Abstract
Purpose
To characterize peripheral nerve stimulation (PNS) of an asymmetric head-only gradient coil that is compatible with a commercial high-channel-count receive-only array.
Methods
Two prototypes of an asymmetric head-only gradient coil set, with 42-cm inner diameter, were constructed for brain imaging at 3T with maximum performance specifications of up to 85 mT/m and 708 T/m/s. 24 volunteer tests were performed to measure PNS thresholds with the transverse (X, left/right; Y, anterior/posterior) gradient coils of both prototypes. 14 volunteers were also tested for the Z-gradient PNS in the second prototype, and were additionally scanned with high-slew-rate EPI immediately after the PNS tests.
Results
For both prototypes, the Y-gradient PNS threshold was markedly higher than the X-gradient. The Z-gradient threshold was intermediate between those for the X- and Y-coils. Out of the 24 volunteer subjects, only two experienced Y-gradient PNS at 80 mT/m, 500 T/m/s. All volunteers underwent the EPI scan without PNS when the readout direction was set to A/P.
Conclusion
Measured PNS characteristics of asymmetric head-only gradient coil prototypes indicate that such coils, especially in the A/P direction, can be used for fast EPI readout in high-performance neuroimaging scans with substantially reduced PNS concerns compared to conventional whole-body gradient coils.
Keywords: peripheral nerve stimulation, PNS, head-only gradient coil, EPI, neuroimaging
Introduction
Peripheral nerve stimulation (PNS) is known to limit the operation of high-amplitude, high slew- rate gradient coils in modern high-field MRI (1). For example, commercial whole-body gradient coils capable of producing 50 mT/m and 200 T/m/s are routinely operated below their maximum concurrent strength and slew rate because of PNS. This prevents use of the gradient coil's full capability in sequences where fast gradient switching is important, such as in the readout of echo planar imaging (EPI). Although MR gradients typically operate far below the cardiac threshold limits, in an extreme case (2), the electric fields produced by strong pulsed gradient fields were reported to be capable of cardiac stimulation at the highest amplitude ranges. Of note, stimulation often occurs away from the imaged anatomy, such as in torso and arms during a brain scan.
Given that the gradient-induced electric field generally increases quadratically with distance from the gradient isocenter (1) in the region of gradient uniformity, it is reasonable to expect that more localized gradient coils centered at a specific anatomic location may reduce nerve stimulation. This has been verified experimentally with a compact head-neck gradient coil (3,4) as well as a uniplanar gradient coil (5), which was operated at up to 200 mT/m and 250 T/m/s without PNS (6). Zhang et al. (7) have derived an empirical relationship between the linear region size (expressed in diameter of spherical volume, or DSV) of multiple commercial and published gradient coils and the PNS threshold parameters, confirming an inverse relationship between the gradient coil's DSV and its PNS thresholds.
In the case of brain imaging, compact gradient coils that can be placed above the subject's shoulder level have been reported, and demonstrated higher switching speed and gradient amplitude efficiency (8–13). Such coils have been used to produce early EPI images of the brain (8,10–12), and were proposed (13) and used (14) as a component in a dedicated neuroimaging MRI scanner. Commercial head gradient inserts have also been successfully used for human brain imaging in whole-body magnets at 3 T (15,16) and higher fields (17–19).
Despite demonstrated advantages of compact neuroimaging gradient coils in terms of PNS and efficiency, such coils have so far not been widely used clinically, perhaps due to two main reasons. First, many early neuroimaging gradient coils (3,8,10–12) had a relatively small region of gradient uniformity (20 cm diameter, or less) which resulted in substantial image distortions in whole-head imaging. Second, to our knowledge, all previously published head-only gradient coils had an inner diameter that was too small for the routine use of commercially available high (8 or more)-channel-count receiver arrays, which are increasingly used clinically for high-speed, high signal-to-noise ratio (SNR) brain scans. We do note that several researchers have successfully demonstrated a 31- or 32-channel receiver array (16,20,21) that was specifically built to fit in an existing narrow-bore head gradient insert. However, most receiver arrays of general availability today are still only usable in a larger, more conventional gradient coil. In terms of the gradient coil design itself, despite much progress in this area (11–13), electromagnetic and mechanical design of a compact, high-power-dissipating gradient coil with simultaneous torque- and eddy-current minimization still remains a challenge. This latter fact appears to have influenced the choice of a whole-body-sized, symmetric design for the 300 mT/m gradient coil that was developed for the Human Connectome Project (2).
Recently we reported brain imaging with an asymmetric head-only gradient coil (22,23) with an inner diameter (42 cm) substantially larger than previous head-only coils, yet with relatively smaller outer dimension (diameter = 59 cm, length = 80 cm) that fits within the bore of a typical whole-body gradient coil. After insertion of the RF transmitter, a net 37 cm diameter aperture for the head remained, which easily accommodated a 32-channel receiver array. The gradient coil had a 26 cm DSV for whole-head coverage. Preliminary in-vivo scan experiments indicated that the transverse (X, left-right; Y, anterior-posterior) gradient coils had PNS thresholds much higher than a typical whole-body gradient coil set (22).
Following successful demonstration of the design concept, we have subsequently built a second version of the asymmetric head-only gradient coil, with identical inner and outer diameters but with improved electromagnetic and thermal management designs (24). The second-generation gradient coil was operated in two different modes. First, PNS tests were performed, on each of the three axes, with a maximum amplitude of 85 mT/m and maximum slew rate of 708 T/m/s. Second, volunteer head imaging scans were performed with reduced parameters of 80 mT/m and 500 T/m/s, for initial image quality assessment. PNS tests in the second generation coil confirmed substantially increased thresholds of the head-only design compared to a conventional whole-body gradient coil. In particular, under the conditions stated above, the anterior-posterior (A/P) gradient coil was nearly PNS-free, allowing high-slew-rate EPI scans on volunteer subjects with A/P readout. In this paper we document the results of PNS threshold measurements on our head-only gradient coil prototypes, and discuss the implications of these results on the development of high-performance neuroimaging gradient coils.
Methods
1. Design and Construction of Head-Only Gradient Coils
Our first generation head-only gradient coil (HG1) was designed as an actively shielded, three-axis coil set with a symmetric Z-coil and asymmetric X, Y coils (23). Here symmetry means that the coil pattern on the service side (z > 0) is the mirror image of that on the patient side (z < 0) so that the gradient isocenter is located at the center of the coil's wire pattern along the z-axis. Radial order of the coil winding layers was the following: from the inside out, Y-primary, X-primary, Z-primary, Y-shield, X-shield, Z-shield. Each axis of the gradient coil was independently force- and torque- balanced, and was designed to produce the maximum gradient amplitude of at least 80 mT/m and the maximum slew rate greater than 500 T/m/s when driven by a standard gradient driver used for a whole-body 3 T scanner (MR750w, GE Healthcare, Waukesha, WI, USA), capable of providing the maximum current and voltage of 660 A and 1650 V, respectively (Table 1). The coil was cooled by continuous flow of chilled water provided by the standard cooling cabinet of MR750w. Typical cooling circuit parameters were: inlet temperature = 17°C, inlet pressure = 5 bars, flow rate = 35 L/min. Estimated (based on experiment) maximum gradient coil heat removal capacity ranged from 2 kW (Y) to 7.5 kW (Z), assuming a maximum acceptable gradient coil internal temperature of 85°C.
Table 1.
Measured (RDC, R1kHz, L) and as-designed (all others) physical parameters of the head-only gradient coils. RDC : DC resistance, R1kHz: resistance at 1 kHz, L: self-inductance, F, τ: maximum force and torque, respectively, η: gradient efficiency, SR/V ≡ η/L: slew rate at zero current at unit voltage, G660A: gradient at 660 A, SR1650V: slew rate at zero current at 1650 V. Gmax and SRmax are the specified maximum gradient amplitude and slew rate, respectively, taking into account any safety margins. These values were actually used in the PNS tests (except for the HG1 Z coil), and constitute “hardware limit” in the data plots (Figs. 3–5)
| para- meter |
unit | HG1 | HG2 | ||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||
| RDC | Ω | 0.091 | 0.085 | 0.126 | 0.124 | 0.114 | 0.087 |
| R1kHz | Ω | 0.251 | 0.234 | 0.239 | 0.178 | 0.165 | 0.133 |
| L | mH | 0.341 | 0.327 | 0.266 | 0.234 | 0.204 | 0.186 |
| F | N | 2 | 10 | 5 | 8 | 10 | 118 |
| τ | Nm | 3 | 2 | 0 | 23 | 48 | 0 |
| η | mT/m/A | 0.133 | 0.133 | 0.133 | 0.129 | 0.129 | 0.132 |
| SR/V | T/m/s/V | 0.391 | 0.408 | 0.501 | 0.550 | 0.631 | 0.709 |
| G660A | mT/m | 88 | 88 | 88 | 85 | 85 | 87 |
| SR1650V | T/m/s | 645 | 673 | 827 | 908 | 1042 | 1169 |
| Gmax | mT/m | 80 | 85 | ||||
| SRmax | T/m/s | 500 | 708 | ||||
The second head-only gradient coil (HG2) (24) differed from HG1 in the following respects.
