Abstract
Objective
To evaluate differences in soluble inflammatory markers between chronically HIV-infected men and women, with or without cognitive impairment, and in response to treatment.
Design
Soluble biomarkers were measured in cryopreserved plasma and cerebrospinal fluid (CSF) of 60 treatment-naïve individuals (25 males and 35 females) with chronic HIV infection and 18 HIV-uninfected controls (9 males and 9 females) from Thailand. Following enrollment, participants began combination antiretroviral therapy (cART) and were evaluated for expression of these markers after 48 weeks.
Methods
Plasma and CSF levels of 19 soluble biomarkers (IFN-γ, TNFα, TNF-RII, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-15, MCP-1, t-Tau, IP-10, neopterin, IFNα, I-FABP, and sCD14) were measured using either a multi-parameter or standard ELISA assay.
Results
Prior to cART, females with impaired cognition had elevated levels of neopterin and TNF-RII compared to females with normal cognition in both the plasma and CSF, however levels did not differ between cognitively impaired or normal males. In a secondary outcome-hypothesis generating analysis, sex differences were also pronounced in plasma levels of MCP-1, IL-10, I-FABP, and sCD14 in response to treatment. Neopterin, IP-10, TNFα, TNF-RII, IFNα, MCP-1, IL-8, I-FABP, and sCD14 plasma levels remained elevated following 48 weeks of therapy in both sexes compared to uninfected controls.
Conclusions
We provide evidence of sustained immune activation after 48 weeks of treatment and identify possible sex differences in biomarkers previously linked to cognitive impairment, chronic inflammation, and gut integrity that may contribute to immunological differences between sexes in relationship to disease progression and response to therapy.
Keywords: HIV, Cognition, Sex, Soluble Factors, sCD14, Neopterin, Cytokines
Introduction
Immune activation is a critical component of HIV pathogenesis and a strong predictor of disease progression [1]. Sex-based differences in HIV-infected individuals have been described previously for viral set point [2, 3], disease progression [4], and cellular activation [5]. These studies revealed women have lower viral loads and higher CD4+ T cell counts than men, however at the same level of viremia, women progressed more rapidly to AIDS (reviewed in [6]). The immunological basis of these sex differences is not well understood, and sex-based comparisons of soluble marker levels have not been thoroughly investigated in chronic HIV infection or following initiation of cART.
In the earliest days following HIV acquisition, CD4+ T cells are depleted from gut associated lymphoid tissue, compromising the integrity of the gut barrier. This event leads to translocation of microbial products, which in turn can drive systemic immune activation [7]. Markers of microbial translocation and enterocyte damage, such as intestinal fatty acid binding protein (I-FABP) and sCD14, are elevated in untreated HIV infection likely due to increased gut permeability and chronic activation of CD4+ T cells [1]. In addition to infecting gut lymphoid tissue, viral RNA is found in other organs, as well as the central nervous system (CNS), as evidenced by cerebrospinal fluid (CSF) HIV RNA immediately following infection [8–10]. In chronic HIV-1 infection, up to 50% of individuals are noted to have cognitive impairment, termed HIV Associated Neurological Disorder (HAND) [11]. There is growing evidence persistent chronic immune activation may contribute to CNS complications [12], as the inflammatory response is considered to be the main mediator of neuronal damage in HAND [13]. As infection progresses, HIV triggers an inflammatory response within the CNS, resulting in macrophage activation and increased expression of neopterin, a surrogate marker of neurocognitive impairment. Treatment-naïve, HIV-infected patients with dementia express high levels of neopterin within plasma and CSF [14]. Furthermore, elevated cell-associated viral reservoir burden in monocyte-enriched peripheral blood cells is associated with cognitive impairment in treatment-naïve patients and is directly associated with markers of immune activation within the CSF [15].
The widespread use of cART to combat HIV has led to a considerable decrease in HIV-associated morbidity and mortality [16, 17]. In response to therapy, expression levels of inflammatory biomarkers decrease, plasma and CSF viral loads decline, and patients with HAND improve cognitively [18–20]. Although survival rates of HIV infected individuals have dramatically improved due to treatment, these individuals are at increased risk for a variety of conditions that lead to early mortality [19, 21] including heart disease, cancer, kidney disease, bone density loss, and cognitive impairment [19, 22]. A growing body of evidence implicates persistent inflammation and immune activation, despite cART, as a contributor to immunosenescence and these age-associated conditions [21, 23, 24].
This study aimed to uncover differences in levels of inflammatory markers that may contribute to sex differences in HIV disease progression and response to cART. We measured an array of soluble factors in plasma and CSF of chronically HIV infected men and women prior to and following treatment to identify variations in these markers that may contribute to differences in disease progression. We compared levels of these soluble factors in HIV-1 infected individuals to those in uninfected individuals within the same region. Most reported studies to date have analyzed HIV-1 subtype B or C infections, and this study is unique to the sex differences in CRF_01 AE infections in Thailand. These efforts propose sex-specific differences in biomarkers previously linked to cognitive impairment, chronic inflammation, and gut integrity that may contribute to immunological differences between sexes in relationship to disease progression and response to therapy.
