Abstract
Aging is associated with declines in cognitive performance and multiple changes in the brain, including reduced default mode functional connectivity (FC). However, conflicting results have been reported regarding age differences in FC between hippocampal and default mode regions. This discrepancy may stem from the variation in selection of hippocampal regions. We therefore examined the effect of age on resting state FC of anterior and posterior hippocampal regions in an adult life-span sample. Advanced age was associated with lower FC between the posterior hippocampus and three regions: the posterior cingulate cortex, medial prefrontal cortex, and lateral parietal cortex. In addition, age-related reductions of FC between the left and right posterior hippocampus, and bilaterally along the posterior to anterior hippocampal axis were noted. Age differences in medial prefrontal and inter-hemispheric FC significantly differed between anterior and posterior hippocampus. Older age was associated with lower performance in all cognitive domains, but we observed no associations between FC and cognitive performance after controlling for age. We observed a significant effect of gender and a linear effect of COMT val158met polymorphism on hippocampal FC. Females showed higher FC of anterior and posterior hippocampus and medial prefrontal cortex than males, and the dose of val allele was associated with lower posterior hippocampus – posterior cingulate FC, independent of age. Vascular and metabolic factors showed no significant effects on FC. These results suggest differential age-related reduction in the posterior hippocampal FC compared to the anterior hippocampus, and an age-independent effect of gender and COMT on hippocampal FC.
Keywords: Functional Connectivity, Aging, Hippocampus, COMT val158met, Gender, Vascular Risk
1. Introduction
Advanced age is associated with low performance on multiple cognitive tasks (Salthouse, 2010), and magnetic resonance imaging (MRI) has been used to explore the neural correlates of this cognitive decline (for example: (Grady, 2012; Gunning-Dixon and Raz, 2000; Raz et al., 1998). Numerous brain characteristics, such as regional brain volumes, structural and diffusion properties of the white matter and task-related activation, exhibit significant age differences and have been linked to cognitive performance, see (Fjell et al., 2014; Kennedy and Raz, 2015) for reviews. Since the introduction of MRI measures of resting brain activity (resting state MRI or rsMRI; (Biswal et al., 1995; Raichle et al., 2001), various measures of functional connectivity within and among brain networks have been used to assess age-related differences on a brain network level.
The core assumption behind applying functional connectivity measures to the study of cognitive aging is that even subtle disruption of physical connections and relatively minor damage to the brain’s regional integrity may affect the flow of information across the brain, which can manifest their effect in age-related differences in functional connectivity (Ferreira and Busatto, 2013). Measures of resting state functional connectivity reflect coherence between temporal fluctuations in the blood oxygen level dependent (BOLD) signal across brain regions organized into distinct networks (Damoiseaux et al., 2006; van den Heuvel et al., 2008; Zuo et al., 2010).
In the extant studies of age-related differences in resting state functional connectivity, the most consistent finding is the association between advanced age and lower functional connectivity within the default mode network (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Ferreira and Busatto, 2013). Default mode network activity is commonly linked to a variety of cognitive processes such as episodic memory, self-referential processing, and mind wandering (Buckner et al., 2008; Raichle et al., 2001), with a positive relationship reported between strength of default mode connectivity and performance on tasks of memory and executive function (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Wang et al., 2010).
Even though both independent component analysis (ICA) and seed-based analyses of the default mode network have consistently revealed age-related differences in functional connectivity, there is no consensus regarding the role of the hippocampus within the default mode network. Consequently, age-related differences in hippocampal functional connectivity remain unclear. Some studies examining age effects on default mode connectivity did not include the hippocampus (Bluhm et al., 2008; Damoiseaux et al., 2008), while others varied considerably in the exact demarcation of the region in question. For example, some (Andrews-Hanna et al., 2007) found lower connectivity in older adults in a posteriorly hippocampal region of interest (ROI), whereas others found either no significant connectivity differences (Koch et al., 2010) or increased functional connectivity (Salami et al., 2014) with older age in more anteriorly located ROIs. These discrepancies in reported findings may, at least partly, be explained by differences in hippocampal ROI selection, since hippocampal connectivity patterns are known to vary across hippocampal regions (Kahn et al., 2008; Zarei et al., 2013). The posterior hippocampus is part of the functional pathway that, via the cingulum bundle, connects to parahippocampal, retrosplenial, posterior cingulate, precuneus, lateral parietal and medial prefrontal cortices - all areas of the default mode network. In contrast, the anterior hippocampus belongs to the functional pathway that via the uncinate fasciculus is connected with the amygdala, perirhinal/entorhinal cortices, and the anterior and lateral temporal lobes (Kahn et al., 2008; Poppenk and Moscovitch, 2011; Ranganath and Ritchey, 2012).
The main goal of this study is to address these discrepancies by determining the effect of age on posterior versus anterior hippocampal functional connectivity to default mode brain regions, and the relationship with cognitive performance, in an adult life-span sample. Based on the extant research outlined above, we hypothesized lower functional connectivity of the posterior hippocampus with older age, and either no age-related differences (Koch et al., 2010) or an increase in connectivity with age (Salami et al., 2014) for the anterior hippocampus.
As characteristics of participants vary across studies, another potential source of discrepancy among studies could be unaccounted variability in the samples with regards to gender, vascular risk burden and genetic risk factors, all of which can affect brain structure and function (Ferreira and Busatto, 2013; Jagust, 2013; Raz and Rodrigue, 2006). Thus, an additional, exploratory aim of this study was to determine the effect of several age-related and age-independent risk factors on hippocampal functional connectivity and cognitive performance. Among the former are risk factors such as vascular and metabolic risk (Friedman et al., 2014; Zhou et al., 2010) whereas the latter include gender and genetic variants that are associated with risk for age-related cognitive and physiological impairment, such as Alzheimer’s disease, diabetes, inflammation and probable dopamine availability in the synapse (for examples see (Barnett et al., 2008; Damoiseaux et al., 2012).
