Abstract
Type 2 diabetes is a rapidly growing disease that poses a significant burden to the United States healthcare system. Despite the many available treatments for the disease, close to half of diagnosed type 2 diabetes cases are not properly managed, largely due to inadequate patient adherence to prescribed treatment regimens. Methods for improving delivery — and thereby easing administration — of type 2 drugs have the potential to greatly improve patient health. This review focuses on two peptide drugs — insulin and glucagon-like peptide 1 (GLP-1) — for treatment of type diabetes. Peptide drugs offer the benefits of high potency and specificity but pose a significant delivery challenge due to their inherent instability and short half-life. The development of insulin and GLP-1 analogs highlights the broad spectrum of drug delivery strategies that have been used to solve these problems. Numerous structural modifications and formulations have been introduced to optimize absorption, residence time, stability, route of delivery and frequency of administration. Continual improvements in delivery methods for insulin and GLP-1 receptor agonists are paving the way towards better patient compliance and improved disease management, and thereby enhanced patient quality of life.
Keywords: Drug delivery, controlled release, peptide, insulin, GLP-1, diabetes
Graphical abstract
INTRODUCTION
Nearly 30 million people in the United States are diabetic, and in 2012 alone, an additional 1.7 million new cases were diagnosed[1], underscoring both the significant health burden and the rapidly growing nature of the disease. Type 2 diabetes accounts for the majority of newly diagnosed cases of diabetes, and is characterized by insulin insensitivity and an inability of pancreatic beta cells to produce enough insulin to adequately control blood glucose levels. Persistent hyperglycemia, a hallmark of both diabetes types, can lead to a host of complications including microvascular damage, cardiovascular disease, retinopathy, neuropathy, and kidney failure[1].
Risk factors for type 2 diabetes include obesity, sedentary lifestyle, and tobacco use; thus, initial treatment commonly consists of lifestyle changes. If hyperglycemia persists, pharmacological therapy is initiated. Treatment regimens for type 2 diabetes aim to reduce blood glucose levels while avoiding hypoglycemia, and will vary depending on the disease pathogenesis as well as family history and health status of the patient. Pharmacological intervention generally begins with metformin, and can progress to include a second or third glucose-lowering agent, including sulfonylureas, thiazolidinediones, sodium-glucose transporter-2 inhibitors, DPP-IV inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists[2]. Type 2 diabetics with persisting severe hyperglycemia will eventually incorporate insulin into their therapeutic regimens[2].
The efficacy of a diabetic treatment strategy is evaluated by the degree of long-term glycemic control achieved and is approximated by the level of glycosylated hemoglobin (HbA1c). Diabetes is diagnosed when HbA1c levels exceed 6.5%[3], and this value remains a stringent goal for adults newly diagnosed with type 2 diabetes, though a 7 – 8% HbA1c target may be more appropriate for patients with advanced disease[2, 4]. However, it is estimated that 40–50% of type 2 diabetics fail to achieve their individualized HbA1c goals[5], suggesting inadequacies in available therapies. In fact, it is lack of patient adherence to devised treatment regimens that remains one of the largest obstacles in achieving glycemic control, and not necessarily a lack of drug efficacy. Until a treatment emerges that can permanently restore effective insulin production and utilization in the body, strategies to improve delivery of available type 2 diabetes drugs, and thus ease administration, have the potential to greatly improve the health of patients.
This review focuses on the two type 2 diabetes drugs that are most relevant to the field of drug delivery: insulin and GLP-1 receptor agonists, and both have interesting stories to tell. Insulin is nearly a century old and yet remains a crucial end-of-the-line treatment for type 2 diabetics, while GLP-1 was recently discovered and has opened up a new avenue for type 2 drugs due to its weight lowering effects and minimal risk of hypoglycemia. Both are peptide drugs, which sets them apart from all other type 2 diabetes drugs, and both provide the benefits of high potency and specificity. Peptide drugs, however, pose a significant delivery challenge due to their instability, short half-life, and susceptibility to degradation. Consequently, a significant amount of effort has been dedicated to design insulin and GLP-1 analogs to enhance their efficacy and safety as well as optimize their route —and frequency— of administration.
INSULINS
In 1921 Frederick Banting and Charles Best first extracted insulin from the pancreas of cows and pigs, and turned type 1 diabetes from a lethal condition into a manageable disease. In an effort to prevent any single entity from monopolizing the supply of insulin, they patented their extraction process, but Banting and Best eventually merged with Eli Lilly when the demand for insulin surpassed their laboratory production limits[6].
While bovine and porcine insulins were monumentally lifesaving, their short duration of action required frequent injections and resulted in alternating states of hyperglycemia and hypoglycemia[7]. The first set of major formulation improvements came from Hans Christian Hagedorn at Nordisk Insulin Laboratories in the 1930s and 40s. Hagedorn's work was based on the principle that a protein is least soluble at a pH equal to its isoelectric point (pI); insulin has a pI of 5.2, and is therefore reasonably soluble at physiological pH (7–7.5). Hagedorn found that combining insulin with the highly basic proteins, protamines, moved the combined pI closer to physiological pH, thus causing the protein mixture to precipitate upon injection[7]. Further experiments found that the addition of zinc resulted in protamine-insulin crystals that could be combined with unmodified fast-acting insulin in the same syringe[7]. This intermediate-acting insulin formulation, later termed neutral protamine Hagedorn (NPH), required less frequent injections and was one of the first examples of a controlled release system in the field of drug delivery[7].
The next notable milestone in insulin formulation came with the advent of recombinant DNA technology. Despite advances in purification techniques, animal insulin continued to elicit mild allergic reactions and occasional immunological responses[8]. In 1978 Genentech was the first to produce a recombinant human insulin[9], and shortly thereafter, Eli Lilly gained Food and Drug Administration (FDA) approval for its recombinant human insulin “Humulin.” Eli Lilly subsequently released a recombinant version of NPH, and Novo Nordisk later followed with its own recombinant human insulin and NPH.
Human insulin and NPH became the standard prandial and basal insulin formulations used in insulin replacement or supplementation regimens for type 1 and type 2 diabetics[10]. However, a prandial bolus of unmodified insulin produced a relatively delayed action onset of 30–60 minutes[10], while NPH failed to adequately mimic endogenous basal insulin secretion due to its peak of action four to seven hours post-administration[11]. Thus, both formulations left room for improvement.
In the last two decades, many structural modifications have been made to control the absorption and residence time of insulin. Ordinarily, drug delivery techniques aim solely to sustain the release and duration of action of a drug; insulin is unique in that it has been engineered to both shorten and lengthen its glucose-lowering action. An overview of currently approved insulin analogs and associated durations of action are provided in Table 1, and expanded upon in the following sections.
Table 1.
Durations of action of currently available standard insulins and insulin analogs
| Brand Name | Generic Name | Manufacturer | Approval Date | Onset of Action | Peak | Duration | |
|---|---|---|---|---|---|---|---|
| Rapid-acting | Humalog | Insulin lispro | Eli Lilly | 1996 | 15 min | 30 – 90 min | 3–5 h |
| NovoLog | Insulin aspart | Novo Nordisk | 2000 | 15 min | 30 – 90 min | 3–5 h | |
| Apidra | Insulin glulisine | Sanofi | 2004 | 15 min | 30 – 90 min | 3–5 h | |
| Short-acting | Humulin R / Novolin R | Regular insulin | Eli Lilly / Novo Nordisk | 1982/1991 | 30–60 min | 2–3 h | 5–8 h |
| Intermediate-acting | Humulin N / Novolin N | Neutral protamine Hagedorn (NPH) | Eli Lilly / Novo Nordisk | 1982/1991 | 2–4 h | 4–10 h | 10–16 h |
| Long-acting | Lantus | Insulin glargine | Sanofi | 2000 | 2–4 h | Peakless | 20–24 h |
| Toujeo | Insulin glargine 300 U/ml | Sanofi | 2015 | 6 h | Peakless | 24 h | |
| Levemir | Insulin detemir | Novo Nordisk | 2005 | 3–4 h | Peakless | 24 h | |
| Tresiba | Insulin degludec | Novo Nordisk | 2015 | 3–4 h | Peakless | >24 h |
Assumes 0.1 – 0.4 units/kg subcutaneous injection. Response will vary depending on injection location and body mass index. Rapid acting analog data retrieved from the NIDDK[12]; regular insulin, NPH, and Lantus data from Dewitt and Hirsch[10]; Toujeo Levemir, and Tresiba data from FDA NDA documents[13–15].
Abbreviations: NPH (neutral protamine Hagedorn)
Fast-Acting Insulin Analogs
Native human insulin is produced by pancreatic beta cells and, before secretion, undergoes post-translational processing to form a 21-amino acid A chain linked via disulfide bonds to a 30-amino acid B chain (Fig. 1). The disulfide-linked monomer is the bioactive form of insulin, but monomers can associate into dimers, and dimers can further associate into hexamers in the presence of zinc ions[16].
Figure 1.
Structure of human insulin. A 21-amino acid A chain is linked via disulfide bonds to a 30-amino acid B chain to form the functional insulin monomer. Shaded residues are commonly mutated in fast-acting insulin analogs to speed up absorption upon s.c. injection.
Endogenous insulin is secreted directly into circulation where it can act on target tissues, primarily muscle and adipose. In contrast, therapeutically administered insulin is injected into the subcutaneous (s.c.) space where it must undergo sufficient absorption and dilution for dissociation into the monomeric (functional) state[17]. Thus, design of fast-acting insulin analogs generally strives to speed up absorption into systemic circulation and subsequent dilution such that their pharmacodynamic profile mimics endogenous prandial insulin as closely as possible. All mutations designed to enhance absorption by preventing higher order insulin monomer associations target amino acid residues in the B chain (Fig.1). This is due to the fact that formation of the insulin hexamer requires interactions between nonpolar surfaces on the molecule, the majority of which consist only of residues from the B chain[16].
Insulin lispro
The first insulin analog designed to speed up absorption was developed by Eli Lilly and approved by the FDA in 1996. Insulin lispro, marketed as Humalog, swaps ProB28 (denoting the proline in position 28 of the B chain) with LysB29, thereby inhibiting the formation of dimers and hexamers. This modification mimics a Lys-Pro motif near the C-terminus of insulin-like growth factor 1, a protein that is structurally similar to insulin but resists dimerization[18]. A 10 unit (U) dose of insulin lispro is absorbed more than twice as quickly and reaches a maximum concentration that is more than two-fold higher than native insulin[19], resulting in a glucose-lowering action onset of approximately 15 minutes[10].
Insulin aspart
The second rapid-acting insulin analog to enter the market was Novo Nordisk's insulin aspart, branded as NovoLog. A ProB28Asp substitution inhibits hexamer formation through charge repulsion between dimers[20], as well as slightly reduces the pI to increase solubility at physiological pH[21]. A 0.1 U/kg dose of insulin aspart displays pharmacokinetic and pharmacodynamic profiles similar to those of insulin lispro[10, 22].
Insulin glulisine
Sanofi's insulin glulisine was the third fast-acting insulin analog to enter the market. A LysB29Glu mutation is employed to slightly decrease the pI, thereby increasing solubility upon injection[21], while an additional AsnB3Lys mutation disrupts a stabilizing conformational change within the hexamer cluster that is ordinarily induced by antimicrobial preservatives present in insulin drug formulations[21, 23]. This destabilizing feature promotes dissociation of the analog into the functional monomer form, which may account for absorption of insulin glulisine occurring approximately 10 minutes faster than absorption of insulins lispro and aspart (each injected at 0.15 U/kg)[24].
Ultra-fast-acting insulin analogs
Despite label instructions to administer insulin analogs 0 to 15 minutes before a meal (0 to 10 minutes for insulin aspart), data suggest that rapid-acting analogs require more than 15 minutes to properly control post-prandial glycemia[25]. Increased dietary planning requirements can introduce patient compliance issues and, therefore, even faster acting analogs may be desirable. Novo Nordisk announced the completion of Phase IIIa trials in March, 2015, for faster-acting insulin aspart (FIAsp)[26]. The formula includes nicotinamide and arginine for the purpose of increasing the rate of absorption upon injection and preventing excess protein degradation during storage[27]. Compared to current fast-acting insulin analogs on the market, FIAsp is intended to provide prandial glucose control closer to that afforded by the endogenous insulin response[28].
