Abstract
Glaucoma challenges the survival of retinal ganglion cell axons in the optic nerve through processes dependent on both aging and ocular pressure. Relevant stressors likely include complex interplay between axons and astrocytes, both in the retina and optic nerve. In the DBA/2J mouse model of pigmentary glaucoma, early progression involves axonopathy characterized by loss of functional transport prior to outright degeneration. Here we describe novel features of early pathogenesis in the DBA/2J nerve. With age the cross-sectional area of the nerve increases; this is associated generally with diminished axon packing density and survival and increased glial coverage of the nerve. However, for nerves with the highest axon density, as the nerve expands mean cross-sectional axon area enlarges as well. This early expansion was marked by disorganized axoplasm and accumulation of hyperphosphorylated neurofilamants indicative of axonopathy. Axon expansion occurs without loss up to a critical threshold for size (about 0.45-0.50 μm2), above which additional expansion tightly correlates with frank loss of axons. As well, early axon expansion prior to degeneration is concurrent with decreased astrocyte ramification with redistribution of processes towards the nerve edge. As axons expand beyond the critical threshold for loss, glial area resumes an even distribution from the center to edge of the nerve. We also found that early axon expansion is accompanied by reduced numbers of mitochondria per unit area in the nerve. Finally, our data indicate that both IOP and nerve expansion are associated with axon enlargement and reduced axon density for aged nerves. Collectively, our data support the hypothesis that diminished bioenergetic resources in conjunction with early nerve and glial remodeling could be a primary inducer of progression of axon pathology in glaucoma.
Keywords: Glaucoma, gliosis, retinal ganglion cell, axonopathy, astrocyte, neurodegeneration
Introduction
Loss of vision in glaucoma is associated with the degeneration of retinal ganglion cell neurons (RGCs) and their axons, which comprise the optic nerve (Quigley, 1999; Whitmore et al., 2005; Nickells, 2007). While age is the greatest risk factor for glaucoma, sensitivity to intraocular pressure (IOP) is the only modifiable risk factor (Tuck and Crick, 1998; Gordon et al., 2002). Nevertheless, neurodegeneration in glaucoma often persists despite clinical management of IOP (Leske et al., 2003). Thus, it is important in deciphering the early events involved in RGC degeneration to discriminate factors associated with IOP from those associated with aging.
Age and IOP combine in different ways to influence the onset and progression of RGC axon degeneration in the optic nerve (Crish et al., 2010; Calkins, 2013). In the nerve head, where RGC axons pass unmyelinated from the retina to the nerve proper, aging stiffens important connective tissues and extracellular matrix, compromises the blood supply, and influences the biochemistry of astrocyte glia that help support axons (reviewed in Hernandez, 2000; Burgoyne, 2011). These factors collectively termed “remodeling” are likely to contribute to normal age-related loss of RGC axons and may increase susceptibility in glaucoma to IOP-related stress (Calkins, 2013).
In terms of key pathogenic events in glaucoma, age is the critical determinant for degradation of anterograde RGC axonal transport from the retina to central brain targets, with IOP serving as an additional stressor (Crish et al., 2010; 2013). We have shown in an inducible model and in the DBA/2J mouse model of hereditary glaucoma that deficits in transport mark a period of functional axonopathy prior to frank degeneration of RGC axons. The DBA2J presents age-dependent elevations in IOP that are normally absent in other mouse strains (Danias et al., 2003; Schuettauf et al., 2004; Zhou et al., 2005; Jakobs et al., 2005; Inman et al., 2006). These elevations arise from closure of the drainage canals in the anterior segment induced by iris atrophy and pigment dispersion caused by mutations in the tyrp1 and gpnmb genes, respectively (John et al., 1998; Chang et al., 1999; Anderson et al., 2002; Howell et al., 2007a). This ocular phenotype presents prominently in a large fraction of animals by 5-8 months of age with phenotypical penetrance of about 50% by 10-11 months (Sheldon et al., 1995; John et al., 1998; Libby et al., 2005a; Scholz et al., 2008). However, a small fraction of animals may demonstrate iris defects and elevated IOP as early as 2-4 months (Inman et al., 2006; Saleh et al., 2007). Conversely, even for older ages, a fraction of animals may retain normal IOPs and RGC and axonal numbers (Schlamp et al., 2006; Inman et al., 2006). Because of this variability across ages, the DBA/2J is a useful model system for probing the differences between age- and IOP-related axonal pathology in the optic nerve.
In the central nervous system, space once occupied by axons that are lost through disease or injury is generally filled by a glial scar, predominantly involving hypertrophic astrocyte processes. So too does reactive astrocyte gliosis contribute to remodeling in the optic nerve, including the DBA2J mouse (Jakobs et al., 2005; Bosco et al., 2008; also this volume; Sun and Jakobs, 2012). Here we ask whether optic nerve remodeling in the DBA2J includes other, perhaps earlier components that might presage overt axon degeneration. We find that with age, the DBA2J optic nerve enlarges, typically coincident with axon loss and increased gliosis. However, before axons are lost, they too expand with loss of cytoskeletal integrity. This pre-degenerative expansion in axon size is accompanied by retraction of astrocyte processes from the inter-axonal space and reduction in local mitochondria.
2. Materials and methods
2.1 Animals
All animal work and experimental procedures were approved by The Vanderbilt University Medical Center Institutional Animal Care and Use Committee. DBA/2J and C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Some DBA/2J mice were bred in a pathogen-free facility and regularly backcrossed with fresh founders from Jackson Laboratories to minimize uncontrolled genetic drift, as described (Inman et al., 2006; Buckingham et al., 2008). In all cases, mice were maintained in a 12h light-dark cycle with standard rodent chow available ad libitum.
