Res. 2014;13(4):1885–95. doi: 10.1021/pr4010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, et al. Proteomics. 2007;7(10):1654–63. doi: 10.1002/pmic.200600579. [DOI] [PubMed] [Google Scholar]
- 58.Takakura D, Hashii N, Kawasaki N. Proteomics. 2014;14(2–3):196–201. doi: 10.1002/pmic.201300332. [DOI] [PubMed] [Google Scholar]
- 59.Abbatiello SE, et al. Mol Cell Proteomics. 2015;14(9):2357–74. doi: 10.1074/mcp.M114.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takakura D, et al. J Proteomics. 2014;101:17–30. doi: 10.1016/j.jprot.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Alpert AJ, et al. J Chromatogr A. 1994;676(1):191–22. doi: 10.1016/0021-9673(94)00467-6. [DOI] [PubMed] [Google Scholar]
- 62.Chen CC, et al. Analyst. 2014;139(4):688–704. doi: 10.1039/c3an01813j. [DOI] [PubMed] [Google Scholar]
- 63.Wohlgemuth J, et al. Anal Biochem. 2009;395(2):178–88. doi: 10.1016/j.ab.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Mysling S, et al. Anal Chem. 2010;82(13):5598–609. doi: 10.1021/ac100530w. [DOI] [PubMed] [Google Scholar]
- 65.Selman MH, et al. Anal Chem. 2011;83(7):2492–9. doi: 10.1021/ac1027116. [DOI] [PubMed] [Google Scholar]
- 66.Zauner G, Deelder AM, Wuhrer M. Electrophoresis. 2011;32(24):3456–66. doi: 10.1002/elps.201100247. [DOI] [PubMed] [Google Scholar]
- 67.Khatri K, et al. J Proteome Res. 2014;13(10):4347–55. doi: 10.1021/pr500506z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmisano G, et al. Nat Protoc. 2010;5(12):1974–82. doi: 10.1038/nprot.2010.167. [DOI] [PubMed] [Google Scholar]
- 69.Nwosu CC, et al. J Proteome Res. 2011;10(5):2612–24. doi: 10.1021/pr2001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hua S, et al. J Proteome Res. 2013;12(10):4414–23. doi: 10.1021/pr400442y. [DOI] [PubMed] [Google Scholar]
- 71.Woo CM, et al. Nat Methods. 2015;12(6):561–7. doi: 10.1038/nmeth.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leymarie N, Zaia J. Anal Chem. 2012;84(7):3040–8. doi: 10.1021/ac3000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morelle W, Michalski JC. Nat Protoc. 2007;2(7):1585–602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor PB, Costello CE. Rapid Commun Mass Spectrom. 2001;15(19):1862–8. doi: 10.1002/rcm.447. [DOI] [PubMed] [Google Scholar]
- 75.Ciucanu IKF. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- 76.Ciucanu I, Costello CE. J Am Chem Soc. 2003;125(52):16213–9. doi: 10.1021/ja035660t. [DOI] [PubMed] [Google Scholar]
- 77.Kang P, et al. Rapid Commun Mass Spectrom. 2005;19(23):3421–8. doi: 10.1002/rcm.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuster B, et al. Anal Biochem. 1997;250(1):82–101. doi: 10.1006/abio.1997.2199. [DOI] [PubMed] [Google Scholar]
- 79.Wada Y, et al. Mol Cell Proteomics. 2010;9(4):719–27. doi: 10.1074/mcp.M900450-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Domon BCCE. Glycoconj J. 1988;5:397–409. [Google Scholar]
- 81.Zhao C, et al. J Am Soc Mass Spectrom. 2008;19(1):138–50. doi: 10.1016/j.jasms.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 82.Wuhrer M, et al. Rapid Commun Mass Spectrom. 2006;20(11):1747–54. doi: 10.1002/rcm.2509. [DOI] [PubMed] [Google Scholar]
