Abstract
Background
Little is known about specific mood symptoms that may confer risk for suicidal ideation (SI) among patients with bipolar disorder. We evaluated prospectively whether particular symptoms of depression and mania precede the onset or worsening of SI, among adults with or without a history of a suicide attempt.
Methods
We examined prospective data from a large (N=2,741) cohort of patients participating in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). We evaluated history of suicide attempts at baseline, and symptoms of depression and mania at baseline and follow-up visits. Hierarchical linear modeling tested whether specific mood symptoms predicted subsequent levels of SI, and whether the strength of the associations differed based on suicide attempt history, after accounting for the influence of other mood symptoms and current SI.
Results
Beyond overall current depression and mania symptom severity, baseline SI, and illness characteristics, several mood symptoms, including guilt, reduced self-esteem, psychomotor retardation and agitation, increases in appetite, and distractibility predicted more severe levels of subsequent SI. Problems with concentration, distraction, sleep loss and decreased need for sleep predicted subsequent SI more strongly among individuals with a suicide attempt history.
Conclusions
Several specific mood symptoms may confer risk for the onset or worsening of SI among treatment-seeking patients with bipolar disorder. Individuals with a previous suicide attempt may be at greater risk in part due to greater reactivity to certain mood symptoms in the form of SI. However, overall, effect sizes were small, suggesting the need to identify additional proximal predictors of SI.
Keywords: Bipolar disorder, depression, mood disorders, suicide/self-harm, cognition
Introduction
Bipolar disorder (BD) is associated with impairment in many areas of functioning1-4. In addition, individuals with BD experience high rates of suicide1, with 25 to 50% of individuals with bipolar disorder having a lifetime history of a suicide attempt5-9, twice the rate of individuals with unipolar depression5,10. However, the clinical features that lead to suicidal ideation (SI) in BD remain understudied9, a notable gap given that SI is a strong predictor of suicide attempts7. A better understanding of specific symptom predictors of SI could allow clinicians to better identify which patients may be most at risk for suicide and could lead to more effective treatments for suicidal individuals with BD.
Relatively few studies have evaluated specific clinical features that may confer risk for SI, particularly in BD. Depression symptom severity has been identified as a strong predictor of SI9,11,12. Of the individual depression symptoms sleep loss, reduced appetite, and guilt each have been associated with SI in major depressive disorder and community samples13-16. In BD, attenuated self-esteem and elevated psychomotor agitation have been identified as correlates of SI in cross-sectional studies17,18. Some evidence has suggested that hypomanic symptoms are associated with SI among individuals with major depressive disorder and BD13,19, but the association between specific manic symptoms and SI has not been reported in BD. Thus, few studies have evaluated whether specific mood symptoms may confer particular risk for SI in BD.
Theoretical models of SI in bipolar disorder propose that affect intensity and poor affect regulation and coping may lead to feelings of defeat and entrapment, paving the way for SI as a means of escape20. These models suggest that some mood symptoms, such as low self-esteem and guilt, may be particularly likely to lead to feelings of defeat. Other symptoms such as distractibility and difficulty concentrating could indicate a lack of prefrontal control and could preclude the ability to effectively regulate strong affect, leading to SI. Sleep loss may further impair cognition and emotion regulation in BD21, resulting in difficulty seeing other solutions or that the current mood will pass. Additionally, past suicide attempts confer risk for future SI22-24, as individuals who have made a previous attempt may have a more readily accessible suicide script that is easily triggered by mood symptoms20,25. Thus, there is also a need to investigate which mood symptoms could confer more proximal risk for SI among bipolar patients who have made previous suicide attempts.
Extant studies of SI in BD primarily have evaluated mood symptoms in relation to SI cross-sectionally, precluding the ability to determine the directionality of these relationships and whether specific symptoms could serve as true risk factors for, rather than just correlates of, SI11,17-19. Additionally, most studies have not evaluated whether specific symptoms predict SI beyond overall symptom severity. Prospective research is needed to evaluate these questions, which could provide helpful information for clinicians seeking indicators of when suicide risk may be most acute.
In this study, we conducted one of the first prospective examinations of whether specific mood symptoms confer risk for future SI in BD, and whether the strength of such associations differs based on suicide attempt history. These questions were evaluated using multi-wave data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD26).
Method
Participants
Participants were patients enrolled in STEP-BD26, a large naturalistic multicenter effectiveness study that evaluated longitudinal outcomes in individuals with bipolar disorder. STEP-BD was approved by the institutional review boards of all participating centers, with oral and written informed consent received from all participants. All patients seeking outpatient treatment who were eligible based on diagnoses were offered participation in STEP-BD. Inclusion criteria included being at least 15 years of age, meeting DSM-IV criteria for bipolar I disorder, bipolar II disorder, cyclothymia, bipolar disorder not otherwise specified, or schizoaffective disorder, bipolar subtype. Exclusion criteria included an inability or unwillingness to adhere to study assessments or inability to provide informed consent, or not being in outpatient treatment at time of enrollment.
