Abstract
Human behavioral response timing is highly variable from trial to trial. While it is generally understood that behavioral variability must be due to trial-by-trial variations in brain function, it is still largely unknown which physiological mechanisms govern the timing of neural activity as it travels through networks of neuronal populations, and how variations in the timing of neural activity relate to variations in the timing of behavior. In our study, we submitted recordings from the cortical surface to novel analytic techniques to chart the trajectory of neuronal population activity across the human cortex in single trials, and found joint modulation of the timing of this activity and of consequent behavior by neuronal oscillations in the alpha band (8–12 Hz). Specifically, we established that the onset of population activity tends to occur during the trough of oscillatory activity, and that deviations from this preferred relationship are related to changes in the timing of population activity and the speed of the resulting behavioral response. These results indicate that neuronal activity incurs variable delays as it propagates across neuronal populations, and that the duration of each delay is a function of the instantaneous phase of oscillatory activity. We conclude that the results presented in this paper are strongly supportive of a general model for variability in the effective speed of information transmission in the human brain and for variability in the timing of human behavior.
Keywords: electrocorticography (ECoG), oscillations, reaction time, broadband gamma
1. Introduction
For many behaviors, neuronal populations must communicate across large areas of the cortex. For example, in a visual reaction-time task, the brain must send neural signals through a spatio-temporal trajectory that connects populations in visual cortex to populations in motor cortex. Hence, some trial-by-trial variations in this trajectory must lead to variance in observed reaction times. Since brain anatomy is practically unchanged on the time scale of seconds, these variations must be due to trial-by-trial differences in function. Neuronal field potential oscillations are prevalent throughout the brain, exhibit rapid dynamics, and are thought to play a role in the long-range coordination of neuronal population activity (Buzsáki and Draguhn, 2004). Their phase has been shown to modulate the amplitude of neuronal population-level activity (Canolty et al., 2006; Voytek et al., 2010; Miller et al., 2012; van der Meij et al., 2012; Voytek et al., 2013) and structure the timing of neuronal firing in visual and motor areas of the brain (Fries et al. (2001b,a) and Haegens et al. (2011); Reimer and Hatsopoulos (2010), respectively), and oscillatory activity is thought to be involved in the gating of neural signal transmission (Hanslmayr et al., 2013; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Fries, 2005; Engel et al., 2001; Schalk, 2015). Furthermore, oscillatory phase in primary motor and visual areas has been shown to alter the timing of behavior (Hanslmayr et al., 2013; Haegens et al., 2011; van Dijk et al., 2008; Dustman and Beck, 1965; Lansing et al., 1959; Bates, 1951). However, it was unknown whether the phase of oscillations modulates the speed of signal propagation across widely distributed populations of neurons that connect a stimulus to a behavior.
To answer this question, we submitted recordings from the cortical surface (electrocorticography (ECoG)) to novel analytic techniques to chart the trajectory of task-related neuronal population activity across the human cortex in single trials. Given this information, we then examined whether the timing of that population activity, and also the timing of consequent behavior, was modulated by the phase of low-frequency oscillations. Our results demonstrate that this was indeed the case for oscillations in the alpha band in all subjects and across all task-related cortical regions. These results support a proposal for a general mechanism that is responsible for producing timing variability in behavior.
2. Methods
2.1. Subjects
Four human subjects (two women: subjects A and B, and two men: subjects C and D) at Albany Medical College (Albany, NY) took part in this study. The subjects were patients with intractable epilepsy who underwent temporary placement of subdural electrode arrays to localize seizure foci prior to surgical resection. The subjects’ clinical profiles are summarized in Table 1. They gave informed consent for the study, which was approved by the Institutional Review Board of Albany Medical College and the Human Research Protections Office of the U.S. Army Medical Research and Materiel Command.
Table 1.
Clinical profiles of the subjects that participated in the study. All of the subjects had normal cognitive capacity and were functionally independent. Language lateralization (LL) was based on the Wada test.
| Subject | Age | Sex | Handedness | LL | Seizure Focus | Grid Locations | # of Elec. |
|---|---|---|---|---|---|---|---|
| A | 29 | F | R | L | Left temporal | Left fronto-parietal | 64 |
| Left temporal | 23 | ||||||
| Left temporal pole | 3 | ||||||
| Left occipital | 6 | ||||||
| B | 26 | F | R | L | Left temporal | Left frontal | 64 |
| Left temporal | 35 | ||||||
| Left temporal pole | 4 | ||||||
| Left occipital | 6 | ||||||
| C | 56 | M | R | L | Left temporal | Left frontal | 35 |
| Left frontal | 56 | ||||||
| Left occipital | 4 | ||||||
| D | 25 | M | R | L | Right frontal | Right frontal | 64 |
| Right posterior frontal | 20 | ||||||
| Right orbito-frontal | 4 | ||||||
| Right fronto-parietal | 4 | ||||||
| Right anterior mesial | 4 | ||||||
| Right posterior mesial | 4 |
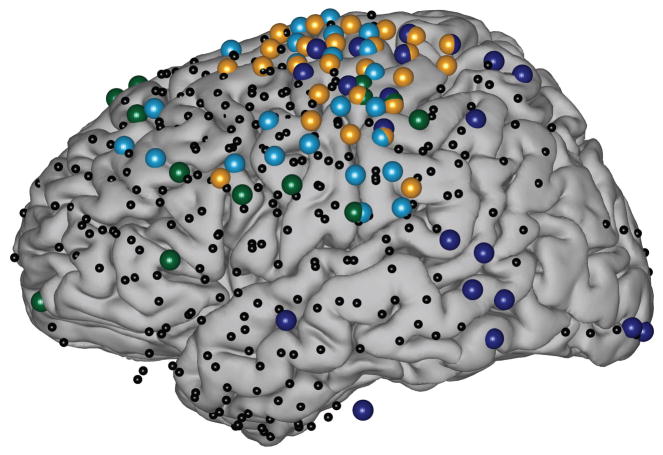
Each subject had several subdural electrode grids/strips (Ad-Tech Medical, Racine, WI) implanted over one hemisphere of the brain. The electrodes were made of platinum-iridium, had a diameter of 3 mm (2.3 mm exposed) and were spaced at a center-to-center inter-electrode distance of 10 mm. The subjects had a total of 97–109 electrodes implanted. The grids were implanted for about one week and covered large areas of frontal, parietal and temporal brain regions, with portions of occipital cortex covered as well (Fig. 1). Subjects A–C had grids implanted over the left hemisphere. Subject D had grids implanted over the right hemisphere. Following placement of the subdural grids, each subject had postoperative anterior-posterior and lateral radiographs, as well as computed tomography (CT) scans to verify grid location.