Methods
Study Design
Sixty treatment-naïve Thais (25 males and 35 females) with chronic HIV infection were recruited to investigate markers of cognitive impairment among cART-naïve HIV-infected individuals who met Thai Ministry of Public Health criteria for initiating cART (CD4 count <350 cell/mm3 or symptomatic disease) as part of the SEARCH 011 study protocol (NCT00782808) [15]. All participants were chronically infected with HIV, cART-naïve, and agreed to begin therapy upon enrollment. Participants were evaluated by a consensus panel of clinical neurology and neuropsychology tests to assign HAND diagnosis as cognitively normal (NL), Asymptomatic Neurocognitive Impairment (ANI), Mild Neurocognitive Disorder (MND) or HIV-Associated Dementia (HAD). Participants then started first-line cART with lamivudine (3TC) + nevirapine (NVP) + either stavudine (d4T), zidovudine (ZDV), or tenofovir (TDF). Participants intolerant to this regimen were changed based on clinical acumen, typically to efavirenz (EFV) for NVP complications. There was no difference in treatment regimens when stratified by sex.
Plasma and CSF samples were collected prior to, and at 48 and 96 weeks following cART initiation. Forty-two out of the 60 participants at enrollment and 14 participants at 48 weeks underwent lumbar puncture. Eighteen uninfected Thai males (N=9) and females (N=9) were enrolled as controls. All participants provided a signed consent, approved by the University of California, (San Francisco, CA), Walter Reed Army Institute of Research (Silver Spring, MD), and the Chulalongkorn University and Phramongkutklao Hospital (Bangkok, Thailand) Institutional Review Boards.
CD4+ T cells and Plasma HIV-1 RNA
HIV RNA and CD4+ T cells counts were measured using Cobas Amplicor (Roche Molecular Diagnostics, Pleasanton, CA), and flow cytometry, respectively, as described previously [15, 25].
Soluble Factor analysis
Matched pre- and post-treatment CSF and citrate-plasma specimens were analyzed for soluble activation markers. A custom multiplex ELISA array was used to quantify thirteen analytes, including IFN-γ, TNFα, TNF-RII, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-15, and MCP-1, according to the manufacturer’s instructions (Quansys Biosciences). Single-analyte ELISAs were performed to measure t-Tau and IP-10 (Life Technologies), neopterin (GenWay Biotech), IFNα, I-FABP and sCD14 (R&D Systems) and analyzed using SoftMax Pro (Molecular Devices).
Statistical analyses
Multiple regression models were used to assess the association between soluble factor levels and sex adjusting for HIV-1 viral load, CD4 counts, and/or severity of neurocognitive disease as covariates using sex as an independent variable. There was no main interaction effect in these models; therefore, we performed a secondary outcome-hypothesis generating analysis by separating the sexes while controlling for viral load and CD4 absolute values. Analysis was also performed without controlling for viral load and CD4 absolute values, and the results were consistent with results controlling for these parameters (data not shown). Independent groups were compared using Mann-Whitney tests. Matched paired data from pre- and post-cART were analyzed by Wilcoxon signed rank test. Spearman’s coefficient was used for correlation analyses. The threshold for statistical significance was set to p < 0.05 for all analyses. Data were graphed using PRISM software (version 6, Graphpad Software, La Jolla, CA, USA).
Results
Study population characteristics
Among the sixty chronically HIV-infected, treatment-naïve Thais, there were no sex differences based on age or plasma or CSF viral load (Table 1, Fig. 1) at the time of enrollment, although females trended towards lower plasma viral load (VL) (p=0.07), as previously reported [4, 6]. Differences in the time of acquisition was unknown; both men and women presented with chronic HIV infection and were immune compromised sufficient to initiate ART. There were no differences in CD4 T cell counts (Table 1, Fig. 1c), which may indicate that infection duration was similar between groups. After 48 weeks of cART, nearly all participants had undetectable viral RNA in the plasma and CSF (Fig. 1a,b) and increased CD4+ T cell counts (Fig. 1c). When stratified by sex, no differences were detected in the HIV RNA levels or CD4+ T cell counts following treatment (Fig. 1), similar to other reports [26].
Table 1.
Demographics and Clinical Characteristics at Enrollment.
| N | Age (years) |
CD4+ Abs (cells/mm3) |
Plasma VL (log10 copies/mL) |
CSF VLb (log10 copies/mL) |
Cognitive Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|
| NL | ANI | MND | HAD | ||||||
| Female | 35 | 35 (22–47)a |
230 (23–553) |
4.68 (3.22–5.88) |
4.43 (1.70–5.26) |
18 | 7 | 7 | 3 |
| Male | 25 | 36 (23–57) |
239 (29–532) |
5.03 (3.15–5.88) |
3.67 (2.12–5.42) |
14 | 7 | 1 | 3 |
| p value (Mann-Whitney test) |
0.4092 | 0.9200 | 0.0712 | 0.1110 | |||||
Data expressed as median (range).