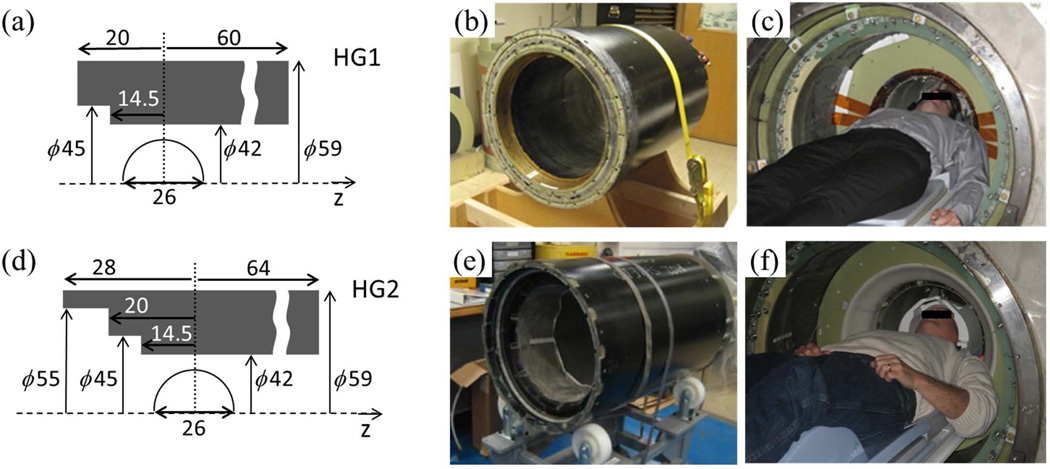
The axial extent of the coil was 8 cm longer than HG1 on the patient side and 4 cm longer on the service side (Fig. 1). The extra 8 cm on the patient side was used for winding the Z-shield coil, which provided more design flexibility for the Z-coil. This introduced a second step in the patient-side profile of HG2 (Fig. 1d), with inner diameter of 55 cm. We note that today a whole-body scanner with a 55 cm bore diameter is generally considered insufficient for many patients. In the head-only case, a difference is that the remainder of the body inferior to the shoulders lies outside the bore. Because of this, and partly because of the curved outline of the shoulders, most volunteers could position their shoulders and upper arms comfortably at the entrance of HG2 (compare Fig. 1c and Fig. 1f).
The coil patterns were simplified on all axes. In particular, torque-balancing of the X- and Y-coils was achieved without employing an additional winding on the service end, as was used in HG1 (Fig. 2 of (25)). As a result the total number of turns for each half of the transverse coil layer was reduced as the following: from HG1 to HG2, X-primary (36→25), X-shield (22→16), Y-primary (36→24), Y-shield (21→15). The total number of windings for the Z-coil was also reduced: Z-primary (38→36), Z-shield (38→16). This resulted in reduced inductances and improved manufacturability.
The new electromagnetic design improved active shielding and reduced the eddy current. Specifically, the calculated maximum leakage field outside the gradient coil was reduced from 16 mT (HG1) to <3 mT (HG2) at a 32 cm radius. Calculated maximum eddy current field, due to a hypothetical conductive cylinder at a 30 cm radius, was reduced from >6% (HG1) of the applied gradient field to <0.2% (HG2), over the 26 cm DSV before eddy current compensation. Here the relatively small radii of 32 cm and 30 cm were selected because of our plan to operate the gradient coils in the future in a compact, head-only magnet, under development in our institute. The reduced eddy current was experimentally verified in a test using an aluminum cylinder with 30 cm inner radius.
All coils in HG2 were made from hollow copper wires, similar to the ones reported in (26). Water flowed continuously through the wires, removing heat by direct contact with the copper. This eliminated need for additional cooling layers in the coil construction, and increased the cooling capacity to about 25 kW.
Figure 1.
Schematics and photos of the two head-only gradient coils: HG1 (a-c) and HG2 (d-f). (a,d) Physical dimensions in centimeters in the RZ plane (not to scale). The half circle indicates the 26 cm DSV. (b,e) The HG1 (b) and HG2 (e) gradient coils prior to mounting. (c,f) Volunteer test setup inside a whole-body 3T magnet.
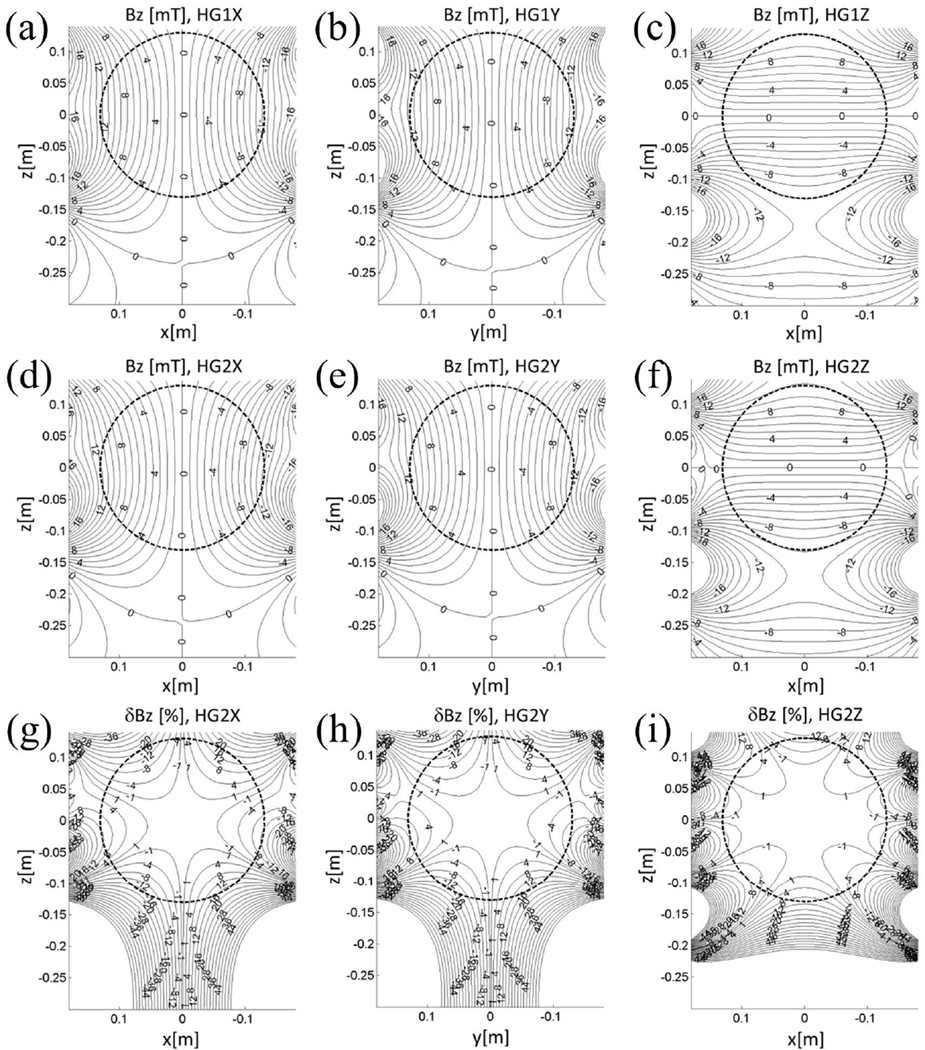
Figure 2.
