Abstract
Background
There is increasing evidence that the pathological overeating underlying some forms of obesity is compulsive in nature, and therefore contains elements of an addictive disorder. However, direct physiological evidence linking obesity to synaptic plasticity akin to that occurring in addiction is lacking. We sought to establish whether the propensity to diet-induced obesity (DIO) is associated with addictive-like behavior, as well as synaptic impairments in the nucleus accumbens core (NAcore) considered hallmarks of addiction.
Methods
Sprague-Dawley rats were allowed free access to a palatable diet for 8 weeks then separated by weight gain into DIO prone (OP) and resistant (OR) subgroups. Access to palatable food was then restricted to daily operant self-administration sessions using fixed (FR1, 3 and 5) and progressive ratio (PR) schedules. Subsequently, NAcore brain slices were prepared and we tested for changes in the ratio between AMPA and NMDA currents (AMPA/NMDA) and the ability to exhibit long-term depression (LTD).
Results
We found that propensity to develop DIO is linked to deficits in the ability to induce LTD in the NAcore, as well as increased potentiation at these synapses as measured by AMPA/NMDA currents. Consistent with these impairments, we observed addictive-like behavior in OP rats, including i) heightened motivation for palatable food (ii) excessive intake and (iii) increased food-seeking when food was unavailable.
Conclusions
Our results show overlap between the propensity for DIO and the synaptic changes associated with facets of addictive behavior, supporting partial coincident neurological underpinnings for compulsive overeating and drug addiction.
Keywords: food addiction, obesity, nucleus accumbens, synaptic plasticity, glutamate, long term depression
Introduction
Obesity is rapidly approaching tobacco use as the leading cause of death in the industrialized world (1). While many factors may underlie obesity, the increasing availability of highly palatable, processed foods is a major contributor. Similar to drugs of abuse, highly palatable foods are powerful reinforcers and interact with brain reward circuitry to promote intake (2-7). As with drug addiction, this can lead to pathological overconsumption in susceptible individuals. Thus, it could be argued that in addition to homeostatic feeding mechanisms, excessive intake of palatable food may be explained by dysfunctions in reward circuitry. Indeed, there is emerging evidence in both humans and rodents that supports a hypothesis that the brain's reward circuitry is dysregulated in certain types of obesity, specifically that which results from compulsive overeating (5, 7-17). This can manifest in symptoms that parallel those observed in drug addiction such as uncontrolled and excessive consumption, unsuccessful attempts to cut back or reduce intake, and the continuation of overeating despite adverse consequences (18-20).
The transition to drug addiction has been strongly linked to changes in prefrontal cortex regulation of basal ganglia circuitry (21, 22). Using animal models of self-administration and relapse, enduring impairments in glutamatergic transmission and synaptic plasticity have been shown on medium spiny neurons in the nucleus accumbens (22). Neuroadaptations in these synapses can be shared between different chemical classes of addictive drugs (22-25). For example, repeated use of drugs such as cocaine and nicotine produces a long-lasting potentiation of these synapses together with a deficit in the ability to induce synaptic plasticity (23, 26-28). Critically, an enduring impairment in the ability to induce long-term depression (LTD) in the accumbens core subcompartment (NAcore) of animals classified as addiction-vulnerable to cocaine, but not as addiction-resilient, has been implicated in the transition to addiction (27). These data point to potentiated glutamatergic synapses in the accumbens with an impaired ability to undergo LTD as a pathology in psychostimulant addiction. Thus, we sought to examine whether rats prone to obesity due to excessive intake of palatable food would exhibit these cardinal synaptic impairments and show similar characteristics towards food that rats classified as addiction-vulnerable show towards drugs. We assessed in rats three addiction-like behaviors, used as hallmarks of both drug addiction and pathological overeating (19, 25, 29, 30): (i) A high motivation to obtain the substance; modeled using a progressive ratio (PR) schedule, whereby the effort required to obtain food increases progressively within the session; (ii) The rapid consumption of significantly larger than normal amounts of the substance; modeled by measuring intake when access to palatable food was limited; (iii) A lack of control to refrain from seeking the substance; modeled by measuring the persistence of lever-pressing during periods that signaled reward unavailability.
Methods and Materials
Experimental Subjects
Experimentally naive, outbred male Sprague-Dawley rats (Charles River Laboratories) weighing 250-300 g at the start of the experiment were housed individually with nesting/enrichment material made available. A 12-h light/dark cycle was maintained at all times, with lights turned off at 6:00 a.m. Experimental procedures were approved by the MUSC Animal IACUC. Rats were given ad libitum access to water and either standard chow (Tekland Global 2018, 18% kcal fat; total density=3.1 kcal g−1, Harlan Laboratories Inc, Indianapolis) or palatable diet (D12451, 45% kcal fat; total density=4.73 kcal g−1; Research Diets Inc, New Brunswick, NJ, USA). Rats were given 7 days to acclimate before experimentation began. A second cohort of Sprague-Dawley rats (Monash University) was housed in similar conditions. Their standard chow diet was obtained from Barastoc (8720610, 9% kcal rat; total density=3.1 kcal g−1, Barastoc, Melbourne, Australia).