- 83.Zubarev RA. Curr Opin Biotechnol. 2004;15(1):12–6. doi: 10.1016/j.copbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Syka JE, et al. Proc Natl Acad Sci U S A. 2004;101(26):9528–33. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desaire H. Mol Cell Proteomics. 2013;12(4):893–901. doi: 10.1074/mcp.R112.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wuhrer M, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;849(1–2):115–28. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 87.Hakansson K, et al. Anal Chem. 2001;73(18):4530–6. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- 88.Madsen JA, et al. Anal Chem. 2011;83(13):5107–13. doi: 10.1021/ac103271w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Racaud A, et al. J Am Soc Mass Spectrom. 2009;20(9):1645–51. doi: 10.1016/j.jasms.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 90.Zhu M, et al. Anal Bioanal Chem. 2009;394(7):1853–67. doi: 10.1007/s00216-009-2865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodin CL, Maxon M, Desaire H. Analyst. 2013;138(10):2793–803. doi: 10.1039/c2an36042j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu H, et al. Glycoconj J. 2015 [Google Scholar]
- 93.Hu H, Khatri K, Zaia J. Mass Spectrom Rev. 2016 doi: 10.1002/mas.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ceroni A, et al. J Proteome Res. 2008;7(4):1650–9. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 95.Goldberg D, et al. Proteomics. 2005;5(4):865–75. doi: 10.1002/pmic.200401071. [DOI] [PubMed] [Google Scholar]
- 96.Apte A, Meitei NS. Methods Mol Biol. 2010;600:269–81. doi: 10.1007/978-1-60761-454-8_19. [DOI] [PubMed] [Google Scholar]
- 97.Bern M, Kil YJ, Becker C. Curr Protoc Bioinformatics. 2012;Chapter 13(Unit 13):20. doi: 10.1002/0471250953.bi1320s40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Z, et al. Anal Chem. 2014;86(18):9212–9. doi: 10.1021/ac502176n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.York WS, et al. Glycobiology. 2014;24(5):402–6. doi: 10.1093/glycob/cwu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Washburn MP, Wolters D, Yates JR., 3rd Nat Biotechnol. 2001;19(3):242–7. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 101.Savas JN, et al. Trends Biochem Sci. 2011;36(7):388–96. doi: 10.1016/j.tibs.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Speers AE, Blackler AR, Wu CC. Anal Chem. 2007;79(12):4613–20. doi: 10.1021/ac0700225. [DOI] [PubMed] [Google Scholar]
- 103.Chen EI, et al. J Proteome Res. 2007;6(7):2529–38. doi: 10.1021/pr060682a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen EI, et al. Anal Chem. 2008;80(22):8694–701. doi: 10.1021/ac800606w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah P, et al. Mol Cell Proteomics. 2015;14(10):2753–63. doi: 10.1074/mcp.M115.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parker BL, et al. Mol Cell Proteomics. 2011;10(8):M110 006833. doi: 10.1074/mcp.M110.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye H, et al. Anal Chem. 2013;85(3):1531–9. doi: 10.1021/ac3026465. [DOI] [PubMed] [Google Scholar]
- 108.Boersema PJ, et al. Mol Cell Proteomics. 2013;12(1):158–71. doi: 10.1074/mcp.M112.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prokhorova TA, et al. Mol Cell Proteomics. 2009;8(5):959–70. doi: 10.1074/mcp.M800287-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z, et al. J Proteome Res. 2010;9(1):227–36. doi: 10.1021/pr900528j. [DOI] [PubMed] [Google Scholar]
- 111.Hakomori S. J Biochem. 1964;55:205–8. [PubMed] [Google Scholar]
- 112.Ruhaak LR, et al. Anal Bioanal Chem. 2010;397(8):3457–81. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee A, et al. Mol Cell Proteomics. 2011;10(9):M900538MCP200. doi: 10.1074/mcp.M900538-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sethi MK, et al. J Proteome Res. 2014;13(1):277–88. doi: 10.1021/pr400861m. [DOI] [PubMed] [Google Scholar]
- 115.Staubach S, et al. J Proteome Res. 2012;11(2):906–16. doi: 10.1021/pr200711w. [DOI] [PubMed] [Google Scholar]
- 116.Sethi MK, et al. Glycobiology. 2015;25(10):1064–78. doi: 10.1093/glycob/cwv042. [DOI] [PubMed] [Google Scholar]
