Abstract
Dopamine (DA) neurons in the mammalian central nervous system are thought to be restricted to the brain. DA-mediated regulation of urinary activity is considered to occur through an interaction between midbrain DA neurons and the pontine micturition center. Here we show that DA is produced in the rat spinal cord and modulates the bladder reflex. We observed numerous tyrosine hydroxylase (TH)+ neurons in the autonomic nuclei and superficial dorsal horn in L6–S3 spinal segments. These neurons are dopamine-β-hydroxylase (DBH)− and some contain detectable dopamine decarboxylase (DDC), suggesting their capacity to produce DA. Interestingly, following a complete thoracic spinal cord injury (SCI) to interrupt supraspinal projections, more TH+ neurons emerged in the lumbosacral spinal cord, coincident with a sustained, low level of DA expression there and a partially recovered micturition reflex. Non-selective blockade of spinal DA receptors reduced bladder activity whereas activation of spinal D2-like receptors increased bladder activity and facilitated voiding. Additionally, depletion of lumbosacral TH+ neurons with 6-hydroxydopamine (6-OHDA) decreased bladder non-voiding contractions and voiding efficiency. Furthermore, injecting the transsynaptic neuronal tracer pseudorabies virus (PRV) into the bladder detrusor labeled TH+ cells in the lumbosacral cord, confirming their involvement in spinal micturition reflex circuits. These results illustrate that DA is synthesized in the rat spinal cord; plasticity of lumbosacral TH+ neurons following SCI may contribute to DA expression and modulate the spinal bladder reflex. Thus, spinally-derived DA and receptors could be a novel therapeutic target to improve micturition recovery after SCI.
Keywords: Dopamine, Spinal cord, Injury, Bladder
1. Introduction
Dopamine (DA) is an important neurotransmitter modulating a broad range of body behaviors. Disrupting DA signaling results not only in motor dysfunction but also in autonomic disorders (Shulman et al., 2001; Sakakibara et al., 2011). For instance, irritable hyperactive bladder symptoms, such as urinary urgency, frequency, and incontinence, often occur when midbrain DA neurons are damaged in Parkinson’s disease (PD) (Winge and Fowler, 2006). Although the underlying mechanism behind bladder hyperreflexia in PD patients is not completely understood, the degeneration of DA neurons in the substantia nigra and resulting deficiency of D1-mediated action upon the pontine micturition center (PMC) are a fundamental cause (Sakakibara et al., 2002; Yoshimura et al., 2003). Interestingly, D2-mediated facilitation of micturition has been reported in normal, cerebrally-infarcted, and chemically-induced Parkinsonian animals (Yoshimura et al., 1998; Yokoyama et al., 1999; Seki et al., 2001; Kitta et al., 2012), indicating that DA in regions other than the brain may modulate micturition.
Recent studies revealed that autonomic neurons in the rat lower spinal cord express DA receptors (Gladwell et al., 1999; Stafford and Coote, 2006), suggesting that DA released within the cord helps regulate autonomic function. Though DA neurons are known to reside in the spinal cord of non-mammalian species, e.g. birds and fish (Roberts and Meredith, 1987; Acerbo et al., 2003), they are thought to be restricted to the brain in mammals (Bjorklund and Dunnett, 2007). Thus, DA in the spinal cord is assumed to come from diencephalospinal pathways that originate mainly from the A11 cell group (Skagerberg et al., 1982; Taniguchi et al., 2011; Sharples et al., 2014). Nevertheless, Mouchet and colleagues observed tyrosine hydroxylase (TH)+ cells in the rat spinal cord (Mouchet et al., 1986). Since TH is expressed in multiple neuron types, including DA-ergic and adrenergic ones, it is not clear if these neurons synthesize neurotransmitter DA. Moreover, the function of these neurons is unknown. Here, we perceived a similar distribution of TH+ cells in the rat spinal cord, confirming the previous observation. The majority of these cells are aggregated in the lumbosacral segments, particularly within the autonomic region and superficial dorsal horn. Importantly, some of them display typical DA-ergic characteristics.
The location of TH+ cells in the lumbosacral cord suggests their involvement in pelvic visceral activity, such as micturition. However, the presence of A11 DA-ergic and other descending catecholaminergic projections that contain DA as a precursor precludes us from determining what function the spinal TH+ neurons have. To specifically identify if these neurons play a role in urinary function, we used a complete spinal cord injury (SCI) model to remove descending control and retain only spinal micturition neural circuitry. Interruption of supraspinal micturition pathways causes acute are flexic bladder paralysis. Yet, over a few weeks, there is usually a partial recovery of urinary function via involuntary bladder and urethral reflexes (Fowler et al., 2008; de Groat and Yoshimura, 2012). In the present study, we observed remarkable plasticity of lumbosacral TH+ neurons after SCI that contributed to a low level of sustained, local spinal DA expression. Furthermore, spinal DA receptors regulating bladder reflex are active, indicating that this spinally-derived DA modulates the recovered micturition function.
2. Materials and methods
2.1. Animals
For these experiments, we used 104 adult female (weigh 200–250 g) and 3 postnatal day 10 (P10) Wistar rats, 4 adult female Sprague Dawley (SD, weigh 200–250 g), and 4 adult female Fischer 344 rats (F344, weigh 150–200 g). Wistar rats were employed for both histology and cystometry whereas SD and F344 rats were used for histological comparison. Institutional Animal Care and Use Committee and National Institutes of Health guidelines on animal care were strictly followed to minimize the number of animals used and any potential suffering.
2.2. Spinal cord surgery
Two SCI animal models were used. Because TH+ neurons were observed throughout the length of the spinal cord, we transected the spinal cord at the higher thoracic level (T4) to study injury-induced intraspinal plasticity, as a high level of injury affects a substantial amount of spinal cord tissue below the injury. To evaluate bladder function, however, we performed T10-transection to complete remove supraspinal control and partially remove some propriospinal projections onto spinal micturition neuronal circuits as descending propriospinal projections may affect bladder function following SCI. Animals were anesthetized with 2% isoflurane. A partial laminectomy was performed at T3 or T9 vertebra to expose the dorsal spinal cord. The spinal cord was completely transected at T4 (T4–Tx; n = 18) or at T10 (T10–Tx; n = 52) using a No.11 blade. Lesion completeness was verified visually at the time of surgery and histologically following perfusion. Overlying musculature and skin were then closed. Animals were administered Lactated Ringer’s solution (Baxter Healthcare, Deerfield, IL), cefazolin (10 mg/kg), and buprenex (0.1 mg/kg; Reckitt Benckiser) post-operatively. Bladders were manually expressed at least twice daily until sacrifice.
2.3. ELISA for DA
DA expression in the rat lumbosacral spinal cord was examined with a DA ELISA kit (Eagle Biosciences, Nashua, NH). Naïve or T10–Tx rats 1, 3, or 6 weeks after injury (n = 3/group) were euthanized with an overdose of Euthasol and then transcardially perfused with 100 ml ice-cold 0.1 M PBS. The dorsal half of L6–S3 spinal cord (~0.5 cm) was quickly dissected and frozen on dry ice. Samples were sonicated in lysate buffer (PBS with 0.25% Triton X-100, 5 mM EDTA, 0.5% BSA, 1 mM PMSF, and 1 μl/ml aprotinin) (Taylor et al., 2006). ELISA plates were incubated with the samples per manufacturer’s instructions. DA levels between groups were compared using a one-way analysis of variance (ANOVA) followed by Fisher’s PLSD post hoc tests (SPSS).
2.4. Fluorogold injection
To retrogradely label sympathetic preganglionic neurons (SPNs) in the intermediolateral cell column (IML) and parasympathetic pre-ganglionic neurons (PPNs) in the lower lumbosacral cord, naïve rats (n = 6) received an intraperitoneal injection of Fluorogold (FG, 0.4 ml of 0.5% in distilled water; Fluorochrome, Denver, Colorado) (Akhavan et al., 2006). Animals were perfused 1 week later.
2.5. Bladder cystometry
We performed cystometry to assess bladder function in rats with T10–Tx (n = 30) 3 weeks after injury. Naïve rats (n = 6) were used as controls. All rats were anesthetized with isoflurane and an incision was made in the lower abdomen to expose the urinary bladder. The apex of the bladder dome was punctured using an 18-gauge needle. One end of a catheter (PE-60; Clay Adams) was inserted into the bladder (Yoshiyama et al., 1999; Mitsui et al., 2005). The other end of the catheter was tunneled subcutaneously and out the back. The abdominal wall was then sutured closed. To intravenously deliver pharmacological agents, a separate cannula (PE-10) filled with heparinized saline (1000 U/ml) was implanted in the right femoral vein. The peripheral end was connected to a blunt, T-shaped 27-gauge needle.