Model of diet-induced obesity
Obesity and drug addiction share the characteristic that when a population is exposed to palatable food or drug, only a subpopulation will develop obesity or addiction. We wanted to model the subpopulation of obese individuals that develop obesity due to excessive overeating of palatable food, as opposed to obesity caused by other factors. We employed a naturalistic diet-induced obesity model that separates outbred rats into obesity prone and resistant groups based on weight gain in response to a palatable diet (31, 32). Diet-induced obese rats in this model exhibit hyperphagia, increased adiposity, as well as the typical metabolic disturbances found in human obesity (33-36). Rats were placed on a highly palatable diet (D12451, 45% kcal fat; total density=4.73 kcal g−1; Research Diets Inc, New Brunswick, NJ, USA) for a period of 8 weeks. Food intake and body weight were determined twice per week. Rats were then separated into diet-induced obese prone (OP, top third) and diet-induced obese resistant (OR, bottom third) groups based on weight gain (Figure 1) (31). Weight gain was determined from weeks 3-8 of the 8 week period in order to avoid weight gain due to normal development during the first 2 weeks. A second group of rats was split into two groups and given access to either standard chow or palatable diet (SF04-001, identical formulation to D12451, 45% kcal fat; total density=4.73 kcal g−1; Specialty Feeds, Glen Forrest, Australia) for a period of at least 8-weeks before being separated by weight gain (also determined from weeks 3-8 of the 8 week period) and utilized for electrophysiology experiments.
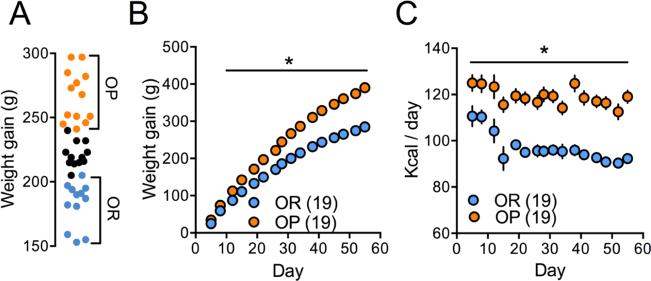
Figure 1.
Free access to palatable food diet causes obesity in some rats but not in others. (A) Weight gain spread of a representative group of rats following 8 weeks of ad libitum palatable food diet in their home cages. Top third – diet-induced obese (OP) rats. Bottom third – diet-induced obesity resistant (OR) rats. (B) OP rats gained more weight than OR rats (two-way ANOVA, F(1,36)=96.64, p<0.0001 for weight gain main effect) during the 8 week diet period. (C) OP rats consumed more calories than OR rats (two-way ANOVA, F(1,36)=69.69 for Kcal consumed main effect) during the 8 week diet period. * - p<0.05. Data represents mean±SEM.
Operant self-administration protocol
After the 8-week diet period rats were placed on standard chow ad libitum in their home cage and their access to palatable food restricted to 45min daily during an operant session. The operant session, a modified version of those previously designed to identify ‘addiction vulnerable’ versus ‘addiction resilient’ subjects (25, 37) consisted of alternating ‘reward available’ (designated S+, 15min × 3) and ‘reward unavailable’ (designated ‘S−’, 5min × 3) periods that were paired with distinct discriminative stimuli. During S+ periods lever pressing on the active lever resulted in the dispensing of a 45 mg palatable food pellet (F06162, 45% kcal from fat, total density=4.6 kcal g−1; Bioserv Inc, Frenchtown, NJ, USA). Responding on the active lever during S−, as well as responding on the inactive lever during either S+ or S− periods also resulted in no programmed consequence.
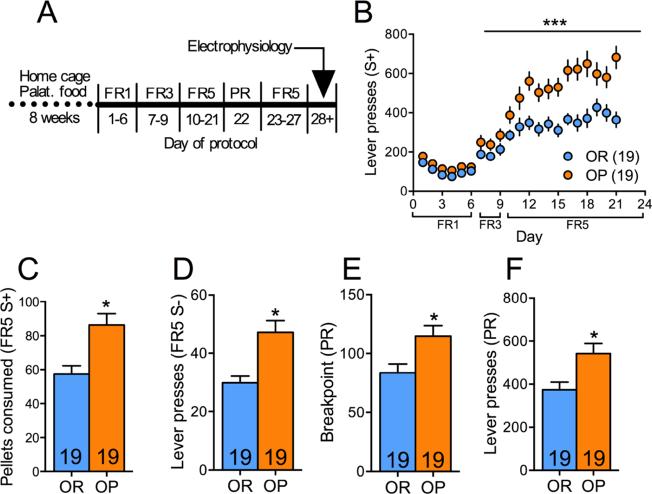
Figure 2 shows the operant protocol whereby rats began on a fixed ratio (FR) of 1 under only S+ conditions for 45min. After 3 d the S− period was introduced and sessions were extended to 60min with alternating S+, S−. Rats experienced FR1 for a further 3 d before the response requirement was increased to FR3 (3d) and then FR5 (remainder of protocol). The progressive ratio (PR) session was conducted in a single session after FR5 responding had been established (typically after 5d FR5). The PR breakpoint was taken as the last step completed prior to a lapse of 1h during which no pellets were earned or the last step completed in 5h, whichever occurred first.
