Abstract
Background
Prescription drugs are a central component of healthcare worldwide. We investigated changes in drug-prescribing patterns over time in the general population.
Methods
Secular trends were analyzed using prescription 1999-2012 data from The Health Improvement Network . Prevalence of receipt of medication prescriptions was computed by age, sex, and therapeutic category for each calendar year. Spearman correlations were computed to assess change over time.
Results
Between 1999 and 2012, the percentage of the population that received at least one medication prescription increased from 64.5% to 69.2% (rho = 0.96, P < 0.001). The percentage of patients receiving prescriptions for one to four unique agents declined from 45.6% to 42.1% (Spearman's rho = −0.98, P < 0.001). Meanwhile, the percentage receiving five to nine and ten or more unique agents increased from 14.1% to 17.5% (rho = 0.996, P < 0.001) and 4.7% to 9.6% (rho = 1.000, P < 0.001) respectively. Largest increases were seen in use of drugs for gastrointestinal disease among women and cardiovascular disease among men. In 2012, the most commonly used agents were for infection or nervous system drugs, with 32.0% and 28.9% of patients receiving at least one prescription, respectively.
Conclusions
Nearly 70% of the United Kingdom population has received prescriptions for one or more medication with increasing proportions receiving prescriptions for 5 or more. The high rates of medication use increase the complexity and cost of healthcare. These data can be used for public health planning and to design pharmacoepidemiology and comparative effectiveness studies.
Introduction
Prescription drugs are a central component of healthcare worldwide, particularly in the developed world1-3. In the United Kingdom, the annual number of prescriptions dispensed has increased 65% from approximately 653 million in 1999 (11 per person) to 1,074 million in 2009 (17.4 per person)4,5. Increased usage has resulted in increased spending on prescription drugs, which reached £10.5 billion in 2009 or approximately 7.59% of total UK expenditures on health and personal social services5. At an individual level, the prevalence of multiple drug use has also increased over the past decade6. This increase could be due to an increase in the number of distinct medications that are available or increased utilization of previously available drugs. However, up to this point, there have been few population-level studies describing the comprehensive epidemiology of drug-prescribing patterns. Most studies have elected to focus on specific classes of drugs7-10, while fewer have characterized issuances from the entire pharmacopeia11,12.
In the United Kingdom, the general practitioner (GP) plays a central role in the coordination of health services and management of long-term prescribing. This, in tandem with the advent of electronic prescribing and large GP databases, means that there are considerable data on prescribing in primary care. The Health Improvement Network (THIN) is a non-interventional, retrospective database of primary care medical records from the United Kingdom. The THIN population is representative of the UK population13. The completeness and accuracy of THIN data have been validated for epidemiological research13,14. Using these data, we sought to investigate changes in drug-prescribing patterns over time in the UK population.
Methods
Study Population
THIN contains the anonymized electronic medical records of over 11 million patients, of which ~3.7 million patients are actively registered in any given year. THIN extracts data from the electronic medical records of over 500 practices covering nearly 6% of the UK population. All participating practices use the Vision general practice computer system. In total, more than 75 million patient-years of data are included in THIN. The demographics of patients in THIN are generally similar to the general population of the United Kingdom. THIN practices include 8.9% of the population in the south of England, 4.6% of London, 4.8% of the east and Midlands, 4.2% of the north of England, 8.5% of Scotland, 7.4% of Wales, and 6.6% of Northern Ireland15.
We conducted a secular trend study describing the prevalence of prescription receipts using THIN data from January 1999 to December 2012. The source population included all individuals with valid records in a THIN practice. Registration status was determined on July 1 of each year. Practices were included in the study for each year that the practice was both using the Vision software and had recorded a minimum of 1,000 prescription records over the course of the calendar year. These criteria helped to assure complete recording of GP prescriptions. Patients with invalid records using THIN data quality metrics (e.g. not permanently registered; out of sequence year of birth or registration date; missing or invalid transfer out date; year of birth missing or invalid; missing sex information) were excluded.
Data on drug prescribing
Within THIN, a new record, along with the date of prescription, is generated with each prescription, including each repeat prescription. Prescriptions are catalogued using Multilex codes issued by First Databank. Drug codes provide detailed information on the drug, dose, and route of administration, and, in most cases, two therapeutic classification codes – British National Formulary (BNF) section heading and Anatomical Therapeutic Chemical (ATC) classification. The BNF section headings comprise a maximum four-level nested classification scheme, whereas the ATC classification system is a maximum five-level nested classification scheme, with the finest distinction identifying the active ingredient. Both classification systems divide drugs into groups according to target organ/system and their therapeutic and chemical characteristics to varying degrees of specificity, making them useful for studying utilization of drugs by purpose and therapeutic properties.
A small minority of medications lack an ATC classification altogether. For the purpose of this study, each unique medication was assigned to a class consisting of a concatenated combination of the first-listed BNF code and ATC code matched by Multilex code. If the BNF code was 00.00.00.00 (unknown) or otherwise missing, the record was excluded from the analysis. Medications did not require an ATC code for inclusion.
We excluded prescription records for miscellaneous devices including appliances, bandages, dressings, garments, and surgical equipment using BNF and ATC codes (see Appendix). We also excluded items such as antiseptics, emollients, sunscreens, supplements, and vitamins. Vaccines were also excluded, as they do not typically require prescription forms and are directly administered during routine care, creating discrepancies between records within THIN and NHS dispensing data11.
Statistical analysis
Period prevalence was measured over 1-year intervals and reported as percentages (numerator was the number of people in strata who received a prescription (i.e., exposed); denominator was the total number of people in strata). Exposure was defined as having received one or more prescriptions from a GP for a unique medication within the calendar year. Using the mid-year estimate of the THIN population of eligible patients, annual prevalence of receipt of at least one medication prescription and exposure to specific therapeutic categories listed by BNF chapters 1 – 13 were calculated both overall and for age-sex specific strata using four age groups: under age 18, 18-44, 45-64, and 65 and over. Secular trends in prescribing were quantified using Spearman correlation coefficients using all years between 1999 and 2012. To assess for possible over-estimation of prescribing prevalence, in a secondary analysis of the secular trends, we excluded over-the-counter (OTC) preparations among the prescription records. For example, we excluded drugs containing paracetamol (unless in combination with a narcotic), and aspirin (unless in combination with a narcotic, nitrates, or dipyridamol). Because the results of this secondary analysis were nearly identical to the primary analysis, all subsequent analyses employed the methods of the primary analysis, including any medications for which a prescription was written.