(a-f) Magnetic field maps (mT) of the two head-only gradient coil sets (a-c, HG1, X to Z coils; d-f, HG2, X to Z coils) at 660 A. The field maps are shown on the coronal (a,c,d,f) and the sagittal (b,e) mid-plane where positive z corresponds to the superior side. (g-i) Linearity error for HG2, X to Z coils, on the corresponding mid-planes. The circle is 26 cm in diameter.
Figure 2(a-f) shows the gradient field maps for both head-only gradient coil sets. Figure 2(g-i) shows the linearity error, defined as the deviation of the field map from the ideal gradient field (85 mT/m for X and Y, 87 mT/m for Z) for HG2 at 660 A, expressed as the percentage of the ideal field 13 cm away (in the direction of the gradient) from the isocenter. The linear region size of HG2 was specified as the DSV in which the linearity error does not exceed 17%. Rounded to the nearest centimeter, this value for the worst axis was 26 cm; this is indicated as circles in Fig. 2. The 17% limit was chosen based on the specification of a representative clinical whole-body gradient coil.
Table 1 shows the physical parameters of the gradient coils. Gmax and SRmax denote the specified maximum gradient amplitude and slew rate that can be used by a scan operator or a sequence developer in practice. They also define the "hardware limit" in the PNS threshold plots in the Results section. They are generally lower than the designed maximum values, G660A and SR1650V (Table 1), due to engineering and operational safety margins assessed through error analyses.
2. PNS measurement
Pulse sequence
The PNS threshold measurement was performed on a single axis at a time. The gradient pulse waveform used for the threshold measurement was similar to that used for previous determination of PNS thresholds (6), and consisted of a train of 128-cycle (256 lobes) bipolar, trapezoidal pulses with a fixed flattop length of 1 ms and a varying rise-time (= fall-time) in the range of Δt = 120 to 424 µs (zero-to-peak). For each rise-time and gradient amplitude, the pulse train was repeated four times, with 1 sec separation between the start of consecutive trains. In our experience, such repetition helped the volunteers to better decide whether a given sensation was induced by mechanical vibration or nerve stimulation, especially for the HG2 Z coil. For each rise-time, the gradient amplitude (ΔG, zero-to-peak) was incremented in steps of between 4 and 8 mT/m to a maximum value (Gmax in Table 1). The temporal gap (pause) between two consecutive amplitude levels ranged from 10 to 20 seconds; larger gaps were used for sufficient cooling of the gradient coil. For HG2, with improved cooling, a pause was not thermally necessary, but we retained a 10 second pause for the consistency of the protocols. The number of amplitude steps ranged from 10 to 16. For HG1, the following three rise-times (zero-to-peak) were used: 160, 248, 400 µs. For HG2, four rise-times, 120, 168, 260, 424 µs, were used. In both cases, the shortest rise-time was chosen as the ratio between the maximum gradient amplitude and the maximum slew rate. All volunteer test sessions (including imaging for HG2) were kept below 1 hour in duration.
Experimental setup
For PNS threshold measurements for both HG1 and HG2, the head-only gradient coils were inserted into a whole-body 3 T scanner (MR 750w, GE Healthcare, Waukesha, WI, USA), from which the body RF coil had been removed. The standard whole-body gradient coil remained in the magnet but was electrically disconnected, and provided a mounting base for the head-only gradient coil. The gap between the inner diameter of the whole-body gradient coil and the outer diameter of the head-only gradient coils was bridged by two, 38-mm-thick custom-made fiberglass mounting rings. A custom-built 16-rung RF birdcage coil (inner diameter = 37cm, outer diameter = 41.5 cm, electrical length = 40 cm) was situated in the head-only gradient coil throughout the tests and used for RF transmission when head imaging was performed. With the RF transmitter in place the head-access bore diameter was reduced to 37 cm. Additionally, volunteer subjects in HG2 placed their head inside a 32-channel RF brain coil (Nova Medical, Wilmington, MA, USA), which was located inside the birdcage coil. The 32-channel coil served as the receiver coil for head imaging following the PNS measurement protocol.
For HG1, PNS thresholds were measured for only X- and Y-gradient coils because of a short that developed on the Z-coil before the tests commenced. For HG2, the thresholds for all gradient axes were measured.
Volunteer test workflow
All volunteer tests were performed at the lead author's institution under an IRB-approved protocol. 10 and 14 healthy volunteer subjects were recruited and provided written informed consent for the PNS tests on HG1 and HG2, respectively. Six subjects volunteered for both gradient coil tests; therefore there were 18 distinct volunteers (17 M, 1 F). The weight and height ranges were 59 – 95 kg and 168 – 188 cm, respectively.
All subjects, including those with relatively short necks, could position their heads with the eye level within 4 cm inferior to the gradient isocenter without the need to press their shoulders firmly against the rim of the compact gradient coil, i.e., there was at most light contact required. Three-plane localizer images (for HG2) confirmed that all volunteers were positioned so that their brains (including the brainstem) were well within the 26 cm DSV.
Once in position, each volunteer was instructed to report immediately any onset of PNS sensation using a squeeze-bulb alarm, as the pulse amplitude was incremented. If a possible sensation was suspected, but the volunteer was unsure upon questioning, pulse trains with the same and/or the adjacent amplitudes were repeated. Once a definite PNS threshold was reported, incrementing the amplitude was discontinued and the nature and location of the sensation were recorded. Then, the next series of pulse trains with a different rise-time or gradient axis was initiated. The resolution of PNS thresholds was limited by the amplitude step size, which was 5% of the maximum amplitude for most cases, and never exceeded 10%. The order of application of different rise-times was not randomized, but was consistently from the highest to the lowest value (i.e., from the lowest to the highest slew rate). A large bias or order effect is not expected because of the small number (3 or 4) of the rise-times, and also because the volunteers were not given prior information about the order of the rise-times, or about how different rise-times might affect PNS. All volunteers wore standard hearing protection devices (earplugs or earmuffs).
3. Head imaging
With the HG2 gradient, the PNS threshold measurements were immediately followed by head imaging. This added less than 10 minutes of test time to each subject, and served two purposes. First, imaging confirmed the position of the head with respect to the gradient coil's isocenter and DSV. Second, it illustrated how increased PNS thresholds of head-only gradient coils can benefit clinical imaging.
The head scan proceeded in two steps. First, a standard 3-plane localizer scan was performed using a 2D single-shot fast spin echo (SSFSE) sequence. The scan parameters were: TR = 667 ms; TE = 80 ms; bandwidth = ±83 kHz; matrix = 256 × 256; FOV = 28 cm; slice thickness = 8 mm; number of slices = 5 (each plane). Second, based on the localizer images, an axial 2D spin echo EPI scan was prescribed with the parameters: TR = 8000 ms; TE = 60 ms; ASSET factor = 2; bandwidth = ±250 kHz; matrix = 128 × 128; FOV = 24 cm; slice thickness = 3.2 mm; number of slices = 20. The readout portion of the EPI sequence was a trapezoidal pulse train similar to the PNS test pulse train, with the following characteristics: echo spacing = 372 µs, Gmax,read = 46 mT/m, SR = 500 T/m/s. For 11 out of the 14 volunteers scanned, the EPI scan was acquired twice with different (X, Y) readout directions. Two out of 14 volunteers who were sensitive to X-axis PNS had an EPI scan with only Y-axis readout. There was one (out of 14) volunteer who had an EPI scan with only X-axis readout, because of a scan workflow issue unrelated to PNS.
4. Other tests and analyses
Tilted head PNS test
As described in the Results section, we observed a marked difference between PNS thresholds for X and Y gradient coils. In order to verify that this difference is governed by the relative orientation of the head and the gradient field, and not the gradient coil construction or other hardware environment, we had one PNS test session in HG2 in which a PNS-sensitive subject was instructed to rotate his head about the z-axis while the applied gradient field direction was fixed. Three different head orientations were investigated while the gradient field was kept at a 45 degree-oblique axial direction. The previously-described PNS threshold measurement protocol for HG2 was followed, with 4 rise-times and the same sets of gradient amplitude () increment steps. Note that in this experiment the hardware limit for the maximum gradient amplitude is times larger than in an individual axis (X or Y) experiment. We did not use the added parameter space but stayed within the single-axis limit () because the main goal of the experiment was to investigate the head orientation dependence.
At each head orientation, a localizer scan was performed to measure the tilt angle in the axial plane. The head remained inside the 32-channel receiver coil, which was rotated along with the head. The volunteer did not report any difficulty with maintaining rotated head positions for 10 minutes at a time.