Participants (N=2,741) were included in the present report if they had completed two or more follow-up assessments of mood symptoms, and if they had valid data on analysis covariates including whether they had a history of making a suicide attempt. The sample was 42.0% male, 90.1% Caucasian/White, and 4.9% Hispanic; the mean age was 40.10 (SD = 12.78); 37.2% were currently married; 45.3% were currently employed; 83.0% had completed some college; and 41.3% had an income < $30,000 per year. The sample was 65.9% bipolar I, 29.7% bipolar II, and 4.4% bipolar NOS. 36.7% had a history of a suicide attempt.
Assessments
The Affective Disorder Examination (ADE)26 (Sachs et al., 2003) was administered by certified psychiatrists to participants at study entry to establish diagnoses. The ADE adapted the mood and psychosis modules from the Structured Clinical Interview for DSM-IV (SCID)27 and also assessed patient reports of history of making suicide attempts.
The Mini International Neuropsychiatric Interview (MINI)28 is a brief structured interview that was administered by different certified study clinicians to confirm bipolar diagnosis at study entry. A consensus diagnosis (on both the ADE and MINI) of one of the eligible bipolar disorders was required for study entry. The MINI has demonstrated acceptable validity and reliability for assessing DSM disorders28.
The Clinical Monitoring Form (CMF)26,29 was administered by certified clinicians at each follow-up visit to assess DSM-IV symptoms of depression and mania. Each symptom is rated on a 0 to 2 scale in increments of 0.25, with higher levels indicating greater symptom severity. Clinicians use all available sources of information when making unscripted ratings of each symptom. The CMF was used to generate variables representing the severity of each symptom of depression and hypomania, including suicidal ideation, at each follow-up visit. Summary scores also were computed representing the sum of symptoms of depression and mania.
All treating psychiatrists were required to complete standardized training in administering the ADE and CMF to establish acceptable inter-rater reliability prior to completing study assessments, and periodic monitoring continued throughout the study to ensure the maintenance of rating standards26,30. Assessments (M=14 per participant) took place across an average of 612 days of follow-up.
Statistical Analysis
Given the nested structure of the data (observations of symptoms at each visit, nested within persons), hierarchical linear modeling (HLM) was used31. Mood symptoms, including SI, served as Level 1 (within-subject) variables, whereas suicide attempt history and baseline symptoms of depression (excluding SI), mania, and SI served as Level 2 (between-subject) variables.
First, to evaluate whether specific symptoms at each visit (at time t-1) predicted SI at subsequent visits (at time t), in separate analyses we entered each t-1 symptom of depression and mania as a predictor of SI at time t, controlling for t-1 SI. Thus, the outcome variable (levels of SI) represented residual changes in SI that occurred relative to the previous time point, with positive values representing relative increases in SI, and negative values representing relative decreases in SI.
Given the likelihood of correlation between specific symptom predictors and total symptom severity, each analysis also controlled for the sum of the other t-1 symptoms of depression or mania. Thus, analyses represented the extent to which specific symptoms of depression and mania at a given visit predicted increases or decreases in SI at the subsequent visit, beyond levels predicted by severity of other mood symptoms. To use a conservative approach, analyses also controlled for suicide attempt history, baseline severity of SI, the focal symptom predictor, and the sum of the other symptoms of depression and mania, as well as number of lifetime episodes of mania and depression, age of onset, duration of illness, number of comorbid diagnoses, and number of comorbid medical conditions.
Second, we explored whether any mood symptoms predicted subsequent SI more strongly among individuals with a suicide attempt history, by examining the interaction between suicide attempt history and specific symptoms at t-1.
Continuous Level 2 variables were centered on the grand (between-subjects) mean. The structure of the models is included in Appendix A.
Results
Preliminary Analyses of Covariates
The intra-class correlation for an empty model predicting SI was .275, indicating that 27.5% of the variance in SI occurred at the between-subjects level (Level 2), whereas 72.5% of the variance in SI occurred at the within-subjects level (Level 1).