A total of 91 DBA/2J mice were used in this study. We measured IOP in a subset of these representing 40 eyes using the Tono-Pen XL from Medtronic Solan (Jacksonville, FL) as described previously (Inman et al., 2006; Crish et al., 2010). Briefly, prior to measurement, the mice were anesthetized (Avertin, 1.3% tribromoethanol, 0.8% tert-amyl alcohol) and proparacaine ophthalmic solution (0.5% proparacaine hydrochloride, Bausch&Lomb, Tampa, FL) was applied topically to the eye. Monthly IOP for each eye was taken as the average of 25-30 Tono-Pen measurements recorded during a session. Previously we demonstrated that IOP measured this way increases nearly monotonically after about 4 months of age until about 10 months so that mean lifetime IOP was an accurate reflection of inter-animal differences across ages (Inman et al, 2006).
2.2 Tissue preparation and histopathology
Mice were perfused with 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB), pH 7.4, and the eyes with the optic nerve attached were carefully dissected from the orbit. A 1-2 mm section of optic nerve proximal (0.3-0.6 mm) to the globe was isolated, post-fixed for 1h in 4% PFA and embedded in Epon resin and semi-thin (light microscopy) and ultra-thin (electron microscopy) cross-sectioned as previously described (Sappington et al., 2003; Inman et al., 2006; Buckingham et al., 2008). Nerve images were obtained using an Olympus Provis AX70 microscope equipped with a motorized X-Y-Z stage, a digital video camera, and 100× oil-immersion, differential interference contrast optics. We collected photomicrographs from 1-2 μm thick cross-sections of nerve en montage so that the entire surface area was represented. Each micrograph was contrast and edge-enhanced using macro-routines written using the ImagePro software package (Media Cybernetics, CA). For ultrastructural analysis, 70 nm cross-sections were photographed at high magnification (5000-15,000×) using a Philips CM-12, 120 keV transmission electron microscope Vanderbilt University Research Electron Microscopy Resource.
For immunochemistry of optic nerve, 6 μm paraffin sections were taken and stained for phosphorylated neurofilaments (SMI31, 1:1000, Covance, Nashville, TN) and glial fibrillary acidic protein (GFAP, 1:500, DAKO, Carpinteria, CA). SMI31 is a mouse monoclonal antibody that reacts with extensively phosphorylated heavy-chain neurofilament with small cross-reactivity with medium-chain neurofilament (Manufacturer’s specification sheet; Sternberger et al., 1982; Sternberger and Sternberger, 1983). It has been shown to react specifically in most mammals and some other vertebrates. Pre-treating the tissue with phosphatase abolished all staining (Sternberger and Sternberger, 1983). The GFAP antibody (Code: Z 0334) was raised in rabbit against GFAP isolated from cow spinal cord. It reacts in most mammals and has shown to be specific to GFAP through crossed immunoelectrophoresis and indirect ELISA (Manufacturer’s specification sheet) and omitting this antibody abolishes staining (Castellano et al., 1991).
2.3 Axon quantification
For measuring cross-sectional nerve area, we used a total of 110 DBA/2J optic nerves and 45 C57 nerves. For a subset of 46 DBA/2J nerves, we measured axon density (axons/mm2) as described previously (Inman et al., 2006; Buckingham et al., 2008). Briefly, from each nerve montage obtained as described above, we randomly choose 25-30 non-overlapping frames encompassing a known area of optic nerve. Additional routines were used to identify and count each axon for which a single, intact myelin sheath could be identified. Nerves with pathology so severe that identification of intact myelin sheaths was ambiguous were excluded from this study. The mean of the axon density measurements within sampling frames was taken as the representative density of the nerve. For these same 46 nerves, we also measured the internal cross-sectional area of each identified axon, exclusive of the myelin sheath. For each nerve, a range of 6000-40,000 axon measurements were used to calculate mean axon area (reported in μm2). For a subset of 29 of these 46 nerves, degenerating axon profiles were counted manually in a representative cross-section; these were identified by densely packed multi-laminar myelin sheaths. For 10 of the 46 nerves, we counted mitochondria in 10 electron micrographs each of area ~ 40 μm2. For these nerves, the number of mitochondria per unit area was expressed as the mean ± standard error. For all data, correlation coefficients were calculated from best-fitting regression lines using Pearson’s coefficient (r).
2.4 Glial area analysis
To measure the fraction of each nerve area covered by glia, the montages from 43 nerves were filtered using a suite of MATLAB routines (MathWorks, Natick, MA) that highlighted glial processes separate from axons. These routines detected glial boundaries in contrast-enhanced binary images of each nerve by edge enhancement and size filtering to exclude axons. Noise from small axons was eliminated from this image based on a roundness and size filter. We calculated the percentage of the nerve occupied by glial processes by summing the area of all identified processes. Finally, the outer boundary of the nerve was outlined from the original montage and eroded to exclude pia and dura mater. Each binary image was segmented into 20 concentric divisions that each represented an interval of 5% of the total nerve area from the outer boundary of the nerve (division 1) to the center (division 20). This was achieved using a structuring element that reduced the area within the outline of the nerve by 5% with each subsequent iteration. This accounted for any irregularities in the shape of the outer boundary of the nerve to produce a consistent area for each division. The percent glial area for each division was calculated to produce a distribution from edge to center. The center of mass of this distribution was computed as the location along the radius of the nerve which delineates 50% of the total glial area on either side.
