Abstract
Nonalcoholic fatty liver disease (NAFLD) is the liver injury most often associated with disordered of insulin resistance, including obesity, diabetes and the metabolic syndrome. The term encompasses several patterns of liver injury, including a relatively benign condition of steatosis without hepatocellular injury, nonalcoholic steatohepatitis (NASH), and a pattern of zone 1 steatosis, inflammation and fibrosis mainly observed in prepubertal children. Staging and grading systems have been developed to characterize the histological changes in NAFLD, mainly as a tool for clinical research. The histological features of NAFLD across these different manifestations and the scoring systems used to evaluate disease severity are discussed.
Keywords: Steatohepatitis, Liver Biopsy, Steatosis, Histology
Introduction
Chronic liver diseases and cirrhosis are the 12th leading cause of death in the United States (1). Within this category, the subgroup of non-alcoholic fatty liver disease (NAFLD) is rapidly increasing in the United States, as well as in the rest of the developed world, concurrent with the global rise in obesity and diabetes. NAFLD is an all-encompassing term for the spectrum of liver diseases linked by the common central feature of steatosis. In adults, NAFLD is typically classified into two categories: nonalcoholic steatohepatitis (NASH), a progressive liver disease characterized by a particular pattern of steatosis, inflammatory changes and hepatocellular injury and steatosis without features of NASH, which we term nonalcoholic fatty liver (NAFL) (2). This latter category, often termed “simple steatosis”, may have inflammation and other features of injury. While NAFL has been generally considered a benign condition, NASH is a progressive disease that can advance to liver cirrhosis and hepatocellular carcinoma (3–5). Nevertheless, it is likely that some cases of NAFL do progress to NASH and the risk factors for progress are incompletely understood (6,7). The classification of NAFL and NASH may seem straightforward, but there remains variation among pathologists when diagnosing NASH. The goal of this review is to provide information on histology, pathophysiology, and diagnostic clues for diagnosing NASH. Additionally, scoring tools for assessing the severity of NASH will be reviewed, as well as special situations in which the diagnosis may be especially problematic (e.g., pediatric cases).
Up until 1980, when Ludwig and colleagues originally coined the term nonalcoholic steatohepatitis (to differentiate it from alcoholic steatohepatitis) the pattern of liver injury in biopsies “caused clinicians to persevere unduly in their attempts to wrench from the patient an admission of excessive alcohol intake or to obtain a confirmation of such habits from relatives of the patient”(8). They examined biopsies taken from 20 patients evaluated at the Mayo Clinic over a 10-year period. These patients had histologic evidence suggestive of alcoholic hepatitis on liver biopsy (i.e., steatosis, lobular inflammation, ballooning injury and Mallory-Denk bodies) but no history of alcohol abuse. Many of these patients were female (60%) and the majority were obese (90%). Since then, studies have shown that NASH is common disorder and an increasingly frequent reason for liver transplantation (9). In addition to the liver disease itself, NASH is also strongly associated with coronary artery disease and metabolic syndrome (diabetes mellitus type 2, insulin resistance, central obesity, dyslipidemia, and hypertension) (10).
Histopathology
In most cases the histological features of NAFLD are indistinguishable from those of alcohol induced liver disease and so the pathologist must rely on the clinician to exclude alcohol use as an etiology. For diagnostic purposes, pathologists divide NAFLD into NAFL (predominantly macrovesicular steatosis with or without nonspecific inflammation) and NASH. The histologic features of NASH include macrovesicular steatosis, ballooning degeneration of hepatocytes, scattered (mainly lobular) inflammation and apoptotic bodies, and Mallory-Denk bodies (MDBs) (Figure 1A). Notably, while some degree of fibrosis is often present, it is not necessary for the diagnosis. As opposed to NAFL, NASH is a specific pattern of liver injury that may be recognized even if present with other liver diseases. At early stages of disease, the histologic changes have a distinctive distribution with the most severe changes in acinar zone 3. The features of steatohepatitis are not present in equivalent proportions in every biopsy and no single feature by itself is diagnostic, making the diagnosis difficult at times. Because of the inherent disease complexity and the wide spectrum of findings, scoring systems were devised to aid pathologists in assessing the severity of NAFLD.