Figure 2.
OP rats show increased addictive-like behavior as compared to OR rats. (A) Experimental protocol. FR – fixed ratio. (B) Timecourse of lever pressing during the S+ periods over the operant protocol schedule. *** - p=0.0003 (2-way ANOVA, F(1,36)=15.70 for main effect of weight gain). (C-F) OP rats showed increased pellet consumption during the FR5 S+ period (unpaired t-test, t(36)=3.49, p=0.0013) and higher lever pressing during the FR5 S− period (unpaired t-test, t(36)=3.755, p=0.0006), higher breakpoint (BP, Mann-Whitney test, U=90, p=0.0063) as well as lever presses (unpaired t-test, t(36)=2.87, p=0.007) during a progressive ratio (PR) task. Numbers in bars – rats. * - p<0.05. Data represents mean±SEM.
Electrophysiology
Slice preparation
Slices were prepared from animals 24h after their final self-administration session approximately the same time each morning for animals still on the homecage diet. Rats were anesthetized with ketamine HCl (1 mg/kg Ketaset, Fort Dodge Animal Health) and decapitated. Brain was removed and coronal accumbens brain slices (220μm) (VT1200S Leica vibratome) were collected into a vial containing aCSF (in mM: 126 NaCl, 1.4 NaH2PO4, 25 NaHCO3, 11 glucose, 1.2 MgCl2, 2.4 CaCl2, 2.5 KCl, 2.0 NaPyruvate, 0.4 ascorbic acid, bubbled with 95% O2 and 5% CO2) and a mixture of 5mM kynurenic acid and 50μM D-(−)-2-Amino-5-phosphonopentanoic acid (D-AP5). Slices were stored at room temperature until recording.
In vitro whole cell recording
All recordings were collected at 32°C (TC-344B, Warner Instrument Corporation) in the dorsomedial NAcore, and the stimulating electrode positioned to optimize activating fibers from prefrontal inputs, although excitatory afferents from other brain regions would also be expected to contribute to the EPSC (38, 39). Medium spiny neurons were visualized with an Olympus BX51WI microscope. Inhibitory synaptic transmission was blocked with picrotoxin (50 μM). Multiclamp 700B (Axon Instruments, Union City, CA) was used to record excitatory postsynaptic currents (EPSCs) in whole cell configuration. Glass microelectrodes (1.3-2 MΩ) were filled with cesium-based internal solution (in mM: 124 cesium methanesulfonate, 10 HEPES potassium, 1 EGTA, 1 MgCl2, 10 NaCl, 2.0 MgATP, and 0.3 NaGTP, 1 QX-314, pH 7.2-7.3, 275mOsm). Recordings started no earlier than 10min after the cell membrane was ruptured, to allow diffusion of internal solution into the cell. Data were acquired at 10kHz, and filtered at 2kHz using AxoGraph X software (AxoGraph Scientific, Sydney). To evoke EPSCs, a bipolar stimulating electrode was placed ~100-200 μm dorsomedial of the cell to maximize chances of stimulating PFC afferents. The stimulation intensity chosen evoked a 30-70% of maximal EPSC. Recordings were collected every 20sec (for AMPA/NMDA experiments) or 10sec (for LTD experiments). Series resistance (Rs) measured with a −2 mV depolarizing step (10 ms) given with each stimulus and holding current were always monitored online. Recordings with unstable Rs, or when Rs exceeded 20 MΩ were aborted. AMPA/NMDA ratios and LTD measurements were never obtained from the same slice.
AMPA/NMDA and LTD
AMPA/NMDA was recorded at +40 mV. Currents composed of both AMPA and NMDA were first obtained, followed by bath application of 50μM (2R)-amino-5-phosphonovaleric acid (D-AP5), an NMDA receptor antagonist, and recording of AMPA currents alone. NMDA current was obtained by subtraction of AMPA current from total current. Time to which the NMDA current decayed to 37% of its peak was used to estimate NMDA decay. LTD was measured at −80 mV. A baseline of 10min was followed by an LTD protocol (26) after which recording continued for 30min.
LTD measurements
Baseline EPSCs were first measured for 10min (0.1Hz). After obtaining a stable baseline, we applied the LTD protocol described in (26). LTD was induced by applying three 5Hz trains, each for 3min, with 5min inter-train interval. Trains were paired with depolarization of the cell to −50 mV while during the inter-train intervals the membrane potential was brought back to −80 mV. After the last train membrane potential was returned to −80 mV and recording at 0.1Hz was resumed for 30min.