Several Level-2 covariates were significantly and positively associated with SI. Higher levels of SI across follow-up were predicted by suicide attempt history (B = 0.073, SE = 0.007, df = 2739, p < .001, R2 = .015, baseline SI (B = 0.267, SE = 0.016, df = 2739, p < .001, R2 = .077), baseline depression symptom severity (B = 0.185, SE = 0.011, df = 2739, p < .001, R2 = .038), baseline mania symptom severity (B = 0.143, SE = 0.018, df = 2739, p < .001, R2 = .009), number of lifetime (hypo)manic episodes (B = 0.009, SE = 0.002, df = 2739, p < .001, R2 = 0.004), number of lifetime depressive episodes (B = 0.014, SE = 0.002, df = 2739, p < .001, R2 = 0.009), age of onset (B = -0.001, SE = 0.0004, df = 2739, p < .001, R2 = 0.002), illness duration (B = 0.001, SE = 0.0003, df = 2739, p < .001, R2 = 0.001), number of comorbid axis I conditions (B = 0.007, SE = 0.001, df = 2739, p < .001, R2 = 0.006), and number of medical conditions (B = 0.010, SE = 0.002, df = 2739, p < .001, R2 = 0.002). Together, these individual Level-2 covariates accounted for up to 28% of the between-subjects variance in SI, or up to 7.7% of the overall variance in SI. Total depression and mania symptom severity at baseline accounted for only 3.8% and 0.9% of the overall variance in SI, respectively.
Additionally, all Level-1 covariates were significantly positively associated with SI, including t-1 SI (B = 0.188, SE = 0.008, df = 38,536, p < .001, R2 = .054), and t-1 symptoms of depression (B = 0.161, SE = 0.007, df = 38,536, p < .001, R2 = .031), and mania (B = 0.090, SE = 0.011, df = 38,536, p < .001, R2 = .011). These individual Level-1 covariates accounted for up to 7.5% of the between-subjects variance in SI, or up to 5.4% of the overall variance in SI. Total depression and mania symptom severity at the previous visit (t -1) accounted for only 3.1% and 1.1% of the overall variance in SI, respectively.
Altogether, Level-1 and Level-2 covariates accounted for 25.9% of the variance in SI.
Primary Analyses: Specific Symptoms Predicting Suicidal Ideation
Results of primary analyses are displayed in Table 1. After accounting for effects of the severity of other mood symptoms and the covariates noted above, higher prospective levels of SI were significantly predicted by several symptoms of depression, including guilt, reduced self-esteem, psychomotor retardation and agitation, and decreases in appetite. Loss of interest, loss of energy, problems with concentration, distraction, increases in appetite, and increases or decreases in sleep did not predict SI. In terms of mania symptoms, distractibility and psychomotor agitation predicted higher prospective levels of SI, whereas talkativeness predicted lower levels of SI. Although statistically significant, the incremental variance explained by individual mood symptoms (beyond overall symptom severity and previous SI) was small, predicting up to 1.6% additional variance in SI at the following visit. There were no overall effects of elevated self-esteem, decreased need for sleep, flight of ideas, goal-directed activity and risky behavior on SI.
Table 1. Results from hierarchical linear models of mood symptoms predicting levels of suicidal ideation at the next wave, alone and in interaction with suicide attempt history.
| Main Effect of Symptom | Interaction with Suicide Attempt History | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Predictor | B | SE | p | B | SE | p | ΔR2 |
| Depression | |||||||
| Loss of Interest | 0.0002 | 0.005 | .955 | 0.012 | 0.007 | .094 | .008 |
| Guilt | 0.010 | 0.005 | .038 | 0.017 | 0.009 | .068 | .015 |
| Self-Esteem (Decrease) | 0.020 | 0.004 | <.001 | 0.009 | 0.007 | .291 | .011 |
| Energy | 0.006 | 0.004 | .133 | 0.008 | 0.007 | .253 | .003 |
| Concentration | 0.001 | 0.004 | .849 | 0.018 | 0.007 | .010 | .012 |
| Distraction | 0.007 | 0.004 | .111 | 0.019 | 0.008 | .006 | .008 |
| Psychomotor Retardation | 0.013 | 0.005 | .011 | 0.015 | 0.010 | .151 | .016 |
| Psychomotor Agitation | 0.012 | 0.005 | .009 | 0.005 | 0.009 | .562 | .011 |
| Sleep (Increase) | -0.002 | 0.004 | .948 | 0.004 | 0.008 | .596 | .005 |
| Sleep (Decrease) | 0.005 | 0.004 | .172 | 0.014 | 0.007 | .045 | .010 |
| Appetite (Increase) | 0.014 | 0.005 | .005 | 0.013 | 0.010 | .179 | .012 |
| Appetite (Decrease) | 0.007 | 0.007 | .130 | -0.009 | 0.010 | .352 | .010 |
| Mania | |||||||
| Self-Esteem (Increase) | -0.017 | 0.009 | .061 | 0.041 | 0.017 | .021 | .002 |
| Need for Sleep (Decrease) | 0.008 | 0.005 | .093 | 0.026 | 0.010 | .010 | .003 |
| Talkativeness | -0.017 | 0.006 | .006 | 0.009 | 0.011 | .381 | .006 |
| Flight of Ideas | 0.001 | 0.006 | .814 | 0.020 | 0.010 | .040 | .008 |
| Distractibility | 0.022 | 0.004 | .001 | 0.018 | 0.008 | .021 | .009 |
| Goal-Directed Activity | -0.010 | 0.006 | .125 | 0.017 | 0.012 | .134 | .007 |
| Psychomotor Agitation | 0.011 | 0.005 | .006 | 0.007 | 0.009 | .409 | .007 |
| High-Risk Behavior | 0.006 | 0.009 | .440 | 0.015 | 0.016 | .373 | .007 |
| Sum of Depression Symptoms | 0.091 | 0.009 | <.001 | 0.033 | 0.013 | .011 | .014 |
| Sum of Mania Symptoms | 0.047 | 0.009 | <.001 | 0.044 | 0.019 | .019 | .007 |
Note: N = 2,741. 36.7% had a history of a suicide attempt. Analyses controlled for severity of other symptoms (in symptom-specific analyses), suicide attempt history, baseline severity of SI, baseline severity of the focal symptom predictor, baseline severity of the sum of the other symptoms of depression and mania, as well as number of lifetime episodes of mania and depression, age of onset, duration of illness, number of comorbid diagnoses, and number of medical conditions.