Only 19 male and 23 females consented to baseline CSF donation.
Fig. 1. HIV-1 related clinical characteristics of study population.
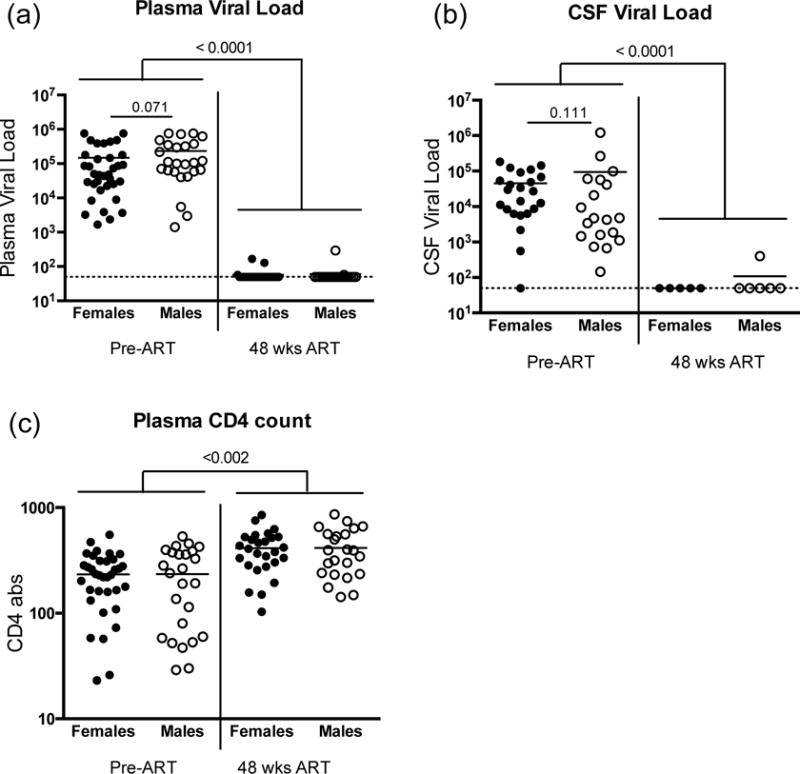
HIV-1 viral load measures in (a) plasma and (b) cerebral spinal fluid at study enrollment (Pre-ART) and following 48 weeks of combination antiretroviral therapy (cART). (c) Absolute CD4+ T cell counts at enrollment and following 48 weeks treatment. Data is stratified by sex, females (closed circles) and males (open circles). Dashed line indicates assay lower limit of detection (LLD), NS indicates not significant.
Sex differences in biomarkers related to cognition
Participants exhibiting ANI, MND or HAD were grouped and labeled as ‘impaired cognition’. While no difference in plasma VL was observed by sex or impaired status in cART-naïve participants (Fig. 2a), females with impaired cognition exhibited higher CSF VL than males with impaired cognition (Fig. 2b). There were also slightly more females (49%) diagnosed with cognitive impairment compared to males (44%), although these values were not statistically significant.
Fig. 2. Relationship of immunological measures with cognitive function.
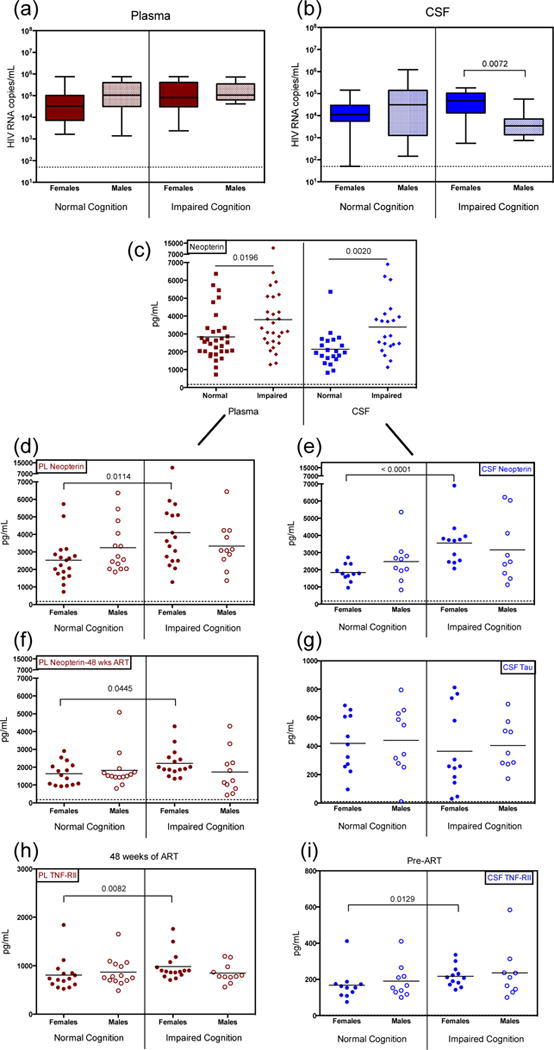
HIV-1 viral load in (a) plasma and (b) cerebral spinal fluid (CSF) at study enrollment, stratified by cognition assessment and sex. (c) Neopterin levels at enrollment in plasma and CSF, comparing levels in participants diagnosed with normal cognition (Normal) verses those with any level of impaired cognition (Impaired). Neopterin levels, further stratified by sex, at enrollment (d–e) and following 48 weeks combination antiretroviral therapy (f). (g) t-Tau levels at enrollment in CSF, comparing levels between cognitive ability and stratified by sex. (h–i) Soluble tumor necrosis factor alpha receptor II (TNF-RII) levels in plasma and CSF at time of enrollment, stratified by cognitive assessment and sex. Participants within the Impaired Cognition grouping include all patients diagnosed with ANI, MND or HAD. Data from plasma, red; CSF, blue; females, closed circles; males, open circles. Dashed line indicates assay LLD.
