Abstract
Objective
Shared decision-making (SDM) is increasingly promoted in the primary care setting, but depressive symptoms, which are associated with cognitive changes, may influence decision-making preferences. We sought to assess whether elevated depressive symptoms are associated with decision-making preference in patients with comorbid chronic illness.
Methods
We enrolled 195 patients ≥18 years old with uncontrolled hypertension from two urban, academic primary care clinics. Depressive symptoms were assessed using the 8-item Patient Health Questionnaire. Clinician-directed decision-making preference was assessed according to the Control Preference Scale. The impact of depressive symptoms on decision-making preference was assessed using generalized linear mixed models adjusted for age, gender, race, ethnicity, education, Medicaid status, Charlson Comorbidity Index, partner status, and clustering within clinicians.
Results
The mean age was 64.2 years; 72% were women, 77% Hispanic, 38% Black, and 33% had elevated depressive symptoms. Overall, 35% of patients preferred clinician-directed decision-making, 19% mostly clinician-directed, 39% shared, and 7% some or little clinician-input. Patients with (vs. without) elevated depressive symptoms were more likely to prefer clinician-directed decision-making (46% versus 29%; p=0.02; AOR 2.51, 95%CI 1.30–4.85, p=0.005). Remitted depressive symptoms (vs. never depressed) were not associated with preference.
Conclusions
Elevated depressive symptoms are associated with preference for clinician-directed decision-making. We suggest that clinicians should be aware of this effect when incorporating preference into their communication styles and take an active role in eliciting patient values and exchanging information about treatment choice, all important components of shared decision-making, particularly when patients are depressed.
Keywords: depressive symptoms, shared decision-making, hypertension
Graphical abstract
Introduction
Shared decision-making (SDM) refers to a collaborative process whereby clinicians and patients make health decisions together by increasing awareness of options, exchanging information about best available evidence, exploring values and preferences, and finally making an informed decision.[1, 2] SDM has been heralded as a central aspect of patient-centered care by several groups, including the Institute of Medicine,[3, 4] and is a key approach to high-quality patient-physician communication, particularly when several evidence-based treatment options exist.
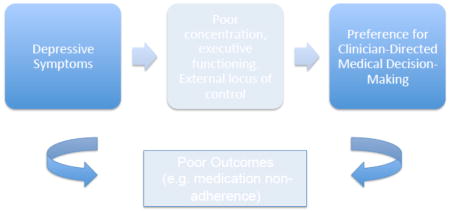
While many patients prefer collaborative styles,[5] some prefer more passive decision-making roles[6] and may require a prolonged deliberation process whereby physicians must balance advocacy for active participation with individual decision-making preferences.[1] Depressive symptoms are particularly prevalent amongst patients with chronic medical illnesses and are associated with worse health outcomes and medication non-adherence.[7–9] Characteristic cognitive aspects of depressive symptoms such as poor concentration and correlates such as low self-efficacy may influence decision-making preference.[10] However, the few studies to assess the impact of depressive symptoms on decision-making preferences have been in small samples of patients with heterogeneous mentally illnesses,[11, 12] and haven’t assessed differing impacts of active and remitted depressive symptoms.[6, 12] A more granular assessment may be crucial to increasing the understanding of decision-making preference in this vulnerable patient group.
We aimed to assess the relationship between depressive symptoms and decision-making preference, and hypothesized that patients with (vs. without) elevated depressive symptoms would prefer clinician-directed decision-making.
Methods
Participants
Between 2011 and 2014, we enrolled a convenience sample of patients with uncontrolled hypertension [13] from two urban, academic hospital-based primary care clinics (Columbia University Medical Center and Mount Sinai Medical Center) as part of a study assessing barriers to antihypertensive medication adherence.[14] Eligible patients were ≥18 years old and prescribed ≥1 blood pressure (BP) medication. All patients who met criteria on chart review and whose clinicians consented were approached at their clinician visit. Key exclusion criteria were inability to self-manage medications (e.g., dementia or severe psychiatric illness), unavailability for follow-up interview (e.g., prolonged travel abroad) and research assistant measured BP at goal (average of the last two of three measurements). After confirming eligibility and obtaining written informed consent, research assistants completed a baseline questionnaire with the patient. This questionnaire included assessments of socio-demographics, depressive symptoms, and decision-making preference. The Institutional Review Board at both institutions approved the study.