Figure 1.
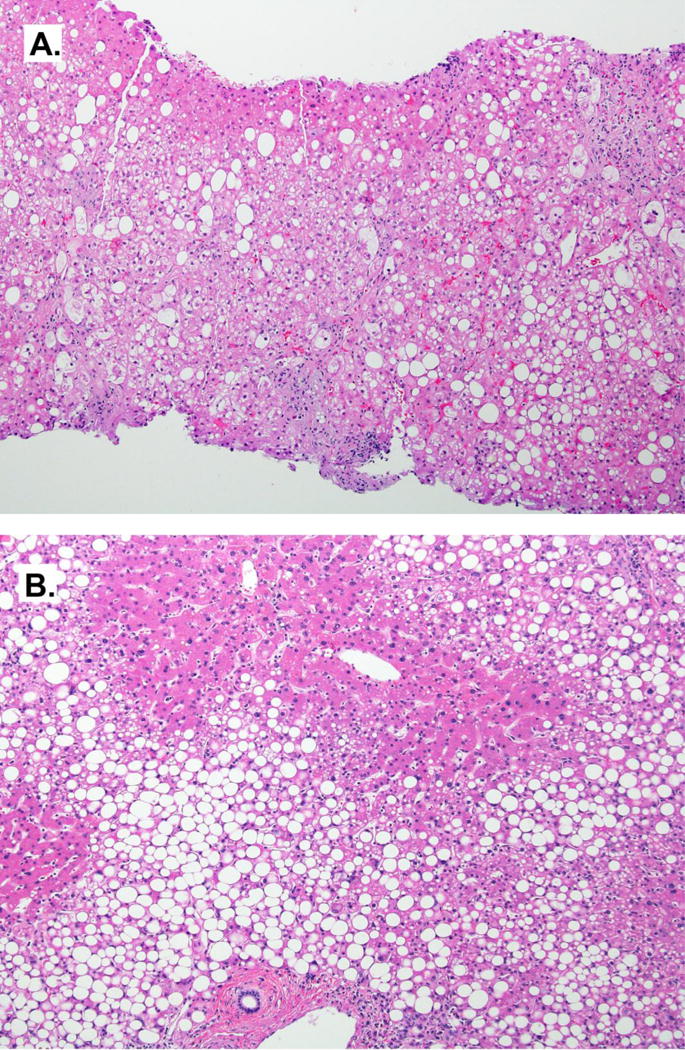
Liver biopsy of Non-Alcoholic Steatohepatitis (NASH). A) Histologic features of NASH include steatosis, ballooning, and lobular inflammation. In severe cases such as this, balloon cells are readily identified. H&E, 4×. B) Zone 1 steatosis in NASH demonstrating variability in the distribution pattern. H&E, 4×
Steatosis is the histological feature that ties together all of the various forms of NAFLD (Figure 2A.), but is a common and completely non-specific lesion that may be seen in the background of many liver diseases. By convention, steatosis should involve at least 5% of hepatocytes (by visual estimate) in order to be considered clinically significant (11). While this is a good general rule, steatosis may become inconspicuous in cirrhosis and a diagnosis of NASH can still be made if the critical features of ballooning and MDBs are seen. The steatosis in NAFLD is typically macrovesicular (12), but may be composed of a mixture of large and small vacuoles. True microvesicular steatosis, with its characteristic foamy cytoplasmic appearance, may be observed in single hepatocytes or in small patches, but is never diffuse. Early in the disease course, the steatosis is most prominent in zone 3, but with progression of disease or severity, the steatosis may spread evenly throughout the hepatic acinus or become irregularly distributed (13).
Figure 2.
