Abstract
Introduction
Cardiovascular events (CE) due to atherosclerosis are preventable. Identification of high-risk patients helps to focus resources on those most likely to benefit from expensive therapy. Atherosclerosis is not considered for patient risk categorization, even though a fraction of CE are predicted by Framingham risk factors. Our objective was to assess the incremental value of combining total plaque area (TPA) with the Framingham risk score (FramSc) using post-test probability (Ptp) in order to categorize risk in patients without CE and identify those at high risk and requiring intensive treatment.
Material and methods
A descriptive cross-sectional study was performed in the primary care setting in an Argentine population aged 22–90 years without CE. Both FramSc based on body mass index and Ptp-TPA were employed in 2035 patients for risk stratification and the resulting reclassification was compared. Total plaque area was measured with a high-resolution duplex ultrasound scanner.
Results
57% male, 35% hypertensive, 27% hypercholesterolemia, 14% diabetes. 20.1% were low, 28.5% moderate, and 51.5% high risk. When patients were reclassified, 36% of them changed status; 24.1% migrated to a higher and 13.6% to a lower risk level (κ index = 0.360, SE κ = 0.16, p < 0.05, FramSc vs. Ptp-TPA). With this reclassification, 19.3% were low, 18.9% moderate and 61.8% high risk.
Conclusions
Quantification of Ptp-TPA leads to higher risk estimation than FramSc, suggesting that Ptp-TPA may be more sensitive than FramSc as a screening tool. If our observation is confirmed with a prospective study, this reclassification would improve the long-term benefits related to CE prevention.
Keywords: hypertension, atherosclerosis, cardiovascular event
Introduction
The prevalence and incidence of cardiovascular disease increase exponentially with age [1, 2]. Although preventable, it remains a leading global cause of death and disability [3]. Even if the only effective approach to restrict this unnecessary loss of life is to prevent the disease from developing in the first place, strategies such as classification based on the Framingham risk score or the European SCORE system are not highly effective.
This lack of effectiveness may be the result of misclassification of patients as low risk and a resulting lack of treatment, or as high risk and corresponding overmedication. These facts remind us that although exposure to causal factors is important, susceptibility to these factors and the disease in question might be more important. Despite its great promise, genetic testing for susceptibility has not yet proven to be useful for risk stratification [4].
Many new approaches to improve risk prediction are in development, using new biochemical and clinical [5, 6] strategies, but these are not appropriate [7–9].
Atherosclerosis, the leading cause of cardiovascular disease, develops over decades silently before symptoms occur. Thus, while there is an opportunity for timely detection and personalized prevention, the period before development of symptoms (preclinical atherosclerosis) is not used efficiently, either to prevent events or to appropriately categorize the risk of patients in primary care. Subclinical atherosclerosis can be detected very accurately and non-invasively by determining carotid total plaque area (TPA) by ultrasound. A recent meta-analysis showed that TPA is a stronger predictor of cardiovascular risk than the more widely used carotid intima-media thickness (IMT) [10], and an accompanying editorial [11] explained why it is more useful for assessing effectiveness of therapy.
Thus, the objective of this study was to assess the incremental value of combining the measurement of TPA with the Framingham risk score (FramSc) using post-test probability (Ptp) for categorizing risk in a population without history of cardiovascular event (CE), and identifying patients at high risk for CE who require intensive treatment.
Material and methods
Study participants
This was a cross-sectional study in a consecutive sample of 2035 physician-referred individuals being followed in an atherosclerosis prevention program (LifeQualityA), conducted by Blossom DMO Argentina, who signed informed consent. The study was approved by the Blossom DMO Argentina ethics committee.
Risk factor assessment
We excluded patients who reported any personal history of cardiovascular disease defined by prior myocardial infarction or coronary/peripheral revascularization or any current symptoms potentially suggestive of angina, defined by self-reports of chest pain, chest pressure, chest tightness, stroke and chronic renal failure. We included patients aged > 18 years with a Framingham score greater than 6% because we focused on patients in whom risk reclassification might change therapy. Very low-risk patients would not be candidates for therapy such as statins [12].