Neopterin, a key marker of cellular immune activation linked to HAND and produced by activated monocytes/macrophages, was measured in both plasma and CSF. Similar to previous studies [27, 28], we observed an increase in neopterin levels in both plasma and CSF in individuals with impaired cognition (Fig 2c). Individuals diagnosed with HAD, the most severe cognitive impairment, displayed the highest levels of neopterin compared to those with normal cognition (data not shown), as described previously [27].
Multiple regression models were used to assess the association between soluble factor levels and sex adjusting for HIV-1 viral load, CD4 counts, and/or severity of neurocognitive disease. In these models that included sex as a covariate, no main interaction effect was observed; therefore, we performed a secondary analysis by separating the sexes while controlling for viral load and CD4 absolute values. In these secondary analyses, when these findings were stratified by sex, only females with cognitive impairment exhibited significantly elevated neopterin levels in both plasma and CSF (Fig. 2d,e) compared to females with normal cognition. In contrast, males with cognitive impairment did not exhibit elevated levels of neopterin compared to males with normal cognition in the plasma (p=0.68) or CSF (p=0.59) (Fig. 2d,e). Plasma neopterin levels remained elevated in impaired females even after 48 weeks of cART (Fig. 2f), yet no significant difference was detected in males between impaired and unimpaired groups (p=0.59). A reduced participation of volunteers for CSF collection did not allow sufficient data for statistical analysis after 48 weeks of cART for this compartment. The severity of impairment improved with treatment, as neither females nor males were diagnosed with HAD after 48 weeks of cART, and instead were diagnosed with less severe forms of cognitive impairment, MND or ANI (data not shown).
As observed previously, TNF-α and TNF-RII were elevated in impaired versus unimpaired individuals when combining sexes into one group prior to the initiation of treatment (Fig. 4, [29]). Although no sex differences were statistically significant in multivariable models, evaluation of TNF-α and TNF-RII stratified by sex revealed that TNF-RII displayed the same trends as neopterin, wherein females with cognitive impairment exhibited significantly elevated TNF-RII levels compared to females with normal cognition in both the plasma and CSF even after 48 weeks of treatment (Fig. 2h,i). Males with impaired cognitive ability did not display elevated levels (Fig. 2h,i). There was no significant difference observed between cognitive impairment and sex in levels of TNFα. Tau, another biomarker investigated for association with HAND [30], was also measured in the CSF. We found no difference in t-Tau levels in HIV-infected females or males with impaired compared to normal cognition (Fig. 2g).
Fig. 4. Impact of combination antiretroviral therapy on expression of immune activation markers.
