Abstract
In this study, a simple, rapid, and eco-friendly green method was introduced to synthesize magnetite nanoparticles (Fe3O4-NPs) successfully. Seaweed Kappaphycus alvarezii (K. alvarezii) was employed as a green reducing and stabilizing agents. The synthesized Fe3O4-NPs were characterized with X-ray diffraction (XRD), ultraviolet-visible spectroscopy (UV-Vis), Fourier transform infrared (FT-IR), and transmission electron microscopy (TEM) techniques. The X-ray diffraction planes at (220), (311), (400), (422), (511), (440), and (533) were corresponding to the standard Fe3O4 patterns, which showed the high purity and crystallinity of Fe3O4-NPs had been synthesized. Based on FT-IR analysis, two characteristic absorption peaks were observed at 556 and 423 cm−1, which proved the existence of Fe3O4 in the prepared nanoparticles. TEM image displayed the synthesized Fe3O4-NPs were mostly in spherical shape with an average size of 14.7 nm.
Keywords: Green synthesis, Fe3O4 nanoparticles, Seaweed, Kappaphycus alvarezii, Transmission electron microscopy
Background
With the recent rapid development and evolvement of technology, human beings have put their faith in nanotechnology and believe that it can ameliorate their current living standard [1]. As a consequence, the nanoparticle has drawn a huge interest from researchers globally due to specific characteristics such as shape, size, and distribution, which could be utilized in a distinct field of applications [2]. Synthesis of Fe3O4-NPs has been carried out because of its unique properties, such as being superparamagnetic [3], biocompatible, biodegradable, and expected to be non-toxic to humans [4–6]. These unique properties allow Fe3O4-NPs to be widely used in different areas of applications, such as catalysis [7, 8], magnetic storage media [9], biosensors [10], magnetic resonance imaging (MRI) [11, 12], and targeted drug delivery [13–15].
Numerous methods of fabrication of Fe3O4-NPs can be employed, such as sol-gel method [16], solid state synthesis [17], and flame spray synthesis [18]. In contrast to the time-consuming chemical and physical methods which involve complicated procedures, green method is much easier and safer to use, and plant-mediated synthesis of nanoparticles is still a new scheme and the outcome is yet to be studied. There are a couple of successful studies in synthesizing Fe3O4-NPs by using plant extract. For instance, fruit extract of Artemisia annua [19], leaf extract of Perilla frutescens [20], Tridax procumbens [21] and caricaya papaya [22], peel extract of plantain [23], and also seed extract of grape proanthocyanidin [24]. However, there are only finite studies on the synthesis of Fe3O4-NPs from marine plants.
Kappaphycus alvarezii (K. alvarezii) is a type of red seaweed from the family of Solieriaceae. It is well-known in the food industries for its gelling properties [25]. Carrageenan gives the thickening characteristic, which can be used as a function of green stabilizer in synthesis of nanoparticles without using hazardous chemicals. Based on the literature review, there are still no specific researches done on seaweed K. alvarezii for the Fe3O4-NPs synthesis, and this inspires and motivates us to work on this. Hence, in this research, a novel green method of synthesizing Fe3O4-NPs using seaweed K. alvarezii is proposed.
Main Text
Methods
Materials
Iron (II) chloride tetrahydrate (FeCl2.4H2O ≥ 99 %) and iron (III) chloride hexahydrate (FeCl3. 6H2O, 97 %) were purchased from Sigma-Aldrich. Sodium hydroxide (NaOH) was obtained from R&M Chemicals. All the chemicals were used without further purification. The seaweed K. alvarezii is a type of red seaweed which was acquired from Sabah, Malaysia. All the aqueous solutions were prepared by deionized water from ELGA Lab Water Purification System, UK. The sensION+ MM374 GLP 2 channel Benchtop Meter was employed to control the pH of the solution. An Esco Isotherm Forced Convection Laboratory Oven was used to dry the washed sample.
Preparation of Kappaphycus alvarezii Extract
The seaweed was washed under running water to remove dirt, salt, and foreign particles. Then, it was soaked overnight (24 h) in deionized water to bleach the yellowish color so that it became colorless. After that, the seaweed was rinsed and dried under sunlight for 3 days. The dried seaweed was chopped into small pieces before being blended using a hammer mill with a 3-mm filter diameter. Finally, the dried seaweed was stored until further processing. The dried seaweed reduced the storage space required and can be stored for a number of years without appreciable loss of the gelling property. In this study, 0.5 g of dried seaweed was weighed and soaked in 50 ml of deionized water for 24 h. The resulting extract was used as a seaweed extract solution.