2. Methods
2.1. Participants
Adult volunteers were recruited from the Metro Detroit, Michigan area through advertisements in local newspapers and flyers for an ongoing longitudinal study of cognitive and neural correlates of aging. Structural and functional MRI data were available on 167 adults (60 men, 107 women) age 18–83 years (mean age 49.1 ± 18.0 years). A subset of this sample, 91 adults aged 18–78 years (mean age 42.2 ± 17.6 years; 33 men, 58 women), completed cognitive testing. The Wayne State University Institutional Review Board approved the study and signed informed consent was obtained from all participants. Participants spoke English as their first language and were right-hand dominant (score over 75% on Edinburgh Inventory, (Oldfield, 1971). They were screened with a health questionnaire for neurological, psychiatric, cardiovascular, and endocrine diseases, diabetes, cancer, and a history of loss of consciousness for more than 5 minutes. In addition, participants were screened for dementia (Mini-Mental State Exam, MMSE ≥ 26; (Folstein et al., 1975) and depression (Center for Epidemiological Study Depression questionnaire, CES-D ≤16; (Radloff, 1977). There was no relationship between age and MMSE (r=−0.053, p=0.498), but older participants had more years of formal education (r=0.173, p=0.025).
2.2. Data acquisition
2.2.1. MRI data
Imaging was performed at the MRI research facility at Wayne State University on a 3-Tesla Siemens Verio (Siemens Medical AG, Erlangen, Germany) full-body magnet with a 12-channel head coil. The scan session included resting state functional MRI and anatomical MRI. For the resting state functional scan, 200 volumes of 43 axial slices were acquired sequential using a T2*-weighted echo planar sequence with the following parameters: repetition time (TR) = 2500 ms, echo time (TE) = 30 ms, flip angle = 90°, pixel bandwidth = 2298 Hz/pixel, GRAPPA acceleration factor PE = 2, field-of-view = 210 mm, matrix size = 64 x 64, voxel size = 3.3 × 3.3 × 3.3 mm. Participants were instructed to lie still with their eyes open. For the anatomical scan a 3D T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence was acquired with the following parameters: TR = 1680 ms, TE = 3.51 ms, inversion time (TI) = 900 ms, flip angle = 9.0°, pixel bandwidth = 180 Hz/pixel, GRAPPA acceleration factor PE = 2; field-of-view = 256 mm, matrix size = 384 x 384, voxel size 0.67 mm × 0.67 mm × 1.34 mm.
2.2.2. Cognitive data
Cognitive performance was assessed using a comprehensive battery of cognitive tests, including: letter comparison, pattern comparison, Woodcock-Johnson-R Memory for Names (immediate and delayed); Stroop, Wisconsin Card Sorting Test (WCST), size judgment span, listening span, spatial recall, and Cattell Culture Fair Test (see (Raz et al., 2009), for a detailed description of the cognitive battery).
2.2.3. Vascular and metabolic risk data
We collected common indicators of vascular and metabolic risk: blood pressure, frequency of exercise, smoking, and waist-to-hip ratio. In addition, a phlebotomist collected blood samples from all participants following a 12-hr overnight fast. The Detroit Medical Center hospital laboratory analyzed these samples to determine levels of cholesterol, glucose and triglycerides.
2.2.4. Genetic data
DNA extraction and genotyping were performed on material obtained from buccal cell cultures that were collected in mouthwash samples. DNA was isolated with Gentra Autopure LS under the standard buccal cell protocol. The Wayne State University Applied Genomics Technology Center performed DNA isolations and genotyping assays using an Applied Biosystems 7900. DNA sequencing reactions were carried out using the 0.5X protocol for ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). The sequencing extension products were purified utilizing Sephadex, and analyzed on an ABI PRISM 3700 DNA analyzer with a 50-cm capillary array. Genetic variants associated with increased risk for Alzheimer’s disease, diabetes, synaptic dopamine degradation and pro-inflammatory response were determined, including: APOE, CLU, TOMM157580, TOMM157581, TOMM207, TOMM5900, GCK, G6PC2, G6PC2G231A, TCF7L2, IL-1β C-511T, IL-6 C-174G and TNFα G308A, and COMT val158met.
2.3. Data analysis
2.3.1. MRI data
Image preprocessing was carried out using tools from FMRIB’s Software Library (FSL, version 4.1; (Smith et al., 2004). For the resting state data the following pre-statistics processing was applied: motion correction (Jenkinson et al., 2002); removal of non-brain structures (Smith, 2002); spatial smoothing using a Gaussian kernel of 6 mm full width at half maximum; and mean-based intensity normalization of all volumes by the same factor (i.e. 4D grand-mean scaling in order to ensure comparability between data sets at the group level). After pre-processing the functional scan was first aligned to the individual’s high resolution T1-weighted image, which was subsequently registered to the MNI152 standard space (average T1 brain image constructed from 152 normal subjects at Montreal Neurological Institute) using affine linear registration (Jenkinson et al., 2002). ICA-based Automatic Removal Of Motion Artifacts (ICA-AROMA; (Pruim et al., 2015) was applied to these normalized images. ICA-AROMA is a data-driven method of identifying and removing motion-related independent components from functional MRI data. As a last step of preprocessing we applied high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=75.0s) and removed the global signal contribution from the data. The anatomical data underwent the following preprocessing: removal of non-brain structures (Smith, 2002), followed by segmentation of the high-resolution images (T1-weighted MPRAGE) into grey matter, white matter, cerebrospinal fluid and background, and lastly calculation of partial volume maps (Zhang et al., 2001). All individual grey matter partial volume maps were transformed into MNI152 standard space using affine linear registration. By averaging every participant’s standard space grey matter image a sample-specific mean grey matter image was created. In addition, individual partial volume estimates were used to calculate grey matter volume for each of the ROIs used in the functional connectivity analyses. The association between grey matter volume of specific ROIs and their functional connectivity was examined using Pearson correlation (significance determined at α=0.003 after applying a Bonferroni correction for multiple comparisons).