Immediately after removal of isoflurane, rats were lightly anesthetized with urethane (1.2 g/kg in naïve rats and 0.8 g/kg in SCI rats, i.p.) (Yoshiyama et al., 2013) and placed in a restraining cage (KN-326, Natsume). The bladder catheter was connected to a pressure transducer (Transbridge, WPI) and a microinjection pump (SP2001, WPI). Room-temperature saline was slowly infused into the bladder (0.1 ml/min). Though micturition cycles usually became stable and fairly regular after approximately 30 min of saline infusion, we waited for at least 1 h before starting the recording to allow for ample adaptation time. At least five continuous stable micturition cycles pre- and post-drug delivery were collected per rat. Experiments lasted no longer than 3 h. Urodynamic parameters including the voiding amplitudes of intravesical bladder pressure (VA), the intervals between two sequential voiding events (VI), and the voiding volumes (VV) were measured (Fig. 1). To measure the VV, expelled urine in each voiding cycle was collected into a small plastic box during cystometry. In rats with SCI, the number of non-voiding contractions (NVC) per micturition episode was also calculated. NVC are defined as rhythmic intravesical pressure increases 5 mm Hg from baseline without a release of fluid from urethra (Mitsui et al., 2005). In each rat, the parameter values in 5 voiding cycles were averaged to determine the mean value for statistical analysis. Animals were sacrificed at the end of this experiment.
Fig. 1.
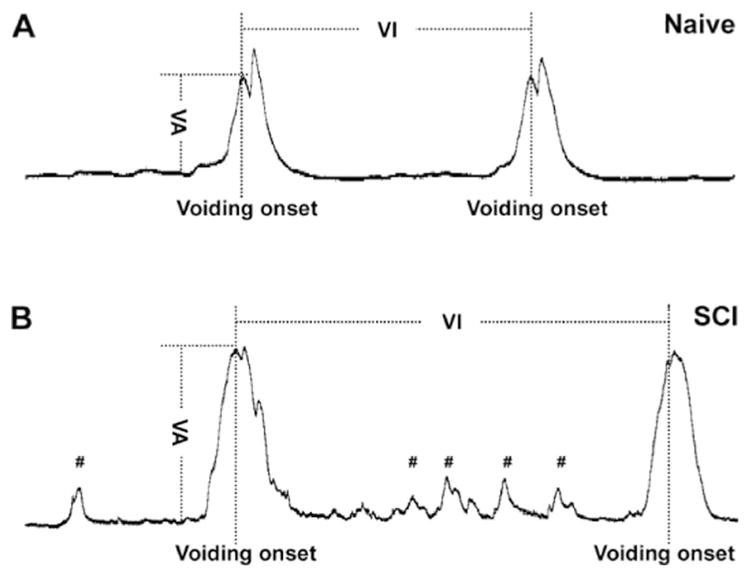
Cartoon illustrating various urodynamic parameters measured during bladder cystometry. In both naïve (A) and complete SCI (B) rats, the voiding amplitudes of intravesical pressure (VA) are always calculated from the peak of voiding onset to the baseline of bladder activity. The voiding intervals (VI) are defined as the time duration between two continuous voiding onsets. Compared to the voiding behavior in naïve rats (A), spinal bladder reflex in SCI rats (B) displays greater amplitudes of voiding pressure and more variable voiding patterns, based on the extent of spontaneous bladder reflex recovery. Notably, non-voiding bladder contractions (#) often occur only in rats with SCI and not in naïve rats.
2.6. Pharmacological interventions of spinal DA receptors
Equivalent volumes of 0.9% saline were delivered intravenously (i.v.) via femoral vein to obtain pre-treatment measurements. Afterwards, DA receptor agonists or antagonists (Tocris Cookson) were given i.v. (Table 1). The doses of the drugs used were chosen on the basis of previously published data (Yoshimura et al., 1998; Seki et al., 2001; Ogawa et al., 2006). All drugs were dissolved in 0.9% saline. Urodynamic data collection was performed within 30 min after drug delivery. The post-drug parameters for each rat, with the exception of NVC, were calculated as a percentage of individual baseline values. To compare normally distributed parameters following saline or single drug application, a paired Student’s t-test was used, to compare the parameters between saline or application of multiple drug doses, a repeated measures one-way ANOVA followed by paired t-test was employed. Wilcoxon signed-rank test was used for nonparametric data.
Table 1.
Drugs and the effects on bladder non-voiding contractions (NVC) in SCI rats.
| Drugs | Types* | Dose (mg/kg) | Rats | Bladder NVC
|
|
|---|---|---|---|---|---|
| Saline | Drugs | ||||
| Flupenthixol | Non-selective DR antagonist | 1.2 2.4 |
N = 6 | 2.3 ± 0.6 | 0.9 ± 0.2* 0.7 ± 0.2** |
| SKF 38393 | D1-like receptor agonist | 3.0 | N = 4 | 1.5 ± 0.7 | 1.9 ± 0.7 |
| SCH 23390 | D1-like receptor antagonist | 3.0 | N = 6 | 1.7 ± 0.6 | 1.5 ± 0.7 |
| Quinpirole | D2-like receptor agonist | 0.1 | N = 6 | 2.4 ± 0.6 | 2.3 ± 0.5 |
| Remoxipride | D2-like receptor antagonist | 1.0 | N = 4 | 2.6 ± 0.7 | 2.6 ± 1.6 |
DR, dopamine receptor.
p = 0.09 compared to saline administration.
p < 0.05 compared to saline administration.
2.7. 6-OHDA injections into L6/S1 spinal cord
To determine the role of lumbosacral TH+ neurons in the spinal bladder reflex, we depleted these cells by injecting the catecholaminergic neurotoxic agent 6-hydroxydopamine (6-OHDA) (Winkler et al., 1996; Kondoh et al., 2005) into the L6/S1 spinal cord. Two weeks after T10–Tx, rats were anesthetized with isoflurane, the L6/S1 spinal cord was exposed by laminectomy and 6 μl 0.2% 6-OHDA was slowly injected into the tissue using a glass micropipette connected to a Hamilton syringe (3 sites per side, 1.5 mm distance between adjacent injections in each side; coordination: 0.5 mm lateral from the midline, 0.5 and 1.0 mm depth, 0.5 μl per depth; n = 5). Some T10–Tx rats (n = 5) were injected only with saline vehicle. Overlying musculature and skin was closed. One week later, the rats underwent bladder catheterization and cystometry assessments, as described above. Animals were then perfused.
2.8. Pseudorabies virus (PRV) transsynaptic tracing
Naïve (n = 8) or T10–Tx rats 3 weeks after injury (n = 8) were anesthetized. An abdominal incision was made to expose the urinary bladder. PRV-152 (Bartha strain, 109 pfu/ml) encoding for green fluorescent protein (GFP) was slowly injected into the ventral bladder detrusor muscle with a Hamilton syringe (3 sites, 2 μl/site, for a total of 6 μl) (Nadelhaft and Vera, 1995). Injection sites were immediately sealed with a small drop of tissue glue. The incision was closed with sutures. Animals were perfused 48 or 72 h later (n = 4 per group) for histological analysis.
2.9. Histology
2.9.1. Tissue processing
Naïve (n = 12 in adult and 3 in P10 Wistar, 4 in SD, 4 in F344) or SCI animals were overdosed with Euthasol and then perfused transcardially with 0.1 M phosphate buffered saline (PBS), pH 7.4, followed by 4% paraformaldehyde in PBS. Spinal cords were dissected, divided into two 3 cm portions of caudal (T13–S4) and rostral (T5–T12) segments, post-fixed, and cryoprotected. Coronal (20 μm) or horizontal (35 μm) sections were obtained using a cryostat (Cameron et al., 2006) and kept in order. To check for possible TH+ efferents, the ventral efferent nerve roots at L1–S3 levels from naïve (n = 3) and T4-transected (n = 3) adult Wistar rats were dissected and cryosectioned longitudinally (10 μm). Brains of naïve rats (n = 6) were cryosectioned sagittally at 35 μm.