Synthesis of K. alvarezii/Fe3O4-NPs
For the synthesis of K. alvarezii/Fe3O4-NPs, firstly, a solution of Fe3+ and Fe2+ with a 2:1 M ratio was added into the seaweed extract to obtain a yellowish colloidal solution. Then, the freshly prepared 1.0 M of NaOH was added drop-wise to the solution under continuous stirring. The pH of the solution was adjusted to pH 11. The solution was then stirred for 1 h to homogenize the solution and also for the completion of reaction. After that, the as-synthesized Fe3O4-NPs were separated by using a permanent magnet. The Fe3O4-NPs were washed for several times by using deionized water. The nanoparticles were dried in an oven at around 70 °C for 24 h. The dried sample was stored in an air-tight container for further characterization. All the experiments were conducted at ambient temperature.
Characterization of K. alvarezii/Fe3O4-NPs
The presence and phase purity of the synthesized K. alvarezii/Fe3O4-NPs were examined by using a PANalytical X’Pert PRO X-ray diffractometer (XRD). The dried sample was performed at an applied current of 20 mA and accelerating voltage of 45 kV with Cu Kα radiation (λ = 1.54 Å) at 2θ angle configuration scanning from 5° to 80° (scanning rate = 2θ/min). The UV-Vis spectrum of Fe3O4-NPs was determined using Shimadzu UV-Visible Spectrophotometer (UV-1800). Fourier transform infrared (FT-IR) spectroscopy was used to study the presence of the biomolecules which are responsible for the synthesis of Fe3O4-NPs. Dried samples were ground with potassium bromide (KBr) to produce pellet, which was examined in a wavelength range of 400–4000 cm−1. The infrared absorption peaks were obtained from Thermo Scientific Nicolet 6700 Spectrometer. The size and morphology of the synthesized Fe3O4-NPs were observed using FEI TECNAI G2 F20 transmission electron microscope (TEM). The microscope had accelerating voltage from 20 to 200 kV and standard magnification from 22 X to 930 KX. The aqueous dispersion of the nanoparticles was dropped on 300-mesh copper grids and air-dried before viewing under a microscope. TEM images were acquired using a SC1000 ORIUS CCD Camera.
Discussion
After addition of NaOH and stirring the solution for 1 h, the color of the reaction mixture of iron chloride salts and seaweed extract changed from light brown (Fig. 1a) to black (Fig. 1b), which indicates the formation of Fe3O4-NPs. Separation of Fe3O4-NPs could be done with the aid of an external permanent magnet. Figure 1c clearly reveals that the synthesized Fe3O4-NPs is able to be attracted by an external permanent magnet quickly, which proved that the nanoparticles possessed magnetic properties. Once the magnet was removed, the nanoparticles were dispersed readily by shaking.
Fig. 1.
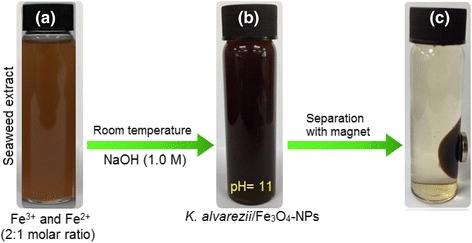
a Solution of Fe3+ and Fe2+ with 2:1 M ratio and seaweed K. alvarezii extract. b K. alvarezii/Fe3O4-NPs. c Separation of synthesized Fe3O4-NPs from reaction mixture using an external magnet
Fe3O4 nanoparticles are prepared by adding a base to an aqueous mixture of Fe3+ and Fe2+ chloride at a 2:1 M ratio. The chemical reaction of Fe3O4 precipitation is given in Eqs. 1 and 2. The overall reaction may be written as follows:
| 1 |
| 2 |
The precipitation occurs because of the Fe3O4-NPs have a high tendency to aggregate into agglomerates as to decrease the energy associated with the large surface area to volume ratio, a phenomenon which is likely deteriorated by the low surface charge [26].