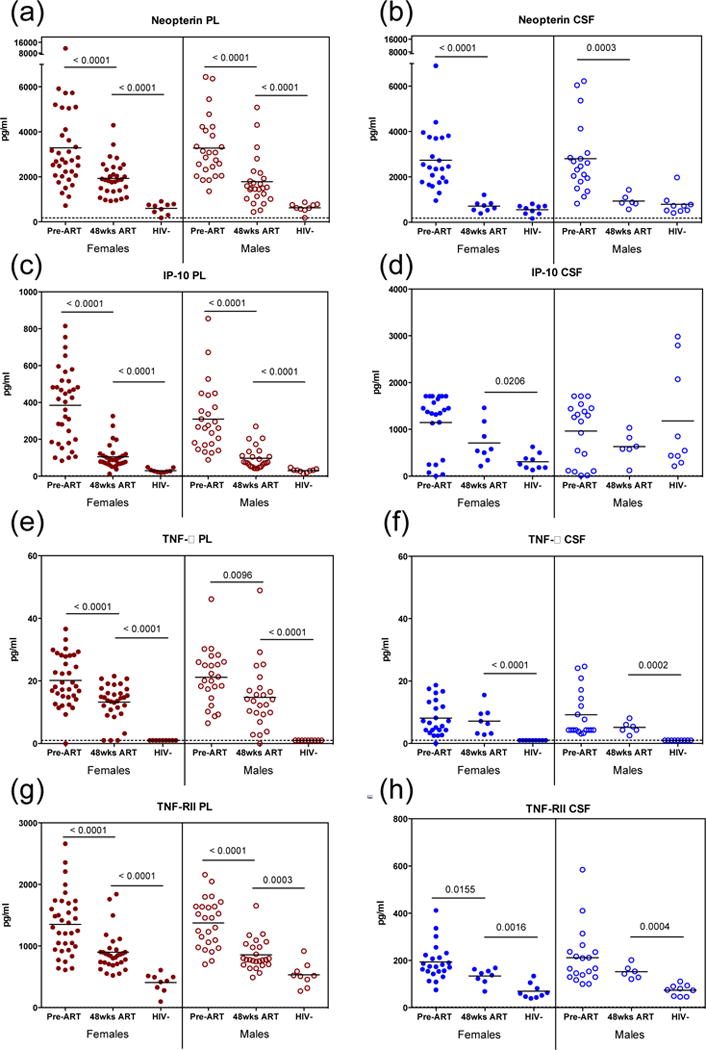
(a–b) Neopterin, (c–d) IP-10, (e–f) tumor necrosis factor alpha (TNFα) and (g–h) TNF-RII were measured at time of enrollment (Pre-ART) and following 48 weeks therapy (48wks ART) in plasma (red) and CSF (blue) of women (closed circles) and men (open circles). All participants are included, regardless of cognitive assessment. Demographically similar uninfected participants (HIV−) were measured as controls. Dashed line indicates assay LLD.
Sex differences prior to and after initiation of treatment
Further analyses were performed to determine the impact of cART on various soluble biomarker levels unrelated to cognition, and to determine if sex differences related to treatment were present. Sex differences in response to treatment were pronounced in expression levels of MCP-1, IL-8, IL-10, I-FABP, and sCD14 (Fig. 3). Interestingly, within this population, uninfected and cART-naïve infected males expressed higher levels of MCP-1 compared to uninfected and cART-naïve infected females in both the plasma (p<0.01) and CSF (p<0.01) (Fig 3a, data not shown). Treatment decreased plasma MCP-1 levels in males, but did not affect plasma MCP-1 levels in females (Fig. 3a). Levels were similar in both sexes after 48 weeks of treatment; however, these plasma levels remained significantly elevated in both sexes compared to uninfected controls (Fig. 3a).
Fig. 3. Sex related differences in soluble factor levels in response to antiretroviral therapy.
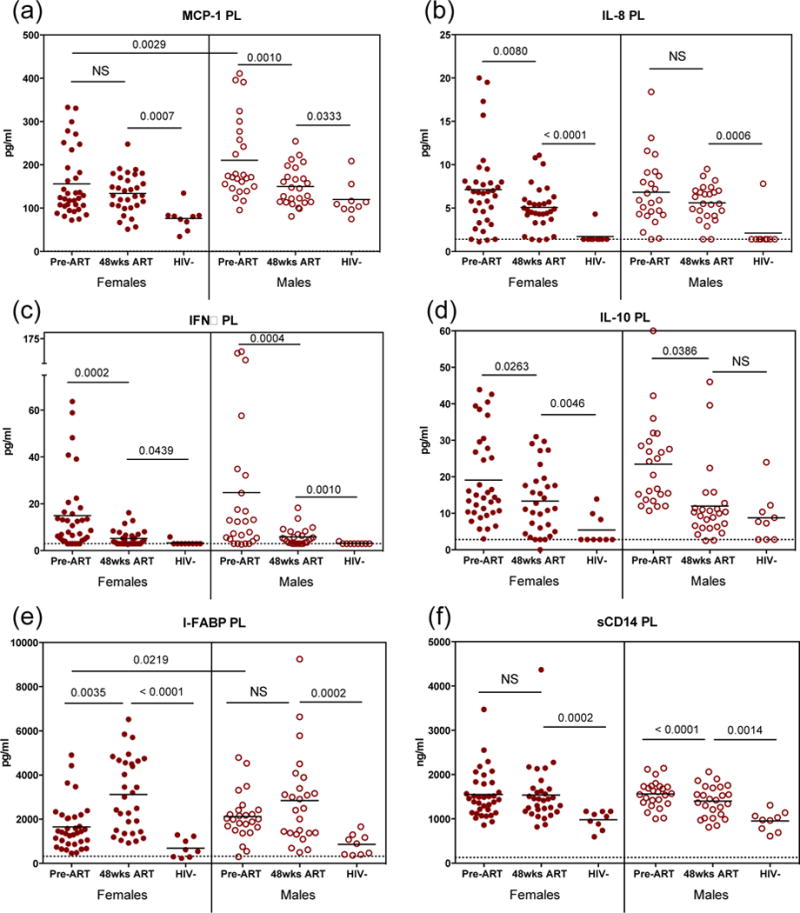
(a–b) Monocyte chemotactic protein 1 (MCP-1) measured at time of enrollment (Pre-ART) and following 48 weeks therapy (48 wks ART) in plasma of women (closed circles) and men (open circles). Additional markers of immune activation were measured only in plasma: (b) interleukin 8 (IL-8), (c) interferon alpha (IFNα), (d) interleukin 10 (IL-10), (e) intestinal fatty acid binding protein (I-FABP), (f) soluble CD14 (sCD14). Dashed line indicates assay LLD; NS, not significant.