2.9.2. Immunohistochemistry
Spinal cord coronal sections 200 μm apart or longitudinal sections 210 μm apart were used in each immunostaining (Hou et al., 2013). Mounted spinal coronal sections or free-floating longitudinal tissue sections were incubated with primary antibodies diluted in blocking solution (Tris-buffered saline, TBS, containing 5% goat serum and 0.5% triton X-100) overnight at 4 °C. We used primary antibodies against tyrosine hydroxylase (TH, rabbit or mouse, 1:1000, Millipore), dopamine-β-hydroxylase (DBH, mouse, 1:1000, Millipore), dopamine decarboxylase (DDC or AADC, rabbit, 1:1000, Abcam), dopamine transporter (DAT, rabbit, 1:500, Synaptic Systems), choline acetyltransferase (ChAT, mouse, 1:200, Millipore), NeuN (mouse, 1:200, Chemicon), D1-like receptors (rabbit, 1:600, Abcam), D2-like receptors (rabbit, 1:200, Millipore), c-fos (mouse, 1:500, Abcam), GFP (mouse, 1:500, Millipore). Longitudinal sections of ventral nerve roots were incubated with antibodies against TH (rabbit, 1:1000) and Tuj1 (chicken, 1:1000, Aves). Following rinsing, sections were by incubated in Alexa 488 or 594-conjugated goat or donkey secondary antibodies (1:500, Invitrogen) for at least 3 h at room temperature. For immunostaining for TH (mouse), DDC, and DAT, antigen retrieval technique was applied. Sections were incubated with 10% Target Retrieval Solution (Dako) prior to performing standard immunohistochemical procedures, as described. Slides were coverslipped with mounting medium containing DAPI except those labeled with FG. Sections were imaged using a Leica DM5500B microscope and a Leica confocal (TCS SP2).
2.9.3. Quantitative analysis
Quantification was conducted by an observer blinded to group identity. All TH+ cells in the medial gray matter including the lateral parasympathetic nuclei and lamina X around the central canal were counted at 200× magnification in each section of one series of coronal lumbosacral cord sections. Only cells displaying typical neuronal morphology with cellular processes and a DAPI+ nucleus were counted. TH+ cells and TH+/DDC+ cells in the lateral parasympathetic nuclei were counted in one series of longitudinal spinal cord sections. The percentage of TH+/DDC+ cells to the total number of TH+ cells per rat was calculated. In 6-OHDA or saline-injected spinal cords, TH+ neurons were counted in 5 mm-long longitudinal sections containing L6/S1 level. To quantify PRV-152 infected TH+ neurons, a series of coronal L6/S1 spinal cord sections were immunostained for TH and GFP. A total of GFP-labeled cells or GFP+/TH+ cells were then counted in these sections. An unpaired Student’s t-test was used to analyze these data.
2.10. Statistics
Statistical analyses were performed in IBM SPSS 22. Significance throughout all experiments was set at p < 0.05. Data were represented as mean ± SEM.
3. Results
3.1. Lumbosacral TH+ cells are interneurons
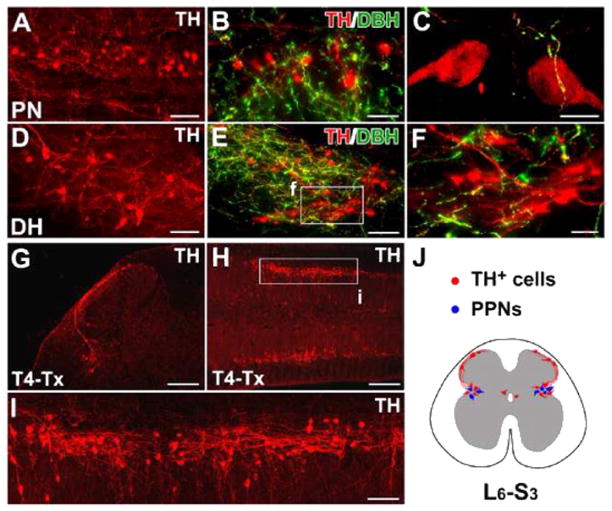
Two different antibodies for TH immunolabeled many cells in the spinal cord of naïve adult Wistar rats. While these TH+ cells were present in the gray matter throughout the spinal cord, the majority resided in the L6–S3 spinal segments (Fig. 2A–F), which differs from the previous observation of TH+ cells mainly at only S1 (Mouchet et al., 1986). These lumbosacral TH+ cells were distributed in the lateral parasympathetic region, lamina X, and superficial dorsal horn (Fig. 2J). Consistent with previous data, spinal TH+ cells did not co-express dopamine-β-hydroxylase (DBH), the enzyme that converts DA to norepinephrine, strongly suggesting that these neurons were DA-ergic and not adrenergic (Fig. 2B–C, E–F). Their presence was not strain-specific as similar TH+ populations were seen in adult Wistar, Sprague Dawley, and Fischer 344 rats (data not shown).
Fig. 2.
The distribution of TH+ cells in the rat lumbosacral spinal cord. A–F, In horizontal sections of the intact rat spinal cord, many TH+ cells are found in the lateral parasympathetic nuclei (PN) (A), lamina X, and superficial dorsal horn (DH) (D). Though a large number of descending fibers are TH+ and DBH+, lumbosacral TH+ cells and their projections are DBH− (B, C, E, F), indicating that they are not noradrenergic/adrenergic. G–I, Three weeks after T4–Tx, spinal TH+ cells and their processes remain in coronal (G) or longitudinal (H, I) sections of lumbosacral cord. Numerous TH+ cells are prominent in the parasympathetic regions of gray matter (H, I). TH+ axons extend segmentally and do not form long ascending projections. J, A schematic diagram illustrates the distribution of TH+ cells (red) in L6–S3 spinal segments in a transverse view of spinal cord. These subpopulations are connected via fibers in lamina I. Notably, TH+ cells are intermingled with parasympathetic preganglionic neurons (PPNs, blue) in the lateral autonomic regions. F and I are higher magnifications of regions boxed in E and H. Scale bars: A, D, 75 μm; B, 50 μm; C, 10 μm; E, I, 100 μm; F, 30 μm; G, 200 μm; H, 400 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To distinguish lumbosacral TH+ cells and projections from supraspinal catecholaminergic pathways, we transected the thoracic rat spinal cord at level T4 to completely interrupt these descending tracts. Three weeks after degradation of distal descending fibers, we observed isolated TH+ cells and projections within the lumbosacral cord (Fig. 2G–J). These prominent TH+ cells extended axons to the contralateral side or across lumbosacral segments. Long ascending projections were not detected.
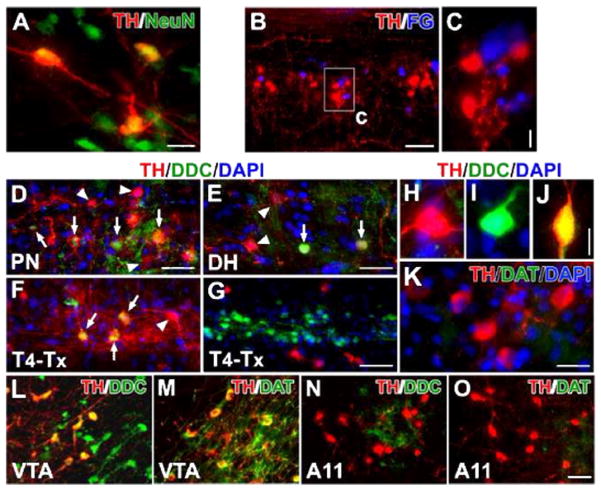
The spinal TH+ cells expressed the mature neuronal marker NeuN, indicating that they are indeed neurons (Fig. 3A). Since the majority of the TH+ neurons were located in the parasympathetic nuclei, we sought to determine whether they are a yet-to-be described part of the parasympathetic efferent system. Fluorogold (FG) was injected i.p. into intact rats to retrogradely label PPNs. One week later, we found TH+ cells juxtaposed to FG-labeled PPNs in the lumbosacral cord; no co-localization was detected (Fig. 3B, C). Furthermore, no Tuj1-immunolabeled efferent fibers in L1–S3 ventral roots were also TH+ (not shown). These results illustrate that lumbosacral TH+ cells are spinal interneurons and are not parasympathetic efferents.
Fig. 3.