UV-Vis Spectral Analysis
The UV-Vis spectroscopy of seaweed K. alvarezii and synthesized Fe3O4-NPs are shown in Fig. 2. Seaweed K. alvarezii does not show any absorption peak (blue). However, the synthesized Fe3O4-NPs reveals continuous absorption in the visible range of 300–800 nm without any strong absorption peak (red). Studies from Basavegowda et al. had reported identical UV-Vis spectra for Fe3O4-NPs synthesized using A. annua and P. frutescens extracts [19, 20].
Fig. 2.
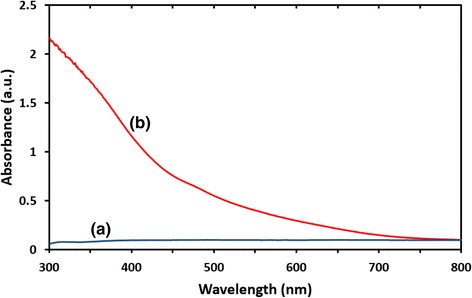
UV-vis absorption spectra of (a) seaweed K. alvarezii. (b) Synthesized Fe3O4-NPs
XRD Analysis
Phase purity and crystallinity of the synthesized Fe3O4-NPs can be identified via XRD analysis. The XRD patterns of seaweed K. alvarezii and synthesized Fe3O4-NPs are shown in Fig. 3. A broad diffraction peak was observed (Fig. 3a) at 21.50°, which is attributed to seaweed K. alvarezii. This is confirmed by a study which showed unanimous XRD patterns of seaweed K. alvarezii that were used in the synthesis of Cu@Cu2O core shell nanoparticles [27]. The diffraction peaks of synthesized Fe3O4-NPs (Fig. 3b) were detected at 2θ = 30.56°, 35.86°, 43.46°, 54.01°, 57.38°, 63.00°, and 74.46°, which are assigned to the crystal planes of (200), (311), (400), (422), (511), (440), and (533), respectively. The analyzed diffraction peaks were matched well with the standard magnetite XRD patterns with JCPDS file no: 00-003-0863, which declared the crystallographic system of cubic structure. Besides, the synthesized nanoparticles were affirmed to be Fe3O4 but not maghemite (γ-Fe2O3) by comparing the XRD patterns with the standard maghemite JCPDS file no.: 01-089-3850. A huge difference can be clearly seen in that the XRD patterns of γ-Fe2O3 consist of many peaks, unlike Fe3O4 which only involves few peaks. Estimation of the crystallite size of synthesized Fe3O4-NPs can be calculated by using the Debye-Scherrer equation [28], which reveals a relationship between X-ray diffraction peak broadening and crystallite size. The Debye-Scherrer equation is shown as follows:
Fig. 3.
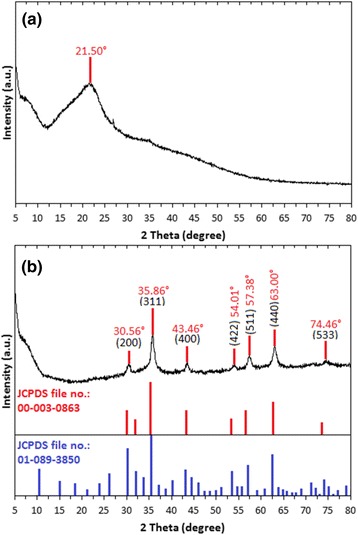
XRD patterns of a seaweed K. alvarezii. b Synthesized Fe3O4-NPs
where d is the crystallite size of synthesized Fe3O4-NPs for (hkl) phase, k is Scherrer constant (0.9), λ is the X-ray wavelength of radiation for Cu Kα (0.154 nm), βhkl is the full-width at half maximum (FWHM) at (hkl) peak in radian, and θhkl is the diffraction angle for (hkl) phase. Using the equation, the estimated crystallite size of synthesized Fe3O4-NPs was 16.79 nm, which was calculated from the full-width at half maximum of the Fe3O4 (311) diffraction peak [29] at 2θ = 35.86°. Based on the X-ray diffraction pattern, the synthesized Fe3O4-NPs were figured out to be high purity crystalline, as no impurity peak was observed.