Another approach for accelerating absorption is to facilitate faster absorption into systemic circulation. Halozyme Therapeutics has completed Phase II clinical trials[29] for a faster-acting insulin formulation that incorporates the hyaluronidase PH20 to degrade hyaluronan in the s.c. space. Preliminary data show respective absorption times reduced by approximately 10 minutes for insulins lispro, aspart, and glulisine, each injected at 0.15 U/kg[24].
Long-Acting Insulin Analogs
Insulin glargine
In 2000, Sanofi gained approval for the first basal human insulin analog. Insulin glargine, marketed as Lantus, has an AsnA21Gly mutation as well as two Arg residues added to the C-terminus of the B chain[11]. Following a similar concept as NPH, these modifications increase the pI such that upon s.c. injection, insulin glargine forms insoluble hexamer aggregates that are slow to absorb[11].
This analog, when injected at 0.4 U/kg, has a flatter pharmacodynamic profile, lacking the 4 to 7 hour time-action peak classically seen with NPH[11]. In an efficacy study comparing once-daily insulin glargine to twice-daily NPH in patients with type 2 diabetes (0.75 U/kg/day), insulin glargine decreased nocturnal hypoglycemia by 25% compared to NPH, while offering an approximately equivalent reduction in HbA1c levels (−0.41% versus −0.59% for NPH group) [30]. A follow-up meta-analysis found a 46% reduction in severe hypoglycemia and a 59% reduction in severe nocturnal hypoglycemia in groups treated with insulin glargine compared to those receiving NPH[31]. Note that incidence of hypoglycemia is defined as percentage of patients reporting at least one hypoglycemic event. It is also worth mentioning that insulin glargine is a clear solution while NPH is a cloudy suspension, implying that all comparison trials have been open label.
Toujeo, a three-fold concentrated version of insulin glargine, was approved by the FDA in February 2015. The increased concentration of Toujeo compared to insulin glargine offers a smaller interfacial area for absorption upon s.c. injection and, theoretically, a slower rate of release. Indeed, Toujeo administered at 0.4 U/kg has a prolonged pharmacodynamic profile, controlling blood glucose in type 1 diabetics for up to 36 hours[32]. Efficacy studies comparing once-daily Toujeo to once-daily insulin glargine treatment in type 2 diabetics (0.2 U/kg starting dose, adjusted to achieve a set fasting glucose level) resulted in equivalent glycemic control (HbA1c reduced by 1.4%), with a 24% reduction in nocturnal hypoglycemia among patients treated with the concentrated formulation[33].
Insulin detemir
The second long-acting insulin analog, insulin detemir, was developed by Novo Nordisk and entered the market in 2005; it features a myristic acid molecule bound to LysB29. Fatty acids radiate out of the hexamer structure and interact with each other to form higher order dihexamer associations[34], further stabilizing the analog. The myristic acid conjugation also allows reversible albumin binding, which can occur when insulin detemir is in the monomer, dimer, hexamer, and dihexamer forms[35]. Interestingly, it is believed that albumin binding in the interstitial space plays a larger role in prolonging the action of insulin detemir than does albumin binding of the analog in circulation[35].
Compared to NPH (both administered at 0.3 U/kg), the time-action profile of insulin detemir is not ideal, showing a distinct peak at 6 to 8 hours[36]. Additionally, there is little correlation between the pharmacokinetic and pharmacodynamic profiles, possibly due to a disproportionate amount of free versus albumin-bound drug[36]. A study comparing twice-daily insulin detemir to twice-daily NPH in type 1 diabetics resulted in 50% and 18% reduction in nocturnal and overall hypoglycemia, respectively, in the insulin detemir group[37]. A similar study involving type 2 diabetics (starting dose 10 U/injection, adjusted to achieve a set fasting glucose level) resulted in 55% and 47% reduction in nocturnal and overall hypoglycemia, respectively, among patients receiving insulin detemir compared to those receiving NPH, as well as less weight gain (1.2 kg compared to 2.8 kg in the NPH group)[38].
Insulin degludec
Insulin degludec is an ultra-long-acting version of insulin detemir that was originally rejected by the FDA in 2013 due to cardiovascular concerns. The drug, branded as “Tresiba,” was resubmitted and received approval in September, 2015[39]. Insulin degludec has a hexadecanedioic acid conjugated to LysB29, allowing albumin binding as well as the formation of multi-hexamers[40]. These higher order structures are designed to remain soluble upon injection with the intention of producing a more predictable time-action curve, since the absorption rate of insulin degludec should primarily depend on dissociation into monomers rather than s.c. blood perfusion[40].
Indeed, this analog, when administered at 0.6 U/kg, has very smooth pharmacokinetic and pharmacodynamic profiles, as well as glucose-lowering effects that extend beyond 24 hours[41]. Meta-analyses comparing once-daily insulin degludec to once-daily insulin glargine in type 1 diabetics showed equivalent glycemic control afforded by the two treatments, with 25% and 30% reduction in nocturnal hypoglycemia among insulin degludec groups in the respective studies[42, 43]. A similar meta-analysis involving type 2 diabetics showed a 17% reduction in overall hypoglycemia and a 32% reduction in nocturnal hypoglycemia among patients receiving insulin degludec compared to those receiving insulin glargine, with comparable improvements in HbA1c levels between the two groups[42].
PEGylated insulin lispro
Eli Lilly is developing an ultra-long acting insulin analog in the form of PEGylated insulin lispro (LY2605541). Conjugation of 20 kDa PEG to LysB28 (resulting from the Pro-Lys residue exchange employed in insulin lispro) reduces renal filtration and produces an analog with an impressive 36 hour time-action profile, when administered at 0.67 U/kg[44]. In an efficacy study involving type 2 diabetics, treatment with PEG-lispro (6 nmol per unit of pre-study basal insulin) resulted in glucose control equivalent to that afforded by insulin glargine (7.0% ending HbA1c for PEG-lispro group compared to 7.2% for insulin glargine)[45]. However, the PEG-lispro group showed a 48% reduction in nocturnal hypoglycemia and a significant reduction in body weight (−0.6 kg compared to +0.3 kg in glargine group)[45]—an especially desirable effect since most insulin therapies cause weight gain. It is postulated that the action of PEG-lispro mimics that of endogenous insulin by preferentially targeting the liver for stimulation of glucose uptake[46]. Unfortunately, in February 2015, Eli Lilly announced a delay in submission of LY2605541 to the FDA in order to thoroughly investigate reported increased clinical incidences of liver fat deposition after treatment with the new formulation[47].
Novel Formulations
Inhaled insulin
A different approach to developing ultra-rapid-acting insulin is a drug and device combination —Afrezza— developed by MannKind in partnership with Sanofi. Powdered human insulin is adsorbed onto “Technosphere” particles consisting of fumaryl diketopiperazine, which serves as an excipient[48]. The particles are 2–5 μm in size[48], allowing entry into the lungs, where the excipient dissolves upon contact with alveoli and insulin diffuses across the membranes[49]. Peak insulin concentrations in the serum occur only 12–17 minutes after inhalation (20, 50, or 100 U doses) and peak glucose-lowering activity occurs in under one hour compared to greater than two hours for 10 U s.c. injected human insulin[48]. A downside to this technology is its lower bioavailability. Afrezza has a bioavailability of 20 to 25%[48] compared to s.c. administered insulin, which has a bioavailability of approximately 70%[50].
Afrezza entered the market in February 2015, and first and second quarter sales have been disappointing. The inhaled insulin product comes with a warning label stating that Afrezza should not be used in patients with lung disease or asthma. As a result, patients are required to take a spirometry test to ensure proper lung function, necessitating a visit to a pulmonologist and inevitably discouraging patient interest. However, Sanofi remains optimistic. Insurance coverage of Afrezza is increasing, as is advertisement of the product. Sanofi is the only major pharmaceutical company pursuing inhaled insulin; Pfizer's Exubera was pulled from the market in 2007 due to lack of demand among patients and physicians, and both Eli Lilly and Novo Nordisk halted development of their respective inhaled insulin products shortly thereafter.
Transbuccal insulin
Generex Biotechnology has developed a liquid spray of human insulin, marketed as Oral-lyn, which is delivered via the inner lining of the cheek. Their “RapidMist” technology aerosolizes emulsified insulin droplets greater than 10 μm in diameter, preventing entry into the lungs[51], while the proprietary formulation contains surfactants that facilitate transport of insulin across the buccal mucosa[52]. Peak insulin concentrations in the serum occur 23 minutes following administration of a 150 U dose compared to 83 minutes for s.c. injected human insulin (0.1 U/kg). Similarly, peak glucose-lowering activity occurs after approximately 44 minutes for Oral-lyn versus 100 minutes for injected insulin[53]. A Phase III clinical trial carried out in India involving type 2 diabetics reported that a 0.48% reduction in HbA1c was achieved after 6 weeks of treatment with Oral-lyn, while a statistically comparable reduction required 12 weeks of injected human insulin treatment[54].
Oral-lyn is currently in Phase III trials in the United States. However, Generex halted clinical trials in March, 2015, while they worked to increase the bioavailability of insulin in their device for the purpose of reducing the required number of puffs[55]. The original formulation had a bioavailability of approximately 10%, necessitating 10 puffs to deliver the standard 10 U prandial dose[56]. The company announced in August, 2015, that they are moving forward with clinical trials after achieving a four- to five-fold increase in the concentration of insulin in the product, as well a five-fold improvement in insulin absorption in dogs compared to the original formulation[57].
Oral insulin
Oral insulin is the Holy Grail that pharmaceutical companies are racing to attain. Not only would oral insulin be needle-free but, like endogenous insulin, it would enter the hepatic portal system before reaching systemic circulation. Subcutaneously injected insulin stimulates glucose uptake by the liver, but the effect is enhanced when insulin is delivered via the portal vein[7]. While oral insulin is a potential therapeutic for type 1 diabetics, the technology is especially geared towards treating type 2 patients. Most type 2 diabetics will eventually require insulin therapy, but this step generally gets delayed, putting the patient at risk for hyperglycemia-induced vascular damage not clinically detectable without further testing. Guidelines, however, are changing to encourage introduction of insulin therapy at an earlier stage of disease progression[4], and an orally administered insulin option could ease resistance on the patient's end.
Attempts at developing oral insulin formulations have been thwarted by the acidic environment of the stomach, peptide-cleaving enzymes in the digestive tract, and the permeation-resistant intestinal wall. Some groups are investigating the use of nanocarriers to protect orally administered peptide drugs during digestion and aid in their absorption via strategically designed coatings and moieties on the nanoparticle corona. Pridgen et al have attempted to enhance permeation of the intestinal wall by targeting nanoparticles to the neonatal Fc receptor (FcRn), which is expressed on epithelial cells in the small intestine and colon. They designed a nanoparticle-forming block copolymer consisting of poly(lactic acid)-b-poly(ethylene glycol) conjugated to the Fc fragment of IgG[58], which binds with high affinity to FcRn at acidic pH. Insulin is loaded into the core of the nanoparticle, which, upon reaching the acidic portion of the intestine, is transported across the epithelium via receptor-mediated endocytosis and subsequently releases its biologically active contents into systemic circulation. Oral administration of 1.1 U/kg of 0.5 wt % insulin-loaded nanoparticles to fasted wild type mice induced a significant hypoglycemic response that persisted for 10 hours[58].
Other nanoparticle-based strategies for enhancing oral delivery of insulin have employed coatings containing the polysaccharide chitosan[59], which interacts with the membrane protein responsible for forming tight junctions, thereby increasing paracellular permeability, as well as nanoparticles displaying goblet cell-targeting peptides to promote endocytosis across the intestinal epithelium[60]. Such technologies, however, continue to suffer from low oral bioavailability. A comprehensive review regarding the current state of nanoparticle-based insulin delivery is provided by Sharma et al[59].