Lumbosacral TH+ cells are spinal interneurons and exhibit DA-ergic characteristics. A, In a coronal section of the lumbosacral spinal cord, TH+ cells co-express the mature neuronal marker NeuN. B, C, Parasympathetic preganglionic neurons (PPNs) were retrogradely labeled by i.p. injections of Fluorogold (FG). Though TH+ cells and FG-labeled cells are juxtaposed in the autonomic nuclei, no cells containing both TH and FG were detected. D–J, In both the parasympathetic nuclei (D) and superficial dorsal horn (E), some TH+ cells co-express DDC (arrows), suggesting the capacity for DA synthesis. The arrowhead highlights a TH+ cell that does not co-express DDC. Three weeks following T4–Tx, after the degeneration of descending catecholaminergic fibers, TH+/DDC+ cells (arrows) are identified more easily in the lateral autonomic region (F). Very few TH−/DDC+ cells are observed in the dorsal and medial gray matter whereas numerous TH−/DDC+ neurons are located in the layers around the central canal and many TH+ neurons reside in the neighboring regions (G). A representative TH+/DDC− (H), TH−/DDC+ (I), or TH+/DDC+ (J) neuron in the lumbosacral spinal cord. K, Dopamine transporter (DAT) is not detected in TH+ neurons. L–O, TH+ neurons in the VTA express strong signals of DDC (L) and DAT (M). However, the majority of TH+ neurons in the A11 nucleus do not contain or express very little DDC (N); A11 TH+ neurons do not express DAT (O). Images B–K are from longitudinal spinal cord sections and L–O are from sagittal brain sections. Scale bars: A, 20 μm; B, 100 μm; C, 10 μm; D, G, O, 50 μm; E, 32 μm; J, 12 μm; K, 25 μm.
3.2. Few spinal TH+ neurons co-express the DA-synthesizing enzyme DDC
The lack of DBH expression in the TH+ neurons is not sufficient to distinguish if these neurons synthesize DA. To more definitively characterize if spinal TH+ neurons are truly DA-ergic, we explored whether they express other markers, i.e. the enzyme DDC and the DA transporter (DAT). TH and DDC are both necessary for DA synthesis. TH converts L-tyrosine to L-DOPA. DDC catalyzes L-DOPA to DA, which is then released into the synaptic cleft. DAT is part of the presynaptic regulatory machinery to recapture DA from the extracellular space (Hoffman et al., 1998; Gabriel et al., 2013).
In the intact spinal cord, a small percentage of TH+ cells were also DDC+ (~4%). They were located in either the lateral autonomic region or the superficial dorsal horn but not in lamina X (Fig. 3D, E). After T4–Tx, TH+/DDC+ cells were apparent in the autonomic nuclei (Fig. 3F). We also observed a few DDC+/TH− neurons in the dorsal and medial gray matter. Throughout the spinal cord, abundant DDC+ cells were present in the ependymal or subependymal layers of the central canal (Fig. 3G), as previously described (Jaeger et al., 1983). Many TH+ neurons and fibers were seen in the adjacent lamina X. In both intact and injured cords, we perceived TH+/DDC−, TH−/DDC+, or TH+/DDC+ neuron in the lumbosacral segments (Fig. 3H–J). However, no spinal TH+ and/or DDC+ neurons were DAT+ (Fig. 3K). As positive controls for more well-characterized DA neurons, brain tissue containing the mesencephalic ventral tegmental area (VTA) and diencephalic A11 cell group was also immunostained. VTA TH+ neurons strongly expressed both DDC and DAT (Fig. 3L, M), whereas A11 TH+ neurons contained no detectable DDC and DAT (Fig. 3N, O). Thus, the characteristics of spinal TH+ neurons appear similar to diencephalic A11 DA neurons that project to the spinal cord.
3.3. Plasticity of lumbosacral TH+ neurons after SCI
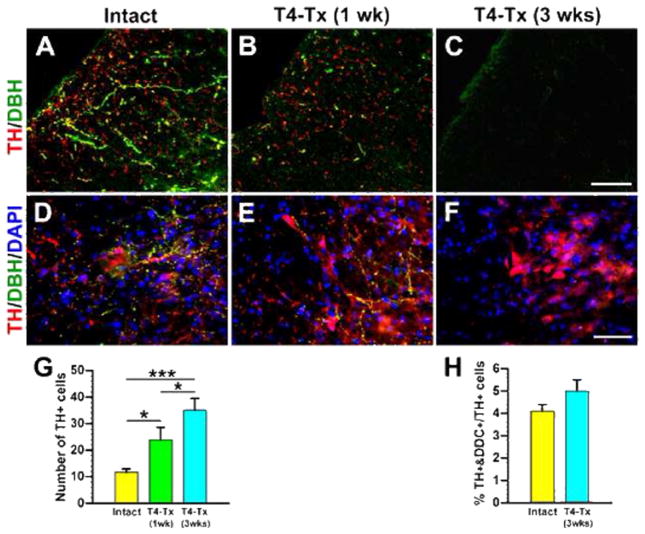
As mentioned above, we transected thoracic spinal cord to better visualize the spinal TH+ neurons. Supraspinal TH+ fibers in the dorsolateral funiculus caudal to a T4–Tx lesion site gradually degenerated and were absent by 3 weeks after SCI (Fig. 4A–C). We noticed that there appeared to be more TH+ neurons in the injured spinal cord than in the naïve cord. Quantitative analysis confirmed a significantly greater number of TH+ neurons in the parasympathetic region 1 and 3 weeks post-injury (Table 2; Fig. 4D–G). Despite this increase, the percentage of TH+/DDC+ cells to all TH+ cells in the lateral autonomic region did not significantly change (Fig. 3D–G, 4H). Similar results were observed following T10–Tx (data not shown). These data suggest that there is injury-induced plasticity of TH+ neurons in the lower spinal cord.
Fig. 4.
Plasticity of lumbosacral TH+ cells after SCI. A–C, Supraspinal catecholaminergic projections gradually degenerate following T4–Tx. In an intact spinal cord (A), TH+/DBH−DA-ergic and TH+/DBH+ noradrenergic/adrenergic fibers descend in the dorsolateral funiculus at L6/S1. One week after T4–Tx (B), the density of these fibers below the lesion drastically decreases. Three weeks post-injury (C), no TH+ profiles are detected in the dorsolateral white matter. D–F, Traumatic SCI induces a dramatic increase in the number of TH+ neuron. Immunolabeling shows some TH+ neurons in the intact L6/S1 cord (D). More TH+ cells were seen 1 week (E) and 3 weeks (F) after SCI. G, Quantitative analysis of TH+ neurons in the medial gray matter of L6–S3 segments indicates significantly more cells in 1 and 3 week post-SCI rats compared to those in the intact (Student’s t-test, *p < 0.05, ***p < 0.001). H, The percentage of TH+/DDC+ neurons to TH+ neurons in lateral parasympathetic nuclei is not significantly different between naïve rats and 3-week post-SCI rats (Student’s t-test, p = 0.15). All images are from coronal sections. Scale bars: C, 75 μm; F, 60 μm.
Table 2.
The number of TH+ cells and the level of DA in the L6–S3 spinal cord.
| Groups | TH+ cell counting
|
DA level by ELISA
|
PRV-GFP-labeled cells
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Rats | Cells | Rats | DA level (ng/g) | Groups | Rats | GFP+/TH+ | GFP+/TH+ to GFP+ (%) | ||
| Naïve | N = 7 | 11.7 ± 1.2 | N = 3 | 391.3 ± 18.8 | Naïve | 48 h | N = 4 | 0.75 ± 0.5 | 4.8 ± 1.2 |
| SCI 1 week | N = 4 | 23.8 ± 4.8 | N = 3 | 230.8 ± 10.9 | 72 h | N = 4 | 3.3 ± 1.7 | 4.7 ± 1.2 | |
| SCI 3 weeks | N = 7 | 35.1 ± 4.4 | N = 3 | 46.6 ± 5.5 | SCI | 48 h | N = 4 | 3.5 ± 0.9 | 12.6 ± 2.9 |
| SCI 6 weeks | N = 3 | 41.7 ± 8.7 | 72 h | N = 4 | 7.3 ± 1.2 | 8.0 ± 1.7 | |||
3.4. DA expression in the spinal cord following SCI
To better assess whether these TH+ neurons produce DA, we measured DA levels in L6–S3 cord in intact and T10–Tx rats using ELISA. Not surprisingly, the level of DA was significantly lower in tissue caudal to a SCI than in the intact because of the interruption and degeneration of descending DA-ergic fibers. The concentration of DA dramatically dropped 1 week after transection (Table 2; Fig. 5; p < 0.001). Notably, a considerable level of DA was still detectable after 3 weeks and persisted at 6 weeks, despite the fact that no supraspinal TH+ fibers were found in the cord at these two time points (Fig. 5C, F). These data demonstrate that DA is indeed synthesized in the spinal cord, even after SCI.
Fig. 5.