All individuals provided details of their demographics, medical history, medication usage, current symptoms, and involvement in leisure time physical activity. A history of cigarette smoking was considered positive if a subject was a current or former smoker. History of hypercholesterolemia was defined as positive for any individual self-reporting a history of high total cholesterol, high LDL, low HDL and/or high triglycerides, or the current use of lipid-lowering therapy. Patients were considered diabetic if they reported using oral hypoglycemic agents, insulin sensitizers, or subcutaneous insulin. Patients were considered hypertensive if they reported a history of this condition or the use of antihypertensive medications. Body mass index (BMI) was calculated from height and weight.
Framingham risk score determination
Framingham sex-specific risk equations were used to predict the 10-year risk of developing myocardial infarction (MI) or cardiovascular death (CVDeath) as previously described [13].
Blood pressure was measured three times in the sitting position after 5 min of rest with the OMRON Hem 705 sphygmomanometer [14]. The average of the three readings was used in the present analyses. The individuals were divided into three groups: low-risk (≤ 10% 10-year risk), intermediate-risk (10–20% risk) and high-risk (> 20% risk).
Carotid plaque area determination
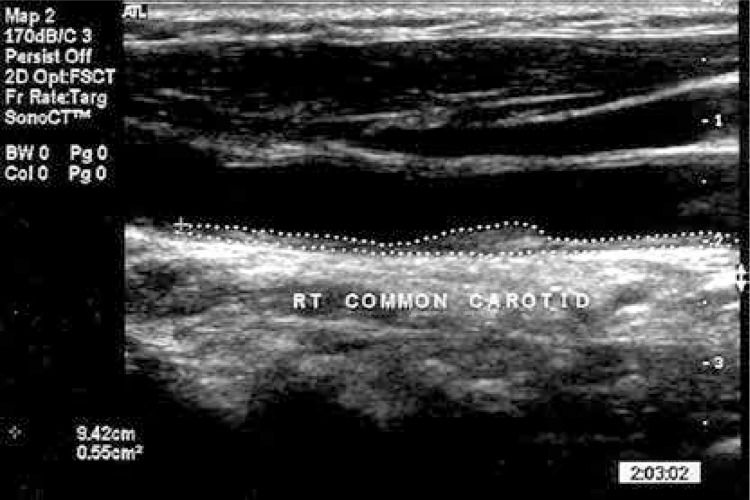
Total carotid plaque area was measured as described previously [15] with a high-resolution duplex ultrasound scanner. Plaque was defined as a local thickening of the intima > 1 mm in thickness. Measurements were made in magnified longitudinal views of each plaque seen in the right and left common, internal, and external carotid arteries. The plane in which each plaque was measured was given by the view showing the largest extent of plaque. The image was then frozen and magnified, and the plaque was measured by tracing around the perimeter with a cursor on the screen (Figure 1). The operator then moved on to the next plaque and repeated the process until all visible plaques were measured. The sum of cross-sectional areas of all plaques seen between the clavicle and the angle of the jaw was taken as TPA. Total plaque area was divided by 2 (to match the risk prediction in the Tromsø study [16], in which plaque was measured only on one side) and this value was used for the post-test analysis. Only patients for whom complete data were available were included in the present study; 46 patients were excluded from the database as a result of morbid obesity obscuring accurate carotid artery evaluation.
Figure 1.
Measurement of carotid plaque area. Each plaque was measured in a longitudinal view in the plane in which the plaque is maximal. The image was frozen and magnified on the screen, and a cursor was traced around the perimeter of the cross section. The microprocessor in the duplex scanner displays the cross-sectional area of the plaque (cm2). The plaque shown is in the right common carotid artery and measures 0.55 cm2 (Reprinted with permission from ref. [8])
Intra-observer reliability (intraclass correlation) was 0.94 for repeated measurements. For the purpose of demonstrating generalizability of our results to other ultrasound laboratories and clinics, we previously carried out a study of interobserver reliability in which plaque area measurements in 25 patients were repeated a week apart by 2 technicians using 2 different machines. The senior technologist, who has been carrying out these measurements for 8 years and who performed all the measurements on which this article was based, used a new, high-resolution TL HDI 5000 scanner; the junior technologist, who has been doing such measurements for 1 year, used an ATL Mark 9 duplex scanner. The reliability (intraclass correlation) was 0.85, with the senior technician using the higher-resolution machine systematically measuring more plaque [8].