Correlation with head size and nose/glabella position
For the volunteers who experienced PNS in the HG2 X-gradient coil, we investigated the correlation between the measured PNS thresholds and several anatomical/geometrical parameters, namely the head size in the A/P direction, and the vertical positions of the nose tip and glabella (a cephalometric landmark slightly superior to the nasion), as measured from the sagittal localizer images. For each parameter, the Pearson correlation coefficient and the null-hypothesis P value (27) were calculated.
Results
PNS thresholds
As notational clarification, in this paper we use zero-to-peak amplitudes ΔG and corresponding time intervals Δt to describe trapezoidal pulse waveforms and to plot the PNS data (Figs. 3–5). Gradient strength parameters Gmax, G660A (Table 1) are similarly defined in terms of zero-to-peak variation. To facilitate comparison with PNS parameters in the literature (7,28), we use ΔGmin and τc τc (defined below) to refer to peak-to-peak changes.
Figure 3.
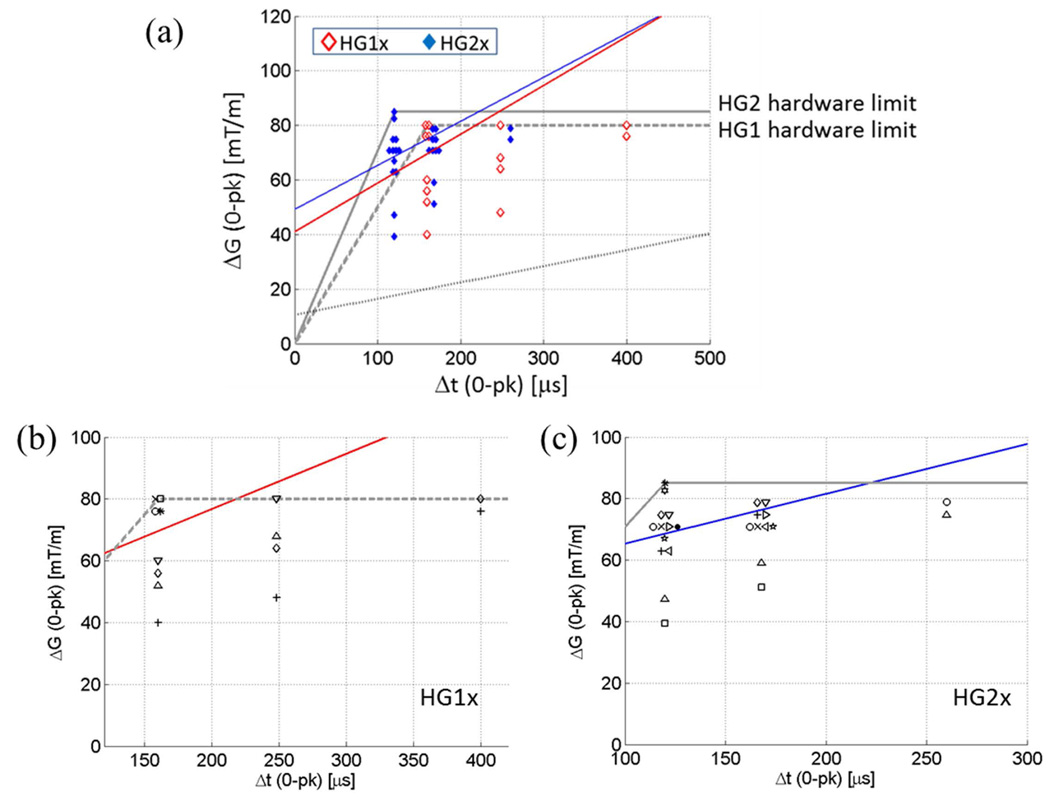
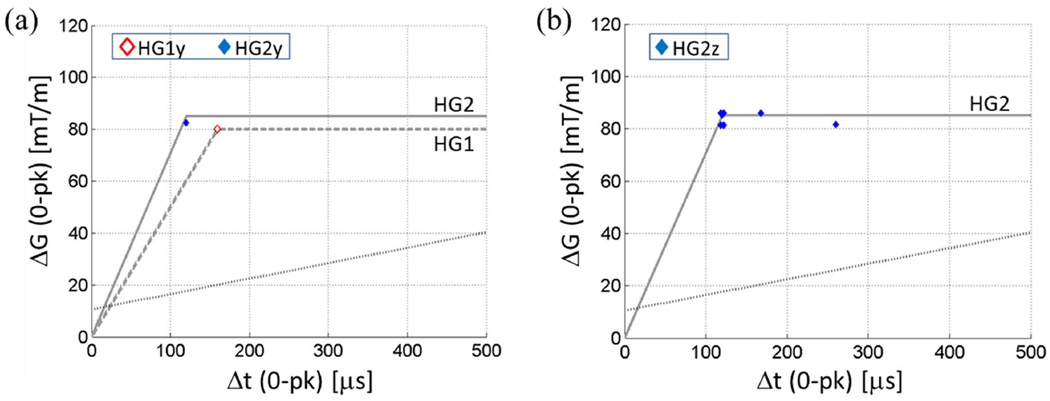
(a) PNS thresholds of the X gradient coil of the head-only gradient coil sets. Markers lightly displaced in the horizontal direction indicate multiple volunteers reporting the same PNS threshold for a given rise time. Red and blue straight lines are PNS threshold curves for HG1x and HG2x, respectively, determined by logistic regression at rise-times where enough data points existed. Solid grey line, HG2 hardware limit; dashed grey line, HG1 hardware limit; dotted black line, representative whole-body gradient PNS threshold. (b) (c) Same data as in (a) plotted with distinct markers for distinct volunteers within the HG1x (b) and HG2x (c) test sessions. Same markers in (b) and (c) do not necessarily indicate the same individual.
Figure 5.
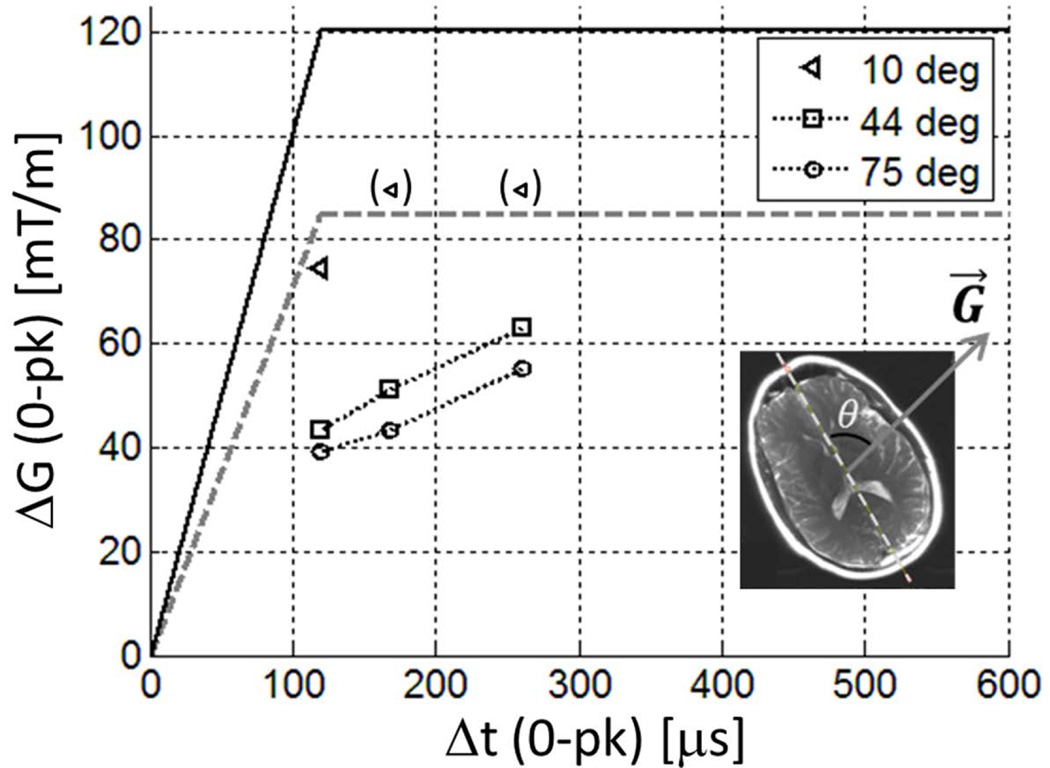
PNS thresholds of a volunteer at different head orientations. The legend indicates the angle θ between the A/P direction of the head and the direction of the gradient field G⃗ (inset); the latter was fixed to a 45 degree-oblique axial (X=Y) direction in the laboratory frame. Markers in parenthesis indicate thresholds above the experimental limit used (= HG2 single-axis hardware limit, dashed grey line). The true hardware limit for the combined-axis (X=Y) gradient test is times larger (black solid line) than this limit.