Figure 1. Electrode coverage and task-related locations in all four subjects.
Location of electrodes from all subjects, projected onto the common MNI template brain. Small black dots show implanted electrode locations. Large colored dots show task-related locations, color coded by subject (dark blue = Subject A; cyan = Subject B; gold = Subject C; dark green = Subject D). For visualization purposes in this figure only, we projected Subject D’s right-hemisphere electrodes onto the left hemisphere of the template brain.
We constructed three-dimensional cortical models of individual subjects using pre-operative structural magnetic resonance imaging (MRI). MRI images were then co-registered with postoperative CT images using Curry software (Compumedics, Charlotte, NC) and transformed into the Talairach coordinate system (Talairach and Tournoux, 1988). Finally, we projected electrode locations onto subject-specific models or onto the three-dimensional cortical template provided by the Montreal Neurological Institute (MNI brain model; http://www.bic.mni.mcgill.ca1) using our NeuralAct toolbox (Kubanek and Schalk, 2014) and MATLAB 2012b (Mathworks, Natick, MA).
2.2. Data collection
We recorded ECoG signals from the four subjects at the bedside using the general-purpose BCI2000 software (Schalk et al., 2004; Schalk and Mellinger, 2010), which interfaced with eight 16-channel g.USBamp biosignal acquisition devices (g.tec, Graz, Austria). A splitter box routed signals simultaneously to the clinical monitoring system and to the BCI2000/g.USBamp system, which allowed for continuous clinical monitoring. Thus, at no time was clinical care or clinical data collection affected by our research. The signals were amplified, digitized at 1200 Hz, and stored by BCI2000. Electrode contacts distant from epileptic foci and areas of interest were used for reference and ground. The recordings were visually inspected offline; channels that did not contain clear ECoG signals (e.g., ground/reference channels, channels with broken connections, presence of environmental artifacts, or interictal activity) were removed from subsequent analyses. This left 67–96 remaining channels that were submitted to further analyses. In addition to recording brain activity, we also recorded the subjects’ behavior using a push button and an eye tracker (Tobii T60, Tobii Technologies). Data collection from the biosignal acquisition devices and behavioral variables, as well as stimulus presentation and control of the experimental paradigm was accomplished simultaneously using BCI2000.
2.3. Task
The behavioral paradigm used in this study was a modified Posner cueing task (Posner, 1980; Posner and Petersen, 1990). This task was originally designed to study visual-spatial attention and encompassed multiple stages and different experimental conditions. The description below describes only those stages of the task that are relevant to the present study. Please see Supplementary Text for additional details and (Gunduz et al., 2011, 2012) for a full description.
In the present study, we were interested in the brain processes that connect the perception of a visual stimulus to an intended behavioral response (a button press). Accordingly, we focused solely on the 1500 ms periods prior to and following the onset of a visual stimulus (“baseline” and “task” periods, respectively). Subjects maintained gaze fixation on a central fixation cross throughout the entire experimental run. All trials began with the presentation of a visual cue. After a random interval (between 3.5 and 4.5 seconds), a visual stimulus prompted the subject to respond with a button press as quickly as possible. All subjects used the hand contralateral to the grid implant to execute the button press. Recordings consisted of 7–13 blocks of 10 to 30 trials each (total of 134–214 trials for the different subjects).
2.4. Data Preprocessing
Our primary interest was in determining the location and time of task-related population-level activity. Thus, we extracted the amplitude envelopes of ECoG broadband activity, which has been shown to correspond closely to the average firing rate of populations of neurons underneath the electrode (Manning et al., 2009; Whittingstall and Logothetis, 2009; Ray and Maunsell, 2011; Miller et al., 2009; Miller, 2010). To do this, we bandpass-filtered signals in the broadband gamma band (70 to 170 Hz; 3rd-order Butterworth filter; zero phase lag, MATLAB filtfilt function) after re-referencing signals to a common average reference (CAR; Schalk et al. (2007); Miller et al. (2012)), and extracted the amplitude envelope of the bandpassed signals using the Hilbert transform. We also bandpass-filtered the ECoG signals to extract oscillatory activity in the delta (1–3 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta1 (12–18 Hz), and beta2 (18–30 Hz) bands. We then low-pass filtered and downsampled all signals to 400 Hz with the MATLAB resample function. Finally, we normalized signals at each channel to the baseline period by subtracting the average baseline amplitude derived from all trials and dividing by the baseline amplitude’s standard deviation.