Differential Associations between Specific Symptoms and Suicidal Ideation among Suicide Attempters vs. non-Attempters
Several mood symptoms also interacted with suicide attempt history to predict subsequent SI (Table 1; Figure 1). Among depressive symptoms, problems with concentration, distraction, and sleep loss each interacted significantly with suicide attempt history. The form of these interactions were such that problems with concentration (t(38,536)=2.766, p=.006), distraction (t(38,536)=1.823, p=.068), and sleep loss (t(38,536)=2.234, p=.026) each predicted higher subsequent levels of SI among individuals with a suicide attempt history; whereas among individuals without an attempt history, concentration (t(38,536)=-0.257, p=.797), distraction (t(38,536)=-1.383, p=.167), and sleep loss (t(38,536)=-0.260, p=.795) did not predict SI. No other individual symptoms of depression predicted SI more strongly among individuals with a suicide attempt history.
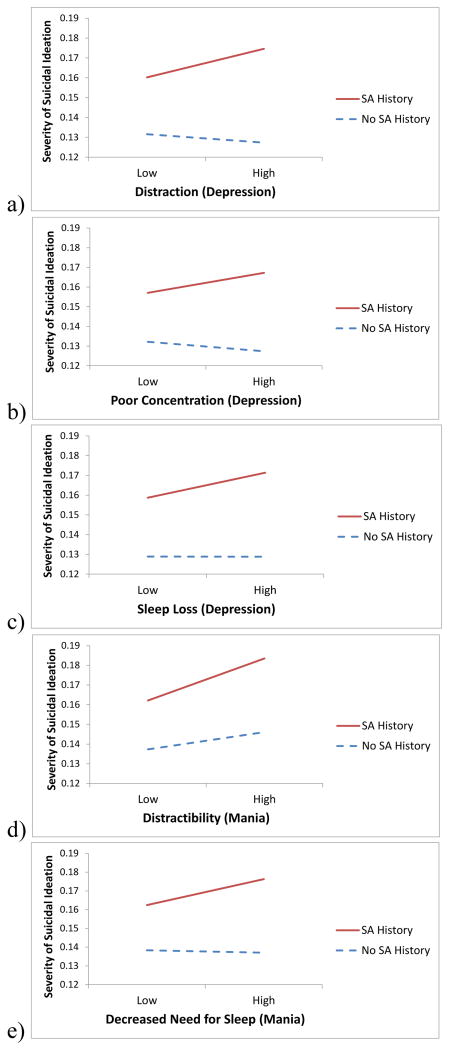
Figure 1.
Interactions between suicide attempt history and (a) distraction, (b) poor concentration, (c) sleep loss, (d) distractibility, and (e) need for sleep, predicting levels of suicidal ideation at the next wave.
In terms of mania symptoms, distractibility and decreased need for sleep also interacted similarly with suicide attempt history. Distractibility predicted higher subsequent levels of SI more strongly among individuals with an attempt history (t(38,536)=4.916, p<.001) than among individuals without an attempt history (t(38,536)=3.049, p<.002). Similarly, decreased need for sleep predicted higher subsequent levels of SI among individuals with an attempt history (t(38,536)=2.724, p=.006), but not among individuals without an attempt history (t(38,536)=-0.409, p=.683). In contrast, elevated self-esteem appeared to serve as a protective factor against SI among individuals without a suicide attempt history (t(38,536)=-3.393, p<.001), but not among individuals with a suicide attempt history (t(38,536)=0.529, p=.597). Flight of ideas interacted with suicide attempt history but did not predict SI among individuals with (t(38,536)=1.485, p=.138) or without a suicide attempt history (t(38,536)=-1.122, p=.262).