ROI-based correlations were used to assess functional connectivity between the anterior and posterior hippocampal regions and default mode regions. Eight spherical ROIs were created using the coordinates specified in (Andrews-Hanna et al., 2007), which were transformed from Talairach to Montreal Neurological Institute (MNI) space using the tal2icbm transform (Lancaster et al., 2007). These ROIs were located in the bilateral posterior hippocampus (L: xyz = −23 −26 −15; R: xyz = 25 −26 −15), posterior cingulate/retrosplenial cortex (xyz = 0 −50 29), medial prefrontal cortex (xyz = 2 44 11), bilateral lateral parietal (L: xyz = −46 −68 30; R: xyz = 57 −65 29), and bilateral parahippocampal gyrus (L: xyz = −25 −41 −12; R: xyz = 28 −41 −12). Two more anteriorly located hippocampal ROIs were created using the coordinates reported by Salami and colleagues (2014) showing an age related increase in functional connectivity (L: xyz = −18 −14 −18; R: xyz = 18 −14 −18). A diameter of 4 mm was used for hippocampal and parahippocampal ROIs, and 8 mm for the others. The sample-specific mean grey matter image was used to constrain the ROIs to grey matter only. The time-series of bilateral ROIs were averaged to calculate posterior and anterior hippocampal functional connectivity with the default mode ROIs. Mean time-series of unilateral ROIs were used to calculate within hippocampal functional connectivity. Pearson correlations among ROIs served as indices of functional connectivity, and their Fisher’s r-to-z transformed values were used as dependent variables in linear regression models to assess age effects while accounting for gender. Within-subjects analysis of variance models were used to assess differences in posterior versus anterior hippocampal functional connectivity to default mode regions and the interaction with age. Significance was determined after correcting for multiple comparisons using the Benjamini-Hochberg procedure with a false discovery rate of q<0.05.
2.3.2. Cognitive data
Cognitive tests scores were available for 91 of the 167 participants. For the Stroop color task, we computed interference scores by residualizing the reading time under the incompatible condition on the time under the neutral condition. Numbers of perseverative responses and perseverative errors that served as indicators of performance on WCST were log-transformed to correct skewness. Listening span scores were log-transformed for the same reason. Stroop interference scores and the numbers of perseverative responses and perseverative errors in WCST were reversely coded, so that for all the variables, higher cognitive scores indicated better performance. All cognitive scores were standardized to avoid scaling problems. Two subjects had missing cognitive data: one missed WCST scores, and the other did not have letter comparison, pattern comparison, spatial recall, Cattell Culture Fair Tests, size judgment span and listening span scores. To determine the main cognitive constructs for analyses, we performed a confirmatory factor analysis (CFA), while taking into account missing observations via the full-Information maximum likelihood (FIML) method. Missing cases were handled under the MAR (missing at random) assumption, which allows missingness to be related to the observed covariates and observed outcomes. The CFA model consisting of three latent factors: processing speed, with scores of letter comparison and pattern comparison as indicators; memory, with scores of Woodcock-Johnson-R Memory for Names (WJR memory, immediate and delayed) as indicators; and executive functions, with the following indicators: Stroop, Wisconsin Card Sorting Test, size judgment span, listening span, spatial recall, and Cattell Culture Fair Test (form 3B, tests 1, 2, 3, 4). The component loadings on common factors are as follows: letter comparison 1.000 and pattern comparison 1.026 onto processing speed; immediate WJR memory 1.000 and delayed WJR memory 0.836 onto memory; for executive functions, the loadings are Stroop tests 1.000, 1.036, size judgment span 0.830, listening span 1.226, WCST perseverative responses 1.077, WCST perseverative errors 1.078, spatial recall 1.010, and 1.245, 0.914, 0.531, 1.072 respectively for the four Cattell subtests. The goodness-of-fit indices are: CFI (Comparative Fit Index) = 0.973, RMSEA (Root mean square error of approximation) = 0.059, SRMR (standardized root mean residual) = 0.049, indicating that the CFA is a valid model. The analyses were conducted using Mplus 6.0 (Muthén and Muthen, 2010), and composite factor scores were calculated for each latent factor. To examine the effect of age on cognitive performance, Pearson correlations between factor scores, which contained information about an individual’s placement on the factor, and age was calculated. In addition, Pearson correlations were calculated to assess the relationship between cognitive performance and functional connectivity. Significance for both correlation analyses was determined at α=0.002 after correcting for multiple comparisons using a Bonferroni correction.
2.3.3. Vascular and metabolic risk data
Composite scores for vascular and metabolic risk were created, based on commonly known clinical risk factors, to measure potential effects of participant’s health on age-related functional connectivity differences. The vascular risk composite score was calculated as 2*diagnosed high blood pressure + smoking − exercise + ((systolic − 120)/10) + ((diastolic − 80)/10)); where diagnosed high blood pressure, smoking and exercise are binary (1 versus 0); and systolic and diastolic are actual blood pressure readings referenced to the hypertension cut-offs. Metabolic risk composite score was calculated as the sum of waist-hip-ratio, triglycerides, systolic blood pressure, glucose and high-density lipoprotein; where the actual values are standardized and high-density lipoprotein is reversely coded so that for all the variables, higher scores indicated increased risk. Individual composite scores were calculated and added as independent variables along with the genetic risk variables described below, age and gender in linear regression models to assess their association with functional connectivity and cognitive performance. Significance was determined after correcting for multiple comparisons using the Benjamini-Hochberg procedure with a false discovery rate of q<0.05.