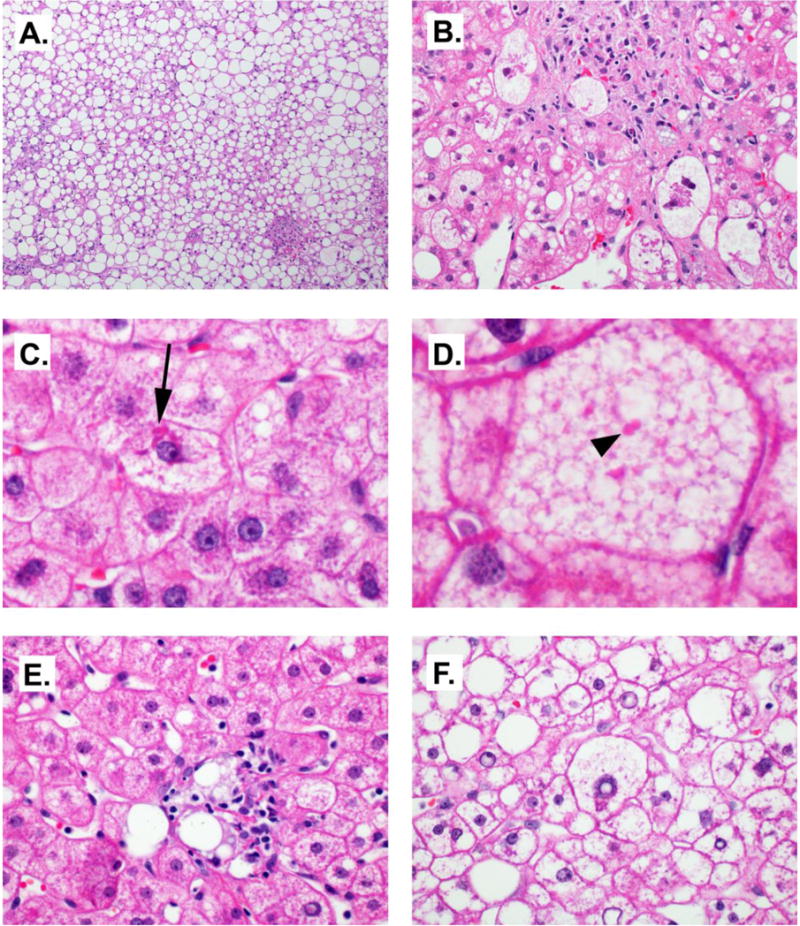
Histologic features commonly seen in NASH. A) Steatosis, 10×. B) Balloon cells. Note wispy cytoplasmic elements. 40×. C) Mallory-Denk bodies (arrow), 60×, cropped. D) Megamitochondria (arrowhead) in a cell with microvesicular steatosis. 60×, cropped. E. Lipogranuloma, 60×. F. Glycogen nuclei, 60×.
The inflammation in NASH consists of a mixed inflammatory infiltrate mainly in a lobular distribution (Figure 2). The lobular infiltrate is composed mainly of a mixture of CD4-(+) and CD8-(+) lymphocytes with scattered Kupffer cell aggregates (microgranulomas) (14). Polymorphonuclear leukocytes (PMNs) may be seen, particularly in the vicinity of Mallory-Denk bodies (MDBs). Eosinophils may be seen in some cases but are not a consistent finding. Lipogranulomas (Figure 2E.) can be seen in portal areas or adjacent to hepatic veins. Portal inflammation in NASH is not uncommon but is usually mild. The portal infiltrate is dominated by CD8-(+) T-cells and macrophages and the severity of portal inflammation has been linked with fibrosis stage (15) (16). However, if the portal tracts are severely inflamed, the possibility of viral hepatitis or autoimmune hepatitis must be considered. While not required for a diagnosis of NASH increased portal inflammation is often associated with progression of disease (17).
The hepatocellular injury seen in NASH can range from ballooning degeneration and apoptosis to less-well characterized reactive changes. Ballooning injury is a feature of major importance in NASH, as one convincing balloon cell can swing the diagnosis towards NASH in the right context. Its presence also has prognostic value associated with increased risk of progression to cirrhosis (18). The typical balloon cell is large (up to several times larger than a non-steatotic hepatocyte), with pale or relatively clear cytoplasm with wispy or feathery eosinophilic strands, often times with a large, hyperchromatic nucleus, with a prominent nucleolus (Figure 2B.). PMNs occasionally surround ballooned hepatocytes, a feature known as satellitosis. Issues can arise when only few balloon cells are present, or they are not significantly larger than the surrounding hepatocytes. Although no specific stain exists that is specific for balloon cells, immunohistochemical staining shows loss of CK 8 and 18 often coupled with reactivity for ubiquitin (19–21).