Statistical analysis
Data were expressed as mean ± SD. Descriptive statistics were used to summarize patient characteristics. We determined FRS, body mass index version (FRS/BMI), for each patient, and it was expressed as the percentage 10-year risk [13]. The post-test probability TPA (Ptp-TPA) was then used as a surrogate marker for the combined outcome of fatal myocardial infarction and stroke, calculated by using the Bayes formula and risk calculator designed by Romanens et al. [17, 18] (http://www.scopri.ch/posttestcalculators1.html). Then, to compare both methods we calculated the Pearson coefficient. To evaluate whether the differences have a clinical impact, risk was divided into three categories, low (< 10%), moderate (10.1% to 20%) and high risk (> 20%), and finally data were evaluated with the κ coefficient. For statistical analysis, the level of significance was set at p < 0.05.
Results
The demographic data of the subjects studied are shown in Table I. In our middle-aged sample mean age + SD was 59 ±0.2 years; 57% were male, 35% hypertensive, 27% had hypercholesterolemia and 14% were diabetic. There were no differences between men and women in the prevalence of cigarette smoking or use of antihypertensive treatment.
Table I.
Characteristics of the study population
| Parameter | Female (n = 860) | Male (n = 1175) | Total (n = 2035) |
|---|---|---|---|
| Age [years] | 63 ±0.4 | 56 ±0.3 | 59 ±0.3 |
| Hypercholesterolemia (%) | 24.5 | 29.3 | 26.9 |
| Hypertension (%) | 32.5 | 37.9 | 35.1 |
| Diabetes mellitus (%) | 13.2 | 15.5 | 14.3 |
| TPA [mm2] | 48.4 ±1.8 | 58.1 ±2.1 | 54.1 ±1.4 |
| FRS (%) | 17.7 ±0.3 | 21.3 ±0.3 | 19.8 ±0.2 |
First, we classified patients based on FRS/BMI to predict their 10-year risk of MI or CVDeath [13]. From our sample of patients studied, the low-risk (< 10%) group represented 20.1% of the subjects. They were 48 ±1 years old, with a TPA of 16.6 ±1.1 mm2. The moderate-risk group of patients represented 28.5% of the subjects, with an average age of 56 ±1 years, and average TPA of 33.0 ±1.5 mm2. The high-risk group represented 51.5%, with an average age of 65 ±1 years, and average TPA of 80.4 ±2.4 mm2. Framingham Risk Score (FRS) increased with age and number of risk factors (r = 0.87 and r = 0.78 respectively).
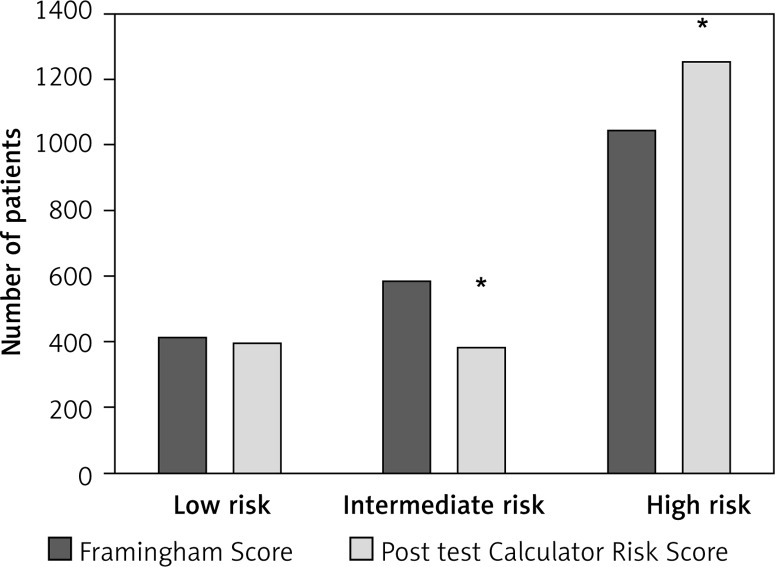
We then reclassified patients (low to high risk FRS) based on the TPA of carotid atherosclerosis; 768 subjects were re-scored to a new risk category: 491 (24.1%) migrated to a higher risk and 277 (13.6%) were reclassified to a lower risk category (κ index = 0.360, SE κ = 0.16) (Table II, Figure 2). The Pearson correlation between both cardiovascular risk methods was 0.813 (p < 0.0001), suggesting that risk did not correlate between the methods in a small fraction of patients.