Although several analyses were statistically significant, the incremental effect sizes for individual symptom predictors of SI were small (1-2% additional variance in SI predicted), as was expected after conservatively controlling for the sum of t-1 mood symptoms and SI. In a separate model, after accounting for t-1 total severity of symptoms of depression and mania, entering all of the significant symptom predictors noted above predicted an additional 11.5% of variance in SI.
In addition to identifying specific symptoms associated with prospective SI, we also examined whether total t-1 symptom severity would predict SI. Symptoms of depression and mania both interacted with suicide attempt history to predict SI (Table 1). When accounting for these significant interactions in symptom-specific analyses, the interactions between suicide attempt history with concentration problems and sleep loss were reduced to non-significance (ps = .10-.11); however, all other interactions reported above remained significant.
Discussion
This study represents the first true prospective examination of whether specific mood symptoms confer risk for future SI in BD. Using a large, naturalistic sample of adults with BD, the present study found that several mood symptoms conferred risk for future SI, albeit with small effect sizes. In particular, guilt, decreases in self-esteem, psychomotor activation and retardation, appetite increases, and distractibility predicted higher levels of SI at the next visit, even after accounting for previous and current SI and overall mood symptom severity. Problems with concentration, distraction, sleep loss, and distractibility were more strongly associated with SI among individuals with a prior suicide attempt, whereas elevated self-esteem appeared to protect against subsequent SI only among individuals without a suicide attempt history.
However, compared to the effects of baseline and current SI, which accounted for 5-8% percent of the variance in SI, the effect sizes for individual symptom predictors were small. Symptoms that significantly predicted SI combined to account for only 11.5% of the variance in SI, suggesting that the clinical utility of identifying individual mood symptoms at each visit may be of limited utility to understand suicide risk in BD. However, in comparison, our data suggest that identifying factors such as suicide attempt history, current SI and symptom severity, and other lifetime illness characteristics when patients present to the clinic also may not account for much variance in SI at future visits as none of these predictors accounted for more than 7.7% of variance in SI (25.9% overall) in our sample. These data are broadly consistent with the ideas that relatively few strong proximal predictors of suicide risk are known32,33 and that the predictive value of more distal predictors is relatively weak34. It is possible that environmental variables that were not evaluated in the present study, such as interpersonal stressors, or clinical variables such as personality, trauma history, attachment style or schemas might be more informative of proximal risk for SI or suicide attempts35. These might confer particular risk when experienced in conjunction with the particular mood symptoms identified here. The large number of operative factors, each of small effect, suggests that large-scale data mining and machine learning algorithms might better capture individual risk, perhaps by combining behavioral and biological indices (for a recent example, see Niculescu et al.41). There is some evidence of the utility of such approaches, in that preliminary data suggests they might better capture suicide risk than clinician ratings36. These are capable of operating in real time on extant electronic health records.
There are several possible mechanisms by which different mood symptoms could contribute to SI in BD. Certain cognitive symptoms such as guilt and low perceived self-worth could result in feelings of defeat and entrapment, which are core risk factors proposed by theoretical models of suicide in BD20. Distractibility, concentration problems, and insomnia also might interfere with the ability to downregulate negative affect or to perceive a future in which symptoms improve, perhaps particularly so among individuals who have made a previous suicide attempt. Many of these symptoms can be targeted with existing psychosocial interventions. For example, cognitive behavioral techniques37 such as cognitive restructuring may be useful for helping patients to think in balanced ways when cognitive symptoms occur rather than considering suicide as a means of escape38, whereas interpersonal and social rhythm therapy39 could help patients to enhance sleep hygiene. Finally, medication regimens may require adjustment to adapt to symptoms that confer risk for SI; for example, antidepressants may worsen psychomotor agitation, whereas mood stabilizers may better control these symptoms, thereby attenuating suicide risk17.
Although this study had several notable strengths, several limitations also must be noted. The effects of individual symptoms were small; nevertheless, even small effect sizes in predicting illness course in BD may have clinical utility40. Our analyses did not predict prospective suicide attempts, an outcome of primary importance; however, SI is important to understand in treatment-seeking samples as it is a major risk factor for later attempts7. Because we were interested in modeling fluctuations in SI following fluctuations in particular mood symptoms, we did not specifically predict the onset of clinically-significant SI, which would require dichotomizing SI and would result in a loss of important contextual information. Finally, it is possible that the time course of the associations between symptoms and SI occurs more quickly than we were able to measure. Future work should consider studying these dynamics with shorter periods between assessments (e.g., using ecological momentary assessment43,44).