To generate time-frequency plots, we de-trended the raw ECoG signals using a high pass filter at 0.1 Hz and re-referenced to a common average. We then convolved the results with complex Morlet wavelets (5-cycle wavelets with 2-second kernel widths) to extract frequency components in the 2–80 Hz range (2 Hz steps) and their amplitude envelopes. Trials were aligned to the onset of the stimulus, normalized relative to the baseline period as described above, and averaged across trials to give one time-frequency plot for each electrode location.
2.5. Electrode selection
We determined which electrode locations responded during the task period using the signal-to-noise (SNR) method described in Schalk et al. (2007). Specifically, we determined, at each electrode the total variance in the broadband gamma amplitude envelope during the task period across all trials. For the same data, we also determined the average variance within each of thirty 50 ms bins. To obtain SNR ratios, we then divided the total variance from all time series in all trials by the average of the thirty variances from the thirty 50-ms bins. Hence, SNR values very close to 1 are indicative of a location whose ECoG broadband activity was not modulated by the task, whereas larger SNR values are indicative of modulation by the task. To determine the statistical significance of the SNR values, we applied a randomization test. In this test, we scrambled the signal by randomly shuffling all samples in the time course of the broadband signal 10,000 times and obtained 10,000 corresponding SNR values. We then calculated a p-value for the true SNR value using the cumulative distribution function. Channels that did not respond to the task (i.e., had p-values >0.001) were excluded from further analyses. This procedure resulted in 42, 50, 33, and 23 locations for subjects A–D, respectively.
2.6. Onset detection and electrode selection pruning
To determine the time of activity onset at each of the task-related locations and in each trial, we identified the first time point in the task period whose broadband amplitude exceeded a channel-specific amplitude threshold. We determined each channel’s threshold by applying different amplitude thresholds (2–6 z-scores, 0.1 z-score increments) and then selecting the amplitude threshold that maximized the difference in the number of detections found in the task period and the number of detections found in the baseline period. We excluded from further analysis any channels that did not exhibit a statistically significant difference (“spread”) in the number of detections between the task period and the baseline period. To do this, we randomly shuffled the labels (“task” and “baseline”) associated with each detection in a location 1000 times to create surrogate distributions of the difference in the number of hits detected in task vs. baseline, modeled the resulting distributions using a Gaussian distribution, and rejected channels whose true spread was not statistically different from the surrogate distribution (p > 0.01). This second stage of the electrode localization procedure resulted in 21, 25, 26, and 13 locations for subjects A–D, respectively, that were distributed across wide areas of occipital, temporal, frontal, and parietal cortices (large colored dots in Figure 1). In contrast, a control analysis that submitted data from catch trials (in which the visual stimulus never occurred) to the same analysis procedure did not identify any task-related locations in any of the four subjects.
Finally, for each of these task-related locations and in each trial, we identified the precise temporal onset of population activity by determining whether or not and when broadband activity exceeded the channel-specific pre-stimulus baseline (i.e., exceeded the channel-specific threshold determined in the optimization procedure described above). This procedure allowed us to determine with unprecedented spatial and temporal precision exactly where and when neuronal population activity occurred in each trial (i.e., the spatiotemporal trajectory of neural activity).
3. Results and Discussion
3.1. The trajectory of neuronal activity across the cortex can be identified in single trials
The procedures described above identified the spatial and temporal location of the onset of population-level activity in single trials. Additional analyses clearly established that this procedure identified physiologically meaningful, task-related events rather than artifacts (see Supplementary Text). Figure 2 and Figure 3 give examples of broadband gamma activity in different locations, its relationship to modulatory low-frequency activity, and detected onset times. Specifically, to illustrate examples of broadband gamma activity, Figure 2 gives exemplary time courses of that activity at five different locations in occipital, temporal, parietal, and frontal locations. The timing of the onset of activity is clearly different in different locations, and indicates a temporal progression from occipital, to temporal, to frontal and finally parietal cortices. Figure 3 shows that, across the different lobes, the timing of the onset of population-level activity appears to be progressively related to the behavior.
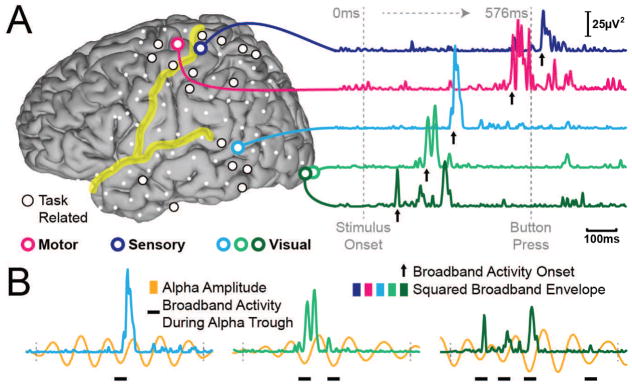
Figure 2. Exemplary spatiotemporal trajectory of task-related neuronal population activity in one single trial.
A) Task-related cortical locations from Subject A (left), and the time course of neuronal population activity in exemplary locations (right). Increases in activity at each location are brief. B) Population-level activity occurs during the trough of alpha oscillations (8–12 Hz).