Paired-pulse ratio measurement
A pair of electrical pulses was administered with an inter-pulse interval of 50ms every 20sec. The paired-pulse ratio (PPR) was calculated as the ratio between the peak amplitude of the second and the first EPSCs.
sEPSC analysis
Spontaneous synaptic activity was measured without stimulation for at least 100sec per cell. Events were detected using Axograph X template detection function. Frequency of sEPSCs (Hz) was calculated as the number of spontaneous events per 100sec divided by 100. sEPSC amplitude was calculated by first averaging all detected events and then calculating average event peak.
Statistical Analysis
Statistics were performed using Graphpad Prizm 6.0 (Graphpad Software Inc, San Diego, CA). Time-course data (weight gain, kcal, lever-pressing) were analyzed by 2way RM ANOVA (time and weight gain as factors). LTD data was analyzed by standard 2-way ANOVA. Bonferroni posthoc analyses were performed where stated. All other behavioral data as well as AMPA/NMDA ratio and NMDA decay data were analyzed by unpaired two-tailed student t-test or one-way ANOVA as they were normally distributed. Breakpoint data was not normally distributed and therefore analyzed by Mann-Whitney test. For correlations, linear regressions were performed on data and F-test determined whether the linear regression slope was different from zero (p values and goodness of fit (r2) values, not F values, are displayed on graphs).
Results
Establishing diet-induced obesity prone and obesity resistant subpopulations
As depicted in Figure 1A, at the end of the 8-week palatable food diet rats showed a normal distribution of weight gain (D'Agostino & Pearson omnibus normality test, K2=0.789, p=0.67), thus allowing their separation into OP (top third) and OR (bottom third) subgroups. OP rats averaged 390±7.1 g of weight gain compared with 284.8±6.2 g for OR rats on the last day of palatable food diet. Importantly, OP rats not only gained significantly more weight over the diet period (p<0.0001 for main effect of weight gain, two-way ANOVA, F(1,36)=96.64; figure 1B), they also ate significantly more (23%), demonstrating a propensity for excessive consumption (p<0.0001 for weight gain main effect, two way ANOVA, F(1,36)=69.69; figure 1C).
Diet-induced obesity confers vulnerability to addictive-like behavior towards palatable food
After 8-weeks of free access to palatable food rats were placed on standard chow and access was restricted to 45min daily during operant sessions. Despite both OP and OR initially responding similarly on FR1 and FR3 schedules, by day 10, when the response requirement increased to FR5, OP rats escalated to pressing over 50% more than OR rats during S+ periods (p=0.0003, F(1,36)=15.7, main effect of group, Figure 2B). This resulted in OP rats consuming 57% more palatable food when it was available, thus constituting more ‘binge-like’ consumption in this limited time period in comparison to their OR counterparts (p=0.0013, t(36)=3.485; Figure 2C).
A core feature of addiction is loss of control over behavior. The persistence of lever-pressing during periods where the lever cue is not associated with reward delivery models this aspect of addiction-like behavior in rodents (25, 27, 40). As expected, OP rats pressed more during this period of reward unavailability (S−) than did OR rats (p=0.006, t(36)=3.755; Figure 2D), and this occurred throughout the entire protocol (supplemental figureS1). Furthermore, OP rats were more highly motivated to obtain a palatable food reward as they exhibited a higher breakpoint and total number of lever presses on the progressive ratio task (p=0.0063, U=90; Figure 2E and p=0.007, t(36)=2.87; Figure 2F).
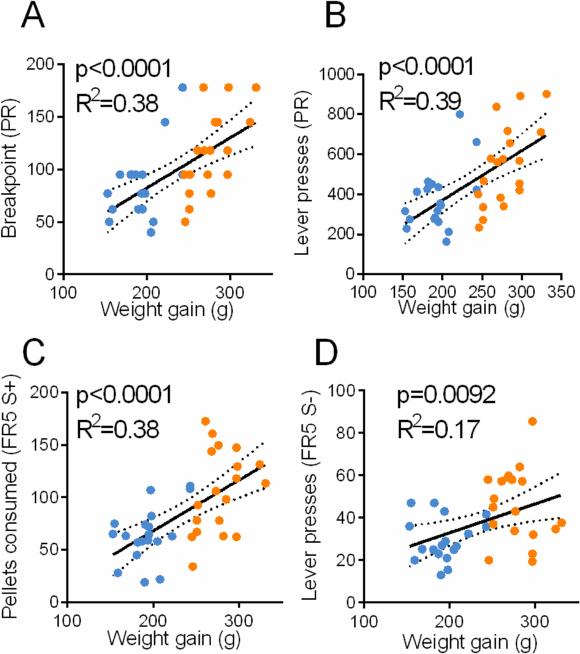
We then tested whether propensity for diet-induced obesity is a predictor of addictive-like behavior towards palatable food. To that end we correlated the weight gain of each rat with its performance on each of the three behavioral parameters described above. We found that all three parameters were positively correlated with weight gain (Figure 3A-D). Thus, the overall results presented in Figures 2-3 strongly indicate that propensity for diet-induced obesity is linked with addictive-like behavior towards palatable food.
Figure 3.