Many attempts by Novo Nordisk to develop an insulin pill have failed during clinical testing, but their lead candidate—Insulin 338— has advanced to Phase II trials[61]. The formulation involves an enteric coating to protect the insulin analog from low pH in the stomach, and employs gastrointestinal permeation enhancement technology (GIPET), which utilizes sodium caprate to promote absorption of insulin in the small intestine[62]. Sodium caprate increases paracellular permeability by inhibiting tight junction formation[63, 64].
An Israeli company, Oramed Pharmaceuticals, is also developing an oral insulin tablet. Their lead candidate, ORMD-0801, which has advanced to Phase IIb trials[65], is intended as a monotherapy for type 2 diabetics. Phase IIa trials showed a trend towards decreased daytime and nighttime glucose levels compared to placebo without incidence of hypoglycemia[66]. Oramed's proprietary formulation involves insulin, protease inhibitors, and absorption enhancers dissolved in an omega-3 fatty acid[67]. The contents are encased in an enteric coating designed to inhibit digestion in the stomach, which then dissolve as the pH becomes increasingly alkaline. The omega-3 fatty acid component protects the insulin from proteases in the small intestine and enables direct absorption across the intestinal lumen with the help of an absorption enhancer – likely EDTA[67]. EDTA affects paracellular permeability by chelating calcium, which is important for tight junction formation[63].
Stimuli-responsive insulin
An interesting pursuit in diabetes treatment is the development of “smart” insulin: insulin that is activated when glucose levels rise, and deactivated when glucose levels return to normal. Chou et al at the Massachusetts Institute of Technology have developed a soluble, circulating, glucose-responsive insulin analog by covalently conjugating insulin to both a dodecanoic fatty acid chain and a phenylboronic acid (PBA) moiety[68]. The fatty acid confers the ability to reversibly bind to serum albumin while PBA is capable of reversibly binding to glucose[68]. In vivo studies in mice demonstrated the ability of “smart insulin” to restore normoglycemia in response to glucose challenges for up to 10 hours following s.c. administration[68], but there is some uncertainty regarding the mechanism of the observed glucose-dependent activity.
Another design of a “smart” insulin delivery system is a glucose-responsive patch, currently in development by Yu et al at the University of North Carolina/North Carolina State University. The technology employs nanoscale vesicles formed by hypoxia-sensitive hyaluronic acid (HA) containing both insulin and glucose oxidase (GOx) in the aqueous core[69]. When glucose levels are elevated in s.c. capillary networks, glucose will theoretically diffuse into the vesicles, react with GOx, simultaneously consume oxygen, and create a local hypoxic environment. A hydrophobic nitroimidazole moiety conjugated to the HA is reduced in hypoxic conditions to a hydrophilic aminoimidazole, resulting in dissociation of the vesicles and release of encapsulated insulin.
The patch consists of a vesicle-loaded microneedle array for painless, transcutaneous glucose-responsive insulin delivery. In vivo studies in a type 1 diabetic mouse model showed efficient penetration of the skin, maintenance of normoglycemia for 4 hours post-administration, and glycemic control within 30 minutes of a glucose challenge without incidence of hypoglycemia[69]. Inflammation at the site of patch application was not observed. If this technology advances to human trials, the insulin load capacity, number of required patches for glycemic control, and degree of microneedle penetration will be of particular relevance.
The same research group that developed the glucose-responsive patch has also created an injectable network that releases insulin in response to ultrasonic waves. Di et al reports suspending insulin within biodegradable nano-sized poly(lactic-co-glycolic acid) beads, then coating the beads with a surfactant – either positively charged chitosan or negatively charged alginate[70]. The oppositely charged nanoparticles are then mixed to create a network of nanoparticles that are held together via electrostatic interactions. While the network will passively release a baseline level of insulin, bolus release is triggered by application of focused ultrasonic pulses to induce cavitation within the network. Insulin accumulates within these cavitations and is then able to diffuse out of the network with near zero-order kinetics. Furthermore, the network displays shear-thinning behavior, which is a desirable property for injectables.
Di et al have evaluated their technology in a type 1 diabetic mouse model. Injection of the nano-network into the s.c. space followed 2 days later by focused ultrasound pulses resulted in a reduction of blood glucose from 500 mg/dL to less than 200 mg/dL, and significant depression in blood glucose were triggered 4, 7,and 10 days post-injection, demonstrating long-term utility of the nano-network[70]. This system provides a less invasive method for long-term insulin delivery; it will be of particular interest if the stimuli-responsive insulin reservoir can be combined with continuous glucose monitoring, perhaps in a closed-loop setting.
A potentially promising product may emerge from Merck & Co. in the next decade. In December 2010, Merck purchased the start-up company SmartCells with the intention of developing a glucose-responsive insulin[71]. The SmartCells technology initially involved an injectable gel containing lectin and an insulin analog with a sugar moiety attached[72]. When blood glucose levels fall, the lectin binds to insulin, inhibiting its action. When blood glucose levels rise, lectin binds to glucose, thereby releasing insulin to stimulate glucose uptake. The technology has evolved to eliminate the need for injected lectin, which is toxic, and instead the insulin analog binds reversibly to a ubiquitous cell receptor[72], possibly the macrophage mannose receptor[73]. Merck's “smart” insulin, now referred to as MK-2640, entered Phase I clinical trials in late 2014[74].
Closed-loop insulin delivery
The ideal technological advancement is the development of a closed-loop artificial pancreas. Although geared primarily towards type 1 diabetics, such a technology would be beneficial to late-stage type 2 diabetics as well. The system would consist of a continuous glucose monitor (CGM) to sense fluctuations in glucose levels, combined with an insulin infusion pump to release an appropriate dose of insulin in response to glucose changes, under the control of a computer-based algorithm.
The current state-of-the-art CGM is the Dexcom G4 Platinum, which consists of a transmitter, a receiver, and an implantable sensor. This device is approved for implantation for no more than 7 days[75] and has an average error of 13% in reference to blood samples measured by a YSI glucose analyzer[76]. Since CGMs measure interstitial glucose levels, there exists an inherent delay in sensing fluctuations in blood glucose, though such a delay is believed to be no more than 5–10 minutes[77]. However, this delay can increase by as much as three-fold upon formation of a fibrous capsule due to a foreign body response initiated by s.c. implantation of the sensor[78–80]. This capsule is a diffusion barrier for small analytes and thus limits utility of a CGM to a week or less.
The CGM is connected to an insulin pump, which connects an insulin cartridge to a catheter that is inserted into the user's s.c. tissue. The majority of patients in the United States choose a Teflon catheter[81]. In terms of lifetime in the body, the catheter will be the limiting component of the artificial pancreas, as even the state-of-the-art catheter and tubing infusion set technology (BD FlowSmart™) is FDA approved for only 3 days[82], at which point it must be replaced to prevent insertion site inflammation or catheter occlusion.
Despite challenges regarding the lifetime of the individual components of a closed-loop insulin delivery system, substantial progress is nevertheless being made in the design of a long-term and user-friendly system. Kovatchev et al at the University of Virginia reported a closed-loop artificial pancreas employing a Dexcom CGM and an Insulet Omnipod insulin pump connected to a smartphone device that afforded 28 hours of glucose control in type 1 diabetic patients in an out-patient setting[83].
Generic Insulin (or lack thereof)
It is estimated that the global insulin market will be worth $32 billion by 2018[84], however the industry is dominated by only three pharmaceutical companies and offers no generic versions—`biosimilars' in the context of biological drugs—for the commonly prescribed insulin analogs. In 2009, the FDA was granted authorization to create an approval pathway for biosimilars with the purpose of giving the original product developers 12 years of market exclusivity, due to the high costs of developing a biologic, before a follow-on biosimilar could be approved[85]. However, once the requisite time passes, the biosimilar approval process is hardly an abbreviated pathway. The issue lies in the fact that there are no clear metrics to prove that two biologics expressed in different cell lines in different factories subjected to different purification methods are, in fact, therapeutically identical. Approval for a biosimilar hence requires exhaustive safety, efficacy, and immunogenicity studies, as well as independent clinical trials[85]. The incentive for a company to produce a biosimilar decreases due to the high capital cost, and the price reduction ultimately seen by the patient may only be 20 to 40% off the branded biologic[86].
Also stymying the introduction of generic insulins to the market is the practice often referred to as “evergreening.” Every time Sanofi, Eli Lilly, and Novo Nordisk modify their branded insulin analog, additional patents are filed. As long as the newer formulation is marginally superior—at least in the opinion of the prescribing physician—the older formulation becomes obsolete, rendering a biosimilar based on the expired version not worth pursuing[8]. Nevertheless, Merck is currently in Phase III clinical trials for its biosimilar of Sanofi's insulin glargine[87]; thus, the diabetes market may see its first biosimilar insulin emerge in 2016.
GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS
GLP-1 Receptor Agonists
The emergence of incretins, a class of insulin-stimulating gut hormones released postprandially, led to the development of the first new peptide drugs for the treatment of type 2 diabetes since insulin. The two primary members of this class are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). GLP-1, a 31 amino acid peptide produced in the L cells of the intestines and released postprandially, was the first incretin pursued as a potential type 2 diabetes drug. Interest was spurred upon cloning and sequencing of the proglucagon gene in 1983[88]. Shortly after discovery of the GLP-1 precursor, Kreymann and colleagues saw enhanced insulin release and reduced peak plasma glucose concentrations in human volunteers receiving a constant infusion of GLP-1[89]. Following Kreymann's findings as well as several other reports of the peptide's effects on metabolic and gastrointestinal regulation[90], interest in the peptide burgeoned and academics and industry alike raced to develop GLP-1 as a drug for type 2 diabetes.
It has since been established that GLP-1 exerts its activity on multiple organs that express GLP-1 receptors (GLP-1Rs) and contribute to the pathogenesis of diabetes. The peptide is also responsible for upregulating insulin expression, protecting beta cells from apoptosis, promoting beta cell proliferation, slowing gastric emptying, inducing satiety, reducing glucagon secretion, and enhancing peripheral glucose absorption[91]. Although GIP has yet to be pursued as a diabetes monotherapy, Roche is currently investigating its use in combination with GLP-1 as a combined dual agonist (RO6811135)[92]. The delay in the development of GIP has been largely due to its convoluted metabolic effects and the fact that it remains controversial whether activation or repression of the GIP receptor is more beneficial to normalizing glycemia[93].
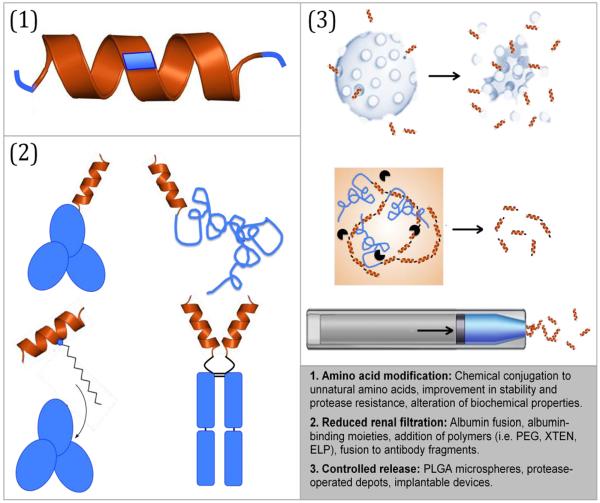
To date, five GLP-1R agonists have been approved by the FDA (Table 2) and several others are in various stages of preclinical and clinical development. Incretin-based therapeutics have received widespread attention and, in 2014, the GLP-1R agonist market was worth an estimated $3 billion[94]. The market size can be attributed to the peptide's attractive qualities from a pharmaceutical development perspective, including a wide therapeutic window and subnanomolar potency. However, these advantages are overshadowed by its rapid degradation and short half-life, making the native peptide unsuitable for clinical application. Research and development efforts have thus aimed to enhance efficacy and therapeutic feasibility of GLP-1 by: 1) reducing proteolytic degradation, 2) enhancing circulation half-life, and 3) developing controlled release formulations of the peptide (Fig. 2).
Table 2.