In conclusion, the present study highlighted the role of several mood symptoms that have small, but significant, associations with prospective risk for SI in BD. In addition, there is a need to identify stronger factors that confer proximal risk for SI, or ways of modeling cumulative effects of multiple small and interacting factors. Future research should evaluate whether various therapeutic approaches to BD can reduce suicide risk via modifying these symptoms. In the meantime, clinicians can be aware that patients, particularly those with past suicide attempts, may be at risk for SI when they experience these symptoms.
Acknowledgments
Funding/Support: STEP-BD was funded in part by contract N01MH80001 from the National Institute of Mental Health (Gary Sachs). Jonathan Stange was supported by National Research Service Award F31MH099761 from NIMH. Evan Kleiman reports no financial conflicts. Louisa Sylvia was employed by Massachusetts General Hospital, served as a Consultant for Bracket Global and Clintara, received research support from NIMH, is a former stockholder in Concordant Rater Systems, and has received support from New Harbinger Publishers. Pedro Vieira da Silva Magalhães reports no relevant financial interests. Michael Berk is supported by a NHMRC Senior Principal Research Fellowship (1059660) and has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Organon, Novartis, MaynePharma and Servier, has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, SanofiSynthelabo, Servier, Solvay and Wyeth, and served as a consultant to Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck Merck and Servier. Andrew Nierenberg has received honoraria or travel expenses from: American Society of Clinical Psychopharmacology, Australasian Society for Bipolar Disorder, Bayamon Region Psychiatric Society, San Juan, Puerto Rico, Belvoir Publishing, Boston Center for the Arts, Corcept, CRICO, Dartmouth, Dey Pharma, L.P./Mylan Inc., Israel Society for Biological Psychiatry, John Hopkins University, National Association of Continuing Education, PAI, Pamlabs, Physicians Postgraduate Press, Ridge Diagnostics, Slack Publishing, Sunovion, Teva Pharmaceuticals, University of Florida, University of Michigan, University of New Mexico, University of Miami, University of Wisconsin, Wolters Klower Publishing. Potential consulting honoraria from Astra Zeneca, Bristol Myers Squibb, Forest, Pfizer, Ridge Diagnostics. Potential support of research at MGH through Biogen Idec, Dey Pharmaceuticals, Pamlabs, Shire, and Sunovian. He owns stock options in Appliance Computing, Inc.(MindSite.com) and Brain Cells, Inc. Additional income is possible from Infomedic.com depending on overall revenues of the company but no revenue has been received to date. Through MGH, Dr. Nierenberg is named for copyrights to: the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). Thilo Deckersbach was supported in part by a K-23 NIMH Career Award 1K23MH074895-01A2. His research has also been funded by NARSAD, TSA, OCF and Tufts University. He has received honoraria, consultation fees and/or royalties from the MGH Psychiatry Academy, BrainCells Inc., Systems Research and Applications Corporation, Boston University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, the Massachusetts Medical Society, Tufts University, NIDA and Oxford University Press. He has also participated in research funded by NIH, NIA, AHRQ, Janssen Pharmaceuticals, The Forest Research Institute, Shire Development Inc., Medtronic, Cyberonics, and Northstar.
Appendix A
Level 1 (Within-Subjects) Model
SIti(t) = π0i + π1i*(SIti(t-1)) + π2i*(SumDeprOrManiaSxti(t-1)) + π3i*(FocalSymptomti(t-1)) + eti
Level 2 (Between-Subjects) Model
π0i = β00 + β01*(HxAttemptsi) + β02*(BaselineDepri) + β03*(BaselineManiai) + β04* (BaselineSIi) + β05*(BaselineFocalSymptomi) + β06*(NumberLifetimeManicEpisodesi) + β07*(NumberLifetimeDepressiveEpisodesi) + β08*(AgeofOnseti) + β09*(IllnessDurationi) + β10*(NumberComorbidDxsi) + β11*(NumberMedicalConditionsi) + r0i
π2i = β20 + r2i
π3i = β30 + β31*(HxAttemptsi) + r3i
Legend
SIti(t) = Suicidal ideation at time t
SIti(t-1) = Suicidal ideation at time t-1
SumDeprOrManiaSxti(t-1) = Depression or mania symptom severity at time t-1
FocalSymptomti(t-1) = Focal symptom severity at time t-1
HxAttemptsi = Lifetime history of suicide attempt(s)
BaselineDepri = Baseline depression severity
BaselineManiai = Baseline mania severity
BaselineSIi = Baseline suicidal ideation
BaselineFocalSymptomi = Baseline severity of focal symptom predictor