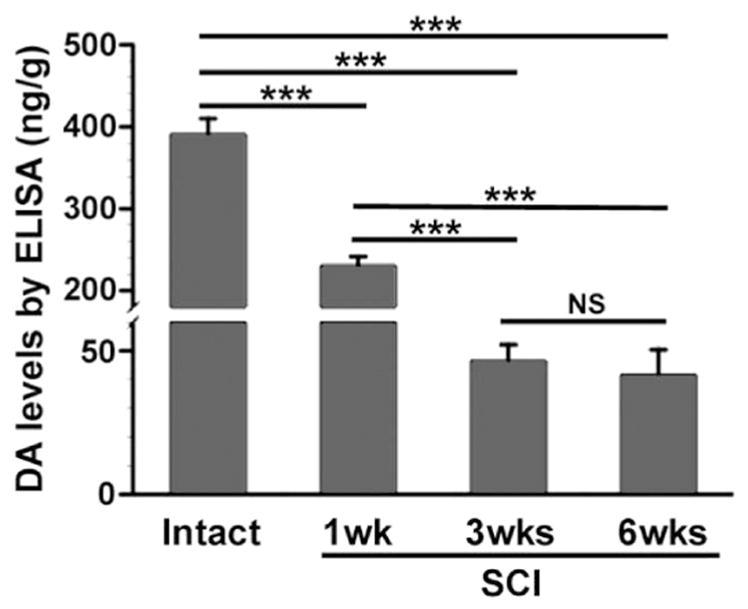
A low level of DA expression sustains in the lumbosacral spinal cord after SCI. DA expression in the dorsal half of L6–S3 spinal segments was measured by ELISA in the intact or T10–Tx spinal cord. DA levels significantly decrease 1 week following injury (one-way ANOVA, followed by Fischer’s PLSD, ***p < 0.001). After 3 weeks, however, an appreciable level of DA (approximately 10% of baseline level) is still detected, even though supraspinal TH+ projections below the lesion have completely degenerated. This low level of DA expression persists in 6 weeks post-injury and is not significantly different than levels found 3 weeks after SCI.
3.5. Pharmacological interventions of spinal DA receptors alter bladder function
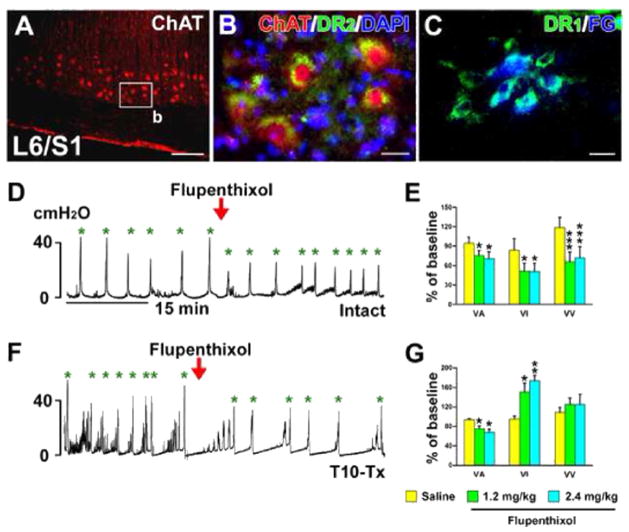
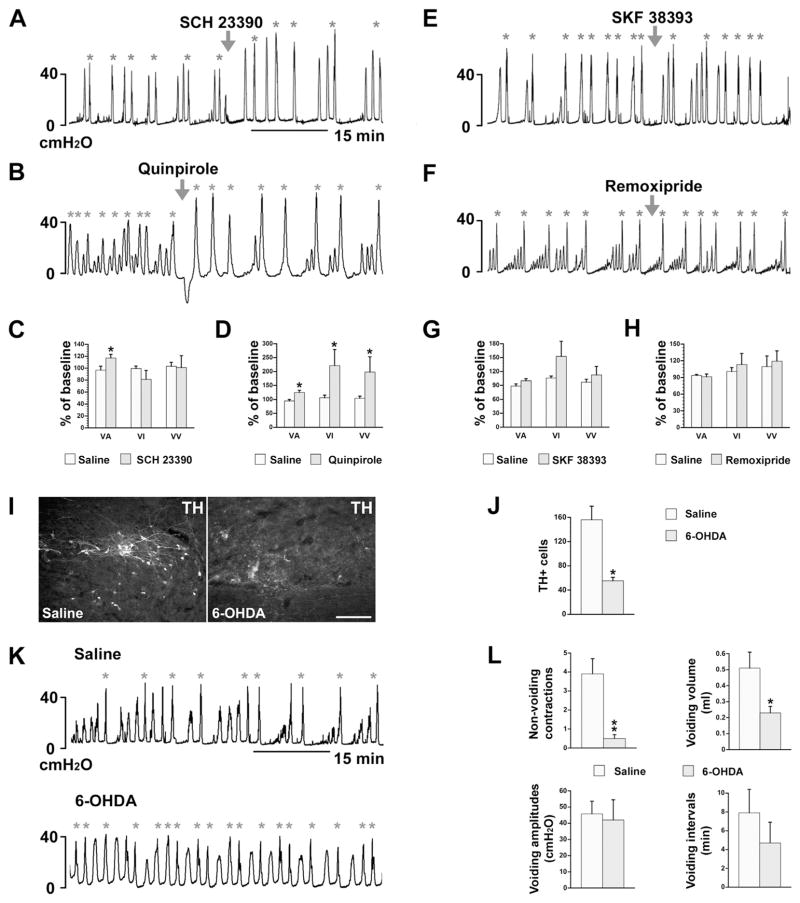
Previous studies demonstrated the expression of DA receptors on SPNs in rat spinal cord (Gladwell et al., 1999; Stafford and Coote, 2006). We also identified both D1-like and D2-like receptors on ChAT+ or FG-retrogradely labeled lumbosacral PPNs (Fig. 6A–C). Thus, it is possible that spinally-derived DA modulates pelvic autonomic function, such as micturition. To begin to elucidate this, naïve rats were administered (i.v.) with the non-selective DA receptor antagonist flupenthixol during bladder cystometry. This significantly decreased voiding amplitudes of intravesical pressure (VA; p < 0.05), shortened voiding intervals (VI; p < 0.05), and reduced voiding volumes (VV; p < 0.001, Fig. 6D, E), suggesting inhibited bladder contraction and decreased bladder capacity.
Fig. 6.
Non-specific blockade of spinal DA receptors inhibits bladder activity in SCI rats. A–C, Lumbosacral parasympathetic preganglionic neurons (PPNs) express D1-like and D2-like receptors. ChAT+ spinal PPNs (A) express D2-like receptors (B) in a longitudinal section of spinal cord. C, Lumbosacral PPNs retrogradely labeled by i.p. injections of Fluorogold (FG) express D1-like receptors. D–G, Representative traces of bladder cystometry in intact (D) or T10–Tx rats (F) after i.v. administration of the non-selective DA receptor antagonist flupenthixol. Inactivating DA receptors significantly reduces VA, VI, and VV in naïve rats (E), but decreases VA and increases VI in SCI rats 3 weeks post-injury (G; repeated measures of one-way ANOVA followed by paired t-test, *p < 0.05, **p < 0.01, ***p < 0.001). This suggests that DA receptors in the lower spinal cord are active and spinally-derived DA modulates bladder activity following SCI. All parameter values (with saline or drugs) were normalized as a percentage to the baseline (red arrowheads represent drug delivery; green asterisks mean voiding). Scale bars: A, 200 μm; B, C, 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
While these data indicate that DA plays a role in micturition, the effects of silencing DA receptors in either the brain or the spinal cord cannot be separated in the naïve rat. To isolate the effects of spinal DA, we again utilized a complete SCI model to disrupt descending projections. Rats were infused with flupenthixol i.v. 3 weeks after T10–Tx. Antagonizing spinal DA receptors after SCI also significantly decreased VA, indicating an inhibitory effect on bladder contraction. However, unlike in naïve rats, flupenthixol in SCI rats substantially prolonged VI (both p < 0.05, Fig. 6F, G), which appeared to facilitate bladder continence. Increasing the dose of flupenthixol produced similar responses and even significantly reduced the number of NVC (Fig. 6G; Table 1). These data strongly indicate that spinal DA receptors actively regulate the bladder reflex after SCI.