Currently available GLP-1R agonists and their mechanisms of sustained delivery and recommended doses
| Brand Name | Generic | Manufacturer | Approval Date | Half-life | Mechanism of Enhanced Delivery | Recommended Dose | |
|---|---|---|---|---|---|---|---|
| Daily | Byetta | Exenatide | Amylin | 2005 | 2.4 h | Protease-resistant analog | 5 or 10 ug, (2x daily) |
| Victoza | Liraglutide | Novo Nordisk | 2010 | 13 h | Albumin binding fatty acid domain | 3 mg | |
| Weekly | Bydureon | Exenatide | Amylin | 2012 | 2 w | Protease-resistant analog, PLGA microspheres | 2 mg |
| Tanzeum | Albiglutide | GlaxoSmithKline | 2014 | 5 d | Fusion to albumin | 30 or 50 mg | |
| Trulicity | Dulaglutide | Eli Lilly | 2014 | 4.5 d | Fusion to IgG4 | 0.75 or 1.5 mg |
All data was obtained from FDA Access Data approval labels[95].
Figure 2.
Overview of strategies for improving the delivery of GLP-1R agonists. Graphic depicting microspheres was adapted from bydureon.com and graphic depicting implantable pump was adapted from intarcia.com.
Reduced Proteolytic Degradation
Native GLP-1 is not a clinically useful drug, as its approximately two minute half-life would require far too frequent administration or constant intravenous infusion to be therapeutically feasible[96]. The short half-life of GLP-1 is largely attributed to rapid inactivation by the enzyme dipeptidyl-peptidase IV (DPP-IV)[97], a ubiquitous cell-surface serine protease that selectively cleaves off N-terminal dipeptides that have alanine or proline in the second position[98]. These N-terminal residues are critical to GLP-1's subnanomolar affinity and their truncation thus yields a biologically inactive peptide[99].
Since its discovery, interest in GLP-1 has led to a plethora of structure-function studies to identify additional contributors to its potency. GLP-1's structure consists of a seven-residue, unstructured N-terminal domain followed by two alpha helical regions separated by a short linker, which enables positioning of the hydrophobic residues along a single face[100]. In 1994, separate studies were published by investigators at Novo Nordisk and by Gallwitz et al on residue-specific contributions to binding and activity using alanine scanning mutagenesis in conjunction with competitive binding and cAMP activity assays. His7 (denoting, by convention, the first residue of GLP-1), Gly10, Phe12, Thr13, and Asp15 were identified as important to GLP-1's receptor affinity while Phe28 and Ile29 were essential for the peptide's activity, likely due to their role in maintaining the alpha helical structure[101, 102].
Identification of protease-sensitive residues has informed the rational design and engineering of analogs incorporating a variety of mutations and modifications to slow degradation and thereby increase the in vivo half-life of GLP-1. The most successful analogs incorporate N-terminal modifications that inhibit recognition and degradation by DPP-IV while maintaining or even increasing GLP-1's potency. Deacon and colleagues have shown that replacing Ala8 with either a glycine or the unnatural amino acid α-aminoisobutyric acid enhances in vivo metabolic stability and increases the peptide's half-life by three- to four-fold, while maintaining a receptor affinity that is comparable to the native peptide[103]. Furthermore, an Ala8Gly mutation has shown improved insulinotropic activity compared with the native peptide: 0.1 nmol of the mutant GLP-1 normalizes fasting hyperglycemia in diet-induced obese mice for several hours whereas a 1.0 nmol injection of native GLP-1 lasts only a few minutes[104]. Similarly, Siegel et al has found that substitution of the endogenous L-isomer of Ala8 with its D-isomer yields an analog that is resistant to proteolytic cleavage with only a three- to four-fold loss in potency[105].
Although the N-terminal His residue has been confirmed as critical for receptor activity, many modifications at this position have demonstrated similar in vivo activity as native GLP-1, with improved resistance against proteolytic degradation. These N-terminal His7 modifications include: desamino, N-imidazole, N-α-methyl, N-methyl, D-His7, N-glucitol, N-acetyl, and N-pyroglutamyl[106–109]. All of the analogs listed have partial or complete resistance to DPP-IV with receptor affinities unchanged or reduced, at most, by 15-fold compared to the native peptide[110].
Incorporation of Cys residues to either cyclize the peptide or generate intermolecular bonds has also shown promise in enhancing circulating half-life. Li et al introduced a cysteine-containing glycine tail at the C-terminus of GLP-1, enabling disulfide bond formation between GLP-1 molecules that stabilized the peptide, protected it from proteolysis, and extended its half-life, while maintaining in vivo insulinotropic and glucoregulatory activity out to 5 days following a single s.c. injection[111].
Taspoglutide
Of the many efforts to create protease-resistant GLP-1 mutants, only one has advanced to phase III clinical trials. Taspoglutide, co-developed by Ipsen and Roche, is a GLP-1-based peptide with both Ala8 and Gly35 replaced by aminoisobutyric acid. These substitutions confer increased stability in vivo without a reduction in potency[112]. In short-term phase II clinical trials, once-weekly Taspoglutide administered at 10 or 20 mg doses induced a mean weight loss of up to 2.3 kg and significantly reduced HbA1c levels (−1.24% for 10 mg and −1.31% for 20 mg dose), conferring a degree of glycemic control superior to twice daily exenatide. However, in September of 2010 the clinical trial was halted due to anti-Taspoglutide antibodies detected in 49% of patients, as well as adverse events reported (nausea, vomiting, and injection site reactions) in greater than 50% of patients[113].
Exenatide
A pivotal point in the history of incretin-based diabetes therapies was the discovery of exendin-4, a naturally occurring peptide hormone first isolated from the saliva of the Gila monster by John Eng in 1992[114]. Although exendin-4 has 53% sequence homology with GLP-1, it is resistant to DPP-IV proteolysis, has a longer therapeutic half-life, and is more potent than native GLP-1. A Gly residue at the second position of the primary amino acid sequence confers DPP-IV resistance and is largely responsible for the extended 2.4-hour half-life of exendin-4. Exenatide, a synthetic analog of exendin-4 and a homolog of GLP-1, is the active ingredient in two FDA approved formulations—Byetta and Bydureon—manufactured by partners Amylin and Eli Lilly.
Byetta
Byetta, the brand name for exenatide, was the first GLP-1R agonist to gain approval for diabetes therapy. On the market since 2005, it is administered twice daily via s.c. injection using pens prefilled with either 5 or 10 μg doses. Byetta is available as a monotherapy or in combination with either oral glucose-lowering agents or insulin glargine. Byetta was compared to insulin glargine in two randomized, open-label trials involving patients whose diabetes had been insufficiently controlled with a metformin and sulfonylurea combination therapy. The treatments yielded similar reductions in HbA1c (−1.36%) while the Byetta group experienced weight loss (−2.3 kg) and superior postprandial glucose control, but inferior fasting serum glucose levels[115]. Bydureon, the long-acting formulation of exenatide, is discussed later in the Novel Formulations for Controlled Release section.
Lixisenatide
Lixisenatide, developed by Zealand Pharma in collaboration with Sanofi, is a modified exenatide analog consisting of a Pro38 deletion and a hexa-lysine tail added to the C-terminus, which increases stability of the peptide. In a series of randomized, placebo-controlled trials, Lixisenatide has been validated for use as a monotherapy or as an add-on therapy to oral antidiabetic agents due to reduction in HbA1c, fasting plasma glucose, and either weight loss or weight neutral effects[116]. Despite approval in Europe, clinical trials in the United States were halted in 2013 due to cardiovascular safety concerns[117]. In September of this year, the FDA accepted Sanofi's New Drug Application (NDA) for Lixisenatide[118], which it reapplied for after seeing results from a study in which a once daily, 20 μg dose of the drug did not increase the frequency of cardiovascular events in a high-risk, type 2 diabetes population. The impending trials, which are currently recruiting, have generated excitement, as they will be the first for this drug class to investigate cardiovascular outcomes. This is an important milestone, as many type 2 diabetes therapies, particularly GLP-1R agonists, are under scrutiny for increased cardiovascular risk factors[119].
By reducing proteolytic degradation via sequence modification, a few drugs have made it to clinical trials and received FDA approval. However, despite resisting degradation, the majority of modified GLP-1R agonists continue to suffer from short circulation half-lives due to rapid renal elimination. Consequently, a second area of GLP-1R agonist development focuses on reduction of renal clearance to further prolong the duration of action of the drug.
Reduced Renal Clearance
The majority of successful incretin therapies to date have relied on novel fusion partners that enhance circulating half-lives by reducing renal clearance. Some of the most common strategies include: 1) increasing the peptide's size by fusing it to a large protein with a long half-life, 2) conjugation of peptides or chemical moieties known to bind molecules in the body with slow turnover rates, and 3) attaching synthetic or biological polymers.
Albiglutide
Albiglutide, developed by GlaxoSmithKline (GSK) and approved by the FDA in 2014, employs an albumin fusion strategy developed by Human Genome Sciences, and the entire 73kDa fusion protein is produced recombinantly. The choice of albumin as a fusion partner is driven by the fact that it has an average half-life of 19 days, making it one of the longest circulating proteins in the body. Beyond its extraordinary half-life, albumin is stable, highly soluble, non-immunogenic, non-toxic, and biodegradable, and distributes to nearly all tissues in the body[120].
Albiglutide consists of two copies of DPP-IV-resistant GLP-1 analogs (Gly8 mutation) in tandem, followed by human serum albumin. The tandem repeat was introduced to mitigate the loss of potency from the bulky albumin protein, but the fusion still suffers from a 100-fold reduction in potency compared to native GLP-1. The loss in activity, however, was deemed an acceptable tradeoff for its long half-life—5-7 days in humans—which allows for weekly or even less frequent dosing with acceptable safety and tolerability[121, 122]. Albiglutide, given in 30 or 50mg doses once weekly, has been validated in a series of HARMONY clinical trials as both a monotherapy and as an add-on to pioglitazone and metformin with overall reductions in HbA1c levels of approximately −0.8%[123–126]. Interestingly, Albiglutide does not induce the same magnitude of weight loss seen with other GLP-1R agonists. This is likely due to albumin's inability to cross the blood-brain-barrier, thereby preventing access to the central GLP-1Rs responsible for the anorectic effect[127, 128].
Liraglutide
Liraglutide is a GLP-1 analog marketed by Novo Nordisk that exploits albumin's extended half-life by non-covalently and reversibly binding the protein via a palmitic acid chain conjugated to Lys26. To ensure that the palmitic acid is only conjugated to Lys26, the only other Lys residue —Lys34—in the native sequence is mutated to an Arg. There are five binding sites for fatty acids on human serum albumin, allowing the C16 fatty acid-modified GLP-1 analog to bind each site with dissociation constants in the submicromolar range. However, because it is not covalently conjugated to albumin, the free peptide retains its potency.
Liraglutide has a half-life of approximately 13 hours when injected s.c., making it suitable for once-daily administration in humans[129]. The analog performed well as a monotherapy in a LEAD-3, double-blind, active parallel control trial. When compared to the sulfonylurea glimepiride after 52 weeks of treatment, the 1.2 and 1.8 mg doses of Liraglutide achieved HbA1c reductions of −0.84% and −1.24%, respectively, compared to −0.51% for the glimepiride group. Treatment with Liraglutide also resulted in greater reduction in fasting plasma glucose and weight loss of approximately 2 kg compared to weight gain commonly associated with glimepiride[130].
Semaglutide
Novo Nordisk is currently conducting Phase III trials for its second albumin-binding GLP-1R agonist, Semaglutide. This formulation is acylated at the same Lys26 residue, but with a stearic diacid in place of Liraglutide's palmitate, which gives it a greater and more stable affinity for albumin[131]. Compared to the lone Glu residue in Liraglutide, Semaglutide has a larger synthetic spacer between the modified Lys site and the acyl chain. In addition, Semaglutide contains the DPP-IV-resistant Ala8 mutation to aminoisobutyric acid, resulting in a half-life of 160 hours, making it suitable for once weekly dosing. In recent clinical trials, weekly s.c. injections of 0.8 or 1.6 mg doses for 12 weeks demonstrated dose-dependent reductions in HbA1c levels by up to −1.69% and weight loss of up to 4.8 kg[132].