Behavior is positively correlated with previous weight gain. (A-B) breakpoint (F(1,36)=21.77) and lever presses (F(1.36)=23.18) in PR test. (C-D) Pellets consumed in FR5 S+ (F(1,36)=22.17) and lever presses during FR5 S−(F(1,36)=7.587) are positively correlated with weight gain. Dashed line represents 95% confidence. Blue – OR. Orange – OP.
Diet-induced obese rats show synaptic properties of addiction in the NAcore
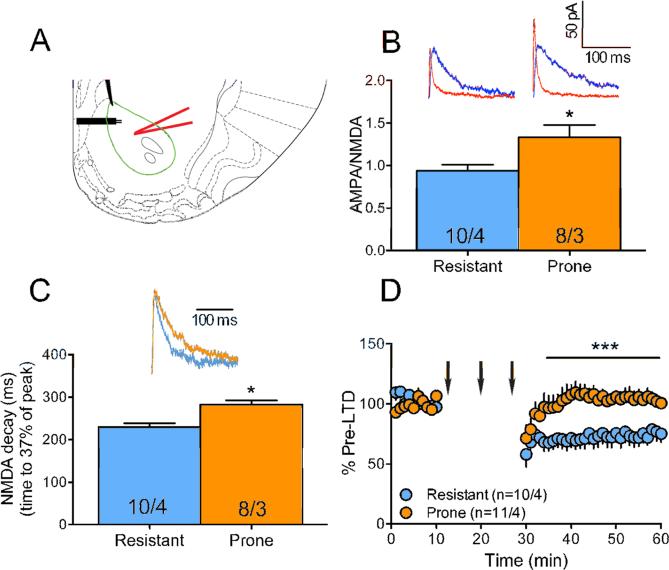
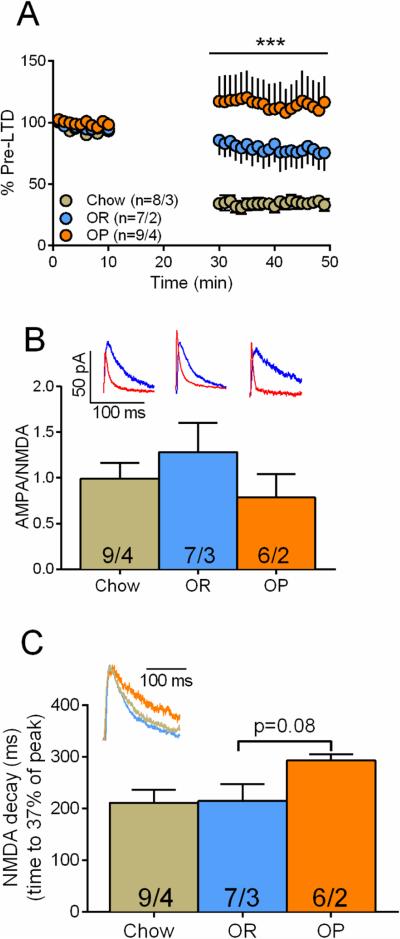
The increased intake, motivation and persistent palatable food-seeking behavior displayed by OP rats resembles behaviors observed in animals that self-administer drugs of abuse. In the latter, the glutamatergic synapses in the NAcore have been shown to change dramatically. Using animal models of self-administration and relapse, enduring impairments in transmission and synaptic plasticity have been shown at glutamatergic synapses in the NAcore. Three changes, among others, are observed: i) the glutamatergic synapses are potentiated after a period of drug self-administration (41-43); ii) loss of the ability of these potentiated synapses to undergo long-term depression (LTD) (26); iii) slower NMDA-mediated currents, consistent with a change in subunit composition (23, 24). If OP rats display addiction-like behavior towards palatable food we would expect to observe similar synaptic changes in their NAcore. To test this we used whole-cell patch-clamp electrophysiology in NAcore brain slices of OP and OR rats (Figure 4A). First, we examined the synaptic strength of the glutamatergic synapses in the NAcore by measuring the ratio between AMPA and NMDA currents (AMPA/NMDA), where a higher ratio indicates potentiated synapses. We found that in the OP rats the glutamatergic synapses in the NAcore reside in an altered state (AMPA/NMDA=1.34±0.39) compared to those in the OR rats (0.94±0.23, p=0.0197, t(16)=2.59; Figure 4B). This is similar to the long-term potentiation (LTP) observed in the NAcore of rats that have self-administered drugs such as cocaine (42) and nicotine (23). Also, akin to nicotine (23) and heroin (24) models of addiction, we found (Figure 4C) that the potentiated glutamatergic synapses of the OP rats showed slower decay of the NMDA current (282±29 ms compared to 230±26 ms in OR rats, p=0.0011, t(16)=3.96), consistent with a change in subunit composition (44, 45). Lastly, using a stimulation protocol that is known to induce LTD in the NAcore (26), we found that the capacity to induce LTD at the glutamatergic synapses in the NAcore is preserved in OR rats but absent in OP rats (p<0.0001, F(1,674)=159.0; Figure 4D). This is akin to what is observed in animal models of drug addiction (27, 28, 42, 46).