Several analyses were conducted to address the number of unique agents being prescribed to patients. First, we estimated the annual prevalence of receipt of one or more prescriptions for different medications using three groupings: one to four medications, five to nine medications, and ten or more medications. Secular trends were similarly quantified using Spearman correlation coefficients. Second, to estimate the extent that patients were being prescribed multiple medications simultaneously, we defined a time window of analysis in which drugs were likely to have been consumed around July 1. We identified prescriptions for unique agents for which July 1 falls between the dates on which the first and last prescriptions were written, or if the date on which the last prescription was written was within 30 days of July 1. Using these prescriptions only, we determined the number of unique medications prescribed to each patient for use on July 1 of each year. Finally, to quantify the spectrum of diseases being treated with prescription medications over the course of a year, we determined how many different therapeutic categories of medications as defined by BNF chapters 1-16 (first level codes) were prescribed to each patient in a year. As with the main analyses, exposure was defined as receipt of one or more prescriptions for a medication within the BNF therapeutic category.
Using all prescription records for the years 2000, 2006, and 2012, we determined the 30 most commonly prescribed medications. The unit of measurement was the number of patients that received at least one prescription of the drug during the study year. New initiation of medications was estimated over three two-year intervals: 1999-2000, 2005-2006, and 2011-2012. New initiation was defined as receipt of a prescription in the later calendar year for a medication not previously prescribed within the past 365 days. The source population was chosen based on the aforementioned inclusion and exclusion criteria; however, for this portion of analysis, to be included in a two-year interval, patients also had to be registered with the same practice both at the beginning (January 1 of the earlier calendar year) and by the end (December 31 of the later calendar year) of the specified timeframe. From this analysis, we determined the 30 most common newly prescribed medications, and the incidence of starting each of these medications was computed for the overall population. Patients who received a prescription for the drug in the prior year were not included in the denominator for the computation of incidence.
All statistical analyses were performed using STATA version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
From the 570 practices that were eligible in THIN, our two inclusion criteria yielded 6206 practice years to analyze. The mid-year number of included practices, the total registered population, and its age-sex composition are shown for each year in Table 1. Consistent with prior research, comparison between the THIN population and the Office for National Statistics mid-year estimates revealed that the elderly, especially those greater than 65 years of age, had a slightly greater representation in THIN than in the total population, while young people under 19 years of age were slightly underrepresented15,16.
Table 1.
Number and proportion of patients in THIN practices for 2000, 2006, 2012
| Percentage of the registered population (%) | Percentage of the registered population (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Eligible Practices | Registered Population | Men 0-18 | Men 19-44 | Men 45-64 | Men 65+ | Women 0-18 | Women 19-44 | Women 45-64 | Women 65+ |
| 2000 | 268 | 2077290 | 10.3 | 18.7 | 13.0 | 7.7 | 9.1 | 18.3 | 12.7 | 10.2 |
| 2006 | 493 | 3701566 | 10.3 | 18.1 | 13.5 | 7.8 | 9.6 | 17.7 | 13.1 | 9.9 |
| 2012 | 500 | 3969150 | 10.2 | 17.3 | 13.3 | 8.5 | 9.8 | 17.3 | 13.2 | 10.3 |
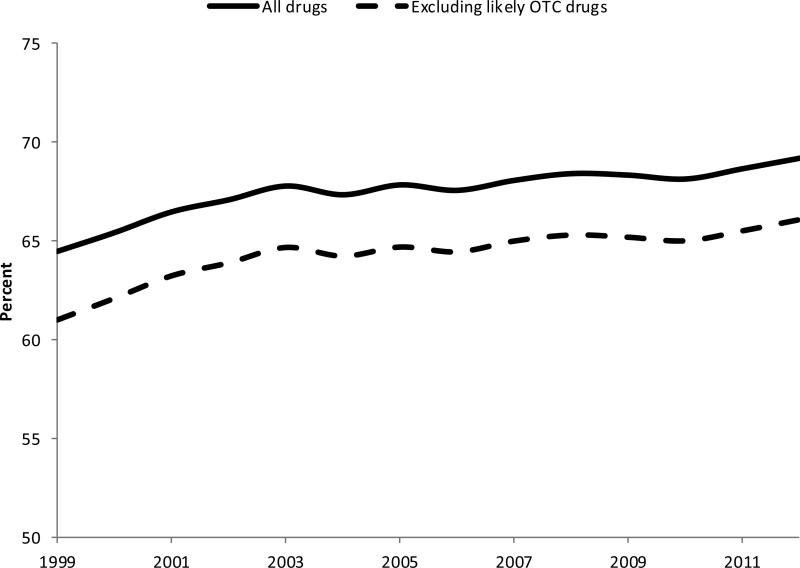
Between 1999 and 2012, the percentage of people in the THIN population having received at least one drug prescription increased from 64.5% to 69.2% (Spearman's rho = 0.96, P < 0.001) (Figure 1). These numbers included patients who received prescriptions for OTC drugs. However, on average, only 4.7% of the prescription-receiving population had received only medications likely to be available OTC, and this was stable over the study period. As such, all drugs were included in subsequent analyses.
Figure 1.