Measures
Depressive symptoms were assessed using the 8-item Patient Health Questionnaire (PHQ-8). A score ≥10 is consistent with elevated depressive symptoms.[15] Remitted depressive symptoms were defined as a PHQ-8 score <10 and history of depressive symptoms based on physician chart review. Preference for decision-making was based on the Control Preference Scale, which ranged from strong clinician-direction to little clinician-input.[16]
Age, gender, race, ethnicity, years of schooling, insurance status, and partner status were based on self-report. Charlson Comorbidity Index was calculated from chart review.[17] Self-reported adherence was based on the 8-item Morisky Medication Adherence Scale.[18]
Statistical Analyses
Chi Square, independent sample t-tests, and Mann Whitney U tests were used to compare demographic, clinical, and behavioral variables by depressive symptoms status. Covariates for adjusted analyses were based on a priori hypotheses.[5, 19–21] Ordinal logistic regression was used to test the association between depressive symptoms (elevated vs. non-elevated) and preference for clinician-directed decision-making (strong clinician-direction to little clinician-input) (Model 1). We used a generalized linear mixed model to account for nesting of patients within providers and adjusted for age, gender, race, ethnicity, years of schooling, Medicaid status, Charlson Comorbidity Index, and partner status (Model 2). The proportional odds assumption was met in the unadjusted and adjusted models. In a subgroup analysis, we compared the association between remitted depressive symptoms (vs. no documented depressive symptoms and PHQ-8<10) and decision-making preference. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Of 522 screened patients, 46 (8.8%) declined participation and 277 were ineligible due to: controlled BP on repeat measurement (35.0%); dementia or severe mental illness (11.9%); unavailability for follow-up interview (9.8%); deemed poor research subject by physician (e.g. extensive psychosocial stressors, unstable medical conditions) (12.3%); and other reasons (non-English and non-Spanish speaking, institutionalization, terminal illness; 31%). This left 199 patients who consented and completed a baseline interview, of which 195 had complete data for our final analyses. Those who refused (vs. consented) were less likely to be Hispanic (50.0% vs. 77.4%, p <0.0001) and female (60.9% vs. 69.9%, p=0.002), but more likely to be Black (65.2% vs. 47.7%, p=0.03).
The mean age was 64.2 (SD 9.1) years; 72% were women, 77% Hispanic, 39% Black, 84% carried Medicaid insurance; 33% had elevated depressive symptoms, 19% remitted depressive symptoms, and 29% low medication adherence. The mean number of comorbidities was 3.2 (SD 2.4) and median PHQ-8 score 7.0 (Interquartile Range 10). Elevated depressive symptoms were associated with low adherence. Overall, 35% of patients preferred strong clinician-directed decision-making, 19% mostly clinician-directed, 39% shared, and 7% some or little clinician-input (Table 1).
Table 1.
Patient Characteristics by depressive symptom status
| Characteristics, N (%) | Total Sample (N=195) | Elevated depressive symptoms (n=65) | Non-elevated depressive symptoms (n=130) | P-value |
|---|---|---|---|---|
| Age, mean (SD), y | 64.2 (9.1) | 62.7 (9.4) | 65.1 (8.6) | 0.08 |
| Female | 141 (72%) | 51 (78%) | 90 (69%) | 0.18 |
| Black | 75 (39%) | 25 (38%) | 51 (39%) | 0.94 |
| Hispanic | 151 (77%) | 53 (82%) | 98 (75%) | 0.28 |
| Grade | 9.2 (4.3) | 8.6 (4.3) | 9.5 (4.2) | 0.13 |
| Medicaid | 162 (84%) | 59 (91%) | 103 (79%) | 0.04* |
| Married | 55 (28%) | 17 (26%) | 38 (29%) | 0.65 |
| Charlson, mean (SD) | 3.2 (2.4) | 3.3 (2.5) | 3.1 (2.3) | 0.69 |
| Low-adherence (SR) | 56 (29%) | 29 (45%) | 27 (21%) | 0.003 |
| BP Medications, mean (SD) | 2.6 (1.0) | 2.6 (0.9) | 2.5 (1.0) | 0.70 |
| Decision Preference | 0.02 | |||
| Strongly clinician-directed | 68 (35%) | 30 (46%) | 38 (29%) | |
| Mostly clinician-directed | 37 (19%) | 11 (17%) | 26 (20%) | |
| Collaborative approach | 77 (39%) | 21 (32%) | 56 (43%) | |
| Some clinician-input | 7 (4%) | 3 (5%) | 4 (3%) | |
| Little clinician-input | 6 (3%) | 0 (0%) | 6 (5%) |
Data presented as N (%) unless otherwise specified.