Figure 4.
OP rats, but not OR rats, show “addiction-like” electrophysiological measures. (A) Schematic drawing of the recording setup. In a coronal section of the NAcore (green line) a medium spiny neuron located dorsomedial to the anterior commissure was patched by a glass pipette (red) and EPSCs were evoked by a stimulating electrode (black) positioned ~300 μm dorsomedial to the recorded neuron. Coronal slice diagram modified from (68). (B) OP rats show higher AMPA/NMDA (unpaired t-test, t(16)=2.59, p=0.02, red trace = AMPA, blue = NMDA) than OR rats. (C) OP rats show longer NMDA current decay (unpaired t-test, t(16)=3.96, p=0.001) than OR rats. Insets: representative traces. (D) OP rats show lack of LTD (two-way ANOVA, F(1,674)=159.0 for rat type main effect). Arrows indicate LTD protocol – three 3-min trains of 5 Hz. Numbers in bars and legend represent number of cells / number of rats. * p<0.05 using unpaired two-tailed student's t-test. *** p<0.0001 using Two-way ANOVA for main effect of group.
These synaptic impairments were not observed in control groups that went through the same behavioral protocols but fed with standard chow instead of palatable diet (supplemental Figure S2 and 3), demonstrating that exposure to palatable food is required in order to observe these impairments. To that end we found that animals that were exposed to the palatable diet alone (no behavior) did not exhibit the potentiation of NAc core synapses observed after the behavioral protocol (one-way ANOVA with bonferroni posthoc analysis; Figure 5B). Thus, the observed potentiation of NAc synapses in OP rats is either the result of switching to a more restricted access of the palatable diet when undertaking the operant protocol and/or the neurophysiological manifestation of the development of the ‘addiction-like’ operant behavior observed. However, induction of LTD was impaired in OP rats compared to OR rats or rats exposed only to standard chow even without the behavioral training (p<0.0001, F(2,833)=129.8; Figure 5A). Also, a trend towards slower NMDA decay in OP rats was observed (p=0.0827, F(2,19)=2.850; Figure 5C). Thus, an effect of diet alone is apparent (chow versus palatable diet), as well as an effect of weight gain within rats fed with palatable food (OP versus OR). The observed differences between OP and OR rats seem to be of postsynaptic origin, as there was no difference in the PPR between the groups (supplemental Figure S4); consistent with this LTD protocol inducing postsynaptic, NMDA-dependent LTD in the NAcore (47). Although a postsynaptic mechanism was not corroborated by a change in sEPSC amplitude, sEPSC measurements account for all the glutamatergic inputs while the AMPA/NMDA or LTD measurements arise primarily from mPFC input (recordings were collected in the dorsomedial NAcore, where the prefrontal inputs are most dense) (39, 48).
Figure 5.
OP rats, but not OR rats or chow rats show some evidence of “addiction-like” electrophysiological measures after the homecage diet alone (no behavior) (A) Schematic drawing of the recording setup. In a coronal section of the NAcore (green line) a medium spiny neuron located dorsomedial to the anterior commissure was patched by a glass pipette (red) and EPSCs were evoked by a stimulating electrode (black) positioned ~300 μm dorsomedial to the recorded neuron. Coronal slice diagram modified from (68). (B) OP rats show lack of LTD and chow rat LTD is significantly greater than OR rat LTD (two-way ANOVA, F(2,833)=129.80 for rat type main effect). Arrows indicate LTD protocol – three 3-min trains of 5 Hz. (C) No difference between AMPDA/NMDA according to diet or weight gain on diet. (D) OP rats show a trend for longer NMDA current decay than OR and chow rats (one-way ANOVA, F(2, 19)=2.850). Insets: representative traces. Numbers in bars and legend represent number of cells / number of rats. *** p<0.0001 using Two-way ANOVA for main effect of group.
Both addiction-like behavior and propensity for diet-induced obesity are positively correlated with dysfunction at excitatory NAcore synapses
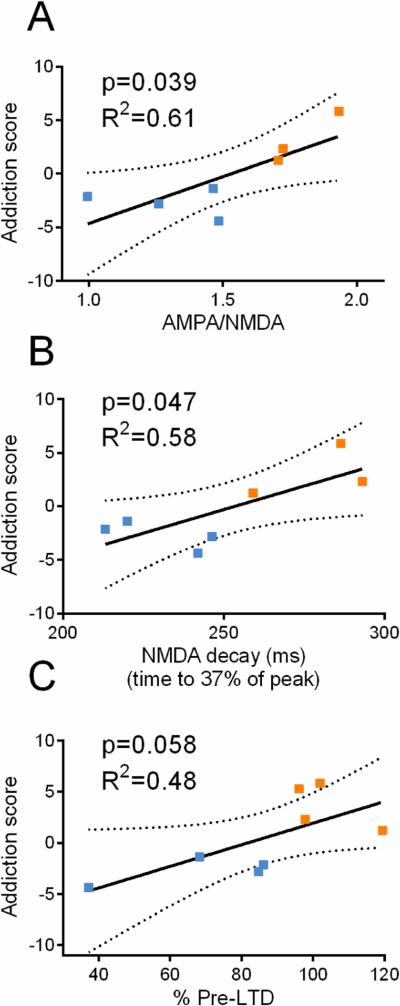
To support that the synaptic changes in the NAcore are linked to the behavior observed in OP rats we correlated each electrophysiological parameter (i.e. AMPA/NMDA, NMDA current decay, magnitude of LTD) with behaviors shown in Figure 2. For simplification we generated for each rat an “addiction score” - the average standardized score of the three behaviors tested (see Supplemental Methods). The three electrophysiological parameters were positively correlated with behavior (Figure 6). Moreover, the electrophysiological parameters were correlated with weight gain (Supplemental Figure S5), thus indicating that the propensity for diet-induced obesity predicts the extent of synaptic dysfunction.