Annual prevalence of receiving a drug prescription: THIN, 1999 - 2012
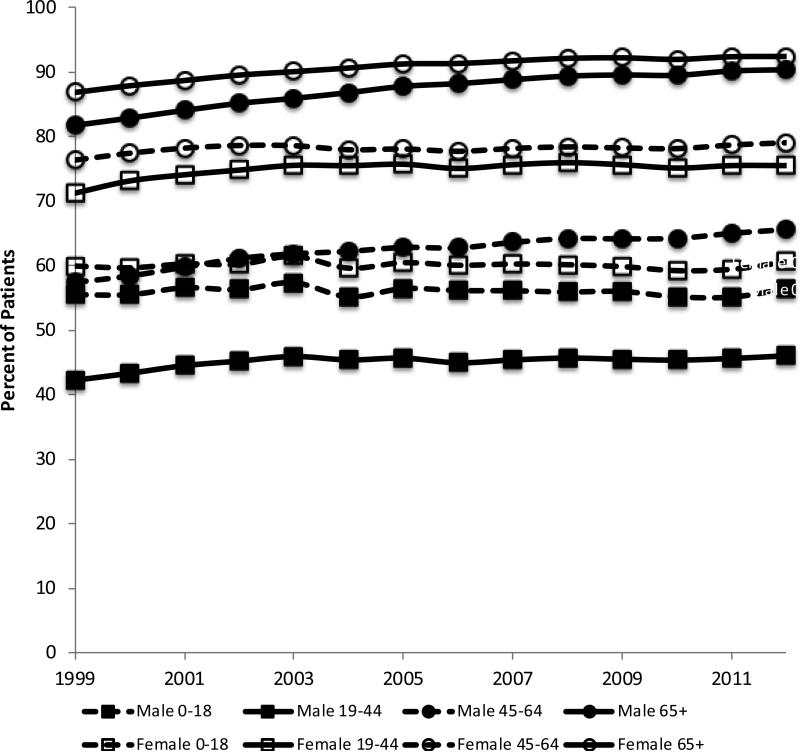
The proportion of patients who received at least one prescription stratified by sex and age is displayed in Figure 2. In general, the prevalence of having been prescribed a medication was higher among older age groups and among women. The largest difference in prescribing patterns between males and females occurred among patients 19 to 44 and 45 to 64 years of age; for instance, women in 2012 were 1.64 times and 1.21 times (determined by the ratio of female prevalence to men prevalence) more likely to be receiving medication than men respectively. With the exception of children, all other age-sex strata exhibited significant increases in the prevalence of receipt of a prescription during the study period; listed in order of magnitude, these are men over the age of 65 (Spearman's rho = 0.996, P < 0.001), men aged 45 to 64 (Spearman's rho = 0.99, P < 0.001), women over the age of 65 (Spearman's rho = 0.99, P< 0.001), men aged 19 to 44 (Spearman's rho = 0.65, P = 0.01), women aged 19 to 44 (Spearman's rho = 0.61, P = 0.02), and women aged 45 to 64 (Spearman's rho = 0.56, P = 0.04).
Figure 2.
Annual prevalence of receiving a drug prescription stratified by sex and age: THIN, 1999 – 2012
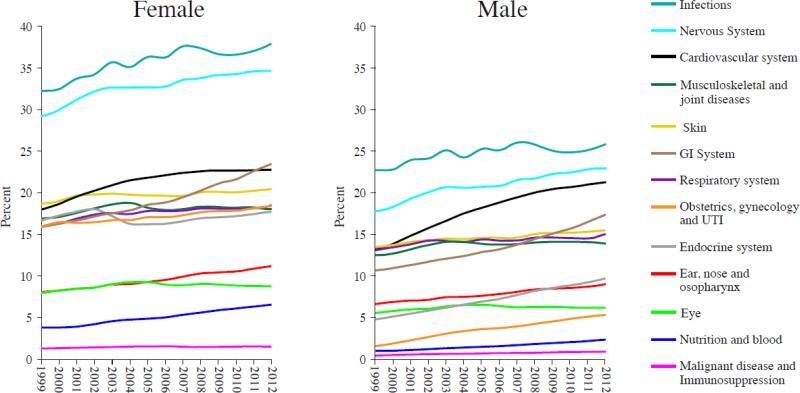
From 1999 to 2012, medications to treat infections and the nervous system were the most commonly prescribed classes of drugs among men and women (Figure 3). In 2012, 32.0% and 28.9% of patients received at least one prescription for an antimicrobial or nervous system drug, respectively. The largest increases were seen in prescribing of drugs for gastrointestinal disease among women and cardiovascular disease among men (Figure 3). Statistically significant increases (Spearman rho across all years, Bonferroni adjusted P < 0.05) were observed for the following categories of drugs among both men and women: GI system, cardiovascular system, respiratory system, nervous system, obstetrics/gynecology and UTI, malignant diseases and immunosuppression, nutrition and blood, ear/nose and osopharynx, and skin; among men only: endocrine system; and among women only: infections. Of particular note, there was a decrease in endocrine system drugs prescribed to women, from 18.0% in 2002 to 16.3% in 2005. Post hoc analyses investigating the types of endocrine medication by most resolved BNF section heading that contributed to this decline confirmed that prescribing of estrogens and HRT to women age 18 year and older, and prescribing of progestogens to women age 45 year and older were among those that exhibited a statistically significant negative trend during the 1999 – 2012 study period. Others included prescribing of drugs affecting gonadotropins to women aged 20 to 64 years, and hypothalamic and anterior pituitary hormones and anti-estrogens to women aged 18 to 44 years (data not shown).
Figure 3.