Dulaglutide
Antibodies, like albumin, have long half-lives, which has led to the development of peptide and protein fusions to the Fc region of antibodies. In particular, the Fc domain of gamma immunoglobin (IgG) has been used as a fusion partner and carrier protein for many peptides because, like albumin, the Fc domain also exploits the neonatal Fc receptor for binding, thus escaping renal filtration and extending the serum half-life of conjugated moieties[133].
Dulaglutide is a once-weekly drug developed by Eli Lilly and approved by the FDA in 2014. Dulaglutide is comprised of a DPP-IV-resistant GLP-1 dimer with each monomer fused to a modified IgG4 Fc fragment. The drug has gone through several iterations since its initial formulation. IgG1 was initially chosen as a fusion partner, but was replaced with a mutated form of IgG4 to improve activity and reduce immunogenicity by preventing interactions with high-affinity Fc receptors. This engineering was critical to Dulaglutide's progression to clinical trials because unmodified Fc molecules are involved in native and adaptive immunity through the activation of complement and antibody-dependent cell-medicated cytotoxicity (ADCC). Mutations have also been introduced into the GLP-1 analog to reduce immunogenicity, and a longer, glycine-rich linker between the peptide and Fc chain was incorporated to improve activity.
In a dose-escalation trial in healthy volunteers, there were reports of gastrointestinal side effects, typical of GLP-1R agonists, but, importantly, there were no reported hypoglycemic events nor developed antibodies to the drug. The final, engineered molecule has a half-life in humans of up to 80 hours, making Dulaglutide a once-weekly option for type 2 diabetics[134]. In a randomized phase III trial to demonstrate non-inferiority, Dulaglutide (1.5 mg, weekly) was compared with Liraglutide (titrated up to 1.8 mg, daily) in patients whose diabetes was inadequately controlled by metformin. After 26 weeks, HbA1c was lowered by −1.42% and −1.36% in the Dulaglutide and Liraglutide groups, respectively. Both treatment groups had similar adverse gastrointestinal events and no severe hypoglycemic events, which demonstrated non-inferiority of Dulaglutide to Liraglutide, although patients on Liraglutide did exhibit a higher degree of weight loss (−2.90 versus −3.61 kg) [135]. A series of AWARD trials have also demonstrated Dulaglutide's superiority in HbA1c reduction over metformin, sitagliptin (a DPP-IV inhibitor), and Byetta[136].
Polymer fusions
Much like fusion to large endogenous proteins, the conjugation of polymers, both biological and synthetic, to peptide drugs is another strategy to reduce renal clearance. Polyethylene glycol (PEG) conjugation is one of the oldest and most widely used methods to enhance a drug's half-life by increasing the size and hydrodynamic radius, thereby reducing its renal clearance. Polymers have additional properties that make them ideal delivery vehicles for peptides, including high solubility, resistance to proteases, and low immunogenicity. However, recent concerns regarding the immunogenicity of PEG and its propensity to cause renal tubular vacuoles have prevented any PEGylated GLP-1R agonists from entering clinical trials[137].
Like synthetic polymers, polypeptides can also be used to enhance the bioavailability and half-lives of GLP-1R agonists. XTEN is an unstructured recombinant polypeptide developed by Amunix that mimics the properties of PEG. It is comprised of a subset of amino acids that are hydrophilic, stable, non-immunogenic, and lack positively charged side chains. The final biopolymer, named XTEN, was selected from a library that was screened for stability, solubility, and aggregation resistance. XTEN consists of randomized Ala, Glu, Gly, Pro, Ser, and Thr residues and is highly expressed in E. coli. XTEN fused to exenatide has 80% bioavailability from the s.c. space and a terminal half-life of 60 hours in cynomolgus monkeys. Additionally, no significant immune response has been observed in mice or rabbits[138]. A phase I trial has demonstrated the safety and tolerability of escalating doses of exenatide-XTEN, and a GLP-1-XTEN is under clinical development as well. The ultimate goal of GLP-1R agonist-XTEN fusions is to reduce injection frequency to once monthly, which would be a welcome feature to patients who must self-administer this class of drug.
Elastin-like polypeptides (ELPs), developed by PhaseBio Pharmaceuticals, are a second example of a recombinant polymer used to enhance the pharmacokinetics of GLP-1R agonists. Like XTEN, ELPs are large, intrinsically disordered, random coil polypeptides. ELPs are comprised of a repeated pentapeptide motif, Val-Pro-Gly-Xaa-Gly, which is derived from tropoelastin and exhibits a lower critical solution temperature (LCST) phase transition behavior [139] in which an ELP fusion goes from a soluble state to an insoluble coacervate. The phase transition is reversible upon dilution so in response to dilution of the coacervate at its margins, the coacervate slowly dissolves to the core, releasing the ELP-drug fusion at a steady rate. This feature of ELPs distinguishes them from other polypeptides such as XTEN and other synthetic polymers developed for drug delivery.
PhaseBio has exploited the unique properties of ELPs to develop recombinant ELP-peptide fusions for delivery of peptide drugs. By engineering the phase transition through its amino acid composition and molecular weight, the ELP fusion is soluble in a syringe but instantaneously coacervates upon s.c. injection, triggered by the increase in temperature from ambient to body temperature. The ability to precisely tune the coacervation temperature of an ELP coupled with its monodispersity and the lack of toxicity and antigenicity of this class of polypeptides make ELPs highly attractive for incretin delivery, and a GLP-1-ELP fusion was developed by PhaseBio for once-weekly treatment[140]. Although a Phase I/IIa trial yielded promising results, PhaseBio's formulation, PB1023, did not meet non-inferiority criteria against the active comparator, Liraglutide in a Phase IIb clinical trial. The company instead plans to pursue this drug as a co-formulation with a fully human, monomeric insulin fusion with an ELP[141].
Novel Formulations for Controlled Release
All of the strategies discussed previously enhance the circulation half-life of GLP-1 and its analogs and hence enable daily or weekly dosing regimens. While these approaches increase half-life and systemic exposure to the drug, they do not provide controlled release. This means that the treatment's duration can only be modulated by adjusting the dose, which is limited for any therapy by its therapeutic window, toxicity, and cost. In order to further reduce the dosing frequency of GLP-1R agonists, industry research and development will need to consider next generation formulations that control release and reduce renal clearance.
Bydureon
Bydureon, a long-acting version of exenatide, is the only controlled released formulation of a GLP1-R agonist on the market. Encapsulation of exenatide into poly (lactic-co-glycolic acid) (PLGA) microspheres using a water-in-oil solvent evaporation method results in an adjustable drug release profile appropriate for once-weekly dosing. Amylin received FDA approval for Bydureon in early 2012.
Trials comparing Bydureon (2 mg, once weekly) to its predecessor, Byetta (10 ug, twice daily), showed improved HbA1c reduction, lower fasting plasma glucose levels, and similar reductions in body weight with fewer gastrointestinal side effects. A DURATION-1 randomized clinical trial was also conducted to investigate the efficacy and safety of a five-year Bydureon treatment regimen in type 2 diabetics. Bydureon was generally well tolerated with sustained improved glycemic control and weight loss, as well as reduced markers of cardiovascular risk[142].
However, this formulation is not without drawbacks. There is a lag in the release of microspheres following the initial, post-administration burst release and the 0.06 mm diameter microspheres require larger, more painful needles for s.c. injection. The drug was initially only available in a powdered form that had to be mixed with a diluent immediately prior to injection, which complicated weekly self-administrations for patients. AstraZeneca has since developed and received FDA approval for a prefilled mixing pen. These devices, forecasted to reach patients by mid-2015, should make the distribution of medications with poor stability more feasible for at-home use[143].
Protease Operated Depots
Another method for controlling GLP-1 delivery, developed by Amiram et al., utilizes ELPs and is termed a protease-operated depot (POD). In a demonstration of the POD technology, six tandem GLP-1 repeats separated by an Arg residue — a peptide that is common to many protease recognition sites — were recombinantly expressed as a fusion to an ELP. The ELP fusion was designed to be soluble in the syringe, but to transition to an insoluble coacervate in the s.c. space upon injection. It was shown that the resulting depot slowly releases DPP-IV-resistant GLP-1 peptides into circulation as the GLP-1 repeats are enzymatically cleaved by proteases in the s.c. space. This system affords glycemic control for up to five days in mice[144].
A similar system has also been designed by Amiram et al, in which a GLP-1 fused to an ELP without a cleavable linker forms a depot upon s.c. injection. They showed that the GLP-1-ELP fusion maintains blood glucose homeostasis in mice for 5 days, similar to the GLP-1 POD[145]. For PODs and GLP-1-ELP fusions, the duration of efficacy can be controlled by adjusting the transition temperature, which is easily tuned by modulating the ELP's molecular weight or guest residue composition (Xaa in the Val-Pro-Gly-Xaa-Gly motif). With an ELP designed to transition to an insoluble coacervate at or below body temperature, the ELP fusion delivery system not only utilizes a carrier polymer —the ELP— for enhanced half-life, but also controls release from the s.c. space by virtue of the formation of a depot upon injection.
Implantable Devices
Implanted devices are another option for longer-term controlled release of GLP-1R agonists. Intarcia is carrying out a series of FREEDOM phase III clinical trials of its matchstick-sized device, ITCA 650, which provides zero-order, continuous release of exenatide from the s.c. space and requires replacement only once every 6–12 months. The device employs a semipermeable membrane coupled with an osmotic pump that forces drug out of the device at a controlled rate. Apart from its exquisite release of a precise dose of drug, ITCA 650 guarantees patient compliance as it eliminates the need for injections. Implantation of the device requires a simple, 15-minute outpatient procedure.
Results from a randomized, open-label 24-week phase II study of ITCA 650, with an optional 24-week extension, were recently published[146]. Patients with type 2 diabetes inadequately controlled by metformin received a device releasing 20, 40, 60, or 80 μg of exenatide per day. At the end of the first 24 weeks, changes in HbA1c ranged from −0.85% to −1.51% and these reductions were maintained throughout the 24-week extension. Adverse gastrointestinal events were mostly mild and transient and declined during the extension period. Reductions in weight were dose dependent and as high as −4.2 kg. More recent phase III studies have been equally exciting, showing a sustained HbA1c reduction of −3.4% in poorly controlled, high baseline patients after 39 weeks of treatment at 40 and 60 ug/day doses[147]. Intarcia's timeline is projecting application for FDA approval in 2016, expecting the device to enter the market in early 2017.
Formulations for Alternative Routes of Administration
Novo Nordisk is pioneering the push for oral delivery of GLP-1R agonists. Until recently, this method of delivery had been infeasible due to membrane impermeability in the gut as well as the harsh acidic and protease-rich environment of the stomach, to which peptides are particularly susceptible. Novo Nordisk has successfully completed phase IIb clinical trials of its once-daily, oral version of their albumin-binding GLP-1 analog, Semaglutide. This proprietary tablet formulation employs an absorption-enhancing excipient for passive transcellular transport across the intestinal lumen.
The Eligen Technology—noncovalent binding of N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC) to a drug—has also been licensed to Novo Nordisk. SNAC increases paracellular permeability by chelating the calcium needed for tight junction formation[62]. While oral delivery using this approach does not compromise the biological activity or pharmacological properties of the drug, much larger doses (over 100-fold) are necessary to achieve therapeutic plasma concentrations, which may challenge the commercial feasibility of oral GLP-1R formulations.
Finally, Novo Nordisk is investigating the use of GIPET (described previously in the Oral Insulin section) to boost the oral bioavailability of their GLP-1R agonist[148]. Future strategies to create orally available incretin formulations may involve active transport of the peptide across the intestinal lumen by conjugation to transportable substrates such as PEG-biotin, which is taken up by sodium-dependent multivitamin transporters [149], and vitamin B12, which is scavenged by the body from the diet by various chaperone proteins[150]. Oramed, a pharmaceutical company headed towards phase IIb trials for its oral insulin capsule, is also developing an orally ingestible exenatide capsule (ORMD-0901)[151]. It is evident from these incretin delivery developments that oral peptide delivery could be an area of immense growth in both academia and industry.