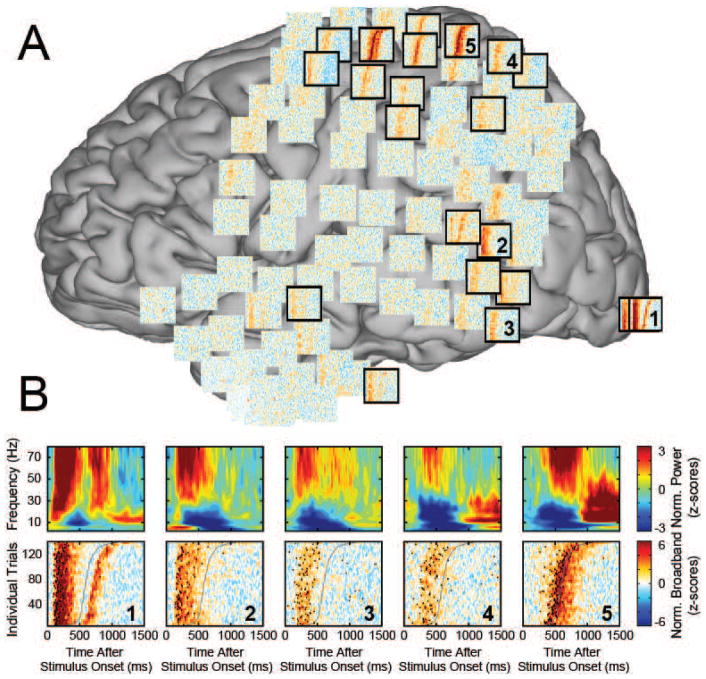
Figure 3. Exemplary time-frequency plots and single-trial broadband gamma plots.
A. Single-trial activity plots for Subject A. Activity in the broadband gamma range (colors) is shown from 0–1500 ms after the onset of the visual stimulus (x-axes). Individual trials (y-axes) are sorted by behavioral response time. Each plot represents data from one electrode location, and is projected onto the corresponding channels’ coordinates on the MNI template brain. Task-related electrode locations identified by our analyses are framed in thick black borders. The top panel in B shows time-frequency plots (averaged across all trials) illustrating that amplitudes at different low frequencies are modulated by the task including the alpha (8–12 Hz) band. The bottom panel gives single-trial broadband activity, blurred with a Gaussian kernel for visualization purposes. Single-trial activity onset times are shown as black circles (i.e., each plot is the same as in A, but with onset times shown on top of broadband activity).
We were surprised at the brevity of the average broadband activation (Figure 4). It is possible that this result could be explained by the trivial finding that we simply detected external physiologic/non-physiologic artifacts. Our control analyses, which are described in full detail in the Supplementary Text, clearly demonstrate that this is not the case. Thus we conclude that the average responses derived by taking inter-trial timing variance into account (and report and show in Figs. 2 and 4), are on average much shorter than those typically reported in the literature, which do not account for that inter-trial variance. This discrepancy is conveniently explained by the large inter-trial latency variations at each location (mean standard deviation of onset latency across all locations: 324ms +/− 74ms on average). In sum, together with the control analyses described in in the Supplementary Text, the results presented here demonstrate that it is possible to accurately identify the locations and onset of population-level activity in single trials and that this activity may be much briefer than previously reported.
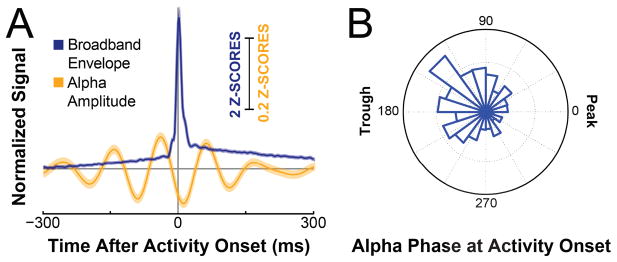
Figure 4.
The onset time of neuronal population activity tends to occur during the falling phase of the trough of alpha oscillations. A. Traces show normalized time courses of the amplitude of neuronal population activity (blue) and the amplitude of activity in the alpha band (orange), averaged across all trials, all task-related locations and across all subjects, and time-locked to the onset of population activity in each corresponding trial (traces show mean ±standard error). B. Polar histogram of alpha phases at the time of detected activity onset from all trials, all locations and all subjects. The distribution has a circular mean of 160.5 degrees, and passed a Rayleigh test for non-uniformity in circular data (p ≪0.01). The counts in the histogram plot range from 400 onsets (origin) to 600 onsets (outer circle).
3.2. Alpha oscillations modulate the onset times of neuronal population activity
We then proceeded to further validating the physiological relevance of the onset times identi-fied by our procedure by establishing their relationship at individual locations with the phase of oscillatory activity.
An increasing number of reports have described a relationship between the phase of low-frequency oscillations and the amplitude of neuronal population activity (phase-amplitude coupling (“PAC”)) (Canolty et al., 2006; Voytek et al., 2010; Miller et al., 2012; van der Meij et al., 2012; Voytek et al., 2013; Haegens et al., 2011; Reimer and Hatsopoulos, 2010). In tasks with visual input similar to ours, oscillatory modulation by activity in the alpha band has been shown to be particularly salient (Voytek et al., 2010; Ward, 2003). We also found task-related modulation in and around the alpha band to be a common feature across task-related locations (Figure 3). Furthermore, our quantitative analyses confirmed the expectation that the onset of population activity occurred preferentially in the trough of alpha oscillations (binomial test for difference in number of onsets between alpha trough and alpha peak; p =5.0x10−9). More precisely, broadband activity onsets occurred with a circular mean of 160.5 degrees (p ≪0.01, Rayleigh test for non-uniformity of circular data; (Figure 2,4)).1
More importantly, our analyses established that onset times preferentially occurred during the falling slope of the trough (binomial test, p = 6x10−12; Figure 4). This effect was visible in each of the four subjects (Suppl. Fig. S5) and was present for each of three subsets of locations representing early-, middle-, and late-responding components of the identified task-related networks (terciles grouped by mean onset latency; 1st tercile p = 0.002, 2nd tercile p = 5x10−6, and 3rd ter-cile p = 0.016 for trough vs. peak; 1st tercile p = 0.001, 2nd tercile p = 2x10−7, and 3rd tercile p = 0.0007 for falling vs. rising slope of trough).