3. Results
3.1 Optic nerve enlargement with age involves axon pathology
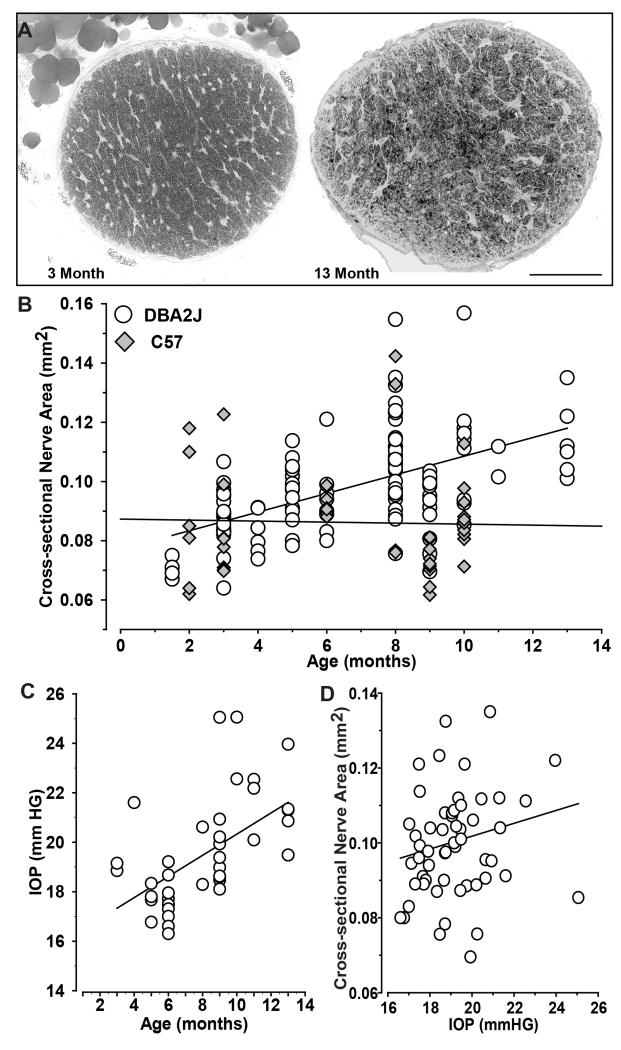
In related studies of the DBA/2J optic projection (e.g., Inman et al., 2006; Bosco et al., 2008; Crish et al., 2010), we noticed an interesting trend. Older DBA2J optic nerves tended to have a larger cross-sectional area. For example, the comparison below shows a 3 month nerve next to a 13 mo nerve with about 20% greater area (Figure 1A). Though variability was quite high at a given age, cross-sectional area increased at a rate of about .01 mm2 every three months (Figure 1B), growing from about 0.07 mm2 at 1.5 months to about 0.12 mm2 at 13 months. In contrast, C57 nerves did not increase with age, though the range of sizes too was highly variable (data not shown). As expected IOP increased with age in our DBA/2J sample (r = 0.57, p < 0.001; Figure 1C), so it is not surprising that nerve size also tended to increase with IOP (Figure 1D). Even so, IOP was not as strong a predictor of nerve size as age was (Figure 1B). This was so not only for the overall group (p = 0.17), but also for restricted age subsets. For example, at 6-8 months IOP ranged from 16-21 mmHG, but did not correlate with nerve size (r = 0.05, p=0.81; data not shown). For older animals (9-13 months), IOP ranged to over 25 mmHG but also did not strongly influence nerve size (r = 0.27, p=0.28; data not shown). Thus, age was the primary determinant of nerve expansion.
Figure 1. Nerve size increases with age in DBA/2J mice.
A. Cross-sections through 3 month (left) and 13 month (right) DBA/2J optic nerve about 0.2 mm proximal to the globe demonstrate different cross-sectional areas (0.083 vs. 0.11 mm2, respectively). Enlargement of the older nerve was accompanied by increased glial coverage and diminished axon packing. B. Cross-sectional area of individual DBA/2J optic nerves compared to C57 nerves across ages. DBA/2J nerve area increases with age (r = 0.54, p<0.001), while C57 does not (r = 0.03, p = 0.84). C. IOP (mmHG) increases with age across our sample (r = 0.57, p < 0.001) D. Nerve size tends to increase also with IOP in the DBA/2J, but this is not significant (r=0.19, p=0.17). Scale = 100 μm (A).
Optic nerves that were larger also demonstrated overt pathology associated with RGC axon loss, as in Figure 1A. A young DBA/2J nerve unaffected by disease can demonstrate a mean axon packing density as high as 5.0–6.0 × 105 axons/mm2 (Inman et al, 2006). This density, when multiplied by nerve area, corresponds to a total number of axons of 40,000 – 60,000, comparable to that in a young C57/B6 nerve (Williams et al., 1996; Scholz et al., 2008). In such nerves, axons are bundled in well-defined fascicles separated by thin astrocyte glial processes (Figure 2A). We found that with expansion, nerves become disorganized, with smaller and more irregular fascicles framed by hypertrophic astrocyte processes and containing notably larger axons (Figure 2B). Many larger nerves also showed increased numbers of degenerating axon profiles identified by their multi-laminar myelin sheaths (Figure 2C,D).
Figure 2. Increased nerve size is associated with pathology.
A. High-magnification light micrograph of cross-section through a small four month DBA/2J optic nerve (0.068 mm2) demonstrates tight packing of axons (5.8 × 105 axons/mm2) interspersed with thin astrocyte processes. B. A larger three month DBA/2J nerve (0.095mm2) with diminished axon packing (2.0 × 105 axons/mm2) and increased astrocyte hypertrophy (arrowheads). Axons also appear larger. C. A 10 month nerve (0.093 mm2) with extensive gliosis and numerous degenerating axon profiles with multi-laminar myelin sheaths (*). D. Electron micrograph through same nerve in C highlights multi-laminar degenerating profiles (*). Scale = 5 μm (A, B, C) or 2 μm (D).
When quantified, the morphological features described in Figure 2 support the idea that enlargement of the optic nerve is indicative of axon pathology. Increased nerve size was associated with significantly diminished axon density and increased numbers of degenerating axon profiles (Figure 3A,B). Similarly, the mean cross-sectional area of axons also increased with nerve size (Figure 3C), a feature we describe in more detail below. While there was a tendency for glial coverage of the nerve to increase, this was not significant (Figure 3D). A better predictor of glial area in the nerve was axon density: as density decreased, glial coverage increased significantly (r = 0.71, p <0.001; data not shown), as shown in parallel studies (Bosco et al., 2016, this issue).