Transmission Electron Microscopy Study
The size and morphology of the synthesized Fe3O4-NPs were analyzed by using TEM. The TEM image of synthesized Fe3O4-NPs (Fig. 4a) showed that majority of the nanoparticles were in spherical shape. The image revealed that most of the particles were agglomerated, which might be due to the thickening properties of seaweed K. alvarezii or the presence of hydroxyl groups from the extract [30]. Besides, the tendency of agglomeration is not surprising as the synthesized Fe3O4-NPs is small in size and possess magnetic characteristics [31]. A histogram of particle size distribution was drawn according to the size of 70 nanoparticles (Fig. 4b). The mean particle size was 14.7 nm with the standard deviation of 1.8 nm. The crystallite size of the synthesized Fe3O4-NPs was found to be 16.7 nm from XRD analysis, which is in an agreement with the result obtained from the TEM that shows a size distribution between 11.0 and 20.0 nm. A few very small spherical objects can be observed from the image which might be due to the residue of seaweed K. alvarezii.
Fig. 4.
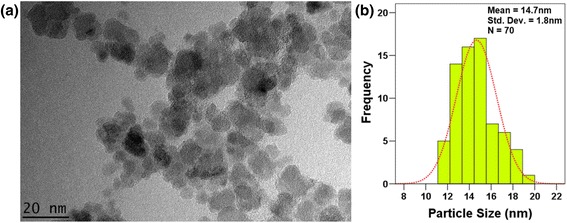
a TEM image of the synthesized Fe3O4-NPs at magnification of 35 KX. b Size distribution histogram of the synthesized Fe3O4-NPs
Fourier Transform Infrared Study
FT-IR spectroscopy was performed to determine the functional groups of seaweed K. alvarezii that acted as a stabilizer and capping agent in the synthesis of Fe3O4–NPs. The spectra of the seaweed K. alvarezii extract revealed strong absorption bands at 3439, 2916, 1636, 1227, 1064, 930, 848, and 704 cm−1 (Fig. 5a), while the absorption bands of synthesized Fe3O4-NPs were observed at 3411, 2917, 1633, 1228, 1065, 928, 846, 556, and 423 cm−1 (Fig. 5b). The absorption peak of 3439 cm−1 in the K. alvarezii extract indicated the O–H stretching vibration. The absorption peaks at 2916 cm−1 contributed to the C–H stretching vibrations of the –CH2 functional group [32], while 1636 cm−1 were attributed to the C–H bending overtone band of aromatic compound. Absorption peak at 1227 cm−1 corresponded to the asymmetric stretching vibration of the sulfate group [33], whereas peak at 1064 cm−1 was assigned to the C–O stretching band related to the C–O–SO3 group [34]. The absorption peaks 930, 848, and 704 cm−1 revealed the existence of aromatic C–H bending band. All the bands were shifted, indicating the participation and interaction of nanoparticles with the seaweed K. alvarezii extract [35, 36]. Two significant new peaks were found at 556 and 423 cm−1 in the spectra of synthesized Fe3O4-NPs, which is associated with the stretching vibration mode of Fe–O. The metal-oxygen band at 556 cm−1 corresponded to intrinsic stretching vibrations of metal at the tetrahedral site, while the metal-oxygen band found at 423 cm−1 was assigned to octahedral-metal stretching of Fe–O [37]. The formation of Fe3O4-NPs was confirmed with these characteristic peaks as the peaks laying in the region between 400 and 600 cm−1 were corresponding to Fe3O4 [28].
Fig. 5.
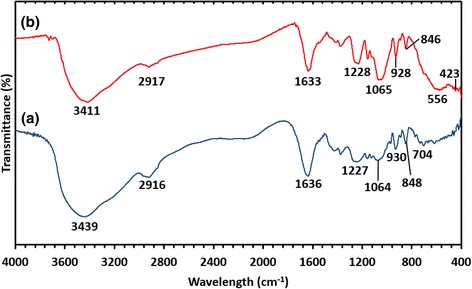
FT-IR spectra of (a) seaweed K. alvarezii. (b) Synthesized Fe3O4-NPs
A presumed schematic diagram of formation of K. alvarezii/Fe3O4-NPs is illustrated in Fig. 6, where the presence of van der Waals forces holds the positively charged Fe3O4-NPs and negatively charged groups presenting in the molecular structure of seaweed K. alvarezii together [38].
Fig. 6.