2.3.4. Genetic data
Genetic variants associated with increased risk for Alzheimer’s disease, diabetes, dopamine availability and pro-inflammatory response were assessed and cumulative risk scores were computed. Such composite scores are frequently computed to evaluate risks conveyed by ensembles of risk alleles rather than single genes (Potkin et al., 2016). Cumulative risk score for Alzheimer’s disease was calculated as the sum of the risk alleles on the following single nucleotide polymorphisms (SNPs): apolipoprotein E (APOE) ε4 variant, clusterine (CLU), and four TOMM40 SNPs (TOMM157580, TOMM157581, TOMM207, TOMM5900). Cumulative diabetes risk was calculated as the sum of the risk alleles on: GCK, G6PC2, G6PC2G231A and TCF7L2; and cumulative pro-inflammatory response propensity was represented as the sum of the risk alleles on: IL-1β C-511T, IL-6 C-174G and TNFα G308A. Probable synaptic dopamine availability (high to low) was evaluated as the dose of the val allele in Cathechol-O-methyltransferase (COMT) val158met SNP. Individual risk scores were added as independent variables along with vascular and metabolic risk, age and gender in linear regression models to assess their association with functional connectivity and cognitive performance. Significance was determined after correcting for multiple comparisons using the Benjamini-Hochberg procedure with a false discovery rate of q<0.05.
3. Results
3.1. Age differences in functional connectivity
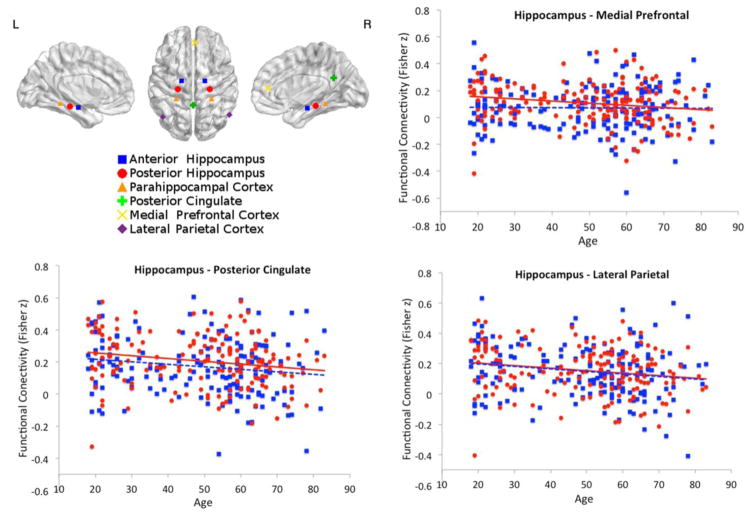
Advanced age was associated with lower functional connectivity of bilateral posterior hippocampus – posterior cingulate/retrosplenial cortex (F(2,164)=4.149, p=0.017, adjusted R2=0.037, age β=−0.198, p=0.010, gender not significant); bilateral posterior hippocampus – medial prefrontal (F(2,164)=8.280, p<0.001, adjusted R2=0.081, age β=−0.198, p=0.009, gender β=0.239, p=0.002); and bilateral posterior hippocampus – bilateral lateral parietal (F(2,164)=4.571, p=0.012, adjusted R2=0.041, age β=−0.199, p=0.010, gender not significant), see figure 1 (plotted in red). Connectivity between the other hippocampal – default mode ROI pairs evidenced no significant association with age. Direct comparison of bilateral anterior versus posterior hippocampal connectivity within participants showed significantly higher connectivity (F(1,165)=10.823, p=0.001) for posterior hippocampus – medial prefrontal cortex and a differential effect of age on these pairs (F(1,165)=5.249, p=0.021), such that connectivity of the posterior hippocampus showed stronger association with age than connectivity of the anterior hippocampus. Connectivity of anterior versus posterior hippocampus to posterior cingulate/retrosplenial and lateral parietal cortices did not show any significant differences (figure 1). Note, that even though only functional connectivity of posterior hippocampal – default mode ROI pairs showed a significant reduction with older age, this association was only significantly different between anterior versus posterior hippocampus for connectivity with the medial prefrontal cortex. The association of age with connectivity of anterior hippocampus to posterior cingulate/retrosplenial and lateral parietal cortices therefore was not significant at the corrected 0.05 threshold.
Figure 1.
Effect of age on hippocampal – default mode functional connectivity. Age-related decreases in functional connectivity between bilateral posterior hippocampus and three other default mode network regions – posterior cingulate/retrosplenial, medial prefrontal and bilateral lateral parietal are shown in red. Functional connectivity of the anterior hippocampus to the same regions is shown in blue. The effect of age on functional connectivity with the medial prefrontal cortex is significantly different between posterior and anterior hippocampus.