Table II.
Category risk distribution FRS vs. Ptp-TPA (number of patients)
| Framingham | Post test category risk | Total | ||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Low category risk | 245 | 130 | 34 | 409 |
| Moderate category risk | 129 | 125 | 327 | 581 |
| High category risk | 19 | 129 | 897 | 1045 |
| Total | 393 | 384 | 1258 | 2035 |
Figure 2.
Distribution of risk category by FRS (black bars) vs. Ptp-TPA (white bars). *p < 0.05 vs. Framingham score
Discussion
We have demonstrated that in a representative sample of patients in primary care, stratification of Ptp-TPA leads to significantly different risk estimation than the FRS. These findings suggest that inclusion of subclinical atherosclerosis may help to better predict cardiovascular event (CE), and thus identify patients who would benefit most from intensive preventive therapies. A prospective study would be needed to confirm this hypothesis.
The FRS is one of the most validated and widely used predictive scores in the medical literature. The FRS was developed in 1998 [12] with robust methods and was intended to offer prospective risk assessment for coronary heart disease risk in men and women without previous heart disease. Risk was calculated based on age, sex, blood pressure, total or low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, smoking status, and presence of diabetes mellitus; however, a simpler FRS version replacing laboratory results with body mass index can be used for more cost-effective and simple stratification by the primary care physician [13]. This method was used in this study.
While atherosclerosis is the main cause of coronary events, and a substantial proportion of strokes, measurement of preclinical burden of atherosclerosis is not used for stratification in any Framingham analysis. Total plaque area determination is a simple and highly reproducible method to quantify atherosclerosis. This may explain why in many studies the FRS does not predict most of the cardiovascular events observed [19]. Spence [20] found that a high FRS identified only 32% of patients who would experience events, whereas 77% of the events occurred among patients in the top quartile of TPA.
As pointed out by Spence [20, 21] and Hackam [21], “Virtually all positive randomized trials of cardiovascular prevention in high-risk patients show relative risk reductions in the range of 9% to 30% [22–26]; this means that 70% to 80% of events are not prevented by guideline-advocated therapies. In the STENO-2 trial, despite a long-term, intensive, multifactorial intervention in diabetic subjects, only 50% of cardiovascular events were prevented during a follow-up of 14 years [27]. In real-world practice, results of therapy tend to be even less effective than in clinical trials.”
Additionally, the Framingham risk score does not assess other factors related to the development of atherosclerosis, such as physical inactivity, hypertriglyceridemia, Lp(a), small LDL particles or family history, and finally, it cannot be applied in all populations.
Because of these limitations, many studies have tried to improve the prediction scores, and some advances have been made. Several European countries evaluated the SCORE model instead of the Framingham model. In Austria, the SCORE model for low-risk regions overestimated mortality rates [28]. In Germany, the SCORE for high-risk regions overestimated absolute risks as compared with the Framingham risk function and mortality statistics [29]. In a third comparison, the SCORE model underestimated risks as compared with the Framingham and FINRISK models in a South Asian population in the United Kingdom [30].
Kivimäki et al. [31] investigated whether the addition of information regarding job strain improved its predictive power in a low-risk working population, but when compared with the Framingham algorithm this maneuver did not improve the model's predictive performance. Scheltens et al. [32] compared the Framingham Heart Study risk model with the SCORE risk prediction model. However, they found that both the SCORE model and the Framingham model functions were insufficient to predict absolute risks.
Wouter de Ruijter et al. [33] evaluated the performance of Framingham risk factors, adding new biomarkers to predict cardiovascular mortality in the elderly. They found that in the very elderly with no history of cardiovascular disease, homocysteine alone is able to identify those at high risk of cardiovascular mortality, whereas classic risk factors included in the Framingham risk score are not. Many other strategies have been used to predict heart dysfunction [34], suggesting that additional information is required in these classical risk stratification algorithms.