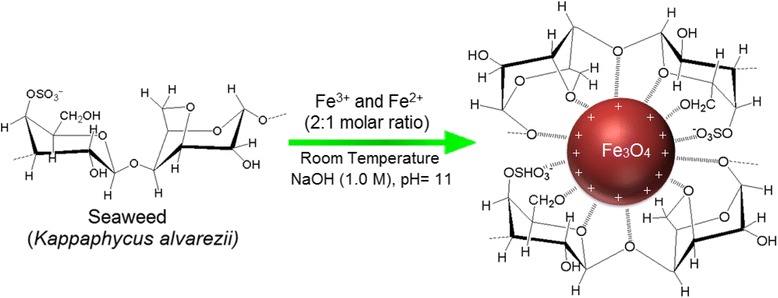
Schematic diagram demostrating the interaction between Fe3O4-NPs-charged groups which are capped by seaweed K. alvarezii
Conclusions
In this study, Fe3O4-NPs were synthesized successfully by a simple and green approach using the seaweed K. alvarezii extract without utilizing any chemical-reducing agent and stabilizer. Based on the XRD analysis studied, a high purity crystalline of Fe3O4-NPs was prepared. FT-IR spectroscopy showed the involvement biomolecules present in the extract of seaweed K. alvarezii, which were verified in the synthesizing process of Fe3O4-NPs. The formation of Fe3O4-NPs was confirmed due to the noticeable absorption peaks at 556 and 423 cm−1. TEM result revealed the size and morphology of the synthesized Fe3O4-NPs. Most of the particles possessed spherical shapes with average particle sizes of 14.7 nm. The non-toxic green synthesized Fe3O4-NPs are expected suitable to be employed in various fields of applications, especially in biomedical applications.
Abbreviations
NPs nanoparticles, K. alvarezii Kappaphycus alvarezii, XRD X-ray diffraction, UV-Vis ultraviolet-visible spectroscopy, FT-IR Fourier transform infrared, TEM transmission electron microscopy
Acknowledgements
This research was supported by the grant funded by the Ministry of education (Reference grant number PY/2015/05547 under FRGS grant). Also, the authors would like to express their gratitude to the Research Management Centre (RMC) of UTM for providing a conducive environment to carry out this research.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
YPY and KS carried out the synthesis and characterization of the compounds. YPY, KS, MM, NK, NBBAK, SEBM, and KXL carried out the acquisition of data, analysis and interpretation of the data collected, were involved in the drafting of the manuscript and revision of the draft for important intellectual content, and gave final approval of the version to be published. All authors read and approved the final manuscript.
References
- 1.Priest S. The North American opinion climate for nanotechnology and its products: opportunities and challenges. J Nanopart Res. 2006;8(5):563–568. doi: 10.1007/s11051-005-9060-7. [DOI] [Google Scholar]
- 2.Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F, Farahani F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 2011;16(8):6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahdavian AR, Mirrahimi MAS. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem Eng J. 2010;159(1):264–271. doi: 10.1016/j.cej.2010.02.041. [DOI] [Google Scholar]
- 4.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv Mater. 2006;18(19):2553–2556. doi: 10.1002/adma.200600385. [DOI] [Google Scholar]
- 5.Zhao H, Saatchi K, Häfeli UO. Preparation of biodegradable magnetic microspheres with poly(lactic acid)-coated magnetite. J Magn Magn Mater. 2009;321(10):1356–1363. doi: 10.1016/j.jmmm.2009.02.038. [DOI] [Google Scholar]
- 6.Zhang L, Dong WF, Sun HB. Multifunctional superparamagnetic iron oxide nanoparticles: design, synthesis and biomedical photonic applications. Nanoscale. 2013;5(17):7664–7684. doi: 10.1039/c3nr01616a. [DOI] [PubMed] [Google Scholar]
- 7.GawandeMB BPS, Varma RS. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev. 2013;42(8):3371–3393. doi: 10.1039/c3cs35480f. [DOI] [PubMed] [Google Scholar]
- 8.Sharad NS, Swapnil RB, Ganesh RM, Samadhan SK, Dinesh KM, Shashikant BB, Anuj KR, Nenad B, Orlando MNDT, Radek Z, Rajender SV, Manoj BG. Iron oxide-supported copper oxide nanoparticles (nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and ullmann-type condensation reactions. ACS Sustainable Chem Eng. 2014;2(7):1699–1706. doi: 10.1021/sc500160f. [DOI] [Google Scholar]