To further parse out which subtype of spinal DA receptor is responsible for micturition control and if various DA receptors play different roles, we administered specific DA receptor agonists or antagonists in the SCI rats during cystometry. Selective blockade of D1-like receptors with SCH 23390 significantly increased VA (p < 0.05). Other urodynamic parameters measured were not affected (Fig. 7A, C). No response was observed when D1-like receptors were activated by SKY 38393 (Fig. 7E, G). Administration of quinpirole, an agonist of D2-like receptors, increased VA, prolonged VI, and increased VV (p < 0.05; Fig. 7B, D). No effect was observed when D2-like receptors were blocked with a low dose of remoxipride (Fig. 7F, H). These results indicate that following SCI, spinal D1-like receptors are tonically active to affect bladder function; spinal D2-like receptors may be silent but their activation can enhance bladder activity and efficiently increase voiding efficiency.
Fig. 7.
Pharmacological interventions of specific spinal DA receptors or ablation of TH+ neurons interfere with recovered bladder function. A–H, Three weeks after T10–Tx, (A, C) administration of the specific D1-like receptor antagonist SCH 23390 significantly increases VA but does not affect any other cystometric parameters (paired t-test, *p < 0.05). (B, D) Delivery of quinpirole, a specific D2-like receptor agonist, significantly increases VA, VI, and VV (paired t-test, *p < 0.05). However, neither (E, G) selectively activating D1-like receptors with SKF 38393 nor (F, H) blocking D2-like receptors with remoxipride significantly affects any measured parameters (paired Student’s t-test, all p > 0.05). I–L, Depletion of lumbosacral TH+ cells inhibits spontaneously recovered bladder function after SCI. (I, J) After 6-OHDA injections into the L6/S1 spinal cord, the number of local TH+ cells is dramatically less when compared to control rats that received saline injections (unpaired t-test, *p < 0.05). (K, L) Bladder cystometry shows that those received 6-OHDA injections exhibit remarkably fewer NVC and reduced VV. No differences in VV or VI between groups were observed (nonparametric Wilcoxon rank-sum test, *p < 0.05, **p < 0.01; arrowheads indicate when drugs were delivered; asterisks indicate when voiding occurred).
3.6. Ablation of spinal TH+ neurons reduces bladder activity
Administering the catecholaminergic neurotoxin 6-OHDA into L6/S1 spinal cord of T10–Tx rats effectively reduced the number of TH+ neurons present there (Fig. 7I). There were dramatically fewer TH+ neurons in rats injected with 6-OHDA than with saline (Fig. 7J; p < 0.05). 6-OHDA mediated-depletion of lumbosacral TH+ neurons significantly reduced bladder NVC and the voiding volume (Fig. 7K, L), reflecting an inhibition of bladder activity and reduced voiding efficiency. Thus, lumbosacral TH+ neurons appear involved in spontaneously recovered bladder function and bladder hyperactivity following SCI.
3.7. Lumbosacral TH+ neurons are an active component of bladder reflex circuitry
Additional evidence supports that lumbosacral TH+ neurons are involved in spinal bladder reflex circuitry. We first examined expression of the immediate early gene c-fos, a marker of neuronal activation (Cohen and Greenberg, 2008), in the lumbosacral spinal cord immediately after cystometry. In both naïve and SCI rats, most TH+ cells in the parasympathetic nuclei were highly immunoreactive for c-fos (Fig. 8A). In another cohort of naïve or T10–Tx rats, we injected the transsynaptic tracer pseudorabies virus (PRV-152) into the bladder detrusor. After 48 h in the naïve rat, some PPNs in the L6/S1 spinal cord were GFP+, yet very few TH+ neurons co-expressed GFP (Table 2; Fig. 8B, F). After 72 h, more GFP+/TH+ neurons were seen in the autonomic region (Fig. 8C, F). In the injured spinal cord, we found significantly more GFP+/TH+ neurons in the region at both 48 h (Fig. 8D, F) and 72 h (Fig. 8E, F) after PRV injections than in the intact condition (p < 0.05). We saw a similar injury-induced increase in the percentage of total GFP+ cells that were also TH+ in SCI rats compared to naïve rats at either 48 h or 72 h (both p < 0.01) post-PRV injection (Table 2). These results suggest the involvement of TH+ neuron plasticity in the reestablished spinal bladder reflex pathways.
Fig. 8.
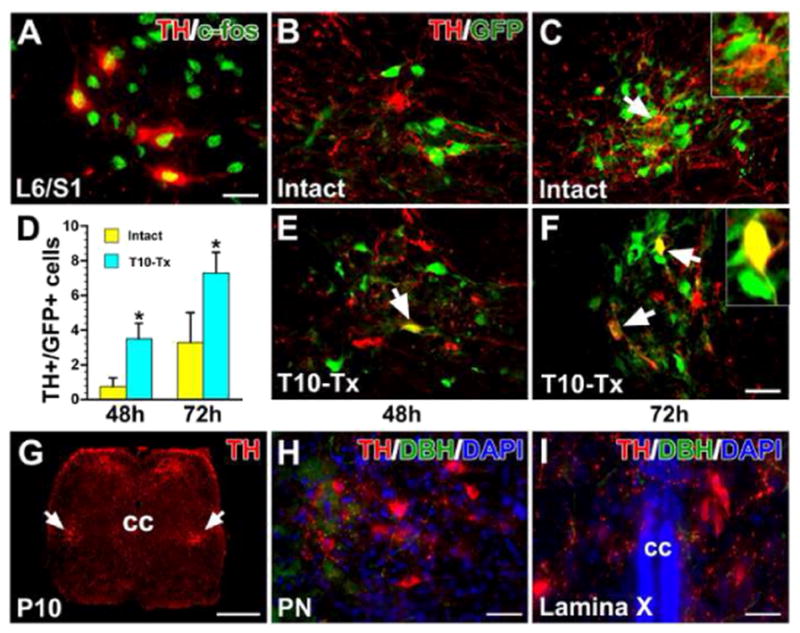
TH+ neurons are involved in the spinal bladder reflex circuitry. A, After cystometry assessment, some lumbosacral TH+ neurons express the immediate early gene c-fos, a marker of related neuronal activity. B–F, PRV-152 was injected into the bladder wall of intact or T10–Tx rats. After 48 h, some parasympathetic preganglionic neurons (PPNs) in the L6/S1 spinal cord are labeled with GFP. Very few TH+ neurons in the intact (B) or injured (E) cord are labeled with GFP (arrow in E). 72 h post-inoculation, some GFP-labeled TH+ neurons (arrows) can be seen in both intact (C) and injured (F) spinal cords. The inset is a high magnification of a double-labeled neuron. D, Quantitative analysis shows that there are significantly more GFP+/TH+ neurons in the injured cord than in the intact one at either 48 h (unpaired Student’s t-test, *p < 0.05) or 72 h after PRV injection. G–I, In a postnatal day 10 rat, immunolabeling reveals many TH+ neurons (arrows) and fibers in a coronal section of the lower spinal cord (G). Prominent TH+/DBH− neurons are located in the lateral parasympathetic nuclei (PN, H) and lamina X (I), suggesting that spinal TH+ neurons may be associated with pelvic visceral activity during development. All images are from coronal sections. cc, central canal. Scale bars: A, I, 20 μm; F, 35 μm; G, 350 μm; H, 30 μm.
Based on the data above, we postulated that lumbosacral TH+ neurons are prominent in neonatal rats, in which descending neuronal pathways controlling micturition are immature. Actually, in postnatal day 10 rats, when spinal bladder reflexes play a dominant role, immunolabeling revealed pronounced TH+ neurons and dense fibers in the lower spinal cord (Fig. 8G). Likewise, these neurons were mainly located in the lateral parasympathetic nucleus and lamina X (Fig. 8H, I), indicating that they may regulate pelvic visceral activity during postnatal development.
4. Discussion
The principal source of DA in the mammalian spinal cord is considered to be the A11 cell group in the diencephalon. Their axons mainly terminate in the dorsal horn, IML, and lamina X (Skagerberg et al., 1982). Here, we report that spinal TH+ neurons are another source of DA in the spinal cord. Interestingly, these cells are predominantly located near where A11 projections terminate in the lower cord. Moreover, they are phenotypically comparable to the TH+ neurons in A11. These TH+ interneurons in the autonomic region make functional synaptic contacts upon PPNs. Following SCI, plasticity of lumbosacral TH+ neurons is correlated to the reestablishment of spinal bladder reflex pathways and appears to at least partly regulate spontaneously recovered urinary function.