Similarly, expression levels of IL-8, IFNα, and IL-10 were elevated in cART-naïve chronically HIV infected males and females compared to uninfected controls (Fig. 3b,c,d). While IL-8 levels decreased after 48 weeks of treatment in females, these levels did not decrease in treated males (Fig. 3b). IFNα and IL-10 plasma levels in both males and females decreased significantly following 48 weeks of treatment (Fig 3c,d). IFNα remained elevated in both males and females compared to uninfected controls after 48 weeks of treatment (Fig. 3c). Likewise, IL-10 remained significantly elevated in HIV-infected females (Fig. 3d), while in infected males, IL-10 decreased to levels found in uninfected controls (Fig. 3d). IFNα levels were not measured in the CSF, there was no significant difference in the levels of IL-8 or IL-10 in the CSF when stratified by sex or cognition (data not shown). No differences were detected in the IL-8, IL-10, or IFNα levels between men and women prior to the initiation of ART (Fig3b,c,d).
Analysis of plasma I-FABP and sCD14 was performed to assess the differences in gut integrity between sexes. In contrast to the other biomarkers, I-FABP, a marker of enterocyte turnover, increased in both sexes following 48 weeks of cART (Fig. 3e), although these increased levels only reached significance in females compared to males (Fig. 3e). Levels of sCD14, a marker associated with early mortality in HIV infection [1], remained unchanged in females throughout the course of treatment, while males significantly decreased sCD14 expression with treatment (Fig. 3f). In both sexes, sCD14 remained elevated compared to uninfected controls (Fig. 3f). Taken together, these findings reveal variations in these biomarkers between sexes in response to treatment.
Inflammatory markers remain elevated after treatment in chronically HIV-infected individuals independent of sex
Subsequent analysis of all infected individuals demonstrated there was a significant decrease in the expression levels of neopterin, IP-10, TNFα, and TNF-RII (Fig. 4) following 48 weeks of cART with no significant difference between men and women when stratified by sex (Fig. 4). Neopterin levels were significantly reduced in the plasma and CSF after 48 weeks of treatment (Fig. 4a,b), but remained elevated compared to uninfected controls. Although these levels in the plasma continued to be elevated, neopterin levels in the CSF reduced to the same level as uninfected controls following 48 weeks of cART (Fig. 4b), consistent with previously published literature [31]. IP-10, TNF-α, and TNF-RII levels decreased with treatment in both sexes, yet these levels remained elevated in the plasma compared to uninfected controls (Fig. 4c–h). Both TNF-α and TNF-RII remained elevated in the CSF after 48 weeks of cART (Fig 4f,h), and IP-10 levels continued to be elevated in the CSF for females (Fig. 4d). CSF IP-10 levels within HIV uninfected control males were increased compared to uninfected females (Fig. 4d). In addition, levels of IL-6 significantly decreased to uninfected control levels following 48 weeks of cART with no significant difference between sexes (data not shown). Other biomarkers measured were not detected at enrollment (IL-1α, IL-1β, IL-4, IL-5, IL-12, IL-15, IFN-γ).
Discussion
Studies comparing the course of HIV infection between men and women have demonstrated considerable sex differences in disease progression [4, 32] and immune activation [5]. Although antiretroviral therapy has dramatically reduced the risk of AIDS-associated opportunistic infections and mortality, chronic inflammation persists despite suppression of plasma HIV RNA leading to immunosenescence and age related diseases [19, 21]. The causes of persistent systemic immune activation when therapy successfully controls viral burden are unclear, but likely result from multiple factors including residual HIV-1 replication within the mucosa or other viral reservoirs, prevalence of other co-infections [33], damage to gut integrity and leakage of gut microbial products [7], damage to the lymphoid tissues, and immunoregulatory cell loss [34–36]. Remarkably, women have an increased risk of early mortality even after treatment with antiretroviral therapy compared to treated men [32]. Therefore, considerations need to be made in regards to the differences between these demographic populations that factor treating persistent chronic immune activation, in addition to HIV infection, that will alleviate the onset of aged-related conditions.