Figure 3. Quantification of axon pathology with nerve expansion.
A. Axon density diminishes with nerve expansion (r = 0.39, p=0.009), while number of degenerating axon profiles (B) increases (r = 0.68, p=0.003). C. For each nerve, mean cross-sectional axon area also increases with nerve size (r = 0.50, p<0.001). D. Portion of each nerve covered by glial processes does not increase significantly with nerve size (r = 0.21, p=0.13).
3.2 Optic nerve and axon expansion precede axon loss
Next we present evidence that nerve and axon expansion occur prior to overt axon loss in the DBA/2J. The electron micrographs below show a 4 month nerve side-by-side with a 9 month nerve that was 32% larger (Figure 4A,B). The 9 month nerve also had a 39% larger mean axon area, 0.40 vs. 0.31 μm2, which is quite evident in the micrographs. Even so, axon density in the 9 month nerve was among the highest in our sample, 6.2 × 105 axons/mm2, comparable to density in the 4 month nerve (5.6 × 105 axons/mm2). Similarly, for two 9 month nerves (Figure 4C,D), axon density was quite high for each, 6.2 vs. 6.0 × 105 axons/mm2, though the nerve in Figure 4D was larger (.095 mm2 vs. 0.08 mm2) with expanded axons (0.40 μm2 vs. 0.35 μm2). At higher magnifications, the axoplasm in the larger nerve appeared highly disordered with poorly articulated microtubules and neurofilaments, compared to axons in the smaller nerve (Figure 4E,F). Also, the axolemma that normally separates the axoplasm from the myelin sheath, quite evident in the smaller 9 month nerve (Figure 4E), was nearly indistinguishable in the larger nerve (Figure 4E). This change suggests that increased axon area prior to loss involves distended axoplasmic volume.
Figure 4. Axons enlarge prior to degeneration.
A. Low magnification electron micrograph of 4 month nerve with tight axonal packing (5.6 × 105 axons/mm2), nerve area of 0.069 mm2, and mean axon area of 0.31 μm2. Arrowheads indicate ramifying astrocyte processes. B. Larger 9 month nerve (0.091 mm2) has larger mean axon area (0.40 μm2) but comparable density (6.0 × 105 axons/mm2). C. Higher magnification electron micrograph of a small 9 month nerve (0.08 mm2) and mean axon area of 0.35 μm2. Axon density was 6.2 × 105 axons/mm2. Mitochondria in axons and astrocyte processes (arrowheads) are indicated (m). D. Larger 9 month nerve (0.095 mm2) has expanded mean axon area of 0.41 μm2 but comparable axon density (6.0 × 105 axons/mm2). E. High power image of nerve in C demonstrates well-ordered packing of neurofilaments and microtubules in axoplasm (dashed square) and prominent axolemma separating the axoplasm and myelin sheath (arrows). F. Enlarged axon from nerve in D shows disordered axoplasm, phagocytic vacuole (*) and little or no distinguishable axolemma. Scale = 5 μm (A, B), 0.5 μm (C, D) and 0.25 μm (E, F).
The comparisons of equal-density nerves in Figure 4 suggest that axonal and nerve enlargement are pre-degenerative, in that expansion can occur without frank loss of axons. To determine the critical threshold at which axon enlargement is associated with axon loss, we ranked the nerves in our sample from highest to lowest axon density. We then compared density with mean cross-sectional axon area for increasingly inclusive density ranges (Figure 5). For the top 20th percentile in density (5.6 – 6.2 × 105 axons/mm2), the span of mean axon areas was modest (0.28-0.40 μm2) and the slope of the best-fitting regression line did not differ from zero (p=0.45). This indicates density did not change with axon size over this range. For the top 40% nerves, the range of axon areas increased to 0.45 μm2, but the slope of the regression line still did not differ from zero (p=0.74). For the 60th percentile group, the range of axon areas increased to nearly 0.50 μm2, and axon density approached a negative correlation with axon area (r = −0.28, p=0.14). By the 70th percentile, axon density and area were negatively correlated (r = −0.36, p=0.03). For the entire sample, axon area ranged to almost 0.75 μm2, and the decrease in axon density with increasing axon size was highly significant (r = −0.60, p<0.001). Thus, axon density can remain high and relatively invariant with increasing axon area up to a certain threshold of approximately 0.50 μm2. This is just about twice the size of the smallest mean axon area in our sample. Figure 5 also shows that as the density range became more inclusive of lower values and the range of axon sizes increased, the average nerve size also increased. This is consistent with the overall negative correlation between axon density and nerve size for the entire sample (Figure 3A).
Figure 5. Axon expansion eventually leads to loss.
Axon density vs. mean axon area for nerves ranging from the highest density group (top 20%) to the entire sample. Best-fitting linear regression is included for each group. Density and axon area become negatively correlated by the 70th percentile (r = −0.36, p=0.03). For each ranking, the average cross-sectional nerve area for the group is given.