- 9.Terris BD, Thomson T. Nanofabricated and self-assembled magnetic structures as data storage media. J Phys D Appl Phys. 2005;38(12):R199–R222. doi: 10.1088/0022-3727/38/12/R01. [DOI] [Google Scholar]
- 10.Kavitha AL, Prabu HG, Babu SA, Suja SK. Magnetite nanoparticles-chitosan composite containing carbon paste electrode for glucose biosensor application. J Nanosci Nanotechnol. 2013;13(1):98–104. doi: 10.1166/jnn.2013.6720. [DOI] [PubMed] [Google Scholar]
- 11.Haw CY, Mohamed F, Chia CH, Radiman S, Zakaria S, Huang NM, Lim HN. Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceram Int. 2010;36(4):1417–1422. doi: 10.1016/j.ceramint.2010.02.005. [DOI] [Google Scholar]
- 12.Qiao RR, Yang CH, Gao MY. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem. 2009;19(35):6274–6293. doi: 10.1039/b902394a. [DOI] [Google Scholar]
- 13.Salem M, Xia Y, Allan A, Rohani S, Gillies ER. Curcumin-loaded, folic acid-functionalized magnetite particles for targeted drug delivery. RSC Adv. 2015;5(47):37521–37366. doi: 10.1039/C5RA01811K. [DOI] [Google Scholar]
- 14.Li XL, Li H, Liu GQ, Deng ZW, Wu SL, Li PH, Xu ZS, Xu HB, Chu PK. Magnetite-loaded fluorine-containing polymeric micelles for magnetic resonance imaging and drug delivery. Biomaterials. 2012;33(10):3013–3025. doi: 10.1016/j.biomaterials.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Wani KD, Kadu BS, Mansara P, Gupta P, Deore AV, Chikate RC, Poddar P, Dhole SD, Kaul-Ghanekar R. Synthesis, characterization and in vitro study of biocompatible cinnamaldehyde functionalized magnetite nanoparticles (CPGF Nps) for hyperthermia and drug delivery applications in breast cancer. Plos One. 2014;9(9):e107315. doi: 10.1371/journal.pone.0107315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Lemine OM, Omri K, Zhang B, El Mir L, Sajieddine M, Alyamani A, Bououdina M. Sol–gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattice Microst. 2012;52(4):793–799. doi: 10.1016/j.spmi.2012.07.009. [DOI] [Google Scholar]
- 17.Paiva DL, Andrade AL, Pereira MC, Fabris JD, Domingues RZ, Alvarenga ME. Novel protocol for the solid-state synthesis of magnetite for medical practices. Hyperfine Interact. 2015;232:19–27. doi: 10.1007/s10751-015-1124-1. [DOI] [Google Scholar]
- 18.Kumfer BM, Shinoda K, Jeyadevan B, Kennedy IM. Gas-phase flame synthesis and properties of magnetic iron oxide nanoparticles with reduced oxidation state. J Aerosol Sci. 2010;41(3):257–265. doi: 10.1016/j.jaerosci.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basavegowda N, Magar KBS, Mishra K, Lee YR. Green fabrication of ferromagnetic Fe3O4 nanoparticles and their novel catalytic applications for the synthesis of biologically interesting benzoxazinone and benzthioxazinone derivatives. New J Chem. 2014;38(11):5415–5420. doi: 10.1039/C4NJ01155D. [DOI] [Google Scholar]
- 20.Basavegowda N, Mishra K, Lee YR. Sonochemically synthesized ferromagnetic Fe3O4 nanoparticles as a recyclable catalyst for the preparation of pyrrolo [3, 4-c] quinoline-1,3-dione derivatives. RSC Adv. 2014;4(106):61660–61666. doi: 10.1039/C4RA11623B. [DOI] [Google Scholar]
- 21.Senthil M, Ramesh C. Biogenic synthesis of Fe3O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on pseudomonas aeruginosa. Dig J Nanomater Biostruct. 2012;7(4):1655–1659. [Google Scholar]
- 22.Latha N, Gowri M. Biosynthesis and characterisation of Fe3O4 nanoparticles using Caricaya papaya leaves extract. Int J Sci Res. 2014;3(11):1551–1556. [Google Scholar]
- 23.Venkateswarlu S, Rao YS, Balaji T, Prathima B, Jyothi NVV. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater Lett. 2013;100:241–244. doi: 10.1016/j.matlet.2013.03.018. [DOI] [Google Scholar]