NumberLifetimeManicEpisodesi = Number of lifetime manic episodes
NumberLifetimeDepressiveEpisodesi = Number of lifetime depressive episodes
AgeofOnseti = Age of onset of bipolar disorder
IllnessDurationi = Duration of bipolar illness
NumberComorbidDxsi = Number of comorbid diagnoses
NumberMedicalConditionsi = Number of comorbid medical conditions
π0i = Level 2 intercept
π1i = Slope for suicidal ideation at time t-1
π2i = Slope for depression or mania symptom severity at time t-1
π3i = Slope for focal symptom severity at time t-1
eti = Level 1 error term
β10 = Intercept for suicidal ideation at time t-1
β20 = Intercept for depression or mania symptom severity at time t-1
β30 = Intercept for focal symptom severity at time t-1
β31 = Intercept for interaction between suicide attempt history and focal symptom severity at time t-1
β01 = Intercept for history of suicide attempts
β02 = Intercept for baseline depression symptom severity
β03 = Intercept for baseline mania severity
β04 = Intercept for baseline suicidal ideation
β05 = Intercept for baseline severity of focal symptom predictor
β06 = Intercept for number of lifetime manic episodes
β07 = Intercept for number of lifetime depressive episodes
β08 = Intercept for age of onset of bipolar disorder
β09 = Intercept for duration of bipolar illness
β10 = Intercept for number of comorbid diagnoses
β11 = Intercept for number of comorbid medical conditions
r0i = Level 2 random effect for intercept
r1i = Level 2 random effect for suicidal ideation
r2i = Level 2 random effect for depression or mania symptom severity
r3i = Level 2 random effect for focal symptom severity
References
- 1.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990. [Google Scholar]
- 3.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004 Aug;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry. 2006 Sep;163(9):1561–8. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YW, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol Psychiatry. 1996 May 15;39(10):896–9. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999 Jul;56(7):617–26. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 7.Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. J Clin Psychiatry. 2003 May;64(5):506–15. doi: 10.4088/jcp.v64n0503. [DOI] [PubMed] [Google Scholar]
- 8.Slama F, Bellivier F, Henry C, et al. Bipolar patients with suicidal behavior: toward the identification of a clinical subgroup. J Clin Psychiatry. 2004 Aug;65(8):1035–9. doi: 10.4088/jcp.v65n0802. [DOI] [PubMed] [Google Scholar]
- 9.Valtonen H, Suominen K, Mantere O, et al. Suicidal ideation and attempts in bipolar I and II disorders. J Clin Psychiatry. 2005 Nov;66(11):1456–62. doi: 10.4088/jcp.v66n1116. [DOI] [PubMed] [Google Scholar]
- 10.Tondo L, Isacsson G, Baldessarini RJ. Suicidal behavior in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- 11.Allen MH, Chessick CA, Miklowitz DJ, et al. Contributors to suicidal ideation among bipolar patients with and without a history of suicide attempts. Suicide Life Threat Behav. 2005 Dec;35(6):671–80. doi: 10.1521/suli.2005.35.6.671. [DOI] [PubMed] [Google Scholar]
- 12.Fagiolini A, Kupfer DJ, Rucci P, et al. Suicide attempts and ideation in patients with bipolar I disorder. J Clin Psychiatry. 2004 Apr;65(4):509–14. doi: 10.4088/jcp.v65n0409. [DOI] [PubMed] [Google Scholar]
- 13.Olgiati P, Serretti A, Colombo C. Retrospective analysis of psychomotor agitation, hypomanic symptoms, and suicidal ideation in unipolar depression. Depress Anxiety. 2006;23(7):389–97. doi: 10.1002/da.20191. [DOI] [PubMed] [Google Scholar]
- 14.Chellappa SL, Araújo JF. Sleep disorders and suicidal ideation in patients with depressive disorder. Psychiatry Res. 2007 Oct 31;153(2):131–6. doi: 10.1016/j.psychres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin RD, Marusic A. Association between short sleep and suicidal ideation and suicide attempt among adults in the general population. Sleep. 2008 Aug;31(8):1097–101. [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper-Patrick L, Crum RM, Ford DE. Identifying suicidal ideation in general medical patients. JAMA. 1994 Dec 14;272(22):1757–62. [PubMed] [Google Scholar]
- 17.Benazzi F. Suicidal ideation and bipolar-II depression symptoms. Hum Psychopharmacol. 2005 Jan;20(1):27–32. doi: 10.1002/hup.649. [DOI] [PubMed] [Google Scholar]
- 18.Daskalopoulou EG, Dikeos DG, Papadimitriou GN, et al. Self-esteem, social adjustment and suicidality in affective disorders. Eur Psychiatry. 2002 Sep;17(5):265–71. doi: 10.1016/s0924-9338(02)00681-8. [DOI] [PubMed] [Google Scholar]