SR self report; SD standard deviation
Forty-six percent of patients with (vs. 29% without) elevated depressive symptoms preferred clinician-directed decision-making (OR 1.91, 95%CI 1.10–3.33; p=0.02; adjusted OR [AOR] 2.51, 95%CI 1.30–4.85, p=0.005). Older age, black race, fewer years of schooling, and less comorbidity were also associated with preference for clinician-directed decision-making (Table 2).
Table 2.
Ordinal Logistic Regression modeling the association between depressive symptoms and preference for clinician-directed decision-makinga
| Variable | Model 1 | P value | Model 2b | P value |
|---|---|---|---|---|
| Depressive symptoms(vs. No depressive symptoms) | 1.91 (1.10, 3.33) | 0.02 | 2.51 (1.30, 4.85) | 0.005 |
| Age (per year increase) | 1.04 (1.00, 1.08) | 0.04 | ||
| Male | 0.98 (0.49, 1.95) | 0.70 | ||
| Black | 2.09 (1.03, 4.23) | 0.04 | ||
| Hispanic | 0.78 (0.30, 2.04) | 0.58 | ||
| Years of Schooling | 0.90 (0.83, 0.98) | 0.009 | ||
| Medicaid | 0.92 (0.39, 2.17) | 0.83 | ||
| Charlson Comorbidity Index | 0.88 (0.77, 0.99) | 0.04 | ||
| Partnered | 0.91 (0.46, 1.84) | 0.36 |
Ordinal Logistic regression is modeling the odds of choosing lower numbers on the Likert scale (e.g. clinician-directed decision-making)
Adjusts for clustering within primary care physicians and all of the covariates listed in the Table.
In a subgroup analysis, 24% of those with remitted depressive symptoms (vs. 31% who never had depressive symptoms) preferred clinician-directed decision-making (OR 0.75, 95%CI 0.46–1.20, p=0.23; AOR 0.62, 95%CI 0.28–1.36 p=0.23).
Sensitivity Analyses
Given the low frequency of participants who preferred “some clinician input” (n=7) and “little clinician input” (n=6), we conducted sensitivity analyses in which we combined these preferences into a single category or excluded both. Whether combining or excluding participants, we found that depressive symptoms remained associated with preference for clinician-directed decision-making [AOR=2.51 (95%CI 1.30–4.83, p=0.007) and AOR=2.07 (95% CI 1.04–4.12, p=0.04), respectively]. Depressive symptoms as a linear variable also remained associated with preference for clinician-directed decision-making (AOR 1.07, 95% CI 1.02–1.13, p=0.01).
Discussion
In this sample of predominantly low-income, minority, urban patients with uncontrolled hypertension, we found that active but not remitted depressive symptoms were associated with preference for clinician-directed decision-making. Our study aligns with prior frameworks that suggest passivity and poor executive functioning may be core components of depressive symptomatology.[22] Our results differ from some previous literature on mental illness and active decision-making preference likely due to prior small samples of patients with heterogeneous mental illnesses lacking non-depressed comparator groups, [11, 12] and inadequate examination of uncontrolled comorbidities and clustering within clinicians.[6] We add to the literature by demonstrating that elevated depressive symptoms themselves not a history of depression affect preference. Further research is needed to understand the pathways linking depressive symptoms with decision-making preferences, which may allow physicians to better tailor communication styles when treating patients with elevated depressive symptoms and multiple comorbidities who face complex options.