Figure 3(a-c) shows the measured PNS thresholds of the X-gradient coils of HG1 and HG2. Markers lying on the same vertical line correspond to PNS thresholds for different volunteers at a given rise-time. Because a discrete set of gradient amplitudes were used, some PNS threshold values for different volunteers coincided with each other. For clarity of display, overlapping threshold values are indicated by markers slightly displaced from one another in the horizontal direction. For example, for the HG2 X gradient, there were four volunteers who reported onset of PNS at ΔG = 71 mT/m at rise-times Δt = 120 µs and 166 µs. For each rise-time, a varying number of subjects did not report any sensation up to the maximum gradient amplitude used. Those subjects are not represented in Fig. 3. Instead, Table 2 shows the number of subjects who did not report PNS.
Table 2.
Number of volunteers who did and did not report PNS for each rise time and gradient coil. Maximum SR (4th row) is the ratio between max ΔG (3rd row) and Δt (2nd row). For HG2z, only sensations that were reported as distinct from vibration were counted.
| Gradient coil | HG1x | HG1y | HG2x | HG2y | HG2z | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rise-time Δt (µs) | 160 | 248 | 400 | 160 | 248 | 400 | 120 | 168 | 260 | 424 | 120 | 168 | 260 | 424 | 120 | 168 | 260 | 424 |
| Max ΔG used (mT/m) | 80 | 80 | 80 | 80 | 80 | 80 | 85 | 85 | 80 | 80 | 85 | 85 | 80 | 80 | 85 | 85 | 82 | 82 |
| Max SR (T/m/s) | 500 | 323 | 200 | 500 | 323 | 200 | 708 | 506 | 308 | 189 | 708 | 506 | 308 | 189 | 708 | 506 | 315 | 193 |
| Volunteers with PNS | 8 | 4 | 2 | 1 | 0 | 0 | 13 | 10 | 2 | 0 | 1 | 0 | 0 | 0 | 4 | 1 | 0 | 1 |
| Volunteers without PNS | 2 | 6 | 8 | 9 | 10 | 10 | 1 | 4 | 12 | 14 | 13 | 14 | 14 | 14 | 10 | 13 | 14 | 13 |
| Total volunteers | 10 | 10 | 14 | 14 | 14 | |||||||||||||
PNS parameters for the X-gradient coils were determined by logistic regression with 25% inclusion standard as described in (7). Here the data from the rise-times where at least 25% of the volunteers reported PNS were fitted to a logistic curve to determine the mean and standard deviation of the underlying threshold distribution. For the X-gradient coils in HG1 and HG2, the two shortest rise-times satisfied the inclusion criterion. A straight line was obtained by connecting the two mean threshold values at those rise-times. These lines are shown in Fig. 3. The PNS parameters obtained from these lines are the following. HG1x: SRmin = 179 ± 104 T/m/s, ΔGmin = 81.9 ± 41.6 mT/m, τc = 457 ± 353 µs; HG2x: SRmin = 161 ± 106 T/m/s, ΔGmin = 98.6 ± 31.2 mT/m, τc = 611 ± 448 µs. Here, the errors are ±1 standard error of the mean. SRmin is the minimum slew rate to induce PNS at any gradient amplitude (28), and is equal to the slope of the PNS threshold line. ΔGmin is the minimum peak-to-peak swing in the gradient amplitude to induce PNS, and τc is the ratio between ΔGmin and SRmin. ΔGmin and τc correspond to twice the magnitudes of the y- and x-axis intercepts of the PNS threshold line. The definitions of ΔGmin, SRmin, τc are consistent with early publications on gradient-induced PNS (6,7,28). The relatively large errors are due to the limited number of PNS data points, which were concentrated on rise-times that were close to one another. Note that in drawing the PNS threshold curve by connecting only two points in the graph, we assumed previously reported linear behavior of PNS thresholds when expressed in the Δt-ΔG coordinates (28).
The PNS thresholds for the Y coils were much higher than the X coils (Fig. 4a). All volunteer subjects, except for one, were free from PNS during each (HG1 and HG2) Y-coil test (Table 2). Lack of sufficient data points prevented determination of the PNS parameters for the Y-coils. From our results it appears that the PNS thresholds are beyond the hardware limit for the Y-coils.
Figure 4.
PNS thresholds of the Y (a) and Z (b) gradient coils. Solid and broken lines are the same as in Fig 3.
The Z-gradient PNS measurement was somewhat confounded by gradient vibration felt by the subjects when their shoulders touched the gradient coil. In our setup, most volunteers had light contact between their clothing and the front cover of the head-only gradient coil, where vibration could be transmitted from the coil. At the same time, unlike in the X- and Y-coils, all suspected PNS sensations for the Z-coil occurred in the shoulder/back area. In an attempt to separate vibration from PNS, we asked the volunteers who reported sensation to try temporarily to slightly gap their shoulders away from contact with the gradient coil while measurement was repeated. After sufficient shoulder displacement was achieved to avoid contact with the gradient coil, a sensation that persisted regardless of the shoulder position was deemed to originate from PNS. Six out of the 14 volunteers tested for the Z-coil PNS in HG2 reported sensation that was believed to be caused by nerve stimulation. The corresponding thresholds are plotted in Fig. 4(b). We note that despite our efforts there were multiple cases where the sensation was not easily distinguishable from vibration. If we assume that the 6 volunteers mentioned were reporting true PNS, the thresholds for the Z-coil appear to fall between those of the X- and Y-gradient coils. In no case was there a sensation that was described as uncomfortable during the Z-coil test.
Figures 3, 4 also compare our data with the PNS limit of a representative whole-body gradient coil. The straight, black dotted line represents the published PNS limit of the most sensitive (Y) axis of the BRM gradient coil (GE Healthcare), reported by Zhang et al (7). Details of the test conditions are described in (7). The PNS parameters of the BRM gradient coil are: SRmin = 59.5 T/m/s, ΔGmin = 20.9 mT/m, τc= 351 µs. We note that SRmin for HG1x and HG2x are larger than this coil by a factor of 3.0 and 2.7, respectively. ΔGmin, on the other hand, is larger by factors of 3.9 (HG1x over BRM) and 4.7 (HG2x over BRM).
PNS locations and severity
The locations of volunteer-reported sensation differed between the transverse (X, Y) coils and the Z-coil. For the X- and Y-coils (for both HG1 and HG2), the sensations were experienced on the face, consistent with those reported by Chronik et al. (4). However, all the sensations for the Z-coil (HG2 only) were in the shoulder/back area. The facial PNS locations were: bridge of the nose (N =5), top of head, sinuses (N =6), as well as upper teeth, cheek, eyelids and eyebrows, and forehead. For the Z-coil, sensations (reported as distinct from vibration) were at the level of the clavicle, upper back, and back of the shoulders. Usually, initial sensation was only reported on the left or right side, but not bilaterally.
Because the subjects reported the onset of the PNS as the pulse amplitude incremented gradually, at which point the increment stopped, it was not expected that any subject would experience a painful sensation. While no subjects experienced painful PNS, one subject reported the onset of PNS in the sinus area as "uncomfortable" during the HG2 X-coil test at the shortest rise-time (120 µs). Of note is that the transition from no sensation to uncomfortable sensation occurred with a small change in the pulse amplitude, from 82.6 mT/m to 85 mT/m. While this was an isolated observation, we feel that the possibility of rapid escalation of facial nerve sensation as a function of the pulse amplitude must be considered in the operation of localized head gradient coils.