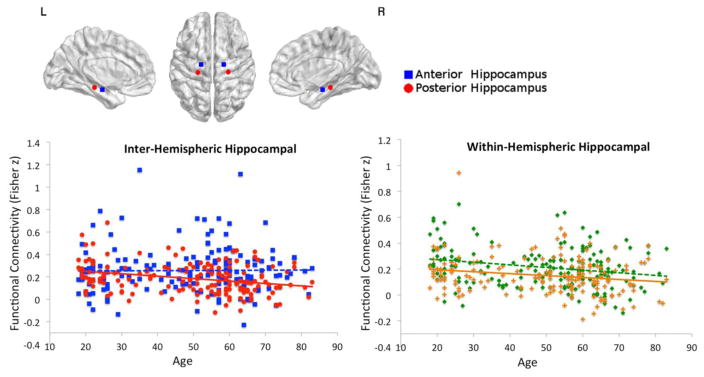
To assess further differences within the hippocampus, we examined functional connectivity between unilateral posterior and anterior hippocampal ROIs. The results showed reduced inter-hemispheric functional connectivity between the left and right posterior hippocampus with advanced age (F(2,164)=9.834, p<0.001, adjusted R2=0.096, age β=−0.298, p<0.001, gender β=0.149, p=0.045). Connectivity between the left and right anterior hippocampus was unrelated to age. Direct comparison of hippocampal inter-hemispheric connectivity showed no significant difference in connectivity strength between anterior and posterior hippocampus, however, it did show a differential effect of age (F(1,165)=5.668, p=0.018), see figure 2. Within-hemispheric functional connectivity along the posterior to anterior axis decreased with age for both left hippocampus (F(2,164)=7.050, p=0.001, adjusted R2=0.068, age β=−0.245, p=0.001, gender β=0.150, p=0.047), and right hippocampus (F(2,164)=4.103, p=0.018, adjusted R2=0.036, age β=−0.194, p=0.012, gender not significant), see figure 2. Direct comparison of this within-hemispheric connectivity revealed significantly higher connectivity strength within the left hippocampus compared to the right hippocampus (F(1,165)=8.941, p=0.005), but no different effect of age, see figure 2. All significant results reported here were significant after correction for multiple comparisons using a false discovery rate of q=0.05. None of the correlations between ROI grey matter volume and functional connectivity of corresponding regions reached significance; therefore grey matter volume was not included as covariate in any of the functional connectivity analyses.
Figure 2.
Effect of age on hippocampal functional connectivity. Age-related decreases in inter-hemispheric functional connectivity for left – right posterior hippocampus are shown in red in the left scatterplot. The anterior hippocampus, which does not show an age effect, is shown in blue. The effect of age on inter-hemispheric functional connectivity is significantly different between posterior and anterior hippocampus. Age-related decreases in within-hemispheric functional connectivity, along the posterior to anterior axis of the hippocampus, are shown in the right scatterplot for both the left (in green) and right (in orange) hemispheres.
3.2. Age differences in cognitive performance and their relationship to functional connectivity
Advanced age was associated with lower performance in all cognitive domains tested: processing speed (r=−0.610, p<0.001), memory (r=−0.548, p<0.001), and executive function (r=−0.665, p<0.001). Functional connectivity between anterior hippocampus and lateral parietal cortex was positively correlated to performance on executive function tasks (r=0.328, p=0.001), meaning that better performance was associated with higher functional connectivity. After controlling for age and gender, this association between functional connectivity strength and cognitive performance no longer remained significant.
3.3. Gender differences in functional connectivity
We observed a relationship between gender and functional connectivity of posterior hippocampus – medial prefrontal cortex (F(2,164)=8.280, p<0.001, adjusted R2=0.081, gender β=0.239, p=0.002, age β=−0.198, p=0.009) and anterior hippocampus – medial prefrontal cortex F(2,164)=3.911, p=0.022, adjusted R2=0.034, gender β=0.213, p=0.006, age not significant). A subsequent one-way ANOVA revealed significantly increased functional connectivity in females compared to males for posterior hippocampus – medial prefrontal cortex (F(1,165)=9.165, p=0.003; females M=0.134, SD=0.148; males M=0.061, SD=0.155) and anterior hippocampus – medial prefrontal cortex (F(1,165)=7.764, p=0.006, females M=0.099, SD=0.166; males M=0.024, SD=0.165).
3.4. Effect of age-related and age-independent risk factors
The examination into the effect of vascular, metabolic and genetic risk on functional connectivity and cognitive function, showed a significant effect of COMT val158met on functional connectivity between the posterior hippocampus and the posterior cingulate/retrosplenial cortex independent of age (F(8,109)=2.449, p=0.018, adjusted R2=0.090, COMT β=−0.346, p<0.001, other variables not significant). A subsequent one-way ANOVA confirmed the observed effect (F(2,129)=7.735, p=0.001) and Tukey post-hoc tests revealed that val homozygotes (M=0.125, SD=0.168) showed significantly lower functional connectivity between the posterior hippocampus and the posterior cingulate/retrosplenial cortex than val heterozygotes (M=0.215, SD=0.144, p=0.013) and met homozygotes (M=0.274, SD=0.161, p=0.001), see figure 3. Our sample contained 38 val homozygotes, 68 val heterozygotes, and 26 met homozygotes, this distribution is consistent with the Hardy – Weinberg principle (χ2=0.199, p=0.655). No differences were found in age, gender, and years of education across allelic groups, and no association was observed between COMT val158met and cognitive performance. None of the other risk factors examined emerged as significant correlates of functional connectivity strength, or cognitive performance.
Figure 3.