3.3 Retraction of astrocyte processes with early axon expansion
The micrographs in Figure 4 hint at another feature of axon expansion prior to outright loss. We noticed that for high axon density nerves, increased nerve and axon size was accompanied by apparent reduction in astrocyte processes within the extra-axonal and inter-fascicular space (e.g., Figure 4A,B). This trend is illustrated more clearly below for two young DBA/2J nerves with comparable axon density: 5.2 × 105 axons/mm2 (Figure 6A) and 5.1 × 105 axons/mm2 (Figure 6B). Compared to the smaller nerve (0.075 mm2; Figure 6A), mean axon size in the larger nerve (0.10 mm2; Figure 6B) was increased: 0.46 μm2 vs. 0.37 μm2. This expansion in axon size was accompanied by diminished ramification of astrocyte processes with less separation between axon bundles. This change was evident also in the next comparison. Figure 6C shows an electron micrograph of a small 9 month nerve (0.076 mm2) with axon density of 5.7 × 105 axons/mm2 and mean axonal area of 0.28 μm2. An adjacent section through the same nerve demonstrated modest levels of phosphorylated neurofilament in axons highlighted by typical ramification of GFAP-labeled astrocyte processes (Figure 6D). Another 9 month nerve that was larger (0.11 mm2) had the same axon density but significantly expanded axons (0.41 μm2) with increased levels of phosphorylated neurofilament and greatly reduced ramification of GFAP-labeled astrocytes (Figure 6E,F). Thus, it appears that axon expansion prior to frank loss of axon density is accompanied by a reduction in glial ramification.
Figure 6. Increased Axon Size is accompanied by astrocyte withdrawal.
A. Light micrograph of cross-section through young (1.5 mo) DBA/2J optic nerve demonstrates tight packing of axons (5.2 × 105 axons/mm2) separated by glial processes (arrowheads). Nerve area is 0.075 mm2 and mean axon area is 0.37 μm2. B. A 5 month nerve has similar axon density (5.1 × 105 axons/mm2) but is larger (0.10 mm2) with increased axon area (0.46 μm2) and diminished astrocyte ramification (arrowheads). C. Electron micrograph of a small 9 month nerve (0.076 mm2) with axon density of 5.7 × 105 axons/mm2 interspersed with glial processes (arrowheads). Mean cross-sectional area of axons was 0.28 μm2. D. Cross-section through same nerve as C with astrocyte processes labeled with antibodies against GFAP (red) and axons labeled for phosphorylated neurofilaments (pNF, green). E. A 9 month nerve with same axon density was larger (0.11 mm2) and had significantly expanded axon area (0.41 μm2) with less inter-axonal space. F. Immuno-labeling of same nerve demonstrates increased phosphorylated neurofilaments in axons and greatly reduced ramification of GFAP-labeled astrocytes. Scale = 5 μm (A, B), 1 μm (C, E) and 10 μm (D, F).
To investigate this phenomenon further, we devised a simple algorithm to characterize the degree and pattern of glial ramification in a subset of nerves. This algorithm applies a series of sequential filters to separate ramifying glial processes from axons in an outline of each nerve. We then superimposed on the resulting binary image 20 concentric divisions, each representing 5% of the total nerve area. Figure 7 shows two examples: an 11 month nerve with glial processes that distribute about evenly across divisions (Figure 7A) and a 6 month nerve with a higher concentration of ramification near the edge (Figure 7B). Accordingly, for the first nerve, each division has about 20% of its area covered by glia (Figure 7C), while for the other nerve, divisions near the edge have increasingly greater glial coverage, rising to over 60% for the first few divisions (Figure 7D). This difference is reflected in the center of mass (CoM) for each distribution, which divides the distribution of glial area per division into halves. While the CoM for the nerve in Figure 7A lies near the 10th division, indicating equivalent glial area between the center and edge of the nerve, the CoM for the other nerve is closer to 8, indicating a shift towards the edge (Figure 7D).
Figure 7. Method to quantify changes in glial distribution.
A. Outline of an 11 month DBA/2J nerve (black) with high axon density and small axons (0.29 μm2) with glial processes highlighted (white). Twenty concentric divisions (red) from the outer edge to the center each delineate an area representing 5% of the nerve. Glial coverage appears nearly uniform from division to division. B. A 6 month DBA/2J nerve with larger mean axon size (0.33μm2) has a higher concentration of glial processes near the edge. C. For the nerve in A, distribution shows the fraction of each division covered by glia from the outer edge (division 1) to the center of the nerve (division 20). Dashed line shows the center of mass (CoM) for the distribution, which represents the location at which glial area is equivalent on either side. D. For the nerve in B, the CoM lies closer to the edge of the nerve, where more glial processes ramify and a greater fraction of each division’s area is covered.
Next we calculated the CoM of the glial distribution for nerves with mean axon areas below 0.50 μm2 (Figure 8, left panel). We chose this value because it represents the approximate threshold above which axon density diminishes (see Figure 5). For nerves with the smallest mean axon size, the CoM is near 10 – indicating an even distribution of glial coverage from center to edge. As axon size increases in this subset of nerves, CoM diminishes. This is consistent with movement of glial processes out of the center and towards the edge of the nerve, as shown for the nerve in Figure 7B. We repeated this analysis for a larger population of DBA/2J nerves, including those with much larger mean axon size (Figure 8, right panel). With increasing axon size, the CoM returns to higher values, though never exceeding 10 or 11. This indicates that as axons continue to expand glial ramification once again covers the nerve about evenly.
Figure 8. Distribution of glial ramification changes as axons expand.
Left: CoM (center of mass) for a subset of nerves with mean axon area below 0.50 μm2 (open symbols) decreases as axon size increases (r = −0.535, p = 0.002). The linear regression was similar for the subset of nerves with axon density > 5.0 × 105 axons/mm2 (filled symbols; p = 0.32). Right: for the complete set of nerves with glial quantification, CoM increases with axon expansion (r = 0.32, p = 0.036). Regression line for nerves with axon area below 0.50 μm2 is repeated from the left panel for comparison (dashed line).