Tilted head PNS
Figure 5 shows how the PNS thresholds varied with differing orientations of the head with respect to the gradient direction in the axial plane. The highest PNS thresholds were observed when the A/P direction of the head was roughly aligned with the gradient direction (θ= 10°), which is analogous to the case when a Y gradient is applied to the head in the usual face-up orientation. As θ increased to 44° the thresholds decreased significantly. Further reduction in the thresholds were observed as the head was tilted further away from the gradient, at θ = 75°. This result, although limited to a single subject, strongly suggests that the gradient direction with the highest PNS thresholds is determined by the orientation of the head, and not the hardware environment specific to our scanner or the gradient coil design, as expected because the X- and Y-gradients shared a very similar electromagnetic design and were manufactured using the same processes. It also suggests that the shoulders play little role in determining the most PNS-sensitive gradient field direction in the axial plane.
Correlation with the head size and nose/glabella position
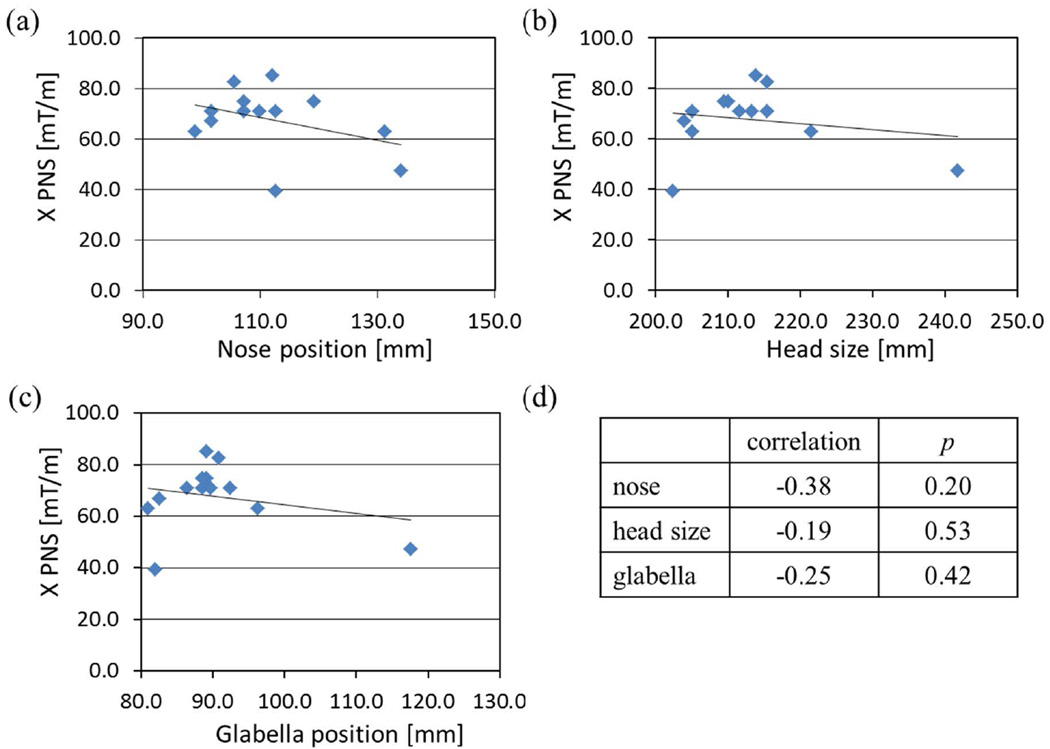
Figure 6 shows the X gradient PNS thresholds as a function of the nose position, head size, and the glabella position in the A/P direction. A positive position corresponds to vertically higher location in the scanner bore. The straight lines correspond to linear fits to the data. As shown in Fig. 6(d), the Pearson correlation coefficients were negative, but all correlations were statistically non-significant, with the nose position being the most significant at P = 0.20. Non-significant correlation between simple anatomical measurements and the PNS thresholds has also been observed in a clinical whole-body gradient coil (29).
Figure 6.
Correlation of the X-gradient PNS threshold and nose position (a), head size (b), and glabella position (c) in the sagittal mid-plane. Data from 13 volunteers who reported PNS with HG2 X-gradient coil were used. Pearson correlation coefficients and the null-hypothesis P values are listed in (d).
EPI imaging
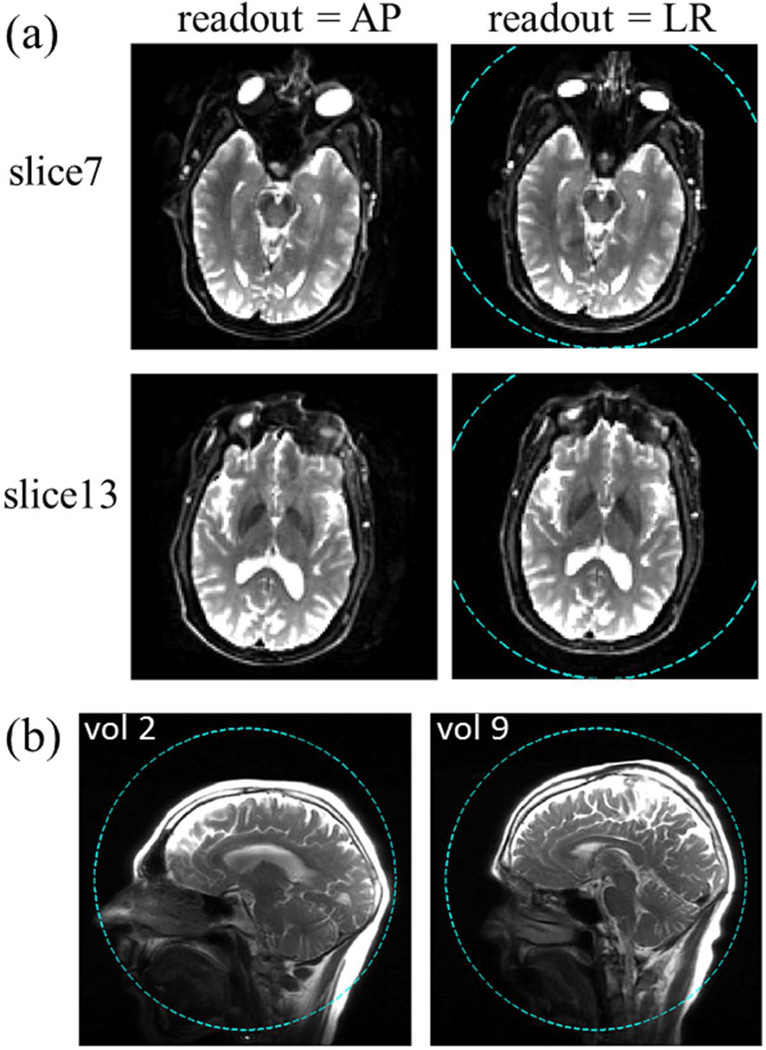
Figure 7(a) shows representative axial EPI images. Dashed circles in Fig. 7(a,b) indicate the 26 cm DSV, showing typical head positions in the axial and sagittal planes with respect to the isocenter. Due to a relatively short echo spacing (372 µs), the image distortion due to susceptibility-induced B0 inhomogeneity near the sinus is reduced compared to that typical in EPI images obtained with conventional whole-body gradient coils (30). Note that in the latter, the typical echo spacing is 600–700 µs. Comparing the two columns of Fig. 7(a), we find that except for the immediate vicinity of the sinus region (including the eyes) the images obtained with different readout directions are very comparable. Because the dominant spatial errors in EPI manifest in the blip phase encoding direction, the relative similarity between the brain images with different readout directions indicates that the magnitude of the pixel shift is only moderate. Consistent with the higher PNS thresholds of the Y gradient coil, there were no reported cases of PNS when the EPI scan was acquired with readout in the A/P direction. This suggests that the A/P gradient coil of an asymmetric head-only gradient coil set can potentially be used for PNS-free, fast EPI readout in clinical scans.
Figure 7.
(a) Axial EPI images of a volunteer with different readout directions. Two representative slices are shown with thickness = 3.2 mm, acquired with a slew rate of 500 T/m/s and gradient amplitude of 46 mT/m in the readout portion of the sequence. Note the relatively mild distortion around the sinus area compared to typical single-shot EPI images acquired with standard whole-body gradients. (b) Sagittal localizer images of two volunteers with a short (left) and a long (right) neck. In both (a) and (b), dashed-line circle shows the 26 cm DSV linearity region.