3.3. Modulation by oscillatory activity explains latency variations in neuronal communication and behavior
Supported by this refined relationship between oscillatory phase and the onset of population-level activity, we arrived at the central conceptual contribution of this paper. This contribution is described in the following paragraphs and describes how oscillatory phase can explain latency variations in neuronal communication and resulting behavior.
In this context, it is important to recognize that information about visual stimuli is propagated across populations of neurons through series of population-level spike volleys (Thorpe, 1990; Meister and Berry, 1999; Van Rullen et al., 1998; Thorpe et al., 1996, 2001; Gautrais and Thorpe, 1998; Reimer and Hatsopoulos, 2010; Takahashi et al., 2015). Repeatedly sending such volleys may ensure that information will be processed by downstream regions. This should prove particularly important if receiving populations are unpredictably and intermittently inhibited by oscillatory activity (Klimesch et al., 2007). Furthermore, it is becoming increasingly clear that communication between populations depends on cortical excitability at the receiving population (Fries, 2005; Jensen and Mazaheri, 2010; Schalk, 2015) and that cortical excitability is modulated by low-frequency oscillations (Haegens et al., 2011; Schalk, 2015).
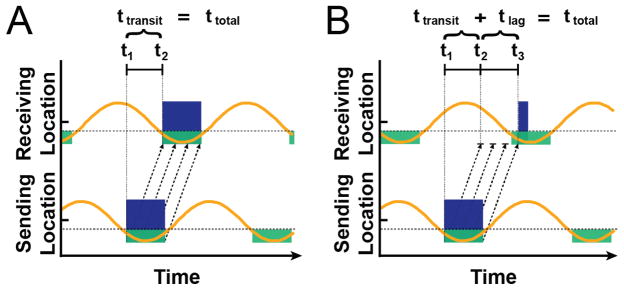
Given these circumstances, we can differentiate two distinct cases of neuronal communication (Figure 5). In the first case, shown in Figure 5a, the phase relationship of oscillatory activity at the sending and receiving neuronal population is such that the first of a series of spike volleys will immediately excite neuronal populations at the receiving location. Thus, the total time between the beginning of neuronal excitation at the sending and receiving site (ttotal) equals the time it takes to transmit a spike volley from the sending to the receiving site (ttransit). In contrast, in the second case, shown in Figure 5b, the phase relationship of oscillatory activity at the sending and receiving sites is such that not the first but only a subsequent volley in a series of spike volleys will result in cortical excitation. Given this model, excitation of neuronal populations at the receiving location may incur a variable lag (tlag) of up to several tens of milliseconds (i.e., the duration of approximately half an oscillatory cycle) after the arrival of the initial spike volley. Under the assumption that the neural trajectory that connects the sensory stimulus to its behavioral consequence contains successive communication between several populations, these variable lags at individual locations will accumulate and thus contribute to the observed behavioral response latency.
Figure 5.
Alpha oscillations may modulate the timing of communication between neuronal populations. As alpha amplitude (orange lines) decreases past a threshold voltage (dotted gray lines), neuronal populations may process and transmit information (i.e., permissive window, green boxes). When one population attempts to signal another, the time it takes for a series of spike volleys (black dotted arrows) to result in excitation of population activity (blue boxes) depends on the phase of the receiving population’s oscillatory activity. In A, the first spike volley immediately excites the receiving population. In B, most spike volleys do not arrive during a permissive window, delaying excitation.
Depending on the task, variations in behavioral response latency can be quite substantial, up to several hundred milliseconds (Teichner and Krebs, 1972; Fox et al., 2007; Jensen, 1992). The hypothesized mechanism outlined above suggests that the relatively long periods of local population inhibition induced by oscillatory activity may readily account for such large variations. Furthermore, important alternative explanations appear to be rather unlikely. First, variations in behavioral response latency resulting from variance in synaptic transmission times should be much smaller than those observed since synaptic transmission timing variance has been estimated to be extremely small (≪ 1 ms; Sabatini and Regehr (1996)), and only relatively few synaptic connections separate visual input from motor output (Thorpe et al., 1996; Delorme and Thorpe, 2001; Reimer and Hatsopoulos, 2010; Takahashi et al., 2015; Miller and Vogt, 1984). Second, our results suggest that the neural trajectory traverses the same locations in both fast and slow trials: we determined for each location whether the number of detected onsets was different for the 33% of the trials with the fastest vs. the slowest reaction times, respectively. The results showed that of the 13–26 locations across the different subjects, none had a different number of detected onsets in the fastest compared to the slowest trials (binomial test, p> 0.05, FDR-corrected for multiple comparisons). Moreover, additional analyses showed that, when we randomly selected two 33% subsets of the trials and performed the same statistics, we were still unable to identify a single electrode whose number of onsets differed across the two subsets. In a complementary analysis, when we summed the number of detections in fast versus slow trials across the task-related network within subjects, we found no difference in the number of activity onsets in the slowest 50% versus the fastest 50% of all trials (paired t-tests, p = 0.69, 0.54, 0.31, and 0.63 for Subjects A-D, respectively; Suppl. Fig. S6). Taken together, these results suggest that variance in reaction times was not due to the recruitment of different cortical networks in fast versus slow trials. Finally, we found no difference in broadband power for the fastest 50% versus the slowest 50% of trials in the 50 ms period after activity onset when summed across detections in the task-related network within each subject (Wilcoxon Rank Sum test, p > 0.05 after Bonferroni correction for multiple comparisons; Suppl. Fig. S7). In sum, these findings practically exclude the possibility that the observed large variations in timing can be accounted for by either synaptic properties, signal transmission through different neuronal populations, or effects of general arousal.