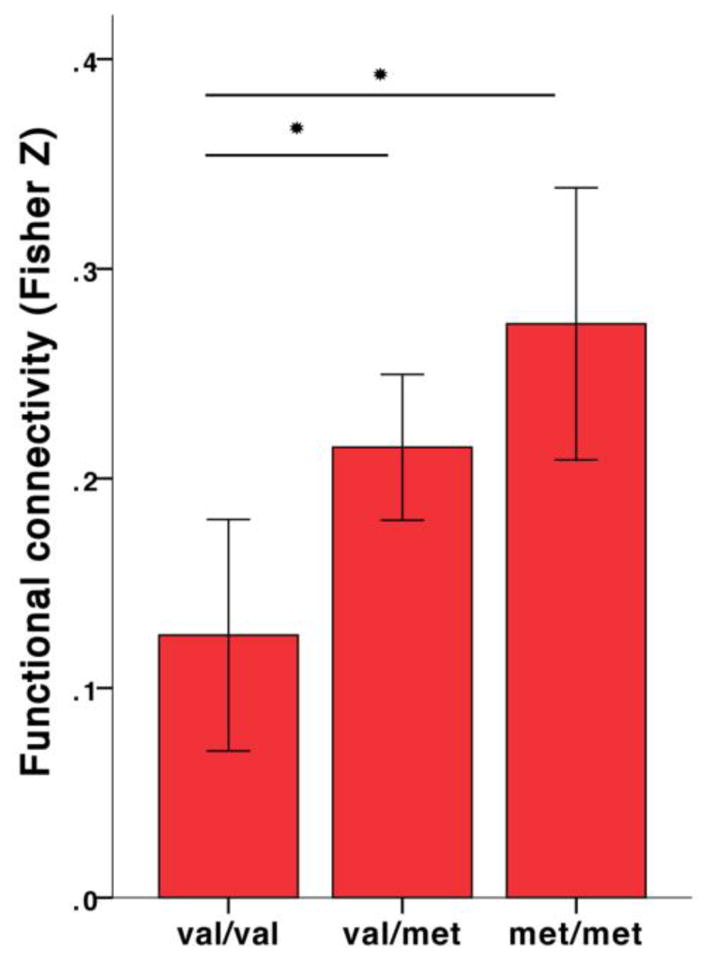
Age-independent linear effect of COMT val158met genotype on functional connectivity strength between posterior hippocampus and posterior cingulate/retrosplenial cortex. The bar graphs show the significant differences between genotypes as revealed by post-hoc comparisons. *Significant at p<0.05
4. Discussion
The main finding of this study is a differential effect of age on functional connectivity of posterior versus anterior hippocampus in healthy adults. Our results show that advanced age is associated with lower functional connectivity between left and right posterior hippocampus and posterior hippocampus to medial prefrontal cortex, and that this is significantly different from connectivity of the anterior hippocampus, which is not affected by age. Advanced age also is linked to lower within-hemispheric, i.e. posterior – anterior, functional connectivity for both left and right hippocampus. Furthermore, we show that advanced age is associated with lower posterior hippocampus – posterior cingulate/retrosplenial, and posterior hippocampus – lateral parietal functional connectivity. However, the magnitude of these age effects was not significantly different between the posterior and anterior hippocampus. The cortical pathway that includes the posterior hippocampus is known to connect with the parahippocampal gyrus and retrosplenial cortex and, via the cingulum bundle, regions of the default mode network, such as the posterior cingulate, precuneus, parietal and medial prefrontal cortex (Ranganath and Ritchey, 2012; Ward et al., 2013). Our findings suggest a partial age-related functional disconnection of the posterior hippocampus from contra- and ipsilateral hippocampal regions and other regions within this pathway. Additional evidence of partial age-related disconnection comes from existing reports on white matter connectivity estimated from diffusion tensor imaging. These studies indicate lower fractional anisotropy in the cingulum bundle with older age (Catheline et al., 2010; Damoiseaux et al., 2009; Yoon et al., 2008). It is plausible, therefore, that disruption of white matter organization of the cingulum underlies the observed decline in functional connectivity. Further research directly comparing structural and functional connectivity measures of this pathway is needed, however, to substantiate this claim. A pattern of reduced hippocampal connectivity has also been observed in patients with mild cognitive impairment and Alzheimer’s disease (Delbeuck et al., 2003; Stoub et al., 2006; Villain et al., 2008). These findings may indicate either an accelerated form of the age-related reduction of hippocampal connectivity, similar to observed in the current study or a distinct process affecting other pathways or separate sub-regions of the cingulum bundle (Catheline et al., 2010; Damoiseaux et al., 2009; Zhang et al., 2007).
In accord with one report (Koch et al., 2010), we found no age differences in functional connectivity of the anterior hippocampus to default mode regions. Nor did we note age differences in inter-hemispheric functional connectivity, as was observed for the posterior hippocampus. As mentioned in the introduction, the anterior and posterior parts of the hippocampus likely belong to distinct functional and vascular systems, and functional differences between the two may play out in various aspects of memory, semantic, social and emotional processing (Kahn et al., 2008; Ranganath and Ritchey, 2012). Nevertheless, for most default mode regions examined here, we found no difference between their functional connectivity to anterior versus posterior hippocampus. Therefore, the role of these separate pathways in the observed differences between anterior and posterior hippocampal functional connectivity needs to be viewed with caution. Although for this study we focused on the default mode network, it must be noted that age-related functional connectivity differences in other resting state networks and in interactions among networks have been also reported (Chan et al., 2014; Ferreira and Busatto, 2013; Geerligs et al., 2015). Our results do not preclude age-related functional connectivity differences across other brain networks, but we did not find them in the anterior hippocampal ROIs, contrary to what was reported elsewhere (Salami et al., 2014). This discrepancy may stem from sample characteristics, such as the age of participants. Although as in our study, the participants included in the study by Salami and colleagues (2014) nominally covered almost the entire adult lifespan, a larger proportion of their participants were older adults, as indicated by the average age of 61.5 years versus 49.1 years in our sample. Perhaps functional connectivity differences across networks other than the default mode become more apparent at an older age. Further research is needed to address this hypothesis.
Including grey matter volume estimates as covariates in functional connectivity studies has become a common practice, in spite of lack of a clear rationale for the relationship between functional connectivity and grey matter volume. In our sample, the correlations between ROI hippocampal volume and functional connectivity of corresponding pairs revealed no significant associations. Further examination of grey matter volume and functional connectivity is needed to more thoroughly assess the need for including grey matter volume in functional connectivity analyses.
Our results replicated the reported negative associations between older age and lower performance on multiple cognitive tests; in addition, we observed a relationship between default mode functional connectivity and performance on tasks of processing speed. However, no links between functional connectivity strength and cognitive performance were found once age was taken into account. This may reflect a well-known problem of cross-sectional mediation, see (Hofer and Sliwinski, 2001; Lindenberger et al., 2011; Lindenberger and Pötter, 1998; Maxwell and Cole, 2007) for discussions. The commonality between age, brain indices and cognitive performance measures is too strong to allow a meaningful representation of developmental dynamics (Raz and Lindenberger, 2011; Salthouse, 2011). Previous cross-sectional studies that did find a relationship between functional connectivity and cognitive function independent of age, worked with more a restricted age range and found these effects predominantly in the older adults (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Future studies using longitudinal designs are needed to investigate the relationship between functional connectivity and cognitive function independent of age.