DA is still detected below a complete spinal cord transection, supporting the notion that DA is also produced in the spinal cord. TH and DDC are two enzymes necessary to synthesize DA. We observed not only TH+/DDC+ bienzymatic neurons but also TH+ or DDC+ monoenzymatic neurons residing in the rat spinal cord. Bienzymatic neurons that do not contain DBH are clearly DA-ergic (Ershov et al., 2002). While we cannot completely exclude the possibility that DDC is expressed at a level below our detection in the neurons we described as TH+/DDC−, these cells may still have the capacity to produce DA, even if truly monoenzymatic. Indeed, DA neuron phenotypes are more complex than originally thought. Besides “canonical DA-ergic neurons” which possess both enzymes needed for DA synthesis (i.e. TH and DDC), studies have described neurons that express only one or the other (Ershov et al., 2002; Karasawa et al., 2007). As a matter of fact, TH+ or DDC+ monoenzymatic neurons are widely distributed throughout the brain in adulthood. These two classes of neurons can cooperatively synthesize DA, that is to say, TH+ cells convert L-tyrosine to L-DOPA; L-DOPA is then released and taken up by the DDC+ neurons, which convert L-DOPA to DA. This is considered to be a compensatory reaction to the failure of bienzymatic neurons in certain neurological disorders (Ugrumov, 2009). Therefore, it might be unnecessary for a DA neuron to possess a full complement of enzymes required for DA synthesis. In adult rat spinal cord, abundant DDC+ neurons were recently disclosed not only near the central canal, but also in the intermediate zone and dorsal gray matter (Jaeger et al., 1983; Wienecke et al., 2014). Further observations revealed that sacral DDC+ neurons began to produce monoamines after SCI (Wienecke et al., 2014). That we found monoenzymatic TH+ or DDC+ neurons adjacent to each other around the central canal may indicate DA synthesis in the spinal cord via this two-step mechanism. Moreover, both spinal cord and A11 TH+ neurons do not express DAT, implying that these two populations use an alternative means to regulate extracellular DA levels (Ciliax et al., 1999).
Spinal interneurons are critical for the micturition reflex in neonatal rats and spinal cord-injured rats. During the first 3 weeks after birth, when supraspinal projections have not yet reached spinal target neurons, rats use an interneuron-mediated, spinal bladder reflex pathway to elicit micturition (Araki and de Groat, 1997). Interneurons in the lumbosacral cord receive afferent input from the lower urinary tract and form excitatory or inhibitory synapses with PPNs involved in the regulation of micturition (de Groat and Yoshimura, 2012). As the animal continues to mature, supraspinal pathways gradually dominate bladder function. Descending axon innervation and synaptic reorganization in the spinal autonomic nuclei eventually leads to the elimination of primitive segmental reflexes and the emergence of mature spinobulbospinal reflexes (Araki and de Groat, 1997; Miura et al., 2003). Following SCI interrupting supraspinal control, a spinal micturition reflex develops due to intraspinal plasticity. This may arise because of newly-formed spinal circuits or a re-emergence of developmental reflex pathways (Zinck and Downie, 2006). Based on our observations, it appears that lumbosacral TH+ neurons are essential for this. The number of TH+ cells substantially increased when diencephalospinal DA-ergic pathways were interrupted, coinciding with the time frame of partial recovery of bladder function. Furthermore, chemical depletion of these neurons reduced bladder hyperactivity and voiding efficiency. Thus, we posit that spinal TH+ neurons are a remnant of spinal circuits involved in pelvic visceral activity during development. In fact, we found numerous TH+ neurons and fibers in the autonomic region of lumbosacral cord in postnatal day 10 rats. While these neurons may not be a key part in micturition regulation in the naïve adult, after the SCI-induced loss of supraspinal DA-ergic control, they undergo plasticity and seem to play a larger role in urinary function.
Our data supports the concept that DA modulation of micturition occurs at both supraspinal and spinal levels. In naïve rats, non-selective blockade of DA receptors with flupenthixol shortened voiding intervals. This result is similar to the symptoms of increased urinary frequency and incontinence in PD patients (Araki et al., 2000). The role of spinally-derived DA in micturition, which acts upon spinal DA receptors, was elucidated by delivering DA receptor agonists or antagonists after a complete thoracic SCI. Non-selective antagonizing DA receptors with flupenthixol inhibited bladder activity, specifying that spinally-derived DA regulates micturition reflex after the loss of descending pathways. The reduction of NVC indicates that spinal DA contributes to SCI-induced bladder hyperactivity. The different actions of DA on bladder reflex are mediated through different DA receptors. In neurologically intact rats, D1-like receptors exert a tonic inhibitory action on the micturition reflex; D2-like receptors do not have tonic activity and their activation facilitates voiding (Yoshimura et al., 1993; Seki et al., 2001; Ogawa et al., 2006). In rats with SCI, spinal cord D1-like receptors appear to exert a tonic inhibitory activity as well, since the selective blockade with SCH 23390 enhanced the bladder activity. Specifically stimulating D1-like receptors had no effect, likely because they were already tonically active (Yoshimura et al., 2003). This illustrates that spinally-derived DA maintains D1-mediated tonic inhibition after removal of supraspinal DA control. On the other hand, activation of spinal D2-like receptors in SCI rats facilitated the bladder reflex whereas suppression with a low dose of a D2 antagonist did not affect any cystometric parameters, similar to what has been observed in naïve rats (Seki et al., 2001). The discrepancy is that non-selectively inhibiting DA receptors with flupenthixol elicited significantly decreased VA and increased VI whereas selectively inhibiting either D1- or D2-like receptors did not induce the same results. As only one dose was chosen for each drug (based on successfully used doses in rats), it is very possible that the discrepancy is dose-dependent. This will be important to follow up in future experiments. Furthermore, given that the sensitivity of spinal DA receptors may be upregulated following SCI, it will also be important for later studies to assess relative levels of DA receptors on lumbosacral autonomic neurons and further elucidate how they shape partial recovery of micturition.
Under normal conditions, effective micturition is achieved by coordinated activity of the bladder detrusor and the external urethral sphincter (EUS) via spinobulbospinal reflex pathways. During voiding, the bladder detrusor contracts while the EUS phasically relaxes, resulting in virtually complete expulsion of urine stored in the bladder. After SCI, disruption of supraspinal inhibitory input to motor neurons controlling the EUS increases tonic activity and decreases phasic activity of the muscle. Additionally, the bladder is overactive when descending projections no longer controls lumbosacral autonomic neurons. As a result, there is a loss of coordination of EUS and bladder detrusor activity and the two muscles can contract simultaneously, a disorder known as detrusor–sphincter dyssynergia (DSD) (Cheng and de Groat, 2004). DSD leads to reduced voiding efficiency and urinary retention. Previous studies demonstrated that D1-like receptors tonically regulate EUS function in the intact rat while activation of D2-like receptors reduces its contraction to assist voiding (Ogawa et al., 2006). Though we found DA derived from the spinal cord elicits D1-mediated control of the bladder detrusor, it is not yet known if the same mechanism mediates increased tonic activity of the EUS after SCI or whether activation of spinal D2-like receptors increases EUS bursting. Thus, we will focus on answering these questions in the subsequent experiments. In addition, we used only one dose of most DA receptor agonists and antagonists to assess the function of these receptors following SCI. To more comprehensively illuminate each of their roles and how they shape the cumulative response, we will repeat these experiments with different drug concentrations and combinations.
One of the striking results presented here is the increased number of neurons expressing TH in the lumbosacral cord after SCI. What is the source of these new TH+ neurons? Previously, more TH+ neurons were observed in the striatum following ablation of DA neurons within the substantia nigra in non-human primates. This was attributed to a phenotypic shift of GABA-ergic interneurons and not neurogenesis (Tande et al., 2006). A similar mechanism may mediate the increase of spinal TH+ neurons after SCI. This will be explored in future experiments. It is possible that lumbosacral DA neurons modulate other pelvic organ functions, as a dysfunctional DA system results in abnormal sexual and bowel function and visceral pain (Shulman et al., 2001; Sakakibara et al., 2011; Jarcho et al., 2012), and is therefore valuable for researchers to pursue.
In conclusion, these experiments demonstrate that DA is synthesized in the rat spinal cord. Plasticity of lumbosacral TH+ neurons may account for the low level of DA expression after SCI. We have identified that spinally-derived DA regulates a recovered bladder reflex in SCI rats. Since multiple neurotransmitters are known to help control micturition (Yokoyama et al., 1999, 2001; Chang et al., 2007), targeting the spinal DA circuits, alone or in combination with other neurotransmitter systems, such as glutamate and serotonin, may prove to be a therapeutic approach to improve micturition and other pelvic functions after SCI.