3.4. The phase of alpha (8–12 Hz) oscillations modulates latency variations in neuronal communication and behavior
The model and resulting hypothesis outlined above prompt two predictions. The experimental testing of these predictions forms the main experimental contribution of this article.
The first prediction is that, in faster trials, the first in a series of spike volleys should tend to arrive at a particular receiving location at any time during the trough of oscillatory activity at that site, since they will immediately result in excitation of neuronal populations. Conversely, in slower trials, initial spike volleys should tend to arrive during the peak of that oscillatory activity, and thus only result in excitation of neuronal populations when the subsequent volley arrives during the oscillatory trough. Hence, onsets of population-level activity should be increasingly biased toward the falling slope of the trough for slow trials compared to fast trials. This effect should be strongest when oscillatory power is high, i.e., when the phase of oscillatory activity has the strongest influence on cortical excitability (Schalk, 2015). Indeed, this was true for trials with high oscillatory power2 in the alpha frequency band (p =0.027, Fisher’s exact test, fastest/slowest 50% of trials), but not for other oscillatory frequency bands (delta (1–3 Hz; p = 0.18), theta (4–8 Hz; p = 0.11), beta1 (12–18 Hz; p = 0.74), or beta2 (18–30 Hz; p = 0.35); Supplementary Table S1) or for trials with low oscillatory power (Supplementary Table S2).
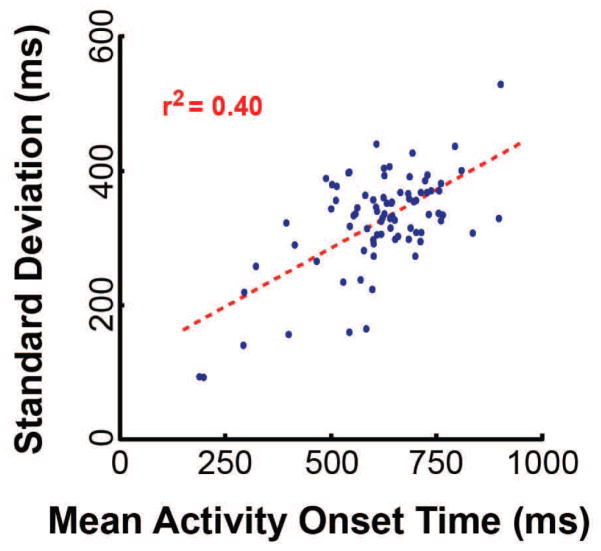
The second prediction is that temporal variance should accumulate across subsequent nodes in the network, i.e., variance in onset times should increase as a function of mean onset latency. This phenomenon is widely recognized, yet not often formally addressed in the literature (but see DiCarlo and Maunsell (2005) and Banerjee et al. (2010)). Our results are consistent with this second prediction as well (r2 = 0.40, p =1.9x10−10, (Figure 6)). These two results strongly support the hypothesis that human reaction time is modulated by the phase relationships of oscillatory activity across communicating locations.
Figure 6.
Variance in neural activity onset times increases as a function of mean onset time. Each dot corresponds to an electrode location. Data are from all four subjects. The dotted line shows the linear trend derived using least squares regression. Pearson’s coefficient of determination, r2, is shown in red inlaid text.
An important point to consider here is that we found evidence for the effect of phase only for oscillations in the alpha band and not for other frequency bands. It is possible that this points to a specific role of alpha in cortical communication. It is also possible that oscillations in the alpha band happen to be more frequent in our task than oscillations in other frequency bands, and thus, our ability to detect an influence of phase on performance in the alpha frequency band simply was greater than for other frequency bands. In this scenario, oscillations in other frequency bands may have similar effects on neuronal communication and its resultant behavior.
3.5. Summary
In this study, we charted the trajectory of neuronal population activity that implements a sen-sorimotor function across large areas of the brain and in single trials. This novel technical ability enabled us to determine how low-frequency oscillations govern the timing of the entire trajectory and the timing of consequent behavior. We found that the phase of neuronal oscillations in the alpha (8–12 Hz) band, but not in other frequency bands, modulated the timing of both. Across all locations, the onset of neuronal population activity occurred preferentially in the trough of alpha oscillations, and deviations from this preferred relationship were related to changes in the timing of the behavioral response. Specifically, for slower response times, the onset of population activity tended to occur more often during the falling phase of the trough compared to the faster response times. Together with the finding that variance in onset times increased as a function of a location’s mean onset latency, these results suggest that reaction time variability results from suboptimal phase synchronization of oscillations across distant populations of neurons.