Novel formulations to deliver GLP-1R agonists are not limited to conventional parenteral routes of administration. There is interest in targeting more patient-friendly routes including transdermal patches and intranasal administration for pulmonary uptake. MannKind is developing an inhalable formulation of native GLP-1 adsorbed to their patented Technosphere microparticles. This drug recently completed a phase Ia clinical trial in which it showed dose-dependent, GLP-1-induced insulin release within six minutes of inhalation in healthy adult males[152].
Insulin and GLP-1R Agonist Combination Therapy
Insulin-incretin combination treatments are also headed towards FDA approval. These two peptide drugs offer complementary features in regulating blood glucose levels. While exquisitely tuned, long-acting basal insulin continues to control fasting glycemia and reduce the stress on pancreatic beta cells, the GLP-1R agonist helps to control postprandial glucose excursions while concomitantly increasing satiety and reducing energy intake. The proposed combination treatment is expected to minimize dose-related adverse effects of each respective drug—nausea from the GLP-1R agonist and hypoglycemia from the insulin analog. Furthermore, GLP-1R agonist treatment promotes desirable weight-lowering effects, which are expected to neutralize the weight gain commonly associated with insulin treatment.
Pharmaceutical companies are currently developing combination insulin/GLP-1R agonist formulations with the intention of utilizing the incretin component in place of prandial insulin. Several GLP-1R agonists are already approved as superior or non-inferior add-ons to basal insulin treatments[153–155], and Novo Nordisk and Sanofi are actively developing their respective fixed-dose combination drugs, Xultophy and LixiLan. Thus far, clinical trials for both formulations are promising with patients achieving significant HbA1c reduction with modest weight loss and no observed increase in hypoglycemia risk[156, 157].
CONCLUSION
Insulin and GLP-1R agonists have each had a fascinating journey from their discovery to their current stage of development and both have had a profound impact on the treatment of diabetes. As the only two major peptide drugs available to treat T2D, insulin and GLP-1R agonists offer high specificity and potency, but have required significant engineering of both the peptides themselves and their modes of delivery. Insulin has been exquisitely optimized to create long-acting analogs that maintain basal plasma concentrations for up to 36 hours, as well as rapid-acting versions that nearly mimic the endogenous insulin response. The GLP-1 sequence has been mutated to protect analogs from proteolysis and prolong circulation to allow for once-daily and once-weekly administration, with monthly and yearly options forthcoming.
Insulin and GLP-1R agonists – and peptide drugs in general – are conventionally delivered via s.c. injection. However, patients are reluctant to adopt treatment regimens requiring this route of administration due to needle phobia, pain, and the association of injections with serious illness. Insulin has a particularly negative social stigma which greatly impacts patient acceptance and adherence[158]. We believe that the future of peptide therapeutics will lie in controlled release modalities that eliminate the variable of patient compliance from the treatment regimen. As such, Intarcia's once- or twice-yearly implantable exenatide pump is likely to have an immense impact on GLP-1R agonist therapy. With the device slated to enter the market in 2017, competing GLP-1 analogs administered via injection could become obsolete. However, we note that clinical adoption is a complex dance that involves companies, physicians, insurers, and patients, and each of the stakeholders in this process often have conflicting agendas that are not solely driven by the impetus of improved healthcare.
For type 2 diabetics requiring basal insulin therapy, the prospect of orally administered insulin also has the potential to drastically affect the market, and the discretion and ease offered by a pill could encourage earlier initiation of insulin therapy, as it is increasingly recommended for optimal diabetes management[4]. For type 1 diabetics, as well as type 2 diabetics with extremely advanced disease, the game-changing innovation will be the advent of the `artificial pancreas,' or a collection of devices that, together, sense glucose fluctuations and release appropriate insulin doses in a completely automated system. However, such a technology is likely decades away from realization.
Whether long-lasting s.c. depot platforms, oral formulations, or implantable devices end up dominating the diabetes peptide drug market remains to be seen. However, it is clear that the discovery and development of insulin and incretins have immensely improved the management of type 2 diabetes and opened avenues for safer, longer-acting, and more patient-friendly treatment options. As pharmaceutical companies and academic innovators alike continue to develop insulin and GLP-1R agonist therapies, the novel strategies for peptide design and controlled release described in this article will be broadly deployed for the development of peptide drugs and delivery systems for the treatment of other diseases.
ACKNOWLEDGMENTS
A.C acknowledges the support of NIH through grants R01-DK091789 and R01-DK092665. K.L. acknowledges the support of the NSF through a graduate research fellowship and the Research Triangle MRSEC (DMR-1121107).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DECLARATION A.C. is a co-founder of and currently serves on the board of directors for PhaseBio Pharmaceuticals in Malvern, PA, USA, that is commercializing elastin-like polypeptides for applications in biotechnology and medicine.
REFERENCES
- [1].Centers for Disease Control and Prevention . In: National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. US Department of Health and Human Services, editor. Atlanta: 2014. [Google Scholar]
- [2].American Diabetes Association Position Statement Standards of medical care in diabetes – 2015. Diabetes Care. 2015;38:S4. [Google Scholar]
- [3].International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, Blonde L, Bray GA, Cohen AJ, Dagogo-Jack S. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocrine Practice. 2015;21:1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bailey CJ. The Current Drug Treatment Landscape for Diabetes and Perspectives for the Future. Clinical Pharmacology & Therapeutics. 2015;98:170–184. doi: 10.1002/cpt.144. [DOI] [PubMed] [Google Scholar]
- [6].Bliss M. The discovery of insulin. University of Chicago Press; Chicago: 2007. [Google Scholar]
- [7].Felig P. Protamine insulin: Hagedorn's pioneering contribution to drug delivery in the management of diabetes. Journal of the American Medical Association. 1984;251:393–396. doi: 10.1001/jama.251.3.393. [DOI] [PubMed] [Google Scholar]
- [8].Greene JA, Riggs KR. Why Is There No Generic Insulin? Historical Origins of a Modern Problem. New England Journal of Medicine. 2015;372:1171–1175. doi: 10.1056/NEJMms1411398. [DOI] [PubMed] [Google Scholar]
- [9].Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. Journal of the American Medical Association. 2003;289:2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- [11].Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644–649. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- [12].National Institute of Diabetes and Digestive and Kidney Diseases . Types of Insulin. In: National Institute of Health, editor. National Diabetes Information Clearinghouse. Bethesda: 2013. [Google Scholar]
- [13].U.S. Food and Drug Administration . Toujeo Solostar NDA 206538 approval label. Feb 25, 2015. Center for Drug Evaluation and Research. [Google Scholar]
- [14].U.S. Food and Drug Administration . Levemir NDA 021536 approval label. Feb 25, 2015. Center for Drug Evaluation and Research. [Google Scholar]
- [15].U.S. Food and Drug Administration . Insulin Degludec and Insulin Degludec/Insulin Aspart Treatment to Improve Glycemic Control in Patients with Diabetes Mellitus NDAs 203314 and 203313 Briefing Document. Center for Drug Evaluation and Research. [Google Scholar]
- [16].Blundell T, Dodson G, Hodgkin D, Mercola D. Insulin: the structure in the crystal and its reflection in chemistry and biology. Advances in Protein Chemistry. 1972;26:279–402. [Google Scholar]
- [17].Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet. 2001;358:739–746. doi: 10.1016/S0140-6736(01)05842-1. [DOI] [PubMed] [Google Scholar]
- [18].Hirsch IB. Insulin analogues. New England Journal of Medicine. 2005;352:174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- [19].Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-Human Insulin: A Rapidly Absorbed Analogue of Human Insulin. Diabetes. 1994;43:396–402. doi: 10.2337/diab.43.3.396. [DOI] [PubMed] [Google Scholar]
- [20].Berenson DF, Weiss AR, Wan Z.-l., Weiss MA. Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Annals of the New York Academy of Sciences. 2011;1243:E40–E54. doi: 10.1111/j.1749-6632.2012.06468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technology & Therapeutics. 2007;9:109–121. doi: 10.1089/dia.2006.0035. [DOI] [PubMed] [Google Scholar]
- [22].Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart. healthy volunteers, European Journal of Clinical Pharmacology. 1999;55:199–203. doi: 10.1007/s002280050618. [DOI] [PubMed] [Google Scholar]
- [23].Rahuel-Clermont S, French CA, Kaarsholm NC, Dunn MF, Chou CI. Mechanisms of stabilization of the insulin hexamer through allosteric ligand interactions. Biochemistry. 1997;36:5837–5845. doi: 10.1021/bi963038q. [DOI] [PubMed] [Google Scholar]
- [24].Morrow L, Muchmore DB, Hompesch M, Ludington EA, Vaughn DE. Comparative pharmacokinetics and insulin action for three rapid-acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care. 2013;36:273–275. doi: 10.2337/dc12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rassam AG, Zeise TM, Burge MR, Schade DS. Optimal administration of lispro insulin in hyperglycemic type 1 diabetes. Diabetes Care. 1999;22:133–136. doi: 10.2337/diacare.22.1.133. [DOI] [PubMed] [Google Scholar]
- [26].Novo Nordisk A/S, Novo Nordisk completes phase 3a trials comparing faster-acting insulin aspart with NovoRapid® in people with type 1 and type 2 diabetes [Press release] 2015. [Google Scholar]
- [27].Olsen HB, Havelund S, Ribel U, Sturis J, Naver H, Schlein M, Ludvigsen S. Google Patents. 2010. Preparation comprising insulin, nicotinamide and an amino acid. [Google Scholar]
- [28].Sorli C. New Developments in Insulin Therapy for Type 2 Diabetes. American Journal of Medicine. 2014;127:S39–S48. doi: 10.1016/j.amjmed.2014.07.006. [DOI] [PubMed] [Google Scholar]
- [29].Safety/Efficacy Study of Subcutaneously Injected Prandial Insulins Compared to Insulin Lispro Alone in Patients With Type 2 Diabetes Mellitus. Halozyme Therapeutics, NCT01194258. [Google Scholar]
- [30].Rosenstock J, Schwartz SL, Clark CM, Park GD, Donley DW, Edwards MB. Basal Insulin Therapy in Type 2 Diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- [31].Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced Hypoglycemia Risk With Insulin Glargine: A meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005;28:950–955. doi: 10.2337/diacare.28.4.950. [DOI] [PubMed] [Google Scholar]
- [32].Shiramoto M, Eto T, Irie S, Fukuzaki A, Teichert L, Tillner J, Takahashi Y, Koyama M, Dahmen R, Heise T, Becker RHA. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes, Obesity and Metabolism. 2015;17:254–260. doi: 10.1111/dom.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bolli GB, Riddle MC, Bergenstal RM, Ziemen M, Sestakauskas K, Goyeau H, Home PD, E.s.i New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3) Diabetes, Obesity and Metabolism. 2015;17:386–394. doi: 10.1111/dom.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Whittingham JL, Havelund S, Jonassen I. Crystal Structure of a Prolonged-Acting Insulin with Albumin-Binding Properties. Biochemistry. 1997;36:2826–2831. doi: 10.1021/bi9625105. [DOI] [PubMed] [Google Scholar]