Mallory-Denk bodies, formerly called Mallory bodies (22), are eosinophilic intracytoplasmic inclusions composed of misfolded intermediate filaments (keratins), chaperone proteins, heatshock proteins, as well as other constituents (22–25) (Figure 2C.). They are most readily identified when found inside balloon cells and they stain positively for ubiquitin and p62 (11). Although not a requirement for the histological identification of NASH, the presence of MDBs suggests poorer outcomes, when paired with steatohepatitis and fibrosis (18). Since MDBs are not specific to NASH, and indeed were first identified with alcoholic hepatitis by Frank Mallory in 1911 (26). They can also be seen in drug-induced steatohepatitis (particularly amiodarone injury), chronic cholestasis, and hepatocellular neoplasms.
Fibrosis is often present in NASH and usually consists of zone 3 perisinusoidal/pericellular fibrosis, demonstrating a “chicken wire” pattern that is typical of NASH (Figure 3). Masson’s trichrome stain highlights deposition of collagen and other extracellular matrix proteins along the sinusoids around the hepatocytes. Portal fibrosis can be seen as the severity of disease increases, and left untreated can progress to bridging fibrosis and cirrhosis. It should be noted that in some cases of obesity-related NASH and in pediatric NASH, only portal fibrosis is present (discussed below). As cirrhosis is an end stage finding, biopsies of late stage disease may lack perisinusoidal/pericellular fibrosis and features of active disease. Therefore, without a prior biopsy of NASH the cirrhosis may only be able to be classified as cryptogenic (27). A meta-analysis of ten longitudinal histological studies of NASH showed the presence of inflammation in the initial biopsy and age emerged as independent predictors of progression to advanced fibrosis in patients with NASH (17). A recent international cross-sectional cohort study found univariate associations between ballooning, portal inflammation and fibrosis with all-cause mortality (28).
Figure 3.
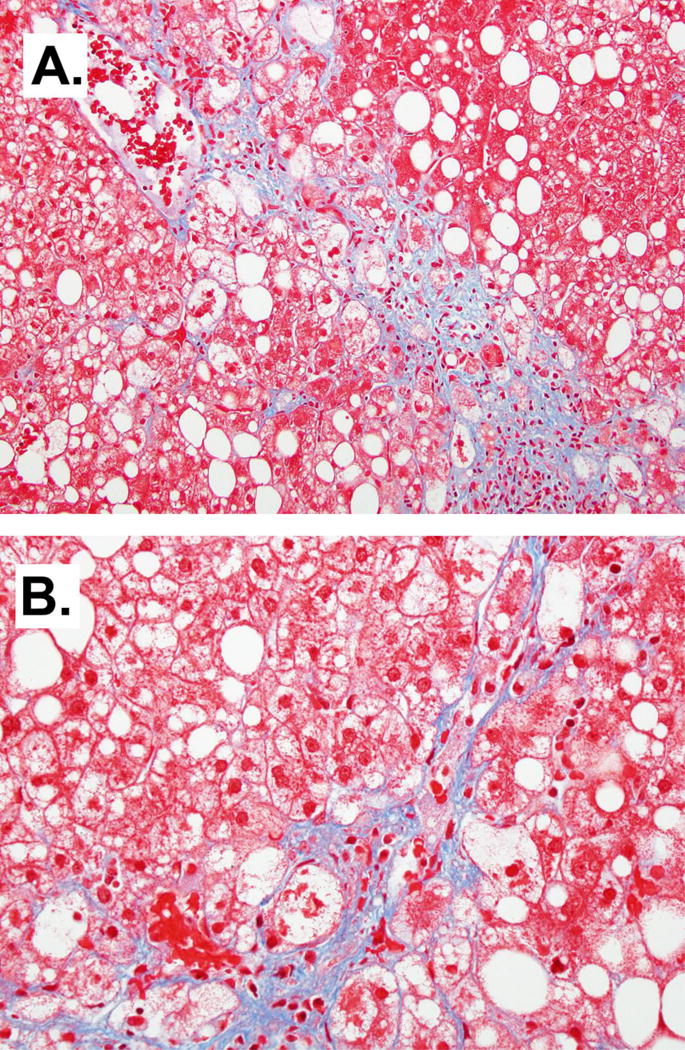
Peri-sinusoidal fibrosis in NASH. Masson’s Trichrome, 10× (A) and 20× (B).