Annual prevalence of receiving a drug prescription by body system: THIN, 1999 – 2012
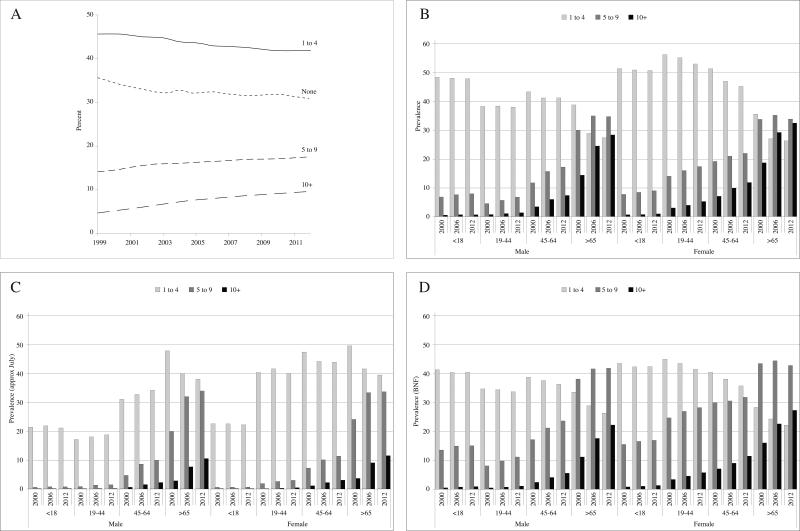
Between 1999 and 2012, the percentage of patients receiving prescriptions for one to four unique agents declined from 45.6% to 42.1% (Spearman's rho = −0.98, P < 0.001) (Figure 4A). Meanwhile, the percentage receiving five to nine and ten or more unique agents has increased from 14.1% to 17.5% (Spearman's rho = 0.996, P < 0.001) and 4.7% to 9.6% (Spearman's rho = 1.000, P < 0.001) respectively. The pattern of increasing number of prescriptions per patient was evident in all age groups in both sexes (10 or more prescriptions, Bonferroni adjusted P < 0.05 for all comparisons; 5-9 prescriptions, Bonferroni adjusted P < 0.05 for all comparisons other than women age 65 or older) (Figure 4B).
Figure 4.
Annual prevalence of receiving a drug prescription stratified by number of different drugs prescribed overall (A), by age and sex (B), on July 1 of each year (C), and by numbers of different medication categories (D): THIN, 1999 – 2012
Analyses of prescriptions on July 1 of each year were similar to those describing annual prescriptions, albeit with lower prevalence. Between 1999 and 2012, the percentage of patients receiving prescriptions for one to four unique agents declined from 33.0% to 32.2% (Spearman's rho = −0.81, P < 0.001), while the percentage receiving five to nine and ten or more unique agents increased from 5.7% to 10.2% (Spearman's rho = 1.000, P < 0.001) and 0.7% to 2.9% (Spearman's rho = 1.000, P < 0.001), respectively. The pattern of increasing number of prescriptions per patient was evident in all age groups in both sexes (Bonferroni adjusted P < 0.05) for 10 or more prescriptions for all comparisons and 5 to 9 prescriptions for all age-sex strata except for males 19 and below (Figure 4C). When comparing Figure 4C to 4B, the most notable feature is the markedly lower prevalence of receiving prescriptions, especially among children, and the disproportionately large prevalence of one to four prescription receivers among people over the age of 65 in the restricted time-window analysis.
Between 1999 and 2012, the percentage of patients receiving prescriptions for one to two BNF categories declined from 38.4% to 35.4% (Spearman's rho = −0.996, P < 0.001). Meanwhile, the percentage receiving prescriptions for three to five and six or more BNF categories has increased from 21.8% to 25.4% (Spearman's rho = 0.987, P < 0.001) and 4.3% to 8.4% (Spearman's rho = 1.000, P < 0.001) respectively. The pattern of increasing number of BNF categories being prescribed was evident in all age groups in both sexes (Bonferroni adjusted P < 0.05) for 6 or more categories for all comparisons and 3 to 5 categories for all age-sex strata except for males and females 19 and below, and women over 65 (Figure 4D).
The 30 most commonly prescribed drugs and newly prescribed drugs, taken either as single- or multiple- component products are listed in Tables 2 and 3, respectively. Anti-infective agents, including amoxicillin (first overall), flucloxacillin, hydrocortisone and antibiotics, clarithromycin, phenoxymethycillin, and erythromycin, were among the most commonly prescribed medications. Twelve drugs have risen to the top 30 in the past decade. Half of them include drugs that lost patent protection during the timeframe, including omeprazole (2002), citalopram (2002), clarithromycin (2004), ramipril (2004), amlodipine (2007), and atorvastatin (2012). Prescriptions for OTC analgesics, such as paracetamol and its multiple-ingredient preparations (fifth and sixth overall respectively), acetylsalicylic acid (seventh overall), and ibuprofen (15th overall) were also frequently issued.
Table 2.
Thirty most commonly prescribed prescription and over-the-counter drugs in 2012
| Rank 2012 | Drug | Rank 2006 | Rank 2000 |
|---|---|---|---|
| 1 | Amoxicillin | 1 | 1 |
| 2 | Salbutamol | 3 | 2 |
| 3 | Omeprazole | 10 | 32 |
| 4 | Simvastatin | 6 | 45 |
| 5 | Paracetamol, combinations excl. psycholeptics | 2 | 3 |
| 6 | Paracetamol | 5 | 4 |
| 7 | Acetylsalicylic acid | 4 | 5 |
| 8 | Flucloxacillin | 9 | 11 |
| 9 | Naproxen | 89 | 58 |
| 10 | Lansoprazole | 17 | 27 |
| 11 | Amlodipine | 21 | 36 |
| 12 | Ramipril | 22 | 78 |
| 13 | Trimethoprim | 15 | 15 |
| 14 | Hydrocortisone and antibiotics | 14 | 12 |
| 15 | Ibuprofen | 11 | 6 |
| 16 | Bendroflumethiazide | 8 | 9 |
| 17 | Levothyroxine sodium | 20 | 26 |
| 18 | Citalopram | 34 | 61 |
| 19 | Beclometasone | 13 | 7 |
| 20 | Prednisolone | 24 | 24 |
| 21 | Amitriptyline | 35 | 33 |
| 22 | Cetirizine | 33 | 43 |
| 23 | Clarithromycin | 55 | 80 |
| 24 | Phenoxymethylpenicillin | 18 | 16 |
| 25 | Atorvastatin | 19 | 74 |
| 26 | Metformin | 37 | 67 |
| 27 | Chloramphenicol | 23 | 18 |
| 28 | Hydrocortisone | 25 | 19 |
| 29 | Tramadol | 40 | 85 |
| 30 | Erythromycin | 16 | 13 |
| ... | ... | ... | ... |
| 32 | Betamethasone | 30 | 25 |
| 34 | Amoxicillin and enzyme inhibitor | 29 | 28 |
| 35 | Furosemide | 26 | 22 |
| 37 | Diclofenac | 7 | 8 |
| 38 | Atenolol | 12 | 14 |
| 40 | Levonorgestrel and ethinlylestradiol | 28 | 17 |
| 42 | Beclometasone | 45 | 29 |
| 43 | Lactulose | 31 | 23 |
| 47 | Alginic acid | 32 | 20 |
| 61 | Loratadine | 52 | 21 |
| 63 | Glyceryl triturate | 41 | 30 |
| 78 | Cefalexin | 27 | 31 |
| 500 | Dextropropoxyphene, combinations excl. psycholeptics | 149 | 10 |
Table 3.