- [35].Havelund S, Plum A, Ribel U, Jonassen I, Volund A, Markussen J, Kurtzhals P. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharmaceutical Research. 2004;21:1498–1504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- [36].Heinemann L, Sinha K, Weyer C, Loftager M, Hirschberger S, Heise T. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabetic Medicine. 1999;16:332–338. doi: 10.1046/j.1464-5491.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- [37].Kolendorf K, Ross GP, Pavlic-Renar I, Perriello G, Philotheou A, Jendle J, Gall MA, Heller SR. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in Type 1 diabetes. Diabetic Medicine: a journal of the British Diabetic Association. 2006;23:729–735. doi: 10.1111/j.1464-5491.2006.01862.x. [DOI] [PubMed] [Google Scholar]
- [38].Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-Week, Randomized, Parallel, Treat-to-Target Trial Comparing Insulin Detemir With NPH Insulin as Add-On Therapy to Oral Glucose-Lowering Drugs in Insulin-Naïve People With Type 2 Diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- [39].Novo Nordisk A/S, Novo Nordisk receives US FDA approval for Tresiba® and Ryzodeg® 70/30. 2015. [Google Scholar]
- [40].Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard D, Wahlund P-O, Ribel U. Design of the Novel Protraction Mechanism of Insulin Degludec, an Ultra-long-Acting Basal Insulin. Pharmaceutical Research. 2012;29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hompesch M, Morrow L, Watkins E, Roepstorff C, Thomsen HF, Haahr H. Pharmacokinetic and Pharmacodynamic Responses of Insulin Degludec in African American, White, and Hispanic/Latino Patients With Type 2 Diabetes Mellitus. Clinical Therapeutics. 2014;36:507–515. doi: 10.1016/j.clinthera.2013.12.014. [DOI] [PubMed] [Google Scholar]
- [42].Ratner RE, Gough SCL, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials, Diabetes. Obesity and Metabolism. 2013;15:175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dżygało K, Golicki D, Kowalska A, Szypowska A. The beneficial effect of insulin degludec on nocturnal hypoglycaemia and insulin dose in type 1 diabetic patients: a systematic review and meta-analysis of randomised trials. Acta Diabetologica. 2015;52:231–238. doi: 10.1007/s00592-014-0604-0. [DOI] [PubMed] [Google Scholar]
- [44].Sinha VP, Choi SL, Soon DKW, Mace KF, Yeo KP, Lim STH, Howey DC. Single-dose pharmacokinetics and glucodynamics of the novel, long-acting basal insulin LY2605541 in healthy subjects. Journal of Clinical Pharmacology. 2014;54:792–799. doi: 10.1002/jcph.276. [DOI] [PubMed] [Google Scholar]
- [45].Bergenstal RM, Rosenstock J, Arakaki RF, Prince MJ, Qu Y, Sinha VP, Howey DC, Jacober SJ. A Randomized, Controlled Study of Once-Daily LY2605541, a Novel Long-Acting Basal Insulin, Versus Insulin Glargine in Basal Insulin–Treated Patients With Type 2 Diabetes. Diabetes Care. 2012;35:2140–2147. doi: 10.2337/dc12-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moore MC, Smith MS, Sinha VP, Beals JM, Michael MD, Jacober SJ, Cherrington AD. Novel PEGylated Basal Insulin LY2605541 Has a Preferential Hepatic Effect on Glucose Metabolism. Diabetes. 2014;63:494–504. doi: 10.2337/db13-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Eli Lilly and Company . Lilly Announces Update on Regulatory Submission Timing for Basal Insulin Peglispro [Press Release] 2015. [Google Scholar]
- [48].Rave K, Potocka E, Boss AH, Marino M, Costello D, Chen R. Pharmacokinetics and linear exposure of AFRESA™ compared with the subcutaneous injection of regular human insulin, Diabetes. Obesity and Metabolism. 2009;11:715–720. doi: 10.1111/j.1463-1326.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- [49].Pfützner A, Forst T. Pulmonary insulin delivery by means of the Technosphere™ drug carrier mechanism. Expert Opinion on Drug Delivery. 2005;2:1097–1106. doi: 10.1517/17425247.2.6.1097. [DOI] [PubMed] [Google Scholar]
- [50].Heinemann L, Traut T, Heise T. Time-action profile of inhaled insulin. Diabetic Medicine: a journal of the British Diabetic Association. 1997;14:63–72. doi: 10.1002/(SICI)1096-9136(199701)14:1<63::AID-DIA298>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [51].Heinemann L, Jacques Y. Oral Insulin and Buccal Insulin: A Critical Reappraisal. Journal of Diabetes Science and Technology. 2009;3:568–584. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bernstein G. Delivery of insulin to the buccal mucosa utilizing the RapidMist™ system. Expert Opinion on Drug Delivery. 2008;5:1047–1055. doi: 10.1517/17425247.5.9.1047. [DOI] [PubMed] [Google Scholar]
- [53].Cernea S, Kidron M, Wohlgelernter J, Modi P, Raz I. Comparison of pharmacokinetic and pharmacodynamic properties of single-dose oral insulin spray and subcutaneous insulin injection in healthy subjects using the euglycemic clamp technique. Clinical Therapeutics. 2004;26:2084–2091. doi: 10.1016/j.clinthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [54].Generex Biotechnology Corporation . Generex Oral-lyn™ Results in More Rapid Reductions of HbA1c in Shreya's Phase III Study of Type 2 Diabetes in India [Press Release] 2013. [Google Scholar]
- [55].Generex Biotechnology Corporation . Generex Provides Update on Buccal Insulin Formulation Enhancement Project [Press Release] 2015. [Google Scholar]
- [56].Modi P, Mihic M, Lewin A. The evolving role of oral insulin in the treatment of diabetes using a novel RapidMist™ System. Diabetes/Metabolism Research and Reviews. 2002;18:S38–S42. doi: 10.1002/dmrr.208. [DOI] [PubMed] [Google Scholar]
- [57].Generex Biotechnology Corporation . Generex Provides Update on Success of Buccal Insulin Formulation Enhancement Project. 2015. [Google Scholar]
- [58].Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC. Transepithelial Transport of Fc -Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Science Translational Medicine. 2013;5:213ra167–213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sharma G, Sharma AR, Nam J-S, Doss GPC, Lee S-S, Chakraborty C. Nanoparticle based insulin delivery system: the next generation efficient therapy for Type 1 diabetes. Journal of Nanobiotechnology. 2015;13:74. doi: 10.1186/s12951-015-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jin Y, Song Y, Zhu X, Zhou D, Chen C, Zhang Z, Huang Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- [61].Trial to Compare NNC0123-0000-0338 in a Tablet Formulation and Insulin Glargine in Subjects With Type 2 Diabetes Currently Treated With Oral Antidiabetic Therapy. Novo Nordisk A/S, NCT02470039. [Google Scholar]
- [62].Maher S, Leonard TW, Jacobsen J, Brayden DJ. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Advanced Drug Delivery Reviews. 2009;61:1427–1449. doi: 10.1016/j.addr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [63].Tomita M, Hayashi M, Awazu S. Absorption-enhancing mechanism of EDTA, caprate, and decanoylcarnitine in Caco-2 cells. Journal of Pharmaceutical Sciences. 1996;85:608–611. doi: 10.1021/js9504604. [DOI] [PubMed] [Google Scholar]
- [64].Aungst BJ. Absorption Enhancers: Applications and Advances. The AAPS Journal. 2012;14:10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oramed Pharmaceuticals Inc . ORAMED COMPLETES RANDOMIZATION OF OVER 50% OF PATIENTS IN PHASE IIB ORAL INSULIN STUDY. 2015. [Google Scholar]
- [66].Oramed Pharmaceuticals Inc . Oramed Pharmaceuticals Presents Data from Phase IIa Trial with ORMD-0801 in Type 2 Diabetes at the 2014 Diabetes Summit [Press Release] 2014. [Google Scholar]
- [67].Kidron M. Google Patents. 2007. Methods and compositions for oral administration of proteins. [Google Scholar]
- [68].Chou DH-C, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2401–2406. doi: 10.1073/pnas.1424684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proceedings of the National Academy of Sciences of the United States of America. 2015 doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Ultrasound-Triggered Regulation of Blood Glucose Levels Using Injectable Nano-Network. Advanced Healthcare Materials. 2014;3:811–816. doi: 10.1002/adhm.201300490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Merck & Co . Merck to Acquire SmartCells, Inc. [Press Release] 2010. [Google Scholar]
- [72].Hurley D. The New York Times Magazine. Arthur Ochs Sulzberger; New York City, New York: 2014. Why Are So Few Blockbuster Drugs Invented Today? [Google Scholar]
- [73].Lancaster TM, Murikipudi S, Zion TC. Google Patents. 2012. Uses of macrophage mannose receptor to screen compounds and uses of these compounds. [Google Scholar]
- [74].Merck Sharp & Dohme Corp., NCT02269735 . A Two Part Study to Evaluate the Safety, Pharmacokinetics and Pharmacodynamics of MK-2640 in Healthy Participants (Part I) and Participants With Type 1 Diabetes Mellitus (Part II) (MK-2640-001) [Google Scholar]
- [75].U.S. Food and Drug Administration . Dexcom G4 PLATINUM Continuous Glucose Monitoring System - P120005/S018. Center for Devices and Radiological Health. [Google Scholar]
- [76].Christiansen M, Bailey T, Watkins E, Liljenquist D, Price D, Nakamura K, Boock R, Peyser T. A New-Generation Continuous Glucose Monitoring System: Improved Accuracy and Reliability Compared with a Previous-Generation System. Diabetes Technology & Therapeutics. 2013;15:881–888. doi: 10.1089/dia.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. American Journal of Physiology - Endocrinology and Metabolism. 1999;277:E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- [78].Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. Journal of Biomedical Materials Research. 1997;37:401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [79].Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. Journal of Biomedical Materials Research Part A. 2008;85A:699–706. doi: 10.1002/jbm.a.31593. [DOI] [PubMed] [Google Scholar]
- [80].Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Analytical and bioanalytical chemistry. 2010;398:1695–1705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Heinemann L, Krinelke L. Insulin Infusion Set: The Achilles Heel of Continuous Subcutaneous Insulin Infusion. Journal of Diabetes Science and Technology. 2012;6:954–964. doi: 10.1177/193229681200600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].U.S. Food and Drug Administration . BD FlowSmart Set 510(k) Premarket Notification. Center for Devices and Radiological Health. [Google Scholar]
- [83].Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Farret A, Pelletier M-J, Place J, Bruttomesso D, Del Favero S, Visentin R, Filippi A, Scotton R, Avogaro A, Doyle FJ. Feasibility of Outpatient Fully Integrated Closed-Loop Control: First studies of wearable artificial pancreas. Diabetes Care. 2013;36:1851–1858. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Letourneau R. Healthcare Finance, HIMSS Media. 2012. Global insulin market expected to reach $32B by 2018. [Google Scholar]
- [85].Engelberg AB, Kesselheim AS, Avorn J. Balancing Innovation, Access, and Profits — Market Exclusivity for Biologics. New England Journal of Medicine. 2009;361:1917–1919. doi: 10.1056/NEJMp0908496. [DOI] [PubMed] [Google Scholar]
- [86].Heinemann L. Biosimilar insulins: how will this story evolve? Diabetes Technology & Therapeutics. 2012;14:986–988. doi: 10.1089/dia.2012.0247. [DOI] [PubMed] [Google Scholar]
- [87].Merck & Co. Merck and Samsung Bioepis Enter Collaboration Agreement to Develop and Commercialize Insulin Glargine Candidate for Diabetes [Press Release] 2014. [Google Scholar]
- [88].Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- [89].Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- [90].Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005, Diabetologia. 2006;49:253–260. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- [91].Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- [92].Roche Hoffmann-La. A Study of Once-Daily RO6811135 in Participants With Type 2 Diabetes (T2D) Inadequately Controlled With Metformin. NCT02205528. [Google Scholar]
- [93].Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nature Reviews Endocrinology. 2013;9:425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- [94].Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discovery Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [95].U.S. Food and Drug Administration . Approval Labels. [Google Scholar]
- [96].Vilsboll T, Agerso H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88:220–224. doi: 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- [97].Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- [98].de Meester I, Lambeir AM, Proost P, Scharpe S. Dipeptidyl peptidase IV substrates. An update on in vitro peptide hydrolysis by human DPPIV. Adv Exp Med Biol. 2003;524:3–17. doi: 10.1007/0-306-47920-6_1. [DOI] [PubMed] [Google Scholar]