A variety of other histological lesions may be identified in NAFLD. Apoptotic or acidophil bodies are another sign of liver injury and often seen in NASH, but are non-specific as they are seen in viral hepatitis, as well as many other liver diseases. When hepatocytes that have undergone programmed cell death or apoptosis, they become small, eosinophilic, and sometimes with either irregular nuclei or scattered nuclear fragments (29). They are generally identifiable on H&E stain and contain CK 18 fragments (19). Megamitochondria are round to irregularly-shaped eosinophilic intracytoplasmic inclusions seen with some frequency in NASH (Figure 2D). Megamitochondria formation in the setting of NASH may be the result of injury from oxidized phospholipid or lipoprotein formation (30,31). However, the presence of megamitochondria is not specific as they are also present in alcoholic steatohepatitis and drug-induced toxicity (32). Glycogenated nuclei are vacuolated nuclei often present in NASH and usually observed in periportal hepatocytes (Figure 2F.). They are non-diagnostic by themselves, but can lend support to a non-alcoholic etiology as they are absent in 90% of alcoholic steatohepatitis cases (33). Lastly, mild iron deposition is often seen in NAFLD biopsies. Iron may accumulate in both hepatocytes and reticuloendothelial cells. The significance of this hemosiderosis is unclear, but studies have suggested that reticuloendothelial iron is associated with advanced fibrosis. Iron-depletion (i.e. phlebotomy) has been tested as an adjunct therapy for NASH (34,35).
Scoring, staging and grading
While making the diagnosis of steatohepatitis can be challenging at times, the grading and staging of NASH has also been problematic. Several systems have been proposed to address this situation and will be reviewed accordingly.
In 1999, Brunt et al proposed a grading and staging system for NASH (36). They incorporated histologic features, such as steatosis, ballooning degeneration, and inflammation to grade the disease and used patterns and location of fibrosis to stage it. The proposed system divided the grade into mild, moderate, and severe categories, combined with 4 possible stages of fibrosis (Table 1). This system has been useful, but was not meant for grading cases not diagnosed as NASH. It is not applicable to pediatric cases that do not show the typical findings of NASH.
Table 1.
Brunt System for Grading and Staging of Steatohepatitis (34)
| Grade of steatohepatitis | Staging Fibrosis | |||||
|---|---|---|---|---|---|---|
| Grade | Steatosis | Ballooning | Lobular Inflam. | Portal Inflam. | Stage | Fibrosis |
| Mild | Involves up to 2/3rds | Occasional, zone 3 | Scattered, mild acute and chronic | None or mild | 0 | None |
| Moderate | Any degree | Obvious, zone 3 | Mild, associated with ballooning | Mild to moderate | 1 | Zone 3 perisinusoidal fibrosis only |
| Severe | Typically more than 2/3rds | Marked, mainly zone 3 | Mild to moderate | Mild to moderate | 2 | Zone 3 perisinusoidal fibrosis and periportal fibrosis |
| 3 | Bridging fibrosis | |||||
| 4 | Cirrhosis | |||||
In 2002, the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) sponsored the multicenter NASH clinical research network (NASH CRN) in order to study the natural history and therapy of NASH. A pathology committee was convened to develop and validate a system appropriate for evaluating the full spectrum of histological changes in adult and pediatric NAFLD. They published a feature scoring system for tracking the histological changes in NAFLD (37). The key features of the system are shown in Table 2. In order to have an aggregate score that would better capture a histological response to therapy, they proposed the NAFLD Activity Score (NAS), which utilizes features of active injury that are at least potentially reversible. The score is defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2); thus ranging from 0 to 8. Like the system proposed by Brunt et al, fibrosis is not included in this semi-quantitative grade, since fibrosis is generally less reversible and thought to be the result of disease activity rather than a potential driver lesion. The NAS was recently validated in a study and shown to be reproducible and easy to use (38) and has been used in numerous clinical trials and cross-sectional studies.
Table 2.