Acknowledgments
This work was supported by the Craig H. Neilsen Foundation (280072) to S.H. and NIH/NINDS R01 NS085426 to V.J.T. The authors are grateful for the assistance of Elizabeth Partida, Theresa Connors, Julien Bouyer, Melisa Semenas, Dr. Chris Haas, Dr. Timothy Himes, and Dr. Jed Shumsky. We thank Dr. Michael Lane for the PRV-152. We thank Dr. Armin Blesch, Dr. Rodrigo España, Dr. Wen-Jun Gao, and Dr. Peter Baas for their constructive comments and Dr. William C. de Groat for the data interpretation. The authors also thank Dr. Itzhak Fischer and Dr. Marion Murray for their support.
Footnotes
Author contributions
S.H., V.J.T., and J.D.H. designed the experiments. S.H., D.M.C., D.W., and M.C.K. carried out the experiments. S.H. and D.M.C. analyzed the data and prepared the figures. S.H., V.J.T., and J.D.H. wrote the paper. V.J.T. and J.D.H. oversaw the project.
Conflicts of interest
The authors declare no competing financial interests.
References
- Acerbo MJ, Hellmann B, Gunturkun O. Catecholaminergic and dopamine-containing neurons in the spinal cord of pigeons: an immunohistochemical study. J Chem Neuroanat. 2003;25:19–27. doi: 10.1016/s0891-0618(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Akhavan M, Hoang TX, Havton LA. Improved detection of fluorogold-labeled neurons in long-term studies. J Neurosci Methods. 2006;152:156–162. doi: 10.1016/j.jneumeth.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki I, Kitahara M, Oida T, Kuno S. Voiding dysfunction and Parkinson’s disease: urodynamic abnormalities and urinary symptoms. J Urol. 2000;164:1640–1643. [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Ren Physiol. 2007;292:F1044–F1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershov PV, Ugrumov MV, Calas A, Krieger M, Thibault J. Differentiation of tyrosine hydroxylase-synthesizing and/or aromatic L-amino acid decarboxylase-synthesizing neurons in the rat mediobasal hypothalamus: quantitative double-immunofluorescence study. J Comp Neurol. 2002;446:114–122. doi: 10.1002/cne.10173. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, Melikian HE. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci. 2013;33:17836–17846. doi: 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwell SJ, Pyner S, Barnes NM, Coote JH. D(1)-like dopamine receptors on retrogradely labelled sympathoadrenal neurones in the thoracic spinal cord of the rat. Exp Brain Res. 1999;128:377–382. doi: 10.1007/s002210050857. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013;33:17138–17149. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger CB, Teitelman G, Joh TH, Albert VR, Park DH, Reis DJ. Some neurons of the rat central nervous system contain aromatic-L-amino-acid decarboxylase but not monoamines. Science. 1983;219:1233–1235. doi: 10.1126/science.6131537. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153:744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa N, Hayashi M, Yamada K, Nagatsu I, Iwasa M, Takeuchi T, Uematsu M, Watanabe K, Onozuka M. Tyrosine hydroxylase (TH)- and aromatic-L-amino acid decarboxylase (AADC)-immunoreactive neurons of the common marmoset (Callithrix jacchus) brain: an immunohistochemical analysis. Acta Histochem Cytochem. 2007;40:83–92. doi: 10.1267/ahc.06019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitta T, Chancellor MB, de Groat WC, Kuno S, Nonomura K, Yoshimura N. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J Urol. 2012;187:1890–1897. doi: 10.1016/j.juro.2011.12.062. [DOI] [PubMed] [Google Scholar]
- Kondoh T, Bannai M, Nishino H, Torii K. 6-Hydroxydopamine-induced lesions in a rat model of hemi-Parkinson’s disease monitored by magnetic resonance imaging. Exp Neurol. 2005;192:194–202. doi: 10.1016/j.expneurol.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Kawatani M, De Groat WC. Excitatory synaptic currents in lumbosacral parasympathetic preganglionic neurons evoked by stimulation of the dorsal commissure. J Neurophysiol. 2003;89:382–389. doi: 10.1152/jn.00180.2002. [DOI] [PubMed] [Google Scholar]
- Mouchet P, Manier M, Dietl M, Feuerstein C, Berod A, Arluison M, Denoroy L, Thibault J. Immunohistochemical study of catecholaminergic cell bodies in the rat spinal cord. Brain Res Bull. 1986;16:341–353. doi: 10.1016/0361-9230(86)90055-9. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Seki S, Masuda H, Igawa Y, Nishizawa O, Kuno S, Chancellor MB, de Groat WC, Yoshimura N. Dopaminergic mechanisms controlling urethral function in rats. Neurourol Urodyn. 2006;25:480–489. doi: 10.1002/nau.20260. [DOI] [PubMed] [Google Scholar]
- Roberts BL, Meredith GE. Immunohistochemical study of a dopaminergic system in the spinal cord of the ray, Raja radiata. Brain Res. 1987;437:171–175. doi: 10.1016/0006-8993(87)91540-x. [DOI] [PubMed] [Google Scholar]
- Sakakibara R, Kishi M, Ogawa E, Tateno F, Uchiyama T, Yamamoto T, Yamanishi T. Bladder, bowel, and sexual dysfunction in Parkinson’s disease. Park Dis. 2011;2011:924605. doi: 10.4061/2011/924605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R, Nakazawa K, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Micturition-related electrophysiological properties in the substantia nigra pars compacta and the ventral tegmental area in cats. Auton Neurosci. 2002;102:30–38. doi: 10.1016/s1566-0702(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Seki S, Igawa Y, Kaidoh K, Ishizuka O, Nishizawa O, Andersson KE. Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats. Neurourol Urodyn. 2001;20:105–113. doi: 10.1002/1520-6777(2001)20:1<105::aid-nau12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits. 2014;8:55. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A, Lindvall O, Schmidt RH. Origin and termination of the diencephalospinal dopamine system in the rat. Brain Res Bull. 1982;9:237–244. doi: 10.1016/0361-9230(82)90136-8. [DOI] [PubMed] [Google Scholar]
- Stafford SA, Coote JH. Activation of D2-like receptors induces sympathetic climactic-like responses in male and female anaesthetised rats. Br J Pharmacol. 2006;148:510–516. doi: 10.1038/sj.bjp.0706763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C. New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- Taniguchi W, Nakatsuka T, Miyazaki N, Yamada H, Takeda D, Fujita T, Kumamoto E, Yoshida M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain. 2011;152:95–105. doi: 10.1016/j.pain.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrumov MV. Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat. 2009;38:241–256. doi: 10.1016/j.jchemneu.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wienecke J, Ren LQ, Hultborn H, Chen M, Moller M, Zhang Y, Zhang M. Spinal cord injury enables aromatic L-amino acid decarboxylase cells to synthesize monoamines. J Neurosci. 2014;34:11984–12000. doi: 10.1523/JNEUROSCI.3838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge K, Fowler CJ. Bladder dysfunction in Parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21:737–745. doi: 10.1002/mds.20867. [DOI] [PubMed] [Google Scholar]
- Winkler C, Sauer H, Lee CS, Bjorklund A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci. 1996;16:7206–7215. doi: 10.1523/JNEUROSCI.16-22-07206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol. 1999;276:R935–R942. doi: 10.1152/ajpregu.1999.276.4.R935. [DOI] [PubMed] [Google Scholar]
- Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Interaction between D2 dopaminergic and glutamatergic excitatory influences on lower urinary tract function in normal and cerebral-infarcted rats. Exp Neurol. 2001;169:148–155. doi: 10.1006/exnr.2001.7639. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Kuno S, Chancellor MB, De Groat WC, Seki S. Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br J Pharmacol. 2003;139:1425–1432. doi: 10.1038/sj.bjp.0705388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Mizuta E, Kuno S, Sasa M, Yoshida O. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuropharmacology. 1993;32:315–321. doi: 10.1016/0028-3908(93)90151-r. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Mizuta E, Yoshida O, Kuno S. Therapeutic effects of dopamine D1/D2 receptor agonists on detrusor hyperreflexia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsonian cynomolgus monkeys. J Pharmacol Exp Ther. 1998;286:228–233. [PubMed] [Google Scholar]
- Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology. 1999;54:929–933. doi: 10.1016/s0090-4295(99)00234-4. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, Takeda M, de Groat WC. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol. 2013;304:F390–F396. doi: 10.1152/ajprenal.00574.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck ND, Downie JW. Plasticity in the injured spinal cord: can we use it to advantage to reestablish effective bladder voiding and continence? Prog Brain Res. 2006;152:147–162. doi: 10.1016/S0079-6123(05)52010-7. [DOI] [PubMed] [Google Scholar]