Figure 6.
Addiction synaptic hallmarks are positively correlated with addictive-like behavior. (A-C) AMPA/NMDA (F(1,5)=7.772), NMDA decay (F(1,5)=6.899), and lack of LTD (F(1,6)=5.486) positively correlate with operant behavior. Operant behavior is presented by an ‘addiction score’ calculated for each rat (see Supplemental Methods). Dashed line represents 95% confidence. Blue – OR. Orange – OP.
Discussion
Here we show that obesity resulting from the inability to regulate consumption of palatable food is linked to plasticity at glutamatergic synapses in the NAcore akin to that which is thought to contribute to drug addiction. Specifically, rats that develop obesity when given access to palatable food exhibit features of addictive behavior. Furthermore, they show changes at NAcore excitatory synapses similar to rats that have self-administered psychostimulants, thus supporting the concept of a shared endophenotype underlying both addiction and overeating.
The presence of features of addictive behavior in OP rats speaks to a pre-existing vulnerability that interacts with exposure to palatable food to elicit the phenotype observed - excessive consumption, the development of ‘binge-like’ intake when access is restricted and increased food-seeking behavior during periods that signal food unavailability. Although the concept of food addiction remains somewhat controversial (49), our results provide preclinical evidence that a food ‘addiction-like’ endophenotype exists in vulnerable subpopulations exposed to a highly palatable diet. A recent study showed a similar food addiction-like phenotype in rats that were screened as highly impulsive (30). These results and our own demonstrate that in outbred rat populations, as with humans, only a proportion of those exposed to palatable food will develop pathological consummatory behavior. This similarity to the human situation provides potential translational relevance to our findings in Binge Eating Disorder (BED)(29), and in individuals classified as ‘food addicted’ using the Yale Food Addiction Scale (20).
We show that OP rats display behavior towards palatable food akin to that displayed towards cocaine by rats classified as ‘addiction vulnerable’, including increased progressive ratio responding (25, 37). Increased responding on a progressive ratio schedule is indicative of heightened motivation, possibly augmented by restricted access to the palatable diet during operant training (54, 55). However, increased motivation has been shown to precede the development of diet-induced obesity in this model, suggesting this is not the case (32). In addition to increased motivation, responses during periods that signal reward unavailability are also elevated in both OP and cocaine ‘addiction vulnerable’ rats, consistent with a contingency learning deficit, increased anticipatory and/or impulsive behavior. Perseveration of behavior constitutes one dimension of compulsive behavior (56-58), and thus models the difficulty individuals experience in limiting substance use/overeating. In addition, we found that when access was limited to 3×15min periods daily OP rats developed ‘binge-like’ consumption (57% greater than OR rats) of palatable food pellets in this limited period. This is consistent with previous work demonstrating the development of binge-like behavior in rats given limited access to a palatable food (59), and is akin to a similar ‘addiction-like’ profile in rats given intermittent access to sucrose in a binge model (60, 61).
Though controversy remains regarding the concept of addiction to palatable food, there is no doubt that drugs of abuse and palatable food interact with similar neurobiological substrates, and that overlap exists in the circuitry underling both drug-seeking and feeding (19). To date, the presence of synaptic plasticity mechanisms known to underlie the transition to compulsive drug use have not been assessed in animal models of compulsive feeding or obesity. Our work provides the first link between obesity and drug addiction at a cellular level by showing that glutamatergic synapses in the NAcore of obese rats resemble those of rats that have self-administered drugs of abuse. Thus, the increased motivation of OP rats to obtain palatable food may be driven by potentiated glutamatergic inputs to the accumbens and/or impaired synaptic plasticity, similar to the potentiated excitatory drive thought to mediate drug-seeking (62).