Thirty most commonly newly-prescribed prescription and over-the-counter drugs in 2012
| Rank 2012 | Incidence 2012 | Drug | Rank 2000 | Rank 2006 |
|---|---|---|---|---|
| 1 | 11.9 | Amoxicillin | 1 | 1 |
| 2 | 5.3 | Paracetamol, combinations excl. psycholeptics | 2 | 2 |
| 3 | 5.0 | Flucloxacillin | 4 | 4 |
| 4 | 4.3 | Salbutamol | 10 | 6 |
| 5 | 4.0 | Naproxen | 47 | 64 |
| 6 | 4.1 | Omeprazole | 58 | 13 |
| 7 | 4.0 | Paracetamol | 11 | 5 |
| 8 | 3.8 | Trimethoprim | 9 | 8 |
| 9 | 3.3 | Hydrocortisone and antibiotics | 8 | 9 |
| 10 | 3.1 | Ibuprofen | 3 | 7 |
| 11 | 2.9 | Clarithromycin | 53 | 33 |
| 12 | 2.9 | Phenoxymethylpenicillin | 7 | 11 |
| 13 | 2.4 | Prednisolone | 21 | 19 |
| 14 | 2.3 | Doxycycline | 42 | 34 |
| 15 | 2.3 | Chloramphenicol | 12 | 12 |
| 16 | 2.3 | Amoxicillin and enzyme inhibitor | 14 | 15 |
| 17 | 2.3 | Erythromycin | 6 | 10 |
| 18 | 2.2 | Cetirizine | 36 | 20 |
| 19 | 2.1 | Hydrocortisone | 13 | 17 |
| 20 | 2.0 | Betamethasone | 18 | 18 |
| 21 | 1.8 | Lansoprazole | 27 | 21 |
| 22 | 1.7 | Diclofenac | 5 | 3 |
| 23 | 1.7 | Amitriptyline | 32 | 32 |
| 24 | 1.7 | Beclometasone (respiratory) | 25 | 35 |
| 25 | 1.6 | Diazepam | 31 | 26 |
| 26 | 1.6 | Nitrofurantoin | 135 | 95 |
| 27 | 1.6 | Tramadol | 75 | 29 |
| 28 | 1.5 | Prochlorperazine | 20 | 22 |
| 29 | 1.6 | Beclometasone (ear, nose, osopharynx) | 19 | 23 |
| 30 | 1.4 | Fusidic acid | 28 | 25 |
| ... | ... | ... | ... | |
| 31 | Citalopram | 55 | 30 | |
| 33 | Lactulose | 26 | 27 | |
| 37 | Clotrimazole | 30 | 39 | |
| 39 | Simvastatin | 89 | 14 | |
| 47 | Loratadine | 15 | 40 | |
| 52 | Cefalexin | 17 | 16 | |
| 53 | Alginic acid | 24 | 37 | |
| 56 | Ciprofloxacin | 43 | 28 | |
| 57 | Acetylsalicylic acid | 22 | 24 | |
| 142 | Bendroflumethiazide | 23 | 56 | |
| 211 | Atenolol | 29 | 109 | |
| 646 | Dextropropoxyphene, combinations excl. psycholeptics | 16 | 263 | |
Discussion
From 1999 to 2012 there was a significant increase in the proportion of the population receiving a prescription for at least one medication and the number of unique agents prescribed per person in the UK. The increase in prevalence of being prescribed 5-9 and 10 or more different medications was evident in nearly all age- and sex-specific strata. In contrast, the increase in prescribing of 1 or more medications was only observed among adults. The largest increases were evident for cardiovascular drugs for men and gastrointestinal drugs for women. Thus, the increased number of prescriptions reflects both an increase in the number of patients, with the exception of children, the number of medications per patient, and differences by sex in terms of the type of medications that are prescribed.
To our knowledge, this is the largest primary care-based study to provide comprehensive information on the prescribing of a broad range of medications in the United Kingdom. Most prior efforts were either focused on specific types of drugs, or derived from sales data or reports of dispensed prescriptions. The available prior results are generally consistent with our observations. For instance, a Scottish study of 300,000 patients noted increases in the mean number of drugs dispensed, from 3.3 in 1995 to 4.4 in 2010, and the prevalence of multiple prescribed medications: 1.8-fold and 3.1-fold increases over the same time period in the numbers of patients receiving respectively 5 or more medications (12 percent to 22 percent) and 10 or more medications (1.9 percent to 5.8 percent)6. A cross-sectional analysis of 2006 data from electronic primary care records on 180,815 adult patients permanently registered in 40 nationally representative Scottish GP surgeries showed that 16.9% of adults were receiving four to nine medications and 4.6% were receiving ten or more medications, increasing with age12. Another recent study conducting a detailed examination of prescribing records of 1,777 patients across 15 general practices in England found that 1,200 (67.5%) received at least one prescription; 299 (17%) were receiving 5 and 9 medications, and an additional 172 (9.7%) were receiving more than 10 medications17.
The current study observed marked difference in prescribing patterns by sex, with the exception of those over age 65. Prior studies from multiple countries have similarly observed that women tend to use more prescription medications than men18-23. For example, studies in the United States ambulatory US adult population and pharmacy claims data reflective of the commercially insured population in 1998 and 1999 both revealed that rates of medication use were greater in women than men in every age group with the exception among persons 65 years or older21,23.