- 24.Narayanan S, Sathy BN, Mony U, Koyakutty M, Nair SV, Menon D. Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl Mater Interfaces. 2012;4(1):251–260. doi: 10.1021/am201311c. [DOI] [PubMed] [Google Scholar]
- 25.Raman M, Doble M. Physicochemical and structural characterisation of marine algae Kappaphycus alvarezii and the ability of its dietary fibres to bind mutagenic amines. J Appl Phycol. 2014;26(5):2183–2191. doi: 10.1007/s10811-014-0241-6. [DOI] [Google Scholar]
- 26.Valentin VM, Svetlana SM, Andrew JL, Olga VS, Anna OD, Igor VY, Michael ET, Natalia OK. Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants. Langmuir. 2014;30(20):5982–5988. doi: 10.1021/la5011924. [DOI] [PubMed] [Google Scholar]
- 27.Khanehzaei H, Ahmad MB, Shameli K, Ajdari Z. Synthesis and characterization of Cu@Cu2O core shell nanoparticles prepared in seaweed Kappaphycus alvarezii media. Int J Electrochem Sci. 2014;9:8189–8198. [Google Scholar]
- 28.Yuvakkumar R, Hong SI. Green synthesis of spinel magnetite iron oxide nanoparticles. Adv Mater Res. 2014;1051:39–42. doi: 10.4028/www.scientific.net/AMR.1051.39. [DOI] [Google Scholar]
- 29.Sofia S, Gawande MB, Alexandre V, João PV, Nenad B, João T, Alexander T, Orlando MNDT, Radek Z, Rajender SV, Paula SB. Magnetically recyclable magnetite-palladium (Nanocat-Fe-Pd) nanocatalyst for the Buchwald-Hartwig reaction. Green Chem. 2014;16(7):3494–3500. doi: 10.1039/c4gc00558a. [DOI] [Google Scholar]
- 30.Venkateswarlu S, Yoon MY. Surfactant-free green synthesis of Fe3O4 nanoparticles capped with 3, 4-dihydroxyphenethylcarbamodithioate: stable recyclable magnetic nanoparticles for the rapid and efficient removal of Hg(II) ions from water. J Chem Soc Dalton Trans. 2015;44(42):18427–18437. doi: 10.1039/C5DT03155A. [DOI] [PubMed] [Google Scholar]
- 31.Manoj BG, Anuj KR, Isabel DN, Rajender SV, Paula SB. Magnetite-supported sulfonic acid: a retrievable nanocatalyst for the Ritter reaction and multicomponent reactions. Green Chem. 2013;15(7):1895–1899. doi: 10.1039/c3gc40457a. [DOI] [Google Scholar]
- 32.Awwad AM, Salem NM. A green and facile approach for synthesis of magnetite nanoparticles. J Nanosci Nanotechnol. 2012;2(6):208–213. doi: 10.5923/j.nn.20120206.09. [DOI] [Google Scholar]
- 33.Mahdavi M, Namvar F, Ahmad MB, Mohamad R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18(5):5954–5964. doi: 10.3390/molecules18055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camara FBG, Costa LA, Fidelis GP, Nobre LTDB, Dantas-Santos N, Cordeiro SL, Costa MSSP, Alves LG, Rocha HAO. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Ma Drugs. 2011;9(1):124–138. doi: 10.3390/md9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkateswarlu S, Kumar BN, Prasad CH, Venkateswarlu P, Jyothi NVV. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Physica B Condens Matter. 2014;449:67–71. doi: 10.1016/j.physb.2014.04.031. [DOI] [Google Scholar]
- 36.Abdullah NIS, Ahmad MB, Shameli K. Biosynthesis of silver nanoparticles using Artocarpus elasticus stem bark extract. Chem Cent J. 2015;9(1):1–7. doi: 10.1155/2015/524056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demir A, Topkaya R, Baykal A. Green synthesis of superparamagnetic Fe3O4 nanoparticles with maltose: its magnetic investigation. Polyhedron. 2013;65:282–287. doi: 10.1016/j.poly.2013.08.041. [DOI] [Google Scholar]
- 38.Elsupikhe RF, Shameli K, Ahmad MB, Ibrahim NA, Zainudin N. Green sonochemical synthesis of silver nanoparticles at varying concentrations of k-carrageenan. Nanoscale Res Lett. 2015;10(1):302. doi: 10.1186/s11671-015-0916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