Here we evaluated the differences in inflammatory markers between chronically HIV-infected men and women that may result in varied responses to antiretroviral therapy and disease progression. We first demonstrate that women with impaired cognitive ability express elevated levels of neopterin and TNF-RII compared to women with normal cognitive ability, however a significant difference was not observed in HIV-1 infected males. It has been documented previously that a greater number of HIV-1+ women develop cognitive deficits than men in Zambia, and the authors of that study suggested this may be due to sex-related social or healthcare disadvantages [37]. Within the current study, cognitive improvement following treatment initiation occurred at the same rate in both genders, although a larger percentage of females (23%) were still diagnosed with cognitive impairment after 48 weeks of treatment compared to males (15%), however this result was not significant.
Sex-specific differences were detected in response to treatment in levels of MCP-1, IL-8, IL-10, I-FABP, and sCD14, while we did not observe a significant different in IFNα levels between men and women. Previous studies have demonstrated that plasmacytoid dendritic cells (pDCs) from women produced significantly higher levels of IFNα in response to HIV than pDCs from men, and these increased levels of IFNα secretion led to stronger activation of CD8+ T cells in vitro [5]. The authors suggested that these increased levels of immune activation may contribute to faster HIV-1 disease progression in females. We did not observe increased systemic IFNα levels between chronically infected men and women in Thailand prior to or following treatment while controlling for viral load and CD4 T cell count. This data may suggest that IFNα levels quantified from pDCs may be more reflective of local responses resulting in long-term chronic inflammation compared to systemic levels.
In stark contrast to the other soluble biomarkers, plasma I-FABP, a marker of enterocyte growth and proliferation, was elevated in females and males within the same demographic on cART, yet only reached significance in females. Furthermore, we found that levels of sCD14, another marker of microbial translocation, only decreased in males following 48 weeks of cART. Previous studies have shown that sCD14 is an independent predictor of disease progression and mortality in HIV infection [1]. We did not detect significant differences between men and women in levels of sCD14 prior to ART, but the continued elevation of sCD14 levels within females may be predictive of increased mortality after treatment as was found with previous studies [23].
Other studies have determined the effect of cART on microbial translocation markers such as I-FABP and sCD14, and similarly found I-FABP levels increased in individuals taking efavirenz (EFV) [38]. In our study, there was no correlation between I-FABP levels and treatment with EFV, and there was no bias of EFV usage in females over males. Overall, there was no difference in the treatment modalities between sexes that would account for these outcomes. Because these individuals were chronically infected, gut integrity is likely impaired at this stage, and increased I-FABP and sCD14 levels in women after treatment may be reflective of local HIV replication and resulting destruction. Evidence of greater gut damage in the female participants may also result from pharmacological side effects not as evident in male participants. The cause of increased I-FABP levels after treatment remains to be determined.
We also provide evidence that after 1–2 years of cART, neopterin, IP-10, TNFα, TNF-RII, and IFNα significantly decreased in both men and women, but remained elevated compared to uninfected controls. MCP-1, IL-8, IL-10, I-FABP, and sCD14 also remain elevated compared to uninfected controls, but levels of these factors in each sex differ in responses to treatment. Remarkably, even in the absence of detectable viral load within the plasma and CSF, these inflammatory signals still persist. Levels of several other soluble factors found to be associated with acute infection [39–41] such as IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-12, IL-15, or IFN-γ were rarely detectable in these chronically infected individuals prior to cART initiation. This data suggests that not all pathways of immune activation continue to be amplified in chronic infection.
In conclusion, we demonstrate chronically HIV-infected individuals manifest elevated levels of inflammatory soluble factors even after 1–2 years of cART compared to uninfected controls. The levels of a subset of these soluble factors vary between males and females before and after treatment, and these sex-specific variations may underlie previously reported sex differences in the outcome of HIV disease progression. Strengths of this work include evaluating soluble factor levels in CRF_01 AE chronic infection where there are few documented studies, available regionally appropriate control specimens, the longitudinal nature of the study, and a reasonable distribution of males and females from a selection criteria that did not include sex. However, the sample size was modest and in our robust multivariable statistical approach, we did not meet statistical significance for sex in these variables, despite prominent differences identified in exploratory approaches. In addition, because these individuals have not been followed longer than 2–3 years following ART initiation, we could not assess how the sustained elevation of these factors impact long-term disease progression and non-AIDS morbidity and mortality within this cohort. Our work could be strengthened by an evaluation of these factors in a larger sample of men and women and following these individuals long-term to determine biological relevance of these inflammatory soluble factors between sex. Understanding sex differences between immune responses during HIV infection, especially differences in biomarkers linked to subclinical cognitive impairment and/or gut integrity, may inform complex decisions surrounding measures to reduce the long-term effects of chronic inflammation.