For men, the greatest increase was in medications to treat cardiovascular disease, likely due in part to increased attention to management of hypertension and hyperlipidemia. Simvastatin was the fourth most commonly prescribed medication. Medications to treat gastrointestinal diseases were increasingly prescribed to both women and men. Omeprazole, a proton pump inhibitor used to treat acid peptic disorders such as gastrointestinal reflux disease, was the third most commonly prescribed medication. Unlike treatment of hypertension and hypercholesterolemia, which is largely aimed at preventing future complications, use of omeprazole and other proton pump inhibitors is typically to treat or prevent gastrointestinal symptoms. These data highlight the need to determine whether gastrointestinal reflux disease is increasing or merely the use of medications to treat acid peptic disease has increased.
One outlier among the increased prevalence of prescribed medications was the striking decline in use of endocrine medications among women from 2002 to 2005. Our post hoc analysis documented that much of this decline was from reduced prescribing of estrogen and progesterone. This likely was in response to the report of the Women's Health Initiative that estrogen plus progesterone does not reduce the incidence of coronary heart disease in postmenopausal women24.
In parallel to the increase in patients being prescribed 5 or more medications within a year, we observed a decline in those receiving 1-4 medications per year. This suggests that the number of medications prescribed to patients has likely increased more than the number of people being prescribed at least one medication. Similar patterns were seen with increased number therapeutic areas (defined by BNF categories) for which patients received prescriptions. Our finding of increased prescribing of multiple medications over time may represent improved delivery of healthcare to patients with multiple chronic diseases. Alternatively, some of this increase may reflect inappropriate prescribing of medications such as antibiotics. It is unlikely that the increase reflects increased access to healthcare, given that nearly all people in the UK are registered with a general practitioner, or is a consequence of switching within therapeutic areas, as the number of unique therapeutic areas treated per patients increased over time as well.
A common consequence of polypharmacy is the potential for drug-drug interactions and adverse drug events, emphasizing the importance of physician vigilance in assessing the need for continued use of each medication. The large number of patients in THIN who are prescribed multiple medications, makes this an ideal data source in which to study drug-drug interactions.
Key strengths of this study include the availability and reliability of complete medical visit information and the representative population. In the UK, nearly all people receive care from a GP who prescribes nearly all of the patient's medications. Thus, THIN provides a near complete picture of prescribing patterns in the UK.
The study used data on prescriptions written by general practitioners. As such, it was not possible to assess patients’ adherence with the prescribed therapy. Consequently, the patterns of prescriptions that we observed may not reflect patterns of actual drug use. In previous studies, rates of filling prescriptions are relatively high, though rates for individual practices and different medications vary widely12,25,26. Similarly, the correlation of prescriptions written and prescriptions filled is stronger when larger time windows are allowed between these two related events. In addition, certain types of medications are difficult to track in THIN, especially commonly used drugs that can be purchased over-the-counter. Selected medications that are prescribed only by specialists, such as biologic therapies for rheumatologic diseases, are also not captured completely within THIN. Thus, we have likely slightly under estimated the prevalence of medications prescribed. Finally, numerous factors may influence whether a physician prescribes a small or large supply of a medication. We did not distinguish between those medications prescribed for 1 month, 3 months, or other durations. This should not bias our results for medications prescribed in the course of a full year but we may have slightly under-estimated the prevalence of medications prescribed on or around July 1 of each year since we assumed a 30 day supply for all prescriptions in this analysis.
In summary, the last decade has witnessed a significant rise in the prescribing of medications. Nearly 70% of the UK population receives one or more prescriptions for medication with increasing proportions having prescriptions for more than 5 unique medications. The ability to link prescription data with diagnoses and other clinical details in the electronic medical records is a unique strength of primary care databases, such as THIN, providing the basis for future utilization and outcome studies requiring information on individual drugs. These data can help investigators plan such studies.
Key points.
Between 1999 and 2012, secular trend analysis of prescription data in THIN, a UK primary care database, showed increased proportions of patients receiving prescriptions for at least one medication and 5 or more medications.
In 2012, nearly 70% of the UK population received one or more prescription, with 27% receiving 5 or more.
The largest increases were seen in prescription of drugs for gastrointestinal disease among women and cardiovascular disease among men.
Primary care databases, such as THIN, allow linkage of prescription data with medical diagnoses, providing the basis for studies of drug utilization, drug-drug interactions, and comparative effectiveness.
Acknowledgments
Grant Support via NIH:
R25-DK066028 (Zhang F), K08-DK095951 (Scott FI), K24-DK078228 (Lewis JD), K08-DK098272 (Goldberg DS), K12 CA 076931, K23-CA187185 (Mamtani R)
Appendix
The following BNF codes and code stems (where unspecified indicates a prematurely terminating code and EC indicates an undefined code) were used to exclude non-drug prescriptions from patient data: 01.08- (stoma care) 01.09.04.02 (EC) 02.13- (local sclerosants) 03.01.05- (peak flow meters, inhaler devices and nebulizers) 03.04.02- (allergen immunotherapy) 03.06- (oxygen) 03.11- (EC) 06.01.01.03 (hypodermic equipment) 06.01.06.00 (diagnostic and monitoring devices for diabetes mellitus) 07.03.03- (spermicidal contraceptives) 07.03.04- (contraceptive devices) 07.04.04- (bladder instillations and urological surgery) 07.05.04- (EC) 09.02- (fluids and electrolytes) 09.03- (intravenous nutrition) 09.04- (oral nutrition) 09.05- (minerals) 09.06- (vitamins) 09.07- (bitters and tonics) 10.03.00- (unspecified drugs for the relief of soft-tissue inflammation and topical pain relief) 11.09- (contact lenses) 13.01- (management of skin conditions) 13.02- (emollient and barrier preparations) 13.03- (topical local anaesthetic and antipruritics) 13.08- (sunscreens and camouflagers) 13.11- (skin cleansers, antiseptics, and preparations for promotion of wound healing) 13.12- (antiperspirants) 13.13- (topical circulatory preparations) 14.01- (active immunity) 14.02- (passive immunity) 14.03- (storage and use) 14.04- (vaccines and antisera) 14.06- (international travel) 15.00- (unspecified anaesthesia) 15.01.02- (inhalational anaesthetics) 15.01.05 (neuromuscular blocking drugs) 15.01.06- (drugs for reversal of neuromuscular blockade) 15.65.30.05 (EC) 17- (non-medicinal substances) 2- (homeopathic remedies, herbal and anthroposophical remedies, unlicensed products, solutions for in-vitro use) 50- (no information available) 51- (no clinical data available) 8- (EC) 9- (trusses, general disinfection, miscellaneous aids & materials for diagnostic procedures, ingredients).