- 19.Umamaheswari V, Avasthi A, Grover S. Risk factors for suicidal ideations in patients with bipolar disorder. Bipolar Disord. 2014 Sep;16(6):642–51. doi: 10.1111/bdi.12179. [DOI] [PubMed] [Google Scholar]
- 20.Malhi GS, Bargh DM, Kuiper S, et al. Modeling bipolar disorder suicidality. Bipolar Disord. 2013 Aug;15(5):559–74. doi: 10.1111/bdi.12093. [DOI] [PubMed] [Google Scholar]
- 21.Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: toward an integrated examination of disorder maintenance and functional impairment. Clin Psychol Rev. 2013 Feb;33(1):33–44. doi: 10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latalova K, Kamaradova D, Prasko J. Suicide in bipolar disorder: a review. Psychiatr Danub. 2014 Jun;26(2):108–14. [PubMed] [Google Scholar]
- 23.Goldberg JF, Garno JL, Portera L, et al. Correlates of suicidal ideation in dysphoric mania. J Affect Disord. 1999 Nov;56(1):75–81. doi: 10.1016/s0165-0327(99)00025-7. [DOI] [PubMed] [Google Scholar]
- 24.Grunebaum MF, Ramsay SR, Galfalvy HC, et al. Correlates of suicide attempt history in bipolar disorder: a stress-diathesis perspective. Bipolar Disord. 2006 Oct;8(5 Pt 2):551–7. doi: 10.1111/j.1399-5618.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 25.Oquendo MA, Waternaux C, Brodsky B, et al. Suicidal behavior in bipolar mood disorder: clinical characteristics of attempters and nonattempters. J Affect Disord. 2000 Aug;59(2):107–17. doi: 10.1016/s0165-0327(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 26.Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003 Jun 1;53(11):1028–42. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 29.Sachs GS, Guille C, McMurrich SL. A clinical monitoring form for mood disorders. Bipolar Disord. 2002;4:323–327. doi: 10.1034/j.1399-5618.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- 30.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006 Feb;163(2):217–24. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 31.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. New York: Sage; 2002. [Google Scholar]
- 32.Cassells C, Paterson B, Dowding D, Morrison R. Long- and short-term risk factors in the prediction of inpatient suicide: a review of the literature. Crisis. 2005;26(2):53–63. doi: 10.1027/0227-5910.26.2.53. [DOI] [PubMed] [Google Scholar]
- 33.Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J of Prev Med. 2014;47(3):S176–80. doi: 10.1016/j.amepre.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Large M, Smith G, Sharma S, et al. Systematic review and meta-analysis of the clinical factors associated with the suicide of psychiatric inpatients. Acta Psychiat Scand. 2011 Jul;124(1):18–19. doi: 10.1111/j.1600-0447.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 35.Bagge CL, Glenn CR, Lee HJ. Quantifying the impact of recent negative life events on suicide attempts. J Abnorm Psychol. 2013 May;122(2):359–68. doi: 10.1037/a0030371. [DOI] [PubMed] [Google Scholar]
- 36.Tran T, Luo W, Phung D, et al. Risk stratification using data from electronic medical records better predicts suicide risks than clinician assessments. BMC Psychiatry. 2014 Mar 14;14:76. doi: 10.1186/1471-244X-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otto MW, Reilly-Harrington NA, Knauz RO, et al. Managing bipolar disorder: A cognitive behavior treatment program workbook. New York: Oxford University Press; 2008. [Google Scholar]
- 38.Stange JP, Sylvia LG, da Silva Magalhães PV, et al. Extreme attributions predict suicidal ideation and suicide attempts in bipolar disorder: prospective data from STEP-BD. World Psychiatry. 2014 Feb;13(1):95–6. doi: 10.1002/wps.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank E. Treating bipolar disorder: A clinician's guide to interpersonal and social rhythm therapy. New York: Guilford Press; 2005. [Google Scholar]
- 40.Strakowski SM. Approaching the challenge of bipolar depression: results from STEP-BD. Am J Psychiatry. 2007 Sep;164(9):1301–3. doi: 10.1176/appi.ajp.2007.07060926. [DOI] [PubMed] [Google Scholar]
- 41.Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015 Nov;20(11):1266–85. doi: 10.1038/mp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychol Assess. 2009 Dec;21(4):463–75. doi: 10.1037/a0017075. [DOI] [PubMed] [Google Scholar]
- 43.Wenze SJ, Miller IW. Use of ecological momentary assessment in mood disorders research. Clin Psychol Rev. 2010 Aug;30(6):794–804. doi: 10.1016/j.cpr.2010.06.007. [DOI] [PubMed] [Google Scholar]