Our examination of the relationship between gender, vascular, metabolic and genetic risk factors and hippocampal functional connectivity yielded two findings: First, a gender difference in hippocampal – medial prefrontal functional connectivity; second, an association between dopamine availability as assessed by COMT val158met and posterior hippocampal – posterior cingulate/retrosplenial functional connectivity. Our findings of gender differences in functional connectivity are in line with previous reports (Gong et al., 2011; Tomasi and Volkow, 2012), and more specifically, with a report of increased functional connectivity of the medial prefrontal cortex in females (Biswal et al., 2010). Because gender is associated with functional connectivity it is important to take it into account in data analysis. Here we included gender in the models examining age effects, and our reported results are significant after controlling for gender.
COMT is an enzyme that catabolizes dopamine in synaptic clefts, mainly of the D1 receptor locations that predominate in the prefrontal cortex. The val allele of the COMT val158met polymorphisms is associated with greater activity of COMT at the synapse and therefore very likely with lesser dopamine availability (Chen et al., 2004; Lotta et al., 1995). The probable increase in dopamine availability associated with the met allele has been linked to better performance on several executive tasks (Bruder et al., 2005; Caldú et al., 2007; de Frias et al., 2005; Raz et al., 2009), although these results have not been replicated consistently, see (Barnett et al., 2008) for a review. The majority of existing neuroimaging literature indicates that the val allele is associated with lower neuronal efficiency, resulting in higher prefrontal and decreased medial temporal lobe activation during working memory tasks (de Frias et al., 2009; Dennis et al., 2010), and lower functional connectivity of hippocampal and other default mode regions (Dennis et al., 2010; Liu et al., 2010). Across a younger part of the life-span, from neonates to middle-aged adults, homozygosity for the val (high activity) allele of COMT val158met polymorphism has been linked to reduced task-related hippocampal activation (Bertolino et al., 2006; Krach et al., 2010), and coupling between the hippocampus and other cortical regions (Bertolino et al., 2006; Dennis et al., 2010). In some samples of young adults, the intermediate levels of COMT activity are associated with greater connectivity of the prefrontal cortex (Dang et al., 2013), and in some instances, the effect of that genetic variant was observed only in conjunction with moderating factors, such as cumulative exposure to stress (Rabl et al., 2014). Our results showing a significant linear relationship between the dose of a met allele and functional connectivity are consistent with these patterns. Although COMT val158met variant is considered of particular relevance to the prefrontal dopaminergic system (Dickinson and Elvevåg, 2009), we found no effect of COMT on the connections involving the medial prefrontal cortex, which showed a relationship in the expected direction that did not reach significance. Notably, this is the first study to examine the effect of COMT val158met on functional connectivity in a sample including older adults and found its influence independent of age.
In addition to its role in cortical dopaminergic D1 synapses, dopamine – via the same receptors – acts as a vasodilator (Jose et al., 2003; Zeng et al., 2004), and the low-activity val allele has been associated with increased vascular risk (Hagen et al., 2007). Thus, in the context of an aging vascular system, probable low availability of dopamine in val homozygotes may impair vasodilatory response, and produce hypoperfusion. The impact of age differences in vasodilatory activity on functional connectivity indices – inferred from the resting state BOLD effect – merit further investigation.
None of the other risk factors (vascular, metabolic, or genetic) significantly affected functional connectivity, nor were they associated with cognitive function. Besides acknowledging the abovementioned problem of variance partitioning in a cross-sectional framework, we can only speculate that the performed health screening could have significantly mitigated the effects of these risk factors. In a sample devoid of most of the age-related disease, the effects of risk factors are quite subtle and elusive. Although the sample size of this study was reasonably large, uncovering such effects may require greater statistical power. Furthermore, it is worth noting that while we examined multiple potential risk factors there may be other factors, such as the presence of amyloid-β or tau, affecting functional connectivity. Further research is needed to examine these additional potential risk factors.
Respiration and cardiac pulsation are known to affect the BOLD signal (Dagli et al., 1999; Shmueli et al., 2007). Monitoring respiration and cardiac pulse during fMRI scan acquisition, and subsequently filtering them out using a priori models (Birn et al., 2006; Glover et al., 2000), can reduce these effects. A limitation of the current study is that no such techniques were applied as respiratory and cardiac data were not collected during scan acquisition. Nevertheless, thorough noise reduction was performed by applying ICA-AROMA, which in addition to motion artifacts also removes other structured noise from the data (e.g. cardiac pulsation artifacts) (Pruim et al., 2015), and global signal removal, which removes global noise, including respiration and pulsation artifacts (Birn et al., 2006).
5. Conclusions
In this report we provide evidence for an age-related partial functional disconnection of the posterior hippocampus to other hippocampal and default mode network regions. This reduction in functional connectivity seems to specifically affect the posterior hippocampus, as functional connectivity of the anterior hippocampus is unrelated to age. In addition, we show that, independent of age, females have higher hippocampal – medial prefrontal functional connectivity than males, and COMT val158met polymorphism is associated with lower posterior hippocampal – posterior cingulate/retrosplenial functional connectivity.
Highlights.