The following ATC codes and code stems (where unspecified indicates a prematurely terminating code) were used to exclude non-drug prescriptions from patient data: A11- (vitamins) A12- (mineral supplements) D02B A- (protective against UV-radiation for topical use) D09- (medicated dressings) G01A (unspecified antibiotics) J07- (vaccines) V03A N- (medical gases) V04C X- (other diagnostic agents) V06- (general nutrients) V07- (all other non-therapeutic products) V08- (contrast media) V20- (surgical dressings).
The following BNF codes and code stems (where unspecified indicates a prematurely terminating code and EC indicates an undefined code) were used to exclude probable non-prescription or over-the-counter drug issuances: 01.01.01- (antacids and simeticone) 01.01.02- (compound alginates and proprietary indigestion preparations) 01.01.03- (EC – dyspepsia and gastro-oesophageal reflux diseases) 01.02.01- (EC – antispasmodics and other drugs altering gut motility) 01.03.06- (EC – antisecretory drugs and mucosal protectants) 01.04.00- (unspecified acute diarrhea) 01.04.01- (adsorbents and bulk-forming drugs) 01.06.00- (unspecified laxatives) 01.06.01- (bulk-forming laxatives) 01.06.02- (stimulant laxatives) 01.06.03- (faecal softeners) 01.06.04- (osmotic laxatives) 01.06.05- (bowel cleansing preparations) 01.07.03- (rectal sclerosants) 01.09.02- (bile acid sequestrants) 02.12.06- (EC – lipid-regulating drugs) 03.04.01.02 (EC – antihistamines) 03.08- (aromatic inhalations) 09.01.02- (drugs used in megaloblastic anaemias) 09.08.01- (drugs used in metabolic disorders) 09.09- (EC) 09.10- (EC) 09.11- (EC) 09.12- (EC) 10.01.05- (other drugs for rheumatic diseases) 10.03- (drugs for the relief of soft-tissue inflammation and topical pain relief) 11.08.02- (ocular diagnostic and perioperative preparations and photodynamic treatment) 12.01- (drugs acting on the ear) 12.02.00- (unspecified drugs acting on the nose) 12.02.02- (topical nasal congestants) 12.03.01- (drugs for oral ulceration and inflammation) 12.03.03- (lozenges and sprays) 12.03.04- (mouthwashes, gargles, and dentifrices) 12.03.05- (treatment of dry mouth) 13.00- (unspecified skin) 13.05.00- (unspecified preparations for eczema and psoriasis) 13.05.01- (preparations for eczema) 13.09- (shampoos and other preparations for scalp and hair conditions) 13.10.01.00 (antibacterial preparations [for skin conditions]) 13.10.01.01 (antibacterial preparations only used topically) 13.10.05- (preparations for minor cuts and abrasions) 13.10.05.01 (EC – preparations for minor cuts and abrasions) 13.14- (EC) 13.15- (EC) 13.31.20.01 (EC) 14.00- (unspecified immunological products and vaccines) 15.02.08- (EC – anaesthesia) 16.02- (treatment for paracetamol poisoning).
The following ATC codes and code stems (where unspecified indicates a prematurely terminating code) were used to exclude probable non-prescription or over-the-counter drug issuances: A01A D- (other agents for local oral treatment) A02A- (antacids) A02B (unspecified drugs for treatment of peptic ulcer) A02B X- (other drugs for treatment of peptic ulcer) A02E A- (antiregurgitants) A02X- (other antacids, drugs for treatment of peptic ulcer and flatulence) A03 (unspecified antispasmodic and anticholinergic agents and propulsives) A03A A- (synthetic anticholinergics, esters with tertiary amino group) A03A X- (other drugs for functional gastrointestinal disorders) A05A X- (other drugs for bile therapy) A06 (unspecified drugs for constipation) A06A (unspecified drugs for constipation) A06A A- (softeners, emollients) A06A B- (contact laxatives) A06A C- (bulk-forming laxatives) A06A D- (osmotically acting laxatives) A06A G- (enemas) A06A X- (other drugs for constipation) A07B- (intestinal adsorbents) A07D- (antipropulsives) A07X- (other antidiarrheals) A09A B- (acid preparations) A16A A- (amino acids and derivatives) B05B A- (solutions for parenteral nutrition) C05A D- (local anesthetics) C05A X- (other agents for treatment of hemorrhoids and anal fissures for topical use) C05B A- (heparins or heparinoids for topical use) C10A (unspecified lipid modifying agents, plain) D01A (unspecified antifungals for topical use) D02- (emollients and protectives) D03 (unspecified preparations for treatment of wounds and ulcers) D03A- (cicatrizants) D04A- (antipruritics, incl. antihistamines, anesthetics, etc.) D05A (unspecified antipsoriatics for topical use) D05A A- (tars) D07A A- (corticosteroids, weak [group i]) D08A G- (iodine products) D11A X- (other dermatologicals) M02A- (topical products for joint and muscular pain) M03B A- (carbamic acid esters) N07B A- (drugs used in nicotine dependence) P03- (ectoparasiticides, incl. scabicides, insecticides and repellents) R01A (unspecified decongestants and other nasal preparations for topical use) R02A (unspecified throat preparations) R02A B- (antibiotics) R02A D- (anesthetics, local) R05D (unspecified cough suppressants, excl. combinations with expectorants) R05D A- (opium alkaloids and derivatives) R05F B- (other cough suppressants and expectorants) R05X- (other cold preparations) R06A A- (aminoalkyl ethers) R06A B- (substituted alkylamines) S02D C- (indifferent preparations) V03A F- (detoxifying agents for antineoplastic treatment).