Discussion
A main goal of the present study was to investigate the degree to which human peripheral nerve stimulation limits the performance of a compact neuroimaging gradient coil, which is capable of high gradient amplitude and slew rate due to the small size, but has a sufficiently large inside diameter so that it can be easily used with a high-channel-count receiver array. Imaging with a receiver phased-array is essential to implement modern brain scan protocols that are very demanding of SNR, parallel imaging acceleration, and simultaneous multi-slice (i.e., multiband) imaging. Given the importance of highly parallelized image acquisition, we decided on the inner diameter of 42 cm in order to accommodate currently available state-of-the-art receive-only arrays as well as future development in receiver coil technology (such as >64 channel coils and those with local B0 shim capability (31)). Compared to previous works reporting enhanced PNS thresholds of localized gradient coils (4,7,32), our work focused on PNS characteristics of head-only gradient coils that were designed to compete favorably with a modern clinical whole-body gradient coil in all aspects of clinical high-performance neuroimaging requirements, such as whole-head coverage, torque and force balancing, and high image SNR, in addition to high gradient amplitude and slew rate. Our results showed that the previously reported PNS advantage of a compact head gradient coil compared to a whole-body gradient coil (4,7,32) extends well into a coil with a sufficient inner diameter (42 cm) to accommodate both an RF transmit coil and a commercial 32-channel phased-array, receive-only head coil. This allowed utilization of a large portion of the enhanced gradient performance parameter space (in terms of the amplitude and slew rate) of a compact gradient coil to accomplish high-SNR, low-distortion EPI images in volunteer subject scans. All our experimental results were obtained with the gradient coil prototypes connected to a standard gradient amplifier and cooling cabinet, with no additional facility power requirements. Therefore, our work suggests that an asymmetric head-only gradient coil with an appropriately-chosen bore size lends itself well as a practical platform for pushing the gradient performance limits in a way that substantially avoids PNS concerns.
Another goal of the present work was to report the observation of a large difference in the PNS thresholds between the anterior-posterior (Y) and the left-right (X) gradient coils of a head-only gradient coil set. A similar observation was made recently by Goodrich et al. (32) in a smaller study with a single rise-time and 10 volunteers. In our setup, the Y gradient field of HG2 appeared to have an average PNS threshold of at least 85 mT/m (zero-to-peak) at a rise-time of 120 µs (corresponding to 708 T/m/s). The average thresholds for the X gradient coil (>60 mT/m at 120 µs), while lower than those of the Y-coil, were still much higher than a typical whole-body gradient coil. We found that the Y-coil thresholds were consistently higher than the X-coil for the two generations of head-only gradient coils (HG1 and HG2) tested, which had different electromagnetic designs. In comparison, most previous works on the PNS of a compact head gradient coil (4,7,33) focused on PNS parameters for the case when the X, Y gradient fields were applied simultaneously, which allowed access to larger combined field amplitudes.
The PNS threshold parameters SRmin, ΔGmin, τc reported here have relatively large uncertainties for two main reasons. First, even for the (most sensitive) X-coils the mean threshold lines passed near the corner of the hardware limit, failing to stimulate many volunteers at rise-times away from the corner point. Second, the number of distinct rise-times used in the test was small due to the scan time limitation. For HG2, for example, the nominal test duration was approximately (4 sec repetition + 10 sec pause) × (16 amplitudes) × (4 rise-times) × (3 axes) = 45 min. Volunteer setup in the magnet and head imaging (with frequent patient communications) added 10 to 15 minutes to this time. Reducing the pause time, which should be possible for a well-cooled coil like HG2, would be a straightforward way to add more rise-times in the protocol. Faster progression in the amplitude sweep would also allow finer resolution in the PNS threshold amplitude determination.
Because many commonly-used, but highly demanding applications in terms of slew rate and PNS, such as axial EPI, concentrate the gradient activity on one axis at a time, we think the independent axis evaluation method described here will be useful for pulse sequence and protocol design. This is especially true in light of the dramatic degree of difference we observed between the PNS produced by X- and Y-coils. We fully acknowledge that other applications, such as spiral imaging, distribute the gradient load more evenly across two or more axes. In those cases a combined-axes measurement may be more appropriate, and this is a limitation of the current study. In the case of simultaneous X- and Y-gradient activity, we speculate from the results presented here that PNS will be dominated by the activity on the X-axis.
The observed difference between the X- and Y-gradient coil PNS limits can be compared with that of a whole-body gradient coil. In the latter, the Y-gradient coil is typically more prone to induce stimulation in the torso than X (32,34,35). This is attributed to the larger cross section in the coronal plane of the torso of a patient in the supine position (34,36), allowing larger induced current perpendicular to the Y-axis. The same reasoning applies to a head-only gradient coil; the larger cross section of the head in the sagittal plane compared to the coronal plane contributes to the observed lower PNS thresholds of the X-gradient coil (36).
Similar to a previous report on the PNS of a whole-body gradient coil (29), we did not find significant correlation between individual volunteers' PNS thresholds and simple anatomical parameters such as the head size. In our work we also did not observe any visual stimulation (2) induced by the pulsed gradient fields. A limitation of the present work is that no attempt was made to find a head position to maximize the PNS, or to systematically study the PNS thresholds as a function of the head position. Whereas the threshold values will likely depend on the exact location of the head in the gradient coil, a brief survey of three volunteers with varying neck lengths found relatively minor changes in the thresholds for the X- and Y-gradient coils as their head position was varied in the S/I direction by about 3 cm. More detailed investigation of potential factors that determine the PNS thresholds for a compact head gradient coil remains as a subject of future studies. A recently reported coupled electromagnetic and neuronal dynamics simulation tool (37) may be useful for numerical studies aimed at better understanding of the nature of nerve stimulation in the face (33).
In conclusion, we have demonstrated that a compact, head-only gradient coil set can achieve PNS thresholds that are much higher than a typical whole-body gradient coil, and that such a design can be used for fast EPI imaging of a human brain with a commercial, high-SNR receiver array with reduced patient safety and discomfort concerns. We observed that the PNS thresholds strongly depended on the orientation of the gradient field. The observed dependence on the physical axis can inform pulse sequence and protocol design to minimize the PNS. Systematic studies of the image quality benefits of the demonstrated head-only gradient coil for the common clinical fast imaging sequences will be a focus of our future research.
Acknowledgement
This work was supported in part by the Grant 5R01EB010065 from the National Institutes of Health. We thank Paul Weavers, Shengzhen Tao, Josh Trzasko, and Erin Gray for their support of this project. Seung-Kyun Lee thanks Brian Rutt, Ph.D. of Stanford University for helpful discussions on the theory of PNS.