3.6. Relationship of our results to current theories of oscillatory function
Current theories of information transmission in the brain, such as the “communication-through-coherence” (CTC) hypothesis (Fries, 2005) and the “gating-by-inhibition” (GBI) hypothesis (Jensen and Mazaheri, 2010) suggest that the brain may facilitate or inhibit neuronal communication by modulating low frequency oscillatory activity (Fries, 2005; Buzsáki and Draguhn, 2004; Jensen and Mazaheri, 2010). CTC suggests that the brain plays an active role in aligning the phases of the oscillations governing a sending and a receiving population to facilitate communication. Thus, matched/mismatched phase relationships are proposed to reflect populations’ ability/impaired ability to communicate, respectively. GBI suggests that disruptive, rhythmic volleys of action potentials at low frequencies (assessed by measuring oscillatory power) inhibit the function of a population of neurons. Thus, low/high alpha power reflects a neuronal population’s ability/impaired ability to communicate with other populations, respectively.
The results presented here support elements of both views, and are consistent with a recently introduced general model of cortical communication, the “function-through-biased oscillations” (FBO) hypothesis (Schalk, 2015). Like CTC, FBO suggests that oscillatory phase modulates communication. Like GBI, FBO suggests that oscillatory power modulates cortical communication as well3. Furthermore, FBO outlines why the modulatory effect of phase observed in our study occurred preferentially for oscillations with high power. Specifically, when oscillatory power is low, neuronal populations are generally excitable, and oscillatory phase has little impact on excitability. In contrast, when oscillatory power is high, neuronal populations can only process and exchange information during permissive phases of the oscillation. This provides an interesting bridge between the CTC and GBI, allowing roles for both phase and power — and for interactions between the two — in modulating signal propagation across populations of neurons.
3.7. Future work
Our results strongly support the hypothesis that oscillatory phase modulates the speed of information transmission in the brain and of resulting behavior. More formally, it suggests that the delay incurred in communication between neuronal populations is a function of oscillatory phase. Testing this hypothesis directly requires knowledge of which of the identified task-related neuronal populations are directly functionally connected.
Our results suggest that existing methods to establish such functional connectivity are not ideally suited to perform this determination. First, as shown in Figs. 2 and 4, broadband gamma activity is highly non-stationary, but stationarity is an important assumption of many methods to estimate functional connectivity such as Granger causality (Granger, 1969). Second, the results shown in Fig. 6 suggest that different neuronal populations have variable timing relative to each other. However, most traditional techniques, including Granger causality, assume that brain signals at different locations have a constant timing relationship (Friston, 2009). Thus, future work is needed to develop techniques that can identify which neuronal populations are functionally connected despite these physiological realities.
4. Conclusion
In summary, our study established the neural trajectory of task-related population-level activity during a simple sensorimotor task in individual trials, and revealed an important role for the phase of alpha oscillations in modulating the timing of that trajectory and of resulting behavior. The work presented in this paper may provide the basis for a general model of variability in the effective speed of information transmission in the human brain and for variability in the timing of human behavior. Our results appear to point to an interesting bridge between theories of oscillatory function by tying together the effects of oscillatory phase and power in modulating cortical signal propagation, as recently proposed and formalized in (Schalk, 2015). More detailed investigations on these and related topics will require novel methods of assessing functional connectivity.
Highlights.
The trajectory of neuronal population activity across the human cortex can be identified in single trials
The phase of high power alpha oscillations modulates latency variations in neuronal communication and behavior
This link between the timing of cortical activity and the timing of consequent behavior occurs only when oscillatory activity has high power
These findings are consistent with a new theory of dynamic brain function, the “Function-through-Biased Oscillations” (FBO) hypothesis.
Acknowledgments
The authors thank Adriana de Pesters for crucial discussion and commentary on the manuscript, and Marcia Sanders for invaluable assistance in editing the manuscript. This work was supported by the NIH (EB00856, EB006356 and EB018783), the US Army Research Office (W911NF-08-1-0216, W911NF-12-1-0109, W911NF-14-1-0440) and Fondazione Neurone.
Footnotes
Statistical tests were performed across all task-related locations from all four subjects.
We tested all onset detection times whose corresponding low-frequency power was higher than the median power in that frequency band across all detections at that location
FBO also explains why oscillatory phase and power, which are mathematically independent measurements, are each related to cortical excitability.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