Drugs containing paracetamol (unless in combination with a narcotic), and aspirin (unless in combination with a narcotic, nitrates, or dipyridamol) were excluded using the following ATC codes (any issuances for paracetamol or aspirin lacking an ATC code were kept): N02B E01 (paracetamol) M01A E51 (ibuprofen, combinations) N02A A58 (dihydrocodeine, combinations) N02A X52 (tramadol and paracetamol) N02B E51 (paracetamol, combinations excluding psycholeptics) N02B E71 (paracetamol, combinations with psycholeptics) N02C (unspecified antimigraine preparations) R01B A51 (phenylpropanolamine, combinations) R01B A52 (pseudoephedrine, combinations) R06A D02 (promethazine) B01A C06 (acetylsalicylic acid) N02B A01 (acetylsalicylic acid) N02B A51 (acetylsalicylic acid, combinations excluding psycholeptics) N02B A71 (acetylsalicylic acid, combinations with psycholeptics).
Footnotes
The authors report no potential conflicts of interest related to this study.
References
- 1.Gu Q, Dillon C, Burt V. Health, United States, 2013: With Special Feature on Prescription Drugs. National Center for Health Statistics; Hyattsville (MD): 2014. Health, United States, 2013: With Special Feature on Prescription Drugs. [PubMed] [Google Scholar]
- 2.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007-2008. NCHS data brief. Sep;2010(42):1–8. [PubMed] [Google Scholar]
- 3.Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy - an individual-based study of the Swedish population 2005-2008. BMC clinical pharmacology. 2010;10:16. doi: 10.1186/1472-6904-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce D, Goldblatt P. United Kingdom Health Statistics. 2000 http://www.ons.gov.uk/ons/rel/ukhs/united-kingdom-health-statistics/2000/index.html.
- 5.Smith M, Sweet D, Holley R. United Kingdom Health Statistics. 2010 http://www.ons.gov.uk/ons/rel/ukhs/united-kingdom-health-statistics/2010/index.html.
- 6.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med. 2015;13(74) doi: 10.1186/s12916-015-0322-7. doi:10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filion KB, Joseph L, Boivin JF, Suissa S, Brophy JM. Trends in the prescription of antidiabetic medications in the United Kingdom: a population-based analysis. Pharmacoepidemiology and drug safety. 2009 Oct;18(10):973–976. doi: 10.1002/pds.1802. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC pediatrics. 2012;12:78. doi: 10.1186/1471-2431-12-78. doi: 10.1186/1471-2431-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. Bmj. 2009;339:b3999. doi: 10.1136/bmj.b3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider-Lindner V, Quach C, Hanley JA, Suissa S. Secular trends of antibacterial prescribing in UK paediatric primary care. The Journal of antimicrobial chemotherapy. 2011 Feb;66(2):424–433. doi: 10.1093/jac/dkq452. [DOI] [PubMed] [Google Scholar]
- 11.The NHS Information Centre, Prescribing and Primary Care Services Prescribing compliance: a review of the proportion of prescriptions dispensed. 2011 http://www.hscic.gov.uk/catalogue/PUB01500/pres-comp-rev-prop-pres-disp-rep.pdf.
- 12.Payne RA, Avery AJ, Duerden M, Saunders CL, Simpson CR, Abel GA. Prevalence of polypharmacy in a Scottish primary care population. European journal of clinical pharmacology. 2014 May;70(5):575–581. doi: 10.1007/s00228-013-1639-9. [DOI] [PubMed] [Google Scholar]
- 13.Bourke A, Dattani H, Robinson M. Feasibility study and methodology to create a quality-evaluated database of primary care data. Informatics in primary care. 2004;12(3):171–177. doi: 10.14236/jhi.v12i3.124. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiology & Drug Safety. 2007 Apr;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 15.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Informatics in primary care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 16.Office for National Statistics Annual Mid-year Population Estimates, 2011 and 2012. 2013 http://www.ons.gov.uk/ons/dcp171778_320900.pdf.
- 17.Avery T, Barber N, Ghaleb M, et al. The PRACtICe Study: PRevalence And Causes of prescrIbing errors in general practiCe. 2012 http://www.gmc-uk.org/Investigating_the_prevalence_and_causes_of_prescribing_errors_in_general_practice___The_PRACtICe_study_Reoprt_May_2012_48605085.pdf.
- 18.Chen YF, Dewey ME, Avery AJ. Self-reported medication use for older people in England and Wales. Journal of clinical pharmacy and therapeutics. 2001 Apr;26(2):129–140. doi: 10.1046/j.1365-2710.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Chrischilles EA, Foley DJ, Wallace RB, et al. Use of medications by persons 65 and over: data from the established populations for epidemiologic studies of the elderly. Journal of gerontology. 1992 Sep;47(5):M137–144. doi: 10.1093/geronj/47.5.m137. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen T, Johansson S, Kennerfalk A, Wallander MA, Svardsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. The Annals of pharmacotherapy. 2001 Sep;35(9):1004–1009. doi: 10.1345/aph.10351. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002 Jan 16;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 22.Loikas D, Wettermark B, von Euler M, Bergman U, Schenck-Gustafsson K. Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. BMJ open. 2013;3(5) doi: 10.1136/bmjopen-2012-002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roe CM, McNamara AM, Motheral BR. Gender- and age-related prescription drug use patterns. The Annals of pharmacotherapy. 2002 Jan;36(1):30–39. doi: 10.1345/aph.1A113. [DOI] [PubMed] [Google Scholar]
- 24.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. The New England journal of medicine. 2003 Aug 7;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 25.Johannesdottir SA, Maegbaek ML, Hansen JG, Lash TL, Pedersen L, Ehrenstein V. Correspondence between general practitioner-reported medication use and timing of prescription dispensation. Clinical epidemiology. 2012;4:13–18. doi: 10.2147/CLEP.S26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mabotuwana T, Warren J, Harrison J, Kenealy T. What can primary care prescribing data tell us about individual adherence to long-term medication?-comparison to pharmacy dispensing data. Pharmacoepidemiology and drug safety. 2009 Oct;18(10):956–964. doi: 10.1002/pds.1803. [DOI] [PubMed] [Google Scholar]