3.4 Early axon expansion involves diminished mitochondrial density
Our micrographs show another feature of associated with early axon expansion. We found that for nerves with axon density among the highest in our sample, as mean cross-sectional axon area increased along with nerve size, the number of discernible mitochondria in our electron micrographs seemed to diminish. For example, a small 4 month nerve with small axon size had mitochondria distributed throughout both axons and glial processes within the inter-axonal space (Figure 9A). In contrast, an older, larger nerve with expanded axons had far fewer mitochondria (Figure 9B). Similar nerves showed other signs of axon pathology, including disorganized axoplasm and swelling of the internal mesaxon in many axons (Figure 9C). When quantified from multiple electron micrographs of nerves with high axon density, we found that axon expansion was strongly associated with decreased density of mitochondria (Figure 9D).
Figure 9. Mitochondrial density decreases as mean axon area increases.
A. Electron micrograph of a small 4 month nerve (0.074 mm2) with axon density of 5.6 × 105 axons/mm2. Note well-ordered packing of neurofilaments and microtubules in axoplasm and multiple mitochondria (m). Mean axon area is small (0.31 μm2). B. A 9 month nerve also has high axon density (6.0 × 105 axons/mm2) but increased mean axon area (0.40 μm2) and nerve size (0.091 mm2). Micrograph shows fewer mitochondria. C. A larger 9 month nerve (0.10 mm2) with high axon density (5.6 × 105 axons/mm2) has disordered axoplasm, few discernible mitochondria, and distended mesaxon compartments in several axons (arrowheads). Mean axon area is 0.40 μm2. D. In a sample of high-density nerves (5.2 – 6.2 × 105 axons/mm2), mitochondrial density decreases as mean axon area increases (r = −0.831, p=0.003). Scale = 0.5 μm.
3.5 Axon expansion and survival are tied to IOP
Earlier we explained that nerve size is predominantly determined by age and not IOP (Figure 1) and that nerve expansion is associated with common measures of pathogenesis (Figures 2, 3). These observations beg the question of what role IOP plays in determining axonal changes for aged animals. In our sample of older nerves (9-13 months), axon size increased significantly with IOP (r = 0.72, p < 0.001), with a commensurate IOP-dependent decrease in axon density (r = −0.69, p = 0.002; Figure 10A). Even within this fixed age group, nerve size varied greatly (0.07 – 0.14 mm2); as it increased, so did axon area (r = 0.57, p =0.01, Figure 10B). Since nerve size for this group was not greatly influenced by IOP (see results section for Figure 1), we conclude that elevations in IOP and enlargement for aged nerves independently influence axon expansion and therefore axon density. This point is reinforced further in Figure 10B, which indicates that those nerves from eyes with IOP ≥ 21 mmHG range in size greatly (filled symbols). Thus, large, aged nerves are not necessarily the same nerves exposed in vivo to the highest IOP. Finally, we asked what factor most influences axon enlargement prior to frank loss, as documented in our micrographs (Figures 4,6 9) and in our comparison with axon density (Figure 5). For the group of highest axon density nerves (top 20%, as in Figure 5), mean axon area was strongly predicted by nerve size (Figure 10C; r = 0.58, p = 0.01). For this group, IOP did not predict axon size (r = 0.31, p = 0.38), nor did age (r = 0.10, p = 0.68). This is most interesting, because for the same top density nerves, age strongly influenced nerve size (r = 0.59, p = 0.008) and IOP (r =0.86, p=0.001; data not shown), consistent with the overall trend in our sample (Figure 1).
Figure 10. IOP is linked to axon expansion and survival for aged nerves.
A. For a sample of aged (9-13 month) nerves, mean axon area expands with increasing IOP (r = 0.72, p < 0.001). Axon density (right panel) accordingly diminishes (r = −0.69, p = 0.002). B. For the same set of aged nerves, axon size also increases with nerve expansion (r = 0.57, p =0.01). Nerves from eyes with IOP ≥ 21 mmHG are indicated (filled circles). Nerve with largest axons (*) has been excluded from regression line. C. For the group of nerves with the highest axon densities (top 20%; 5.6 – 6.2 × 105 axons/mm2), mean axon area increases with nerve expansion (r = 0.58, p = 0.01).
4. Discussion
Intrinsic variability in optic nerve pathology is a signature feature of the DBA/2J mouse model of glaucoma (Libby et al., 2005a; Schlamp et al., 2006; Inman et al., 2006; Saleh et al., 2007; Scholz et al., 2008). A DBA/2J nerve unaffected by disease will demonstrate a mean axon packing density of 5.0 − 6.0 × 105 axons/mm2 (Inman et al, 2006). When multiplied by the cross-sectional nerve area delineated within the layers of pia and dura mater, this leads to an estimated total number of axons of 50,000 – 60,000, comparable to that in C57/B6 nerve (Williams et al., 1996; Scholz et al., 2008). Nerves with the worst pathology often demonstrate a packing density less than about 100,000 axons/mm2 (Scholz et al., 2008). Here, nerves with pathology so severe that identification of intact myelin sheaths was too ambiguous to quantify were excluded, since we are interested in early changes associated with progression.
Based on this restricted sample, we found that with age, the cross-sectional area of the myelinated segment of the DBA/2J optic nerve tends to increase (Figure 1). Nerve size ranges from about 0.06 mm2 at 2 months of age to as high as 0.16 mm2 at 10-12 months. Since IOP increased with age in our sample as expected, nerve size also tended to increase with IOP, but not significantly (Figure 1D). A previous study using a smaller sample of nerves hinted at a modest difference in nerve size between ages that was not systematic, though the range was highly variable (0.07 - 0.11 mm2, p=0.08; Inman et al., 2006). We now interpret this variability as indicative of the age-dependent increase in size. Expansion of the nerve generally was associated with increased pathology, including diminished axon packing density and increased incidence of degenerating profiles (Figures 2,3). Glial coverage of the nerve, indicative of gliosis, also tended to increase, though variability was high (Figure 3D). A similar result can be gleaned from Scholz et al. (2008), in which an 11 month DBA/2J nerve with severe axonal pathology was roughly 25% larger than a 10 month nerve with none (their Figure 4).