Our study is important because research has shown that passive decision-making preference may be associated with worse outcomes,[23] which may partially explain why patients with elevated depressive symptoms have worse adherence,[7–9] as in our sample. Regardless of decision-making preference, amongst individuals with elevated depressive symptoms, SDM can be associated with increased probability of receiving guideline-concordant care and depressive symptom resolution.[24] How then should clinicians approach patients with depressive symptoms who prefer clinician-directed decision-making? Given potential benefits, we believe that clinicians should make special efforts to elicit preference and engage depressed patients in SDM, which begins with information exchange and value assessment prior to embarking on final decision-making.[25] Preference for passive final medical decision-making does not preclude preference for discussion of treatment choices, equally important for SDM and thus outcomes.[1, 25–27] Given that patients with elevated depressive symptoms may seek out less information or use fewer resources to support decision-making,[22] physicians should consider taking a more active role in information exchange. One study found using decision aids may mitigate the relationship between depressive symptoms and a patient’s perception of their decision-making ability and thus comfort level.[22] Physicians can also consider using problem-solving therapy to improve depressive symptoms and thus SDM engagement. If patients continue to defer final decision-making, physicians should consider aligning their styles to patient preference [26] and strive to incorporate patient values into decision-making processes or involve family members [1], all resulting in true shared decision-making. In fact, congruence between decision-making preference and actual communication style of the physician may improve satisfaction and adherence.[28, 29] This strategy may also be used for subgroups of mostly older, minority, lower socioeconomic status patients who prefer clinician-directed decision-making,[6] as was also demonstrated in our study. In all, educational campaigns are integral to improving clinician proficiency in SDM communication styles.
There were several limitations to our study. Our sample of low-income, urban patients may not be generalizable to the broader population of primary care patients. Nevertheless, our study allowed for a granular analysis of multiple uncontrolled illnesses in the primary care setting while examining remitted depressive symptoms and adjusting for physician-patient relationships, which has rarely been available previously. Another limitation is that our main outcome was assessed using a single item based on the Control Preference Scale, which provides a limited view of a patient’s actual preference.[30] This measure of SDM preference, however, continues to be a widely used and validated scale of patient decision-making preference.[31]
In conclusion, amongst primary care patients with uncontrolled hypertension, most with elevated depressive symptoms preferred clinician-directed decision-making. Future studies should identify approaches that improve SDM in patients with elevated depressive symptoms.
Highlights.
Patients with elevated depressive symptoms prefer clinician-directed medical decision-making
Cognitive symptoms of depression may affect decision-making preferences
Physicians should be mindful of this association when incorporating patient preferences into the shared decision-making process
Physicians should consider taking an active role in information exchange, value assessment, and review of treatment choices, all hallmarks of shared decision-making
Acknowledgments
Funding: This research was funded by the National Heart, Lung, and Blood Institute (NHLBI) (K23 HL-098359) and the American Heart Association (SDG 10SDG2600321). Dr. Ye received support from NHLBI (K23 HL121144). Dr. Moise received support from the Health Resources and Services Administration (T32HP10260). Dr. Alcántara received support from NHLBI (HL115941-01S1). Dr. Davidson received support from PCORI, NHLBI (5K24HL084034, R01 HL115941, R01 HL114924) and from ECRIP.
Footnotes
Financial Disclosures: none
Conflict of Interest: None
The authors have no competing interests to report.
Author Contributions: Dr. Nathalie Moise had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Moise, Kronish, Ye
Acquisition of data: Kronish, Moise
Analysis and interpretation of data: Moise, Kronish, Davidson, Ye, Alcantara
Drafting of the manuscript: Moise, Kronish
Critical revision of manuscript for important intellectual content: Moise, Kronish, Davison, Ye, Alcantara
Statistical analysis: Moise
Obtained funding: Kronish
Study supervision: Kronish
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared Decision Making: A Model for Clinical Practice. Journal of general internal medicine. 2012;27(10):1361–7. doi: 10.1007/s11606-012-2077-6. 05/23 08/22/received 01/03/revised 04/03/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry MJ, Edgman-Levitan S. Shared Decision Making — The Pinnacle of Patient-Centered Care. New England Journal of Medicine. 2012;366(9):780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 1995 May 1;152(9):1423–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): 2001. [Google Scholar]
- 5.Swenson SL, Buell S, Zettler P, White M, Ruston DC, Lo B. Patient-centered communication: do patients really prefer it? Journal of general internal medicine. 2004 Nov;19(11):1069–79. doi: 10.1111/j.1525-1497.2004.30384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora NK, McHorney CA. Patient Preferences for Medical Decision Making: Who Really Wants to Participate? Medical care. 2000;38(3):335–41. doi: 10.1097/00005650-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Katon W, Sullivan MD. Depression and chronic medical illness. The Journal of clinical psychiatry. 1990 Jun;51( Suppl):3–11. discussion 2–4. [PubMed] [Google Scholar]
- 8.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosomatic medicine. 1999 Jan-Feb;61(1):6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Katon WJ, Rutter C, Simon G, Lin EHB, Ludman E, Ciechanowski P, et al. The Association of Comorbid Depression With Mortality in Patients With Type 2 Diabetes. Diabetes Care. 2005 Nov 1;28(11):2668–72. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 10.Benassi VA, Sweeney PD, Dufour CL. Is there a relation between locus of control orientation and depression? Journal of abnormal psychology. 1988 Aug;97(3):357–67. doi: 10.1037//0021-843x.97.3.357. [DOI] [PubMed] [Google Scholar]
- 11.Adams JR, Drake RE, Wolford GL. Shared decision-making preferences of people with severe mental illness. Psychiatric services (Washington, DC) 2007 Sep;58(9):1219–21. doi: 10.1176/ps.2007.58.9.1219. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Bakken S. Preferences for participation in decision making among ethnically diverse patients with anxiety and depression. Community mental health journal. 2010 Oct;46(5):466–73. doi: 10.1007/s10597-010-9323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.Measuring adherence to control hypertension (MATCH) [Internet] 2010 cited September 11, 2015 Available from: https://clinicaltrials.gov/ct2/show/NCT01257347.