- [99].Deacon CF, Nauck MA, Toftnielsen M, Pridal L, Willms B, Holst JJ. Both Subcutaneously and Intravenously Administered Glucagon-Like Peptide-I Are Rapidly Degraded from the Nh2-Terminus in Type-Ii Diabetic-Patients and in Healthy-Subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- [100].Thornton K, Gorenstein DG. Structure of glucagon-like peptide (7-36) amide in a dodecylphosphocholine micelle as determined by 2D NMR. Biochemistry. 1994;33:3532–3539. doi: 10.1021/bi00178a009. [DOI] [PubMed] [Google Scholar]
- [101].Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. Structure-activity studies of glucagon-like peptide-1. J Biol Chem. 1994;269:6275–6278. [PubMed] [Google Scholar]
- [102].Gallwitz B, Witt M, Paetzold G, Morys-Wortmann C, Zimmermann B, Eckart K, Folsch UR, Schmidt WE. Structure/activity characterization of glucagon-like peptide-1. Eur J Biochem. 1994;225:1151–1156. doi: 10.1111/j.1432-1033.1994.1151b.x. [DOI] [PubMed] [Google Scholar]
- [103].Deacon CF, Knudsen LB, Madsen K, Wiberg FC, Jacobsen O, Holst JJ. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia. 1998;41:271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- [104].Burcelin R, Dolci W, Thorens B. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism: Clinical and Experimental. 1999;48:252–258. doi: 10.1016/s0026-0495(99)90043-4. [DOI] [PubMed] [Google Scholar]
- [105].Siegel EG, Gallwitz B, Scharf G, Mentlein R, Morys-Wortmann C, Folsch UR, Schrezenmeir J, Drescher K, Schmidt WE. Biological activity of GLP-1-analogues with N-terminal modifications. Regulatory Peptides. 1999;79:93–102. doi: 10.1016/s0167-0115(98)00155-4. [DOI] [PubMed] [Google Scholar]
- [106].Gallwitz B, Ropeter T, Morys-Wortmann C, Mentlein R, Siegel EG, Schmidt WE. GLP-1-analogues resistant to degradation by dipeptidyl-peptidase IV in vitro. Regul Pept. 2000;86:103–111. doi: 10.1016/s0167-0115(99)00095-6. [DOI] [PubMed] [Google Scholar]
- [107].Siegel EG, Scharf G, Gallwitz B, Mentlein R, Morys-Wortmann C, Folsch UR, Schmidt WE. Comparison of the effect of native glucagon-like peptide 1 and dipeptidyl peptidase IV-resistant analogues on insulin release from rat pancreatic islets. Eur J Clin Invest. 1999;29:610–614. doi: 10.1046/j.1365-2362.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- [108].O'Harte FP, Mooney MH, Kelly CM, McKillop AM, Flatt PR. Degradation and glycemic effects of His(7)-glucitol glucagon-like peptide-1(7-36)amide in obese diabetic ob/ob mice. Regul Pept. 2001;96:95–104. doi: 10.1016/s0167-0115(00)00125-7. [DOI] [PubMed] [Google Scholar]
- [109].Green BD, Mooney MH, Gault VA, Irwin N, Bailey CJ, Harriott P, Greer B, O'Harte FP, Flatt PR. N-terminal His(7)-modification of glucagon-like peptide-1(7-36) amide generates dipeptidyl peptidase IV-stable analogues with potent antihyperglycaemic activity. J Endocrinol. 2004;180:379–388. doi: 10.1677/joe.0.1800379. [DOI] [PubMed] [Google Scholar]
- [110].Green BD, Gault VA, O'Harte F P, Flatt PR. Structurally modified analogues of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) as future antidiabetic agents. Curr Pharm Des. 2004;10:3651–3662. doi: 10.2174/1381612043382774. [DOI] [PubMed] [Google Scholar]
- [111].Li Y, Zheng X, Tang L, Xu W, Gong M. GLP-1 analogs containing disulfide bond exhibited prolonged half-life in vivo than GLP-1. Peptides. 2011;32:1303–1312. doi: 10.1016/j.peptides.2011.04.001. [DOI] [PubMed] [Google Scholar]
- [112].Sebokova E, Christ AD, Wang HY, Sewing S, Dong JZ, Taylor J, Cawthorne MA, Culler MD. Taspoglutide, an Analog of Human Glucagon-Like Peptide-1 with Enhanced Stability and in Vivo Potency. Endocrinology. 2010;151:2474–2482. doi: 10.1210/en.2009-1459. [DOI] [PubMed] [Google Scholar]
- [113].Rosenstock J, Balas B, Charbonnel B, Bolli GB, Boldrin M, Ratner R, Balena R, Grp T-ES. The Fate of Taspoglutide, a Weekly GLP-1 Receptor Agonist, Versus Twice-Daily Exenatide for Type 2 Diabetes The T-emerge 2 trial. Diabetes Care. 2013;36:498–504. doi: 10.2337/dc12-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- [115].Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29:2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [116].Bolli GB, Owens DR. Lixisenatide, a novel GLP-1 receptor agonist: efficacy, safety and clinical implications for type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2014;16:588–601. doi: 10.1111/dom.12253. [DOI] [PubMed] [Google Scholar]
- [117].Sanofi Provides Update on Lixisenatide New Drug Application in U.S. 2013. Sanofi. [Google Scholar]
- [118].Sanofi New Drug Application for Lixisenatide Accepted For Review By FDA. 2015. Sanofi. [Google Scholar]
- [119].Petrie JR. The cardiovascular safety of incretin-based therapies: a review of the evidence. Cardiovascular Diabetology. 2013;12:130. doi: 10.1186/1475-2840-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. Journal of Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [121].Bush MA, Matthews JE, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, Holland MC, Gutierrez M, Stewart MW. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes, Obesity and Metabolism. 2009;11:498–505. doi: 10.1111/j.1463-1326.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- [122].Matthews JE, Stewart MW, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, Holland MC, Bush MA, Albiglutide G. Study, Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:4810–4817. doi: 10.1210/jc.2008-1518. [DOI] [PubMed] [Google Scholar]
- [123].Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN, H.S. Group HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–2148. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- [124].Home PD, Shamanna P, Stewart M, Yang F, Miller M, Perry C, Carr MC. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes, Obesity and Metabolism. 2015;17:179–187. doi: 10.1111/dom.12414. [DOI] [PubMed] [Google Scholar]
- [125].Blair HA, Keating GM. Albiglutide: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2015;75:651–663. doi: 10.1007/s40265-015-0370-5. [DOI] [PubMed] [Google Scholar]
- [126].Trujillo JM, Nuffer W. Albiglutide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Ann Pharmacother. 2014;48:1494–1501. doi: 10.1177/1060028014545807. [DOI] [PubMed] [Google Scholar]
- [127].Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- [128].Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Meece J. Pharmacokinetics and pharmacodynamics of liraglutide, a long-acting, potent glucagon-like peptide-1 analog. Pharmacotherapy. 2009;29:33S–42S. doi: 10.1592/phco.29.pt2.33S. [DOI] [PubMed] [Google Scholar]
- [130].Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, L.-S. Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- [131].Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- [132].Nauck MA, Petrie JR, Sesti G, Mannucci E, Courreges JP, Atkin S, During M, Jensen CB, Heller S. The once-weekly human GLP-1 analogue semaglutide provides significant reductions in HbA(1c) and body weight in patients with type 2 diabetes. Diabetologia. 2012;55:S7–S7. [Google Scholar]
- [133].Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- [134].Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK. Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Current opinion in molecular therapeutics. 2010;12:790–797. [PubMed] [Google Scholar]
- [135].Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- [136].Kuritzky L, Umpierrez G, Ekoe JM, Mancillas-Adame L, Lando LF. Safety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetes. Postgrad Med. 2014;126:60–72. doi: 10.3810/pgm.2014.10.2821. [DOI] [PubMed] [Google Scholar]
- [137].Sroda K, Rydlewski J, Langner M, Kozubek A, Grzybek M, Sikorski AF. Repeated injections of PEG-PE liposomes generate anti-PEG antibodies. Cellular and Molecular Biology Letters. 2005;10:37–47. [PubMed] [Google Scholar]
- [138].Schellenberger V, Wang CW, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WP. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- [139].Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr Opin Chem Biol. 2006;10:652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Christiansen M, Matson M, Brazg R, Georgopoulos L, Arnold S, Kramer W, Shi L, Strange P. Weekly Subcutaneous Doses of PB1023 a Novel GLP-1 Analogue Reduce Glucose Exposure Dose Dependently. PhaseBio Pharmaceuticals; 2014. [Google Scholar]
- [141].PhaseBio Pharmaceuticals . PhaseBio Initiated Phase 1 Study of Investigational Once-Weekly Basal Insulin for Potential Treatment of Diabetes. 2013. [Google Scholar]
- [142].Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five-year efficacy and safety data of exenatide once weekly: long-term results from the DURATION-1 randomized clinical trial. Mayo Clin Proc. 2015;90:356–365. doi: 10.1016/j.mayocp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- [143].Ginsberg BH. Mixing Pens and the Future of Diabetes Drugs. Journal of Diabetes Science and Technology. 2015 doi: 10.1177/1932296815585873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. Injectable protease-operated depots of glucagon-like peptide-1 provide extended and tunable glucose control. Proc Natl Acad Sci U S A. 2013;110:2792–2797. doi: 10.1073/pnas.1214518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. Journal of Controlled Release. 2013;172:144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Henry RR, Rosenstock J, Logan D, Alessi T, Luskey K, Baron MA. Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin-treated subjects with type 2 diabetes. Journal of Diabetes and its Complications. 2014;28:393–398. doi: 10.1016/j.jdiacomp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- [147].Therapeutics I. FREEDOM-1 and FREEDOM-1 High Baseline (HBL) Study Results. 2014. Intarcia Announces Two Positive Phase 3 Trials for ITCA 650 in Type 2 Diabetes. [Google Scholar]
- [148].Leonard TW, Lynch J, McKenna MJ, Brayden DJ. Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET. Expert Opinion on Drug Delivery. 2006;3:685–692. doi: 10.1517/17425247.3.5.685. [DOI] [PubMed] [Google Scholar]
- [149].Chae SY, Jin CH, Shin HJ, Youn YS, Lee S, Lee KC. Preparation, characterization, and application of biotinylated and biotin-PEGylated glucagon-like peptide-1 analogues for enhanced oral delivery. Bioconjug Chem. 2008;19:334–341. doi: 10.1021/bc700292v. [DOI] [PubMed] [Google Scholar]
- [150].Clardy-James S, Chepurny OG, Leech CA, Holz GG, Doyle RP. Synthesis, characterization and pharmacodynamics of vitamin-B(12)-conjugated glucagon-like peptide-1. ChemMedChem. 2013;8:582–586. doi: 10.1002/cmdc.201200461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Eldor R, Kidron M, Greenberg-Shushlav Y, Arbit E. Novel glucagon-like peptide-1 analog delivered orally reduces postprandial glucose excursions in porcine and canine models. Journal of Diabetes Science and Technology. 2010;4:1516–1523. doi: 10.1177/193229681000400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Marino MT, Costello D, Baughman R, Boss A, Cassidy J, Damico C, van Marle S, van Vliet A, Richardson PC. Pharmacokinetics and pharmacodynamics of inhaled GLP-1 (MKC253): proof-of-concept studies in healthy normal volunteers and in patients with type 2 diabetes. Clin Pharmacol Ther. 2010;88:243–250. doi: 10.1038/clpt.2010.85. [DOI] [PubMed] [Google Scholar]
- [153].Rhinehart AS. Adding GLP-1 Receptor Agonist Therapy to Basal Insulin for Postprandial Glucose Control. Clinical Diabetes. 2015;33:73–75. doi: 10.2337/diaclin.33.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180–2181. doi: 10.1016/S0140-6736(14)61409-4. [DOI] [PubMed] [Google Scholar]
- [155].Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- [156].Rosenstock J, Diamant M, Silvestre L, Souhami E, Zhou T, Fonseca V. Benefits of a fixed-ratio formulation of once-daily insulin glargine/lixisenatide (LixiLan) vs glargine in type 2 diabetes inadequately controlled on metformin. Diabetologia. 2014;57:S108–S108. [Google Scholar]
- [157].Gough SCL, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, Damgaard LH, Buse JB, Invest N-D-IT. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes & Endocrinology. 2014;2:885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- [158].Abu Hassan H, Tohid H, Mohd Amin R, Long Bidin MB, Muthupalaniappen L, Omar K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Family Practice. 2013;14:164. doi: 10.1186/1471-2296-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]