- 117.An HJ, et al. Mol Cell Proteomics. 2012;11(4):M111 010660. doi: 10.1074/mcp.M111.010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park D, et al. Mol Cell Proteomics. 2015;14(11):2910–21. doi: 10.1074/mcp.M115.053983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jensen PH, et al. Nat Protoc. 2012;7(7):1299–310. doi: 10.1038/nprot.2012.063. [DOI] [PubMed] [Google Scholar]
- 120.Hamouda H, et al. J Proteome Res. 2014;13(12):6144–51. doi: 10.1021/pr5003005. [DOI] [PubMed] [Google Scholar]
- 121.Rudd PM, et al. J Biol Chem. 1997;272(11):7229–44. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- 122.Parry S, et al. Glycobiology. 2006;16(7):623–34. doi: 10.1093/glycob/cwj110. [DOI] [PubMed] [Google Scholar]
- 123.Powlesland AS, et al. Glycobiology. 2009;19(8):899–909. doi: 10.1093/glycob/cwp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sano M, et al. Glycobiology. 2012;22(5):714–24. doi: 10.1093/glycob/cws010. [DOI] [PubMed] [Google Scholar]
- 125.Soto AG, et al. Proc Natl Acad Sci U S A. 2015;112(27):E3600–8. doi: 10.1073/pnas.1508838112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kariya Y, Gu J. PLoS One. 2011;6(11):e27084. doi: 10.1371/journal.pone.0027084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitazume S, et al. J Biol Chem. 2010;285(9):6515–21. doi: 10.1074/jbc.M109.073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lazar IM, et al. Electrophoresis. 2015;36(1):225–37. doi: 10.1002/elps.201400400. [DOI] [PubMed] [Google Scholar]
- 129.Pan S, et al. Mol Cell Proteomics. 2011;10(1):R110 003251. doi: 10.1074/mcp.R110.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leymarie N, et al. Mol Cell Proteomics. 2013;12(10):2935–51. doi: 10.1074/mcp.M113.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wada Y, et al. Glycobiology. 2007;17(4):411–22. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 132.Trinidad JC, et al. Mol Cell Proteomics. 2013;12(12):3474–88. doi: 10.1074/mcp.M113.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Medzihradszky KF, Kaasik K, Chalkley RJ. Mol Cell Proteomics. 2015;14(8):2103–10. doi: 10.1074/mcp.M115.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J, et al. Biochemistry (Mosc) 2015;80(3):260–75. doi: 10.1134/S0006297915030025. [DOI] [PubMed] [Google Scholar]
- 135.Parker BL, et al. J Proteome Res. 2013;12(12):5791–800. doi: 10.1021/pr400783j. [DOI] [PubMed] [Google Scholar]
- 136.Yin X, et al. Mol Cell Proteomics. 2013;12(4):956–78. doi: 10.1074/mcp.M112.024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Treuheit MJ, Costello CE, Kirley TL. J Biol Chem. 1993;268(19):13914–9. [PubMed] [Google Scholar]
- 138.Thaysen-Andersen M, et al. Electrophoresis. 2011;32(24):3536–45. doi: 10.1002/elps.201100294. [DOI] [PubMed] [Google Scholar]
- 139.Zhou L, et al. Carbohydr Res. 2015;402:180–8. doi: 10.1016/j.carres.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 140.Duffin KL, et al. Glycobiology. 1993;3(4):365–80. doi: 10.1093/glycob/3.4.365. [DOI] [PubMed] [Google Scholar]
- 141.Matsushita T, et al. Biochemistry. 2013;52(2):402–14. doi: 10.1021/bi3013142. [DOI] [PubMed] [Google Scholar]
- 142.Wu SL, et al. Mol Cell Proteomics. 2013;12(5):1239–49. doi: 10.1074/mcp.M112.024554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanda M, et al. Mol Cell Proteomics. 2013;12(5):1294–305. doi: 10.1074/mcp.M112.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanda M, et al. Electrophoresis. 2013;34(16):2342–9. doi: 10.1002/elps.201200658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goldman R, Sanda M. Proteomics Clin Appl. 2015;9(1–2):17–32. doi: 10.1002/prca.201400152. [DOI] [PMC free article] [PubMed] [Google Scholar]