References
- 1.Schaefer DJ, Bourland JD, Nyenhuis JA. Review of patient safety in time-varying gradient fields. J Magn Reson Imaging. 2000;12:20–29. doi: 10.1002/1522-2586(200007)12:1<20::aid-jmri3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, Keil B, Tisdall MD, Hoecht P, Dietz P, Cauley SF, Tountcheva V, Matschl V, Lenz VH, Heberlein K, Potthast A, Thein H, Van Horn J, Toga A, Schmitt F, Lehne D, Rosen BR, Wedeen V, Wald LL. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage. 2013;80:220–233. doi: 10.1016/j.neuroimage.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronik BA, Alejski A, Rutt BK. Design and fabrication of a three-axis edge ROU head and neck gradient coil. Magn Reson Med. 2000;44:955–963. doi: 10.1002/1522-2594(200012)44:6<955::aid-mrm18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Chronik BA, Rutt BK. A comparison between human magnetostimulation thresholds in whole-body and head/neck gradient coils. Magn Reson Med. 2001;46:386–394. doi: 10.1002/mrm.1202. [DOI] [PubMed] [Google Scholar]
- 5.Aksel B, Marinelli L, Collick BD, Von Morze C, Bottomley PA, Hardy CJ. Local planar gradients with order-of-magnitude strength and speed advantage. Magn Reson Med. 2007;58:134–143. doi: 10.1002/mrm.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman RE, Hardy CJ, Aksel B, Schenck J, Chronik BA. Experimental determination of human peripheral nerve stimulation thresholds in a 3-axis planar gradient system. Magn Reson Med. 2009;62:763–770. doi: 10.1002/mrm.22050. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Yen YF, Chronik BA, McKinnon GC, Schaefer DJ, Rutt BK. Peripheral nerve stimulation properties of head and body gradient coils of various sizes. Magn Reson Med. 2003;50:50–58. doi: 10.1002/mrm.10508. [DOI] [PubMed] [Google Scholar]
- 8.Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- 9.Roemer PB. Transverse gradient coils for imaging the head. 5177442. US patent. 1993
- 10.Abduljalil AM, Aletras AH, Robitaille PM. Torque free asymmetric gradient coils for echo planar imaging. Magn Reson Med. 1994;31:450–453. doi: 10.1002/mrm.1910310415. [DOI] [PubMed] [Google Scholar]
- 11.Alsop DC, Connick TJ. Optimization of torque-balanced asymmetric head gradient coils. Magn Reson Med. 1996;35:875–886. doi: 10.1002/mrm.1910350614. [DOI] [PubMed] [Google Scholar]
- 12.Bowtell R, Peters A. Analytic approach to the design of transverse gradient coils with co-axial return paths. Magn Reson Med. 1999;41:600–608. doi: 10.1002/(sici)1522-2594(199903)41:3<600::aid-mrm24>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Crozier S, Luescher K, Hinds G, Roffmann WU, Doddrell DM. Designs for an asymmetric gradient set and a compact superconducting magnet for neural magnetic resonance imaging. Review of Scientific Instruments. 1999;70:4062–4066. [Google Scholar]
- 14.Meier C, Zwanger M, Feiweier T, Porter D. Concomitant field terms for asymmetric gradient coils: consequences for diffusion, flow, and echo-planar imaging. Magn Reson Med. 2008;60:128–134. doi: 10.1002/mrm.21615. [DOI] [PubMed] [Google Scholar]
- 15.Kimmlingen R, Eberlein E, Gebhardt M, Hartinger B, Ladebeck R, Lazar R, Reese T, Riegler J, Schmitt F, Sorensen GA, Wedeen V, Wald LL. Proceedings of the 12th Annual Meeting of ISMRM. Kyoto, Japan: 2004. An easy to exchange high performance head gradient insert for a 3T whole body MRI system: First results; p. 1630. [Google Scholar]
- 16.Cohen-Adad J, McNab JA, Benner T, Descoteaux M, Mareyam A, Wedeen V, Wald LL. Proceedings of the 18th Annual Meeting of ISMRM. Stockholm, Sweden: 2010. Improving high-resolution Q-Ball imaging with a head insert gradient: Bootstrap and SNR analysis; p. 1606. [Google Scholar]
- 17.Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele PF, Garwood M, Ugurbil K. 9.4T human MRI: preliminary results. Magn Reson Med. 2006;56:1274–1282. doi: 10.1002/mrm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadzynski GL, Pohmann R, Shajan G, Kolb R, Bisdas S, Klose U, Scheffler K. In vivo proton magnetic resonance spectroscopic imaging of the healthy human brain at 9.4 T: initial experience. MAGMA. 2015;28:239–249. doi: 10.1007/s10334-014-0460-5. [DOI] [PubMed] [Google Scholar]
- 19.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS one. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert KM, Gati JS, Kho E, Klassen LM, Zeman P, Menon RS. Proceedings of the 23rd Annual Meeting of ISMRM. Toronto, Canada: 2015. A parallel-transmit, parallel-receive coil for routine scanning on a 7T head-only scanner; p. 623. [Google Scholar]
- 21.Shajan G, Kozlov M, Hoffmann J, Turner R, Scheffler K, Pohmann R. A 16-channel dual-row transmit array in combination with a 31-element receive array for human brain imaging at 9.4 T. Magn Reson Med. 2014;71:870–879. doi: 10.1002/mrm.24726. [DOI] [PubMed] [Google Scholar]
- 22.Lee S-K, Mathieu J-B, Piel JE, Hardy CJ, Schenck JF, Tan ET, Budesheim E, Fiveland E, Park K, Rohling K, Hua Y, Lin J, Bernstein MA, Huston J, III, Shu Y, Foo TK-F. Proceedings of the 22nd Annual Meeting of ISMRM. Milan, Italy: 2014. Brain imaging with a Dedicated Asymmetric Head-only Gradient Coil without Peripheral Nerve Stimulation at 500 T/m/s; p. 310. [Google Scholar]
- 23.Mathieu J-B, Lee S-K, Budesheim EG, Hua Y, Lin J, Immer C, Lechner-Greite S, Piel J, Schenck JF, Bernstein MA, Foo TK-F. Proceedings of the 21st Annual Meeting of ISMRM. Salt Lake City, UT, USA: 2013. Preliminary Evaluation of a High Performance Gradient Coil for 3T Head Specialty Scanner; p. 2708. [Google Scholar]
- 24.Mathieu J-B, Lee S-K, Graziani D, Lin J, Budesheim E, Piel J, Thiagarajan N, Hardy CJ, Schenck JF, Tan ET, Fiveland E, Park K, Hua Y, Bernstein MA, Huston J, III, Shu Y, Foo TK-F. Proceedings of the 23rd Annual Meeting of ISMRM. Toronto, Canada: 2015. Development of a Dedicated Asymmetric Head-only Gradient Coil for High-Performance Brain Imaging with a High PNS Threshold; p. 1019. [Google Scholar]
- 25.Lechner-Greite S, Mathieu J-B, Lee S-K, Amm BC, Foo TK-F, Schenck J, Bernstein MA, Huston J., III . Proceedings of the 21st Annual Meeting of ISMRM. Salt Lake City, UT, USA: 2013. AC resistance predictions vs experimentally measured values for a high performance head gradient coil; p. 2711. [Google Scholar]
- 26.Wade TP, A A, J B, D T, Rutt BK, McKenzie CA. Proceedings of the 23th Annual Meeting of ISMRM. Toronto, Canada: 2015. Thermal characterization of an all hollow copper insertable head gradient coil; p. 1021. [Google Scholar]
- 27.Bevington PR, Robinson DK. Data reduction and error analysis for the physical sciences. Boston: McGraw-Hill; 1992. [Google Scholar]
- 28.Chronik BA, Rutt BK. Simple linear formulation for magnetostimulation specific to MRI gradient coils. Magn Reson Med. 2001;45:916–919. doi: 10.1002/mrm.1121. [DOI] [PubMed] [Google Scholar]
- 29.Chronik BA, Ramachandran M. Simple anatomical measurements do not correlate significantly to individual peripheral nerve stimulation thresholds as measured in MRI gradient coils. J Magn Reson Imaging. 2003;17:716–721. doi: 10.1002/jmri.10300. [DOI] [PubMed] [Google Scholar]
- 30.Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage. 2010;50:175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truong TK, Darnell D, Song AW. Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain. NeuroImage. 2014;103:235–240. doi: 10.1016/j.neuroimage.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodrich KC, Hadley JR, Kim S-E, Kaggie JD, Handler WB, Chronik BA, Bolster BD, Jr, Parker DL. Peripheral nerve stimulation measures in a composite gradient system. Concepts in Magnetic Resonance Part B. 2015;44B:66–74. [Google Scholar]
- 33.Feldman RE, Odegaard J, Handler WB, Chronik BA. Simulation of head-gradient-coil induced electric fields in a human model. Magn Reson Med. 2012;68:1973–1982. doi: 10.1002/mrm.24188. [DOI] [PubMed] [Google Scholar]
- 34.IEC. IEC 60601-2-33: International Electrotechnical Commission. 2013. Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. [Google Scholar]
- 35.Schaefer DJ, Bourland JD, Nyenhuis JA, S FK, F WW, A GL, E RM. Proceedings of the 2nd Annual Meeting of SMRM. San Francisco, CA, USA: 1994. Determination of gradient-induced, human peripheral nerve stimulation thresholds for trapezoidal pulse trains; p. 101. [Google Scholar]
- 36.Schmitt F, Irnich W, Fischer H. Physiological Side Effects of Fast Gradient Switching. In: Schmitt F, Stehling MK, Turner R, editors. Echo-planar imaging: theory, technique, and application. 1 ed. Heidelberg: Springer; 1998. pp. 212–213. [Google Scholar]
- 37.Oikonomidis IV, Neufeld E, Wolf J, Sharma D, Hamnerius Y, Kuster N. Proceedings of the 22th Annual Meeting of ISMRM. Milan, Italy: 2014. Coupled Electromagnetic and Neuronal Dynamics Simulation of Gradient Coil Switching Induced Nerve Stimulation; p. 1388. [Google Scholar]