Essential Elements of the NASH CRN Scoring System (35)
| Numerical Grade or Stage | Feature |
|---|---|
| Fibrosis
| |
| 0 | None |
| 1 | Perisinusoidal or periportal fibrosis; 3 substages defined |
| 2 | Perisinusoidal and periportal fibrosis |
| 3 | Bridging fibrosis |
| 4 | Cirrhosis |
|
| |
| Ballooning
| |
| 0 | None |
| 1 | Few |
| 2 | Many |
|
| |
| Lobular Inflammation
| |
| 0 | No foci |
| 1 | <2 foci per 20× field |
| 2 | 2–4 foci per 20× field |
| 3 | >4 foci per 20× field |
|
| |
| Portal Inflammation
| |
| 0 | None |
| 1 | Mild |
| 2 | More than mild |
| 3 | |
|
| |
| Steatosis
| |
| 0 | <5% |
| 1 | 5% to 33% |
| 2 | 33% to 67% |
| 3 | >67% |
A third system has been recently proposed by Bedossa et al. This system uses similar scoring to the NASH CRN but has proposed an algorithm for diagnostic categorization of NAFLD into NAFL and NASH. A steatosis score of at least 1 is required to diagnose NAFL or NASH. The distinction of NAFL vs NASH then depends on the lobular inflammation and ballooning scores. NASH cases have at least 1 point in both scales, with NAFL cases not meeting these criteria (39).
Special situations: children, bariatric surgery, lipodystrophy
While diagnosis of nonalcoholic fatty liver disease in adults continues to be intensely studied, there are some situations that deviate from established patterns and criteria, which may yield difficulties in diagnosis. Pediatric steatohepatitis can be challenging for pathologists, while fatty liver disease in bariatric surgery and lipodystrophy patients continues to be understudied. In this section we will briefly review the available data concerning these unusual situations.
Several studies have attempted to characterize the histological changes in pediatric NAFLD. Children may or may not mimic patterns seen in adults, including various combinations of cirrhosis, steatosis, and inflammation. Schwimmer et al (40) described 2 types of steatohepatitis in pediatric fatty liver disease. Type 1 contains features of the adult pattern, with zone 3 of steatosis and is more common in girls, but least common overall. Type 2 is more common in boys and features either zone 1 or panacinar steatosis (Figure 1B). Ballooning degeneration is rarely present and fibrosis is generally mild or absent. Other reports (41,42) have since found this categorization less useful in that an overlap pattern between the two types is a more common presentation. Again, the typical features of adult steatohepatitis, such as zone 3 steatosis and ballooning degeneration, were rare. The NASH CRN has investigated pediatric NAFLD in their population. Cases with a zone 1 pattern of steatosis and inflammation, including periportal fibrosis were categorized separately as the “zone 1 borderline pattern”. These cases showed similar features as Schwimmer’s Type 2 cases in that they lack ballooning or the zone 3 injury pattern of typical adult NASH. These cases were mainly identified in prepubertal children, with boys outnumbering the girls. About a quarter of the cases showed advanced (bridging) fibrosis, although none were cirrhotic (43).
Most obese patients that undergo bariatric surgery have significant steatosis in liver biopsies (44,45). Unfortunately, most studies are underpowered and do not specifically address histologic concerns when studying the effect of weight loss surgery on NASH (46,47). A large meta-analysis of cross-sectional surveys of biopsies from 1620 bariatric surgery patients found a high prevalence of steatosis (91%) and steatohepatitis (37%) (48). Despite the high risk of liver disease, biopsies are not routinely performed. A normal aminotransferase level is no guarantee of the absence of significant disease. A study by the Longitudinal Assessment of Bariatric Surgery Consortium demonstrated that 8% of patients with strictly defined normal aminotransferases had steatohepatitis and about 5% had advanced fibrosis (49). Lassailly et al (50) recently reported that biopsy-proven NASH completely disappeared after bariatric surgery in 85% of cases, associated with reductions in body mass index and aminotransferases. The resolution of NASH was even higher if the NASH was only mild prior to surgery. Ballooning degeneration was reduced by 84% and lobular inflammation was absent in 67%, post treatment. While impressive, it remains to be seen if these affects are long lasting, as previous studies report significant weight gain despite the initial loss (51–53).