The accumbens serves as a gateway through for motivationally relevant information to access motor circuitry (63). It is thought that the transition to compulsive drug seeking arises from an impaired ability of the accumbens to process information about negative environmental contingencies, leading to an inability to inhibit prepotent drug-associated responses (21, 22, 64). This impairment has been proposed to result from dysregulated glutamatergic signalling at cortico-accumbens synapses. We and others have demonstrated enduring impairments in glutamatergic transmission and synaptic plasticity on medium spiny neurons in the accumbens which underlie relapse vulnerability in animal models of drug addiction (23, 28, 46, 65, 66). The presence of similar synaptic dysfunction at NAcore synapses in OP rats suggests similar neurobiological mechanisms lead to overeating beyond energy requirements. In particular a deficit the ability of excitatory synapses in the NAcore to undergo LTD speaks to the lack of behavioral flexibility observed in people who cannot refrain from overeating or using drugs (28). As what has been found with models of cocaine addiction (28), it may be that reversing this impairment (pharmacologically or otherwise) restores eating in obese individuals to a normal adaptive behavior.
Many questions remain for future investigation. Our findings provide evidence for addiction-like synaptic impairments in the NAcore of diet-induced obese rats, but whether the mechanisms leading to these impairments are similar to those implicated in drug addiction is not known. For example, it is thought that increased motivation in addicted individuals is translated to uncontrollable action by changes in a common final pathway that includes the accumbens (67). Although we presented three examples of synaptic changes occurring in obese rats and rats trained to self-administer cocaine or nicotine, other changes may not be shared. The persistence of these synaptic changes also remains to be determined. Both palatable food and drugs of abuse are strong reinforcers, but drugs of abuse have specific pharmacological effects whereas food does not. Another question that remains open do these synaptic impairments represent a ‘biomarker’ for an addiction-like endophenotype that that can be generalized to other compulsive disorders (e.g. compulsive gambling, internet gaming addictions)? Future studies focused on these questions are needed to better understand how addiction-like plasticity may be involved in the development of obesity and more generally, addictive behavior.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by NIH Grant DA032898 (TCJ), NIH Grants DA003906, DA012513, DA015369 (PWK) and Melbourne Research Grant Support Scheme, University of Melbourne (RMB). RMB is supported by National Health & Medical Research Council, Austraila (NHMRC) and the American Australian Association. AJL holds an NHMRC Principal Research Fellowship (1020737). We acknowledge the Victorian Government's Operational Infrastructure Support Program. The assistance of Rachel Smith with Med Associates PC programming and study design is gratefully acknowledged. The assistance of Vidya Narayanaswami with the diet-induced obesity model is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors declare no biomedical financial interests and no potential conflicts of interest.
REFERENCES
- 1.NIDDK . Network WW-cI. NIDDK; 2005. Overweight and Obesity Statistics. [Google Scholar]
- 2.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013;73:811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold MS, Avena NM. Animal models lead the way to further understanding food addiction as well as providing evidence that drugs used successfully in addictions can be successful in treating overeating. Biological psychiatry. 2013;74:e11. doi: 10.1016/j.biopsych.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 17.Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, et al. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 19.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gipson CD, Kupchik YM, Kalivas PW. Rapid, Transient Synaptic Plasticity in Addiction. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 27.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 28.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature neuroscience. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.APA . Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; 2013. [Google Scholar]
- 30.Velazquez-Sanchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2463–2472. doi: 10.1038/npp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 32.Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2013;37:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 34.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 35.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 36.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R941–948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 37.Brown AL, Flynn JR, Smith DW, Dayas CV. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol. 2011;14:1099–1110. doi: 10.1017/S1461145710001367. [DOI] [PubMed] [Google Scholar]
- 38.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- 40.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature neuroscience. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 44.Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science's STKE : signal transduction knowledge environment. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 46.Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacol. 2012:1–3. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 48.Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal–ventral divide of the striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- 50.Gearhardt AN, White MA, Potenza MN. Binge eating disorder and food addiction. Curr Drug Abuse Rev. 2011;4:201–207. doi: 10.2174/1874473711104030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gearhardt AN, Boswell RG, White MA. The association of “food addiction” with disordered eating and body mass index. Eat Behav. 2014;15:427–433. doi: 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57:711–717. doi: 10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Imperatori C, Innamorati M, Contardi A, Continisio M, Tamburello S, Lamis DA, et al. The association among food addiction, binge eating severity and psychopathology in obese and overweight patients attending low-energy-diet therapy. Compr Psychiatry. 2014;55:1358–1362. doi: 10.1016/j.comppsych.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 2013;37:1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- 55.Pickering C, Alsio J, Hulting AL, Schioth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology. 2009;204:431–443. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 56.Vanes LD, van Holst RJ, Jansen JM, van den Brink W, Oosterlaan J, Goudriaan AE. Contingency learning in alcohol dependence and pathological gambling: learning and unlearning reward contingencies. Alcohol Clin Exp Res. 2014;38:1602–1610. doi: 10.1111/acer.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins T, Clark L. Behavioral addictions. Curr Opin Neurobiol. 2014;30C:66–72. doi: 10.1016/j.conb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 60.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Experimental and clinical psychopharmacology. 2007;15:481–491. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 61.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 63.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 64.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 65.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone Restores Glutamate Homeostasis and Prevents Relapse to Cocaine Seeking. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 67.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 68.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Academic Press; San Diego, CA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.