- 15.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders. 2009 Apr;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. The Canadian journal of nursing research = Revue canadienne de recherche en sciences infirmieres. 1997 Fall;29(3):21–43. [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of clinical hypertension. 2008 May;10(5):348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.McKinstry B. Do patients wish to be involved in decision making in the consultation? A cross sectional survey with video vignettes. BMJ (Clinical research ed) 2000 Oct 7;321(7265):867–71. doi: 10.1136/bmj.321.7265.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krupat E, Bell RA, Kravitz RL, Thom D, Azari R. When physicians and patients think alike: patient-centered beliefs and their impact on satisfaction and trust. The Journal of family practice. 2001 Dec;50(12):1057–62. [PubMed] [Google Scholar]
- 21.Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE., Jr Characteristics of physicians with participatory decision-making styles. Annals of internal medicine. 1996 Mar 1;124(5):497–504. doi: 10.7326/0003-4819-124-5-199603010-00007. [DOI] [PubMed] [Google Scholar]
- 22.Leykin Y, Roberts CS, DeRubeis RJ. Decision-Making and Depressive Symptomatology. Cognitive Therapy and Research. 2011 May 04;35(4):333–41. doi: 10.1007/s10608-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beach MC, Duggan PS, Moore RD. Is patients’ preferred involvement in health decisions related to outcomes for patients with HIV? Journal of general internal medicine. 2007 Aug;22(8):1119–24. doi: 10.1007/s11606-007-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clever SL, Ford DE, Rubenstein LV, Rost KM, Meredith LS, Sherbourne CD, et al. Primary care patients’ involvement in decision-making is associated with improvement in depression. Medical care. 2006 May;44(5):398–405. doi: 10.1097/01.mlr.0000208117.15531.da. [DOI] [PubMed] [Google Scholar]
- 25.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Social science & medicine (1982) 1997 Mar;44(5):681–92. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 26.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Social science & medicine (1982) 1999 Sep;49(5):651–61. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 27.Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. American journal of preventive medicine. 1999 Nov;17(4):285–94. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Chan CM, Azman WA. Attitudes and role orientations on doctor-patient fit and patient satisfaction in cancer care. Singapore medical journal. 2012 Jan;53(1):52–6. [PubMed] [Google Scholar]
- 29.De Las Cuevas C, Penate W, de Rivera L. To what extent is treatment adherence of psychiatric patients influenced by their participation in shared decision making? Patient preference and adherence. 2014;8:1547–53. doi: 10.2147/PPA.S73029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Entwistle VA, Watt IS, Gilhooly K, Bugge C, Haites N, Walker AE. Assessing patients’ participation and quality of decision-making: insights from a study of routine practice in diverse settings. Patient education and counseling. 2004 Oct;55(1):105–13. doi: 10.1016/j.pec.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Kryworuchko J, Stacey D, Bennett C, Graham ID. Appraisal of primary outcome measures used in trials of patient decision support. Patient education and counseling. 2008 Dec;73(3):497–503. doi: 10.1016/j.pec.2008.07.011. [DOI] [PubMed] [Google Scholar]