We also found that the mean cross-sectional area of surviving axons increased as the nerve expanded (Figure 3C). Perhaps our most important and novel result is that many nerves retained their high axon packing density (> 5.0 × 105 axons/mm2) even as nerve area increased. For these nerves – matched for high axon density – axon size also increased. This correlation was highly significant for nerves with the top 20% of axon densities (Figure 10C). We found that axon density remained relatively invariant up to a mean axon area of 0.45-0.50 μm2 (Figure 5). In this sense, modest axonal enlargement with nerve expansion presages degeneration; once this threshold is reached, axon density decreases rapidly with increasing mean axon size up to an average size of 0.7-0.8 μm2 (Figure 5). The axon expansion noted here is gradual, which leads us to believe that the mechanism of expansion differs from the relatively quick axonal swelling and distension observed after trauma (Povlishock and Becker, 1985). Even so, expanded axons showed signs of early axonopathy. These features included disorganized and expanded axoplasm, poorly articulated microtubules and neurofilaments, deformation of the mesaxon and axolemma, and increased accumulation of hyperphosphorylated neurofilaments (Figures 4,6, 9). The latter is indicative of challenged axonal transport (Goldstein et al., 1987; Shea and Chan, 2008), which is one of the earliest pathogenic events in DBA/2J glaucoma (Crish et al., 2010; Calkins, 2012). Thus, axon expansion and diminished anterograde transport probably go hand-in-hand with accumulation of phosphorylated neurofilaments and disordered axoplasm (Jakobs et al., 2005; Howell et al., 2007b; Soto et al., 2008).
Hypertrophy of glial processes in the optic nerve, especially of astrocyte processes, is a prominent characteristic of substantial axon loss in glaucoma (Hernandez, 2000), including the DBA/2J mouse (Jakobs et al., 2005; Bosco et al., 2008, Bosco et al., this volume). Importantly, our results demonstrate that as axons expand early prior to frank loss, the ramification of astrocyte processes in the inter-axonal space actually diminishes (Figures 4, 6). This finding is consistent with two studies, both involving acute elevations in IOP. In the first, short-term elevations in IOP (7 days) in a rat model reduced GFAP label in the optic nerve head concurrent with axonal enlargement and increased levels of phosphorylated neurofilaments; significant axon loss occurred later (Johnson et al, 2000). In another acute model, an early phase of reactive astrocyte gliosis involves retraction of processes from the center of the nerve prior to re-extension (Sun et al., 2013). In the DBA/2J, we found that with early axon expansion, the center of mass for glial ramification changed from an even distribution across the nerve to one skewed towards the edge of nerve (Figures 7,8). As axons continued to expand beyond the threshold for overt loss, the center of mass returned to an even distribution (Figure 8, right panel). This result stands in contrast to those from another inducible model in which astrocyte processes in the optic nerve head actually appear to detach from the edge of the nerve (Dai et al., 2012).
Some features of the early pathogenesis we describe can be gleaned as well from other optic nerve injury or disease models. RGC axonal diameter increases in a mouse model of Batten disease concordant with loss of axon density and an overall loss of uniformity and integrity (Sappington et al., 2003). Additionally, hints of swelling mesaxons, poor articulation of microtubules and neurofilaments within the axoplasm, and astrocyte remodeling are evident in a rat model of chronic secondary degeneration after partial optic nerve transection (Payne et al., 2012). Nerves vulnerable to secondary degeneration also demonstrate increased markers of oxidative stress and upregulated levels of myelin basic protein throughout the partially transected nerve (Fitzgerald et al., 2010). Thus, our findings may be relevant to the degeneration of retinal ganglion cell axons more generally.
What might be the significance of astrocyte retraction with early axon and nerve expansion? We found that nerves with axon expansion prior to frank loss demonstrated diminished numbers of mitochondria per unit area of tissue with increasing axon size (Figure 9). One estimate suggests that astrocytes normally account for more than 70% of the mitochondria in the optic nerve (Perge et al, 2009). Thus, reduced ramification in the extra-axonal space could induce a local depletion of available energy for axons already challenged by glaucomatous stressors. This is consistent with our earlier work in the DBA/2J showing an age- and IOP-related decrease in optic nerve ATP (Baltan et al., 2010). Ganglion cell axons are generally small compared to other white matter tracts (Perge et al., 2009), and there are numerous features that render them energy-demanding (Calkins, 2012). Diminished bioenergetic resources could be a primary inducer of progression in glaucoma (Li et al., 2015). Our data certainly support this hypothesis, as does new evidence for reduced oxidative capacity for mitochondria in DBA/2J optic nerve axons (Coughlin et al., 2015).
A previous study proposed that mechanisms of degeneration in glaucoma differ between the center of the nerve, based on vascular insufficiency, and the edge, where IOP may have the most robust compressive effect (Quigley et al., 1980). While we do not address the spatial specificity of disease-relevant stressors in our study, our data do hint at two independent mechanisms of early progression. Our data show that both IOP and nerve expansion are associated with axon enlargement independently (Figure 10), though age is the predominant determinant of both (Figure 1). However, for nerves in our sample with the highest axon densities, ironically only nerve size itself and not age or IOP was a strong predictor of axon size (Figure 10C), as was astrocyte center of mass (Figure 8). Thus, prior to loss of axons, the retreat of astrocyte processes from the nerve center and nerve expansion both predict very well axon enlargement (Figure 8, left panel; Figure 10C). The question becomes which change occurs first – axon expansion, nerve enlargement, or astrocyte remodeling? For the highest density nerves, astrocyte center of mass tends to decrease with increasing nerve size, though not significant (r = −0.48; p = .08, data not shown), and was not predicted by age (r = 0.14, p=0.61). In this case, we are left with a model in which age-related nerve expansion drives critical interactions between axons and astrocyte processes that ultimately undermine axon survival.