W. G. Coon, Email: wcoon@neurotechcenter.org.
A. Gunduz, Email: agunduz@bme.ufl.edu.
P. Brunner, Email: pbrunner@neurotechenter.org.
A. L. Ritaccio, Email: ritacca@mail.amc.edu.
B. Pesaran, Email: bijan@nyu.edu.
G. Schalk, Email: gschalk@neurotechcenter.org.
References
- Banerjee A, Dean HL, Pesaran B. A likelihood method for computing selection times in spiking and local field potential activity. Journal of neurophysiology. 2010 Dec;104(6):3705–3720. doi: 10.1152/jn.00036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JAV. Electrical activity of the cortex accompanying movement. The Journal of physiology. 1951 Apr;113(2–3):240–257. doi: 10.1113/jphysiol.1951.sp004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006 Sep;313(5793):1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Thorpe SJ. Face identification using one spike per neuron: resistance to image degradations. Neural Networks. 2001 Jul-Sep;14(6–7):795–803. doi: 10.1016/s0893-6080(01)00049-1. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Maunsell JHR. Using neuronal latency to determine sensory–motor processing pathways in reaction time tasks. Journal of Neurophysiology. 2005;93(5):2974–2986. doi: 10.1152/jn.00508.2004. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Beck EC. Phase of alpha brain waves, reaction time and visually evoked potentials. Electroen Clin Neuro. 1965;18(5):433– 440. doi: 10.1016/0013-4694(65)90123-9. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001 Oct;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005 Oct;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nature neuroscience. 2001a Feb;4(2):194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science (New York, NY) 2001b Feb;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009 Feb;7(2):e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautrais J, Thorpe S. Rate coding versus temporal order coding: a theoretical approach. Bio Systems. 1998 Sep-Dec;48(1–3):57–65. doi: 10.1016/s0303-2647(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Granger C. Investigating causal relationships by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
- Gunduz A, Brunner P, Daitch A, Leuthardt EC, Ritaccio AL, Pesaran B, Schalk G. Neural correlates of visual–spatial attention in electrocorticographic signals in humans. Front Hum Neurosci. 2011;5:89. doi: 10.3389/fnhum.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz A, Brunner P, Daitch A, Leuthardt EC, Ritaccio AL, Pesaran B, Schalk G. Decoding covert spatial attention using electrocorticographic (ecog) signals in humans. NeuroImage. 2012 May;60(4):2285–2293. doi: 10.1016/j.neuroimage.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences. 2011;108(48):19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Dalal SS, Greenlee MW. Prestimulus oscillatory phase at 7 hz gates cortical information flow and visual perception. Current Biology. 2013;23(22):2273– 2278. doi: 10.1016/j.cub.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The importance of intraindividual variation in reaction time. Pers Indiv Diff. 1992;13:869–881. [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010 Nov;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. Eeg alpha oscillations: the inhibition–timing hypothesis. Brain Research Reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kubanek J, Schalk G. NeuralAct: a tool to visualize electrocortical (ECoG) activity on a three-dimensional model of the cortex. Neuroinformatics. 2014:1–8. doi: 10.1007/s12021-014-9252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing RW, Schwartz E, Lindsley DB. Reaction time and eeg activation under alerted and nonalerted conditions. Journal of experimental psychology. 1959 Jul;58(1):1–7. doi: 10.1037/h0041016. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Berry MJ. The neural code of the retina. Neuron. 1999 Mar;22(3):435–450. doi: 10.1016/s0896-6273(00)80700-x. [DOI] [PubMed] [Google Scholar]
- Miller KJ. Broadband spectral change: evidence for a macroscale correlate of population firing rate? The Journal of Neuroscience. 2010;30(19):6477–6479. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, Ojemann JG, Fetz EE. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Computational Biology. 2012;8(9):e1002655. doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, Den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;5(12):e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 1984 Jun;226(2):184–202. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980 Feb;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biology. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Hatsopoulos NG. Periodicity and evoked responses in motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Aug;30(34):11506–11515. doi: 10.1523/JNEUROSCI.5947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996 Nov;384(6605):170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Schalk G. A general framework for dynamic cortical function: the function-through-biased-oscillations (fbo) hypothesis. Frontiers in Human Neuroscience. 2015;9(352) doi: 10.3389/fnhum.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk G, Kubánek J, Miller KJ, Anderson NR, Leuthardt EC, Ojemann JG, Limbrick D, Moran D, Gerhardt LA, Wolpaw JR. Decoding two-dimensional movement trajectories using electrocortico-graphic signals in humans. J Neural Eng. 2007 Sep;4(3):264–275. doi: 10.1088/1741-2560/4/3/012. [DOI] [PubMed] [Google Scholar]
- Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004 Jun;51(6):1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Schalk G, Mellinger J. A Practical Guide to Brain–Computer Interfacing with BCI2000. Springer; New York: 2010. [Google Scholar]
- Takahashi K, Kim S, Coleman TP, Brown KA, Suminski AJ, Best MD, Hatsopoulos NG. Large-scale spatiotemporal spike patterning consistent with wave propagation in motor cortex. Nature communications. 2015 May;6:7169. doi: 10.1038/ncomms8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Sterotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Teichner WH, Krebs MJ. Laws of the simple visual reaction time. Psychol Rev. 1972;79(4):344–358. doi: 10.1037/h0032946. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Delorme A, Van Rullen R. Spike-based strategies for rapid processing. Neural networks : the official journal of the International Neural Network Society. 2001 Jul-Sep;14(6–7):715–725. doi: 10.1016/s0893-6080(01)00083-1. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996 Jun;381(6582):520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ. Spike arrival times: A highly efficient coding scheme for neural networks. Parallel processing in neural systems. 1990:91–94. [Google Scholar]
- van der Meij R, Kahana M, Maris E. Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse. The Journal of neuroscience : the official journal of the Society for Neuro-science. 2012 Jan;32(1):111–123. doi: 10.1523/JNEUROSCI.4816-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008 Feb;28(8):1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rullen R, Gautrais J, Delorme A, Thorpe S. Face processing using one spike per neurone. Bio Systems. 1998 Sep-Dec;48(1–3):229–239. doi: 10.1016/s0303-2647(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Voytek B, Canolty RT, Shestyuk A, Crone N, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4(191) doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, D’Esposito M, Crone N, Knight RT. A method for event-related phase/amplitude coupling. NeuroImage. 2013 Jan;64:416–424. doi: 10.1016/j.neuroimage.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences. 2003;7(12):553– 559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-band coupling in surface eeg reflects spiking activity in monkey visual cortex. Neuron. 2009;64(2):281–289. doi: 10.1016/j.neuron.2009.08.016. [DOI] [PubMed] [Google Scholar]