Patients with lipodystrophy have an abnormal subcutaneous distribution of adipose tissue. This change may be generalized or only affect a part of the body and it may be congenital or acquired from medications or conditions. The loss of peripheral adipose tissue is often accompanied by insulin resistance and diabetes, which may be severe (54,55). Patients are hypoleptinemic, which has led to an effort to correct the metabolic consequences of lipodystrophy with exogenously administered leptin. A recent study examined baseline biopsies from patients with lipodystrophy and compared them to follow-up biopsies (56). Fifty patients had baseline biopsies prior to starting leptin therapy, 43 of whom demonstrated NASH. Fibrosis was also common, with 13 showing bridging fibrosis and 8 having cirrhosis at baseline. Patients with acquired generalized lipodystrophy and those with congenital lipodystrophy and mutations of the BSCL2 gene constituted the majority of cases with advanced fibrosis. Following leptin therapy, there were significant improvement in steatosis and ballooning, with fibrosis generally remaining unchanged.
Summary
NAFLD is a complex liver disease, with several distinct manifestations. NASH is a subtype of NAFLD in which there is a specific injury pattern characterized by ballooning hepatocellular injury, in combination with macrovesicular steatosis and inflammation. Fibrosis and MDBs are also often seen, but not required for the diagnosis. Children, particularly pre-pubertal children, have a pattern of NAFLD characterized by a zone 1 distribution of steatosis, inflammation and fibrosis. Several scoring systems are available for following patients over time, and for assessing histological change in clinical trials.
Acknowledgments
This work was funded by the Intramural Research Program of the National Institutes of Health, National Cancer institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no financial conflicts of interest to disclose.
Contribution of Authors: Drs Brown and Kleiner both contributed significantly to the main text of the manuscript. Dr Kleiner captured histopathologic images.
References
- 1.d’Assignies G, Fontes G, Kauffmann C, Latour M, Gaboury L, Boulanger Y, Van Beers BE, Soulez G, Poitout V, Tang A. Early detection of liver steatosis by magnetic resonance imaging in rats infused with glucose and intralipid solutions and correlation to insulin levels. Metabolism. 2013;62:1850–1857. doi: 10.1016/j.metabol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams LA, Ratziu V. Non-alcoholic fatty liver - perhaps not so benign. Journal of hepatology. 2015;62:1002–1004. doi: 10.1016/j.jhep.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clinics in liver disease. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 5.Karagozian R, Derdak Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–617. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V, Group LS. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. Journal of hepatology. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 7.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. Journal of hepatology. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic proceedings. 1980;55:434–438. [PubMed] [Google Scholar]
- 9.Malik SM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2009;15:1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wree A, Schlattjan M, Bechmann LP, Claudel T, Sowa JP, Stojakovic T, Scharnagl H, Kofeler H, Baba HA, Gerken G, Feldstein AE, Trauner M, Canbay A. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism. 2014;63:1542–1552. doi: 10.1016/j.metabol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A, Network NCR. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with nonalcoholic fatty liver disease. Journal of hepatology. 2008;48:829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefkowitch JH, Haythe JH, Regent N. Kupffer cell aggregation and perivenular distribution in steatohepatitis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2002;15:699–704. doi: 10.1097/01.MP.0000019579.30842.96. [DOI] [PubMed] [Google Scholar]
- 15.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA, Appendix, N. C. R. N. l. o. m. o. t. N. S. C. R. N. c. b. f. i. t. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. Journal of hepatology. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 19.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 20.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy CD, Suzuki A, Burchette JL, Brunt EM, Abdelmalek MF, Cardona D, McCall SJ, Unalp A, Belt P, Ferrell LD, Diehl AM, Nonalcoholic Steatohepatitis Clinical Research, N. Costaining for keratins 8/18 plus ubiquitin improves detection of hepatocyte injury in nonalcoholic fatty liver disease. Human pathology. 2012;43:790–800. doi: 10.1016/j.humpath.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Experimental cell research. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Franke WW, Denk H, Schmid E, Osborn M, Weber K. Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Laboratory investigation; a journal of technical methods and pathology. 1979;40:207–220. [PubMed] [Google Scholar]