Advanced age is related to lower posterior hippocampal functional connectivity
Higher hippocampal – medial prefrontal connectivity in females vs. males
COMT val alleles are linked to lower posterior hippocampal functional connectivity
No association between functional connectivity and vascular or metabolic risk
Acknowledgments
This work was supported by a grant R37 AG011230 from the National Institutes of Health to NR. We thank Cheryl Dahle, Institute of Gerontology, for her assistance in data collection and management and Susan Land, Applied Genomics Technology Center, Wayne State University School of Medicine, for genotyping.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. BPS. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RWJ, Théberge J, Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008;19:887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. BPS. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007 doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Catheline G, Periot O, Amirault M, Braun M, Dartigues JF, Auriacombe S, Allard M. Distinctive alterations of the cingulum bundle during aging and Alzheimer’s disease. Neurobiol Aging. 2010;31:1582–1592. doi: 10.1016/j.neurobiolaging.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences. 2014;111:E4997–5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli MS, Ingeholm JE, Haxby JV. Localization of cardiac-induced signal change in fMRI. NeuroImage. 1999;9:407–415. doi: 10.1006/nimg.1998.0424. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD Alzheimer’s Disease Neuroimaging Initiative. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SARB. White matter tract integrity in aging and Alzheimer’s disease. Human brain mapping. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC, O’Neil JP, Jagust WJ. Genetic effects on behavior are mediated by neurotransmitters and large-scale neural networks. NeuroImage. 2013;66:203–214. doi: 10.1016/j.neuroimage.2012.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias C, Marklund P, Eriksson E, Larsson A, Oman L, Annerbrink K, Bäckman L, Nilsson L, Nyberg L. Influence of COMT Gene Polymorphism on fMRI-assessed Sustained and Transient Activity during a Working Memory Task. Journal of cognitive neuroscience. 2009 doi: 10.1162/jocn.2009.21318. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. Journal of cognitive neuroscience. 2005;17:1018–1025. doi: 10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van Der Linden M, Collette F. Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev. 2003;13:79–92. doi: 10.1023/a:1023832305702. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters-Metenier S, Cirulli ET, Kragel J, Goldstein DB, Cabeza R. COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cereb Cortex. 2010;20:672–683. doi: 10.1093/cercor/bhp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Elvevåg B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Neuroscience and Biobehavioral Reviews. Neuroscience and Biobehavioral Reviews. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative1 ADN. Progress in Neurobiology. Progress in Neurobiology. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang CY, de Haas HJ, Changchien L, Goliasch G, Dabas P, Wang V, Fayad ZA, Fuster V, Narula J. Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC Cardiovasc Imaging. 2014;7:1039–1053. doi: 10.1016/j.jcmg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb Cortex. 2015;25:1987–1999. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain Connectivity: Gender Makes a Difference. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2011 doi: 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart J-A. High systolic blood pressure is associated with Val/Val genotype in the catechol-o-methyltransferase gene. The Nord-Trøndelag Health Study (HUNT) Am J Hypertens. 2007;20:21–26. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding Ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Jagust W. Vulnerable Neural Systems and the Borderland of Brain Aging and Neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002 doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol. 2003;95:p19–27. doi: 10.1159/000073676. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct Cortical Anatomy Linked to Subregions of the Medial Temporal Lobe Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Normal Aging of the Brain. In: Toga AW, editor. Brain Mapping: an Encyclopedic Reference. Elsevier; 2015. [DOI] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde ALW, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Krach S, Jansen A, Krug A, Markov V, Thimm M, Sheldrick AJ, Eggermann T, Zerres K, Stöcker T, Shah NJ, Kircher T. COMT genotype and its role on hippocampal-prefrontal regions in declarative memory. NeuroImage. 2010;53:978–984. doi: 10.1016/j.neuroimage.2009.12.090. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human brain mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What’s change got to do with it? Psychol Aging. 2011;26:34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Pötter U. The complex nature of unique and shared effects in hierarchical linear regression: Implications for developmental psychology. Psychological Methods. 1998;3:218–230. doi: 10.1037/1082-989X.3.2.218. [DOI] [Google Scholar]
- Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T. Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J Neurosci. 2010;30:64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthen B. Mplus 6.0. Los Angeles: 2010. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72:931–937. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Potkin SG, van Erp T, Ling S, Macciardi F, Xie X. Identifying unanticipated genes and mechanisms in serious mental illness: GWAS-based imaging genetics strategies. In: Bigos KL, Hariri A, Weinberger DR, editors. Neuroimaging Genetics: Principles and Practices. Oxford University Press; 2016. pp. 141–156. [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, Popovic A, Scharinger C, Huemer J, Kalcher K, Pail G, Haslacher H, Perkmann T, Windischberger C, Brocke B, Sitte HH, Pollak DD, Dreher JC, Kasper S, Praschak-Rieder N, Moser E, Esterbauer H, Pezawas L. Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci. 2014;34:9917–9926. doi: 10.1523/JNEUROSCI.3113-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: cross-sectional studies offer no solution to the age-brain-cognition triangle: comment on Salthouse (2011) Psychological Bulletin. 2011;137:790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1410233111. 201410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002 doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Human brain mapping. 2012;33:849–860. doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PLoS ONE. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC, Eustache F, Chetelat G. Relationships between Hippocampal Atrophy, White Matter Disruption, and Gray Matter Hypometabolism in Alzheimer’s Disease. J Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KRA, Pihlajamäki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KRA, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Human brain mapping. 2013;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: a diffusion-tensor analysis. Arch Gerontol Geriatr. 2008;47:129–138. doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Zarei M, Beckmann CF, Binnewijzend MAA, Schoonheim MM, Oghabian MA, Sanz-Arigita EJ, Scheltens P, Matthews PM, Barkhof F. Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer’s disease. NeuroImage. 2013;66:28–35. doi: 10.1016/j.neuroimage.2012.10.071. [DOI] [PubMed] [Google Scholar]
- Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lu W, Shi Y, Bai F, Chang J, Yuan Y, Teng G. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neuroscience. 2010 doi: 10.1016/j.neulet.2009.12.057. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]