Highlights.
Optic nerve in DBA/2J mice expands with age
As nerve expands, so do individual axons prior to outright degeneration
Axon expansion is accompanied by local retraction of astrocyte processes to the nerve edge also prior to degeneration
Glial retraction with early axon expansion involves diminished numbers of mitochondria
Acknowledgements
This research was supported by the Melza M. and Frank Theodore Barr Foundation through the Glaucoma Research Foundation (DJC, PJH), Departmental Unrestricted and Senior Investigator Awards from Research to Prevent Blindness, Inc. (DJC), and the Vanderbilt Vision Research Center NEI Core Grant (5P30EY008126-19, DJC). The authors would like to thank John Collyer and Megan Flint for help with programing and image processing and Brian Carlson and Wendi Lambert for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–5. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010;30:5644–52. doi: 10.1523/JNEUROSCI.5956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–46. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- Bosco, et al. this volume. [Google Scholar]
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Ex Eye Res. 2011;93:120–32. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Horner PJ, Roberts R, Gradianu M, Berkowitz BA. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Invest Ophthalmol Vis Sci. 2008;49:5083–8. doi: 10.1167/iovs.08-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–19. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Age-related changes in the visual pathways: blame it on the axon. Invest Ophthalmol Vis Sci. 2013;54:ORSF37–41. doi: 10.1167/iovs.13-12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano B, González B, Jensen MB, Pedersen EB, Finsen BR, Zimmer J. A double staining technique for simultaneous demonstration of astrocytes and microglia in brain sections and astroglial cell cultures. J Histochem Cytochem. 1991;39:561–8. doi: 10.1177/39.5.1707903. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–9. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Coughlin L, Morrison RS, Horner PJ, Inman DM. Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2015;56:1437–46. doi: 10.1167/iovs.14-16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:5196–201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Dapper JD, MacNamee SE, Balaram P, Sidorova TN, Lambert WS, Calkins DJ. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 2013;229:55–70. doi: 10.1016/j.neuroscience.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–62. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia. 2012;60:13–28. doi: 10.1002/glia.21242. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA. Early events of secondary degeneration after partial optic nerve transection: an immunohistochemical study. J Neurotrauma. 2010;27:439–52. doi: 10.1089/neu.2009.1112. [DOI] [PubMed] [Google Scholar]
- Goldstein ME, Cooper HS, Bruce J, Carden MJ, Lee VM, Schlaepfer WW. Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve. J Neurosci. 1987;7:1586–94. doi: 10.1523/JNEUROSCI.07-05-01586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John S. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007a;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007b;179:1523–37. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:986–96. doi: 10.1167/iovs.05-0925. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–25. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–42. [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, Early Manifest Glaucoma Trial Group Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Li Y, Li D, Ying X, Khaw PT, Raisman G. An energy theory of glaucoma. Glia. 2015;63:1537–52. doi: 10.1002/glia.22825. [DOI] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005a;22:637–48. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–87. [PubMed] [Google Scholar]
- Payne SC, Barlett CA, Harvey AR, Dunlop SA, Fitzgerald M. Myelin sheath decompaction, axon swelling, and functional loss during chronic secondary degeneration in rat optic nerve. Invest Ophthalmol Vis Sci. 2012;53:6093–101. doi: 10.1167/iovs.12-10080. [DOI] [PubMed] [Google Scholar]
- Perge JA, Koch K, Miller R, Sterling P, Balasubramanian V. How the optic nerve allocates space, energy capacity, and information. J Neurosci. 2009;29:7917–28. doi: 10.1523/JNEUROSCI.5200-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP. Fate of reactive axonal swellings induced by head injury. Lab Invest. 1985;52:540–52. [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Flower RW, Addicks EM, McLeod DS. The mechanism of optic nerve damage in experimental acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 1980;19:505–17. [PubMed] [Google Scholar]
- Saleh M, Nagaraju M, Porciatti V. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4564–72. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Pearce DA, Calkins DJ. Optic nerve degeneration in a murine model of juvenile ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2003;44:3725–31. doi: 10.1167/iovs.03-0039. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2 mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz M, Buder T, Seeber S, Adamek E, Becker CM, Lütjen-Drecoll E. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest Ophthalmol Vis Sci. 2008;49:613–21. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Walski M, Frontczak-Baniewicz M, Voelker M, Blatsios G, Shinoda K, Zagorski Z, Zrenner E, Grieb P. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol. 2004;107:352–8. doi: 10.1007/s00401-003-0816-9. [DOI] [PubMed] [Google Scholar]
- Shea TB, Chan WK. Regulation of neurofilament dynamics by phosphorylation. Eur J Neurosci. 2008;27:1893–901. doi: 10.1111/j.1460-9568.2008.06165.x. [DOI] [PubMed] [Google Scholar]
- Sheldon WG, Warbritton AR, Bucci TJ, Turturro A. Glaucoma in food-restricted and ad libitum-fed DBA/2NNia mice. Lab Anim Sci. 1995;45:508–18. [PubMed] [Google Scholar]
- Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–61. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger LA, Harwell LW, Sternberger NH. Neurotypy: regional individuality in rat brain detected by immunocytochemistry with monoclonal antibodies. Proc Natl Acad Sci U S A. 1982;79:1326–1330. doi: 10.1073/pnas.79.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Jakobs TC. Structural remodeling of astrocytes in the injured CNS. Neuroscientist. 2012;18:567–88. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Qu J, Jakobs TC. Reversible reactivity by optic nerve astrocytes. Glia. 2013;61:1218–35. doi: 10.1002/glia.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck MW, Crick RP. The age distribution of primary open angle glaucoma. Ophthalmic Epidemiol. 1998;5:173–83. doi: 10.1076/opep.5.4.173.4192. [DOI] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci. 1996;16:7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–62. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005;280:31240–8. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]