- 24.Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, Denk H. p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases. The American journal of pathology. 2002;160:255–263. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadrin M, Marceau N, French SW. Cytokeratin of apparent high molecular weight in livers from griseofulvin-fed mice. Journal of hepatology. 1992;14:226–231. doi: 10.1016/0168-8278(92)90162-i. [DOI] [PubMed] [Google Scholar]
- 26.Mallory FB. Cirrhosis of the Liver. Five different types of lesions from which it may arise. Johns Hopkins Hospital Bulletin. 1911;22:69–75. [Google Scholar]
- 27.Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL. NASH and cryptogenic cirrhosis: a histological analysis. Annals of hepatology. 2009;8:346–352. [PMC free article] [PubMed] [Google Scholar]
- 28.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397. e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klion FM, Schaffner F. The ultrastructure of acidophilic “Councilman-like” bodies in the liver. The American journal of pathology. 1966;48:755–767. [PMC free article] [PubMed] [Google Scholar]
- 30.Wakabayashi T. Megamitochondria formation - physiology and pathology. Journal of cellular and molecular medicine. 2002;6:497–538. doi: 10.1111/j.1582-4934.2002.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clinics in liver disease. 2004;8:595–617. x. doi: 10.1016/j.cld.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Matsuhashi T, Liu X, Nishizawa Y, Usukura J, Wozniak M, Wakabayashi T. Mechanism of the formation of megamitochondria in the mouse liver induced by chloramphenicol. Toxicology letters. 1996;86:47–54. doi: 10.1016/0378-4274(96)83964-6. [DOI] [PubMed] [Google Scholar]
- 33.Pinto HC, Baptista A, Camilo ME, Valente A, Saragoca A, de Moura MC. Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Digestive diseases and sciences. 1996;41:172–179. doi: 10.1007/BF02208601. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV, Nonalcoholic Steatohepatitis Clinical Research, N. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World journal of gastroenterology: WJG. 2014;20:3002–3010. doi: 10.3748/wjg.v20.i11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 37.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research, N. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 38.Hjelkrem M, Stauch C, Shaw J, Harrison SA. Validation of the non-alcoholic fatty liver disease activity score. Alimentary pharmacology & therapeutics. 2011;34:214–218. doi: 10.1111/j.1365-2036.2011.04695.x. [DOI] [PubMed] [Google Scholar]
- 39.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 40.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 41.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, Sartorelli MR, Angulo P. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 42.Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, Xanthakos SA, Whitington PF, Charatcharoenwitthaya P, Yap J, Lopez R, McCullough AJ, Feldstein AE. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50:1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J, Nonalcoholic Steatohepatitis Clinical Research, N. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. e1962. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moretto M, Kupski C, Mottin CC, Repetto G, Garcia Toneto M, Rizzolli J, Berleze D, de Souza Brito CL, Casagrande D, Colossi F. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obesity surgery. 2003;13:622–624. doi: 10.1381/096089203322190853. [DOI] [PubMed] [Google Scholar]
- 45.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, Romon M, Pattou F. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 46.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. The Cochrane database of systematic reviews. 2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 48.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. Journal of hepatology. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Kleiner DE, Berk PD, Hsu JY, Courcoulas AP, Flum D, Khandelwal S, Pender J, Pomp A, Roerig J, Machado LL, Wolfe BM, Belle SH, Consortium L. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Seminars in liver disease. 2014;34:98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, Altattan M, Balasubramaniam M, Gibbs DS, Krause KR, Chengelis DL, Franklin BA, McCullough PA. Behavioral predictors of weight regain after bariatric surgery. Obesity surgery. 2010;20:349–356. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 52.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Annals of surgery. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obesity surgery. 2008;18:648–651. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 54.Garg A. Acquired and inherited lipodystrophies. The New England journal of medicine. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 55.Akinci B, Koseoglu FD, Onay H, Yavuz S, Altay C, Simsir IY, Ozisik S, Demir L, Korkut M, Yilmaz N, Ozen S, Akinci G, Atik T, Calan M, Secil M, Comlekci A, Demir T. Acquired partial lipodystrophy is associated with increased risk for developing metabolic abnormalities. Metabolism. 2015;64:1086–1095. doi: 10.1016/j.metabol.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. Journal of hepatology. 2013;59:131–137. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