Acknowledgments
The authors thank the SEARCH 011 Study Group volunteers for their participation, and the physicians who cared for the volunteers. The SEARCH 011 Study group includes Nitiya Chomchey, Somprartthana Rattanamanee, James L.K. Fletcher, Duanghathai Sutthichom, and Pairoa Praihirunkit from SEARCH/TRCARC; Nijasri Charnnarong and Sukalaya Lerdlum from Chulalongkorn University; Yotin Chinvarun from Phramongkutklao Medical Center; Mark de Souza, Alexandra Schuetz, and Rapee Trichavaroj from the Armed Forces Research Institute of Medical Sciences; Supunee Jirajariyavej from Taksin Hospital; Elijah Mun, Stephanie Chiao, Akash Desai, Edgar Busovaca, Nicholas Hutchings, Collin Adams, Katherine Clifford, and Lauren Wendelken from UCSF. The authors also thank Matthew Creegan and Roger Faubel for technical support and Michael Eller for critical review of this manuscript. The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense. This work was supported by R01NS061696 (NIH) and a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the U.S. Department of Defense.
Footnotes
Author Contributions: Conceived and designed the experiments: JA, VV, MM, JK; Performed the experiments: BS, LJ, PS; Analyzed the data: SK, BS, EA, LJ, MM; Contributed reagents/materials/analysis/clinical tools: PS, TC, ST, NP; Wrote the paper: SK, BS; Edited the manuscript: VV, JA, JK, MM
Conflicts of Interest
There are no relevant conflicts of interest.
References
- 1.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans JS, Nims T, Cooley J, Bradley W, Jagodzinski L, Zhou S, et al. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis. 1997;175:795–800. doi: 10.1086/513973. [DOI] [PubMed] [Google Scholar]
- 3.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–672. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 4.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 5.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis. 2014;209(Suppl 3):S86–92. doi: 10.1093/infdis/jiu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 8.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Annals of internal medicine. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw GM, Harper ME, Hahn BH, Epstein LG, Gajdusek DC, Price RW, et al. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985;227:177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- 11.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. The Journal of infectious diseases. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 14.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma The Journal of experimental medicine. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8:e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 17.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55:1242–1251. doi: 10.1093/cid/cis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggers C, Hertogs K, Sturenburg HJ, van Lunzen J, Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–1906. doi: 10.1097/00002030-200309050-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 22.Longenecker CT, Jiang Y, Yun CH, Debanne S, Funderburg NT, Lederman MM, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013;168:4039–4045. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut Epithelial Barrier Dysfunction and Innate Immune Activation Predict Mortality in Treated HIV Infection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalayjian RC, Spritzler J, Matining RM, Fiscus SA, Gross BH, Francis IR, et al. Older HIV-infected patients on antiretroviral therapy have B-cell expansion and attenuated CD4 cell increases with immune activation reduction. AIDS. 2013;27:1563–1571. doi: 10.1097/QAD.0b013e32835fabc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS one. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murri R, Lepri AC, Phillips AN, Girardi E, Nasti G, Ferrara S, et al. Access to antiretroviral treatment, incidence of sustained therapy interruptions, and risk of clinical events according to sex: evidence from the I.Co.N.A. Study. J Acquir Immune Defic Syndr. 2003;34:184–190. doi: 10.1097/00126334-200310010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis. 1996;174:294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs D, Chiodi F, Albert J, Asjo B, Hagberg L, Hausen A, et al. Neopterin concentrations in cerebrospinal fluid and serum of individuals infected with HIV-1. AIDS. 1989;3:285–288. doi: 10.1097/00002030-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Nolting T, Lindecke A, Koutsilieri E, Maschke M, Husstedt IW, Sopper S, et al. Measurement of soluble inflammatory mediators in cerebrospinal fluid of human immunodeficiency virus-positive patients at distinct stages of infection by solid-phase protein array. J Neurovirol. 2009;15:390–400. doi: 10.3109/13550280903350192. [DOI] [PubMed] [Google Scholar]
- 30.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz A, Yiannoutsos CT, Fuchs D, Price RW, Crozier K, Hagberg L, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62. doi: 10.1186/1742-2094-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemly DC, Shepherd BE, Hulgan T, Rebeiro P, Stinnette S, Blackwell RB, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs A, Karim R, Mack WJ, Xu J, Chen Z, Operskalski E, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201:823–834. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS pathogens. 2013;9:e1003471. doi: 10.1371/journal.ppat.1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bixler SL, Mattapallil JJ. Loss and dysregulation of Th17 cells during HIV infection. Clin Dev Immunol. 2013;2013:852418. doi: 10.1155/2013/852418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, et al. Paucity of IL-21-producing CD4(+) T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood. 2012;120:3925–3935. doi: 10.1182/blood-2012-04-420240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR, Jr, Imasiku ML, Kalima K, et al. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. The Journal of nervous and mental disease. 2012;200:336–342. doi: 10.1097/NMD.0b013e31824cc225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesterbacka J, Nowak P, Barqasho B, Abdurahman S, Nystrom J, Nilsson S, et al. Kinetics of microbial translocation markers in patients on efavirenz or lopinavir/r based antiretroviral therapy. PloS one. 2013;8:e55038. doi: 10.1371/journal.pone.0055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 40.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. Journal of virology. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts L, Passmore JA, Williamson C, Little F, Bebell LM, Mlisana K, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]