Thus the Framingham score has important limitations, and we hypothesized that the addition of a measurement of preclinical atherosclerotic burden would enhance prediction of absolute risk.
For this purpose we selected TPA, because it is noninvasive, is highly reproducible, requires minimal training, is not expensive, can identify both calcified and non-calcified atherosclerotic plaques with an axial resolution accuracy of < 0.1 mm, and can be used to guide management and monitor therapy aimed at decreasing atherosclerotic plaque area [21].
There are other techniques to evaluate subclinical atherosclerosis such as non-contrast computed tomography imaging of coronary arteries for calcification using the coronary artery calcium (CAC) score, B-mode ultrasound to measure carotid intima-media thickness (CIMT), and the ankle brachial index (ABI). TPA, however, has many advantages over these techniques. The CAC score serves as a noninvasive measure of coronary plaque burden [35–37], and it has prognostic value, independent of and incremental to that of the Framingham Risk Score, demonstrated in multiple studies [36, 37]. The major limitations of CAC scoring are radiation exposure and cost.
B-mode ultrasound to measure CIMT requires special technical expertise in performance and interpretation of the results [38, 39]. With this method, prospective studies also showed incremental prognostic information for traditional risk factor scoring, and improved risk classification [40, 41]. However, comparative studies in asymptomatic subjects have shown that the CAC score provides greater incremental prognostic information compared to CIMT [42, 43].
Finally, an ABI of < 0.9 is considered diagnostic for lower extremity peripheral arterial disease (flow-limiting atherosclerosis) and is associated with a high risk of developing other atherosclerotic manifestations. The limitations of this method include the indirect measure of obstruction only assumed to be due to atherosclerosis and its inability to determine treatment efficiency.
Taking these issues into consideration, addition of TPA to the FRS may help to better predict cardiovascular events compared to the FRS. This is easily explained based on the following example: Mrs. CEB is a 57-year-old without hypertension or diabetes mellitus, with a systolic blood pressure of 116 mm Hg, a small carotid plaque area of 20 mm2 and a BMI of 22.73 kg/m2. Her FRS is 8.99%, indicating low risk and a vascular age of 66 years. These parameters do not directly evaluate the vascular tree; it is possible that this patient would receive general lifestyle advice on a cardioprotective dietary pattern, physical activity and smoking cessation or another non-pharmacological approach to treat multiple risk factors. If we evaluate this patient based on TPA, however, the result changes. Now her post-test is 23%, reclassifying the patient to a high risk score, and so the patient will be treated differently.
Thus, the post-test procedure provides the physician with a method to identify vascular disease early and the possibility to treat patients appropriately before they experience a cardiovascular event. It has been shown previously that coronary artery calcium scoring is of value in the general population when combined with traditional risk factors using a post-test classification: In the MESA study [43], coronary artery calcium (CAC) scoring improved the prediction of coronary events including revascularization. In the Heinz Nixdorf Recall study, CAC scoring improved the prediction of hard coronary events, i.e. cardiac death and myocardial infarction [37]. In the Rotterdam Study, hard coronary event prediction was significantly improved in an elderly cohort [44].
Today, fewer than 15% of patients hospitalized with a first atherosclerotic event are taking preventive lipid-lowering treatment before admission, and the majority of these have only been treated for a few years [45]. Additionally, recent reports have highlighted the importance of “optimal” medical therapy with antiplatelet drugs in reducing both first-ever and recurrent stroke [46] as well as optimal doses of statins [47, 48]. If patients destined for symptomatic disease can be identified and treated at an earlier stage by means of a combination of biomarkers and noninvasive imaging, prevention of cardiovascular events will be more successful. These issues were highlighted in 2011 by Sillesen and Falk [49], Puz et al. [50] and by Hecht [51].
In our study, 36% of the population evaluated changed risk status, 62% of whom notably migrated from the intermediate-risk group to the high-risk group. These patients are now on intensive treatment to prevent a cardiovascular event.
Lastly, it is important to note that in this study we only included patients with > 6% FRS from a primary prevention network center in Argentina, and thus its results may not apply to secondary prevention or other countries. However, the findings of Spence et al. [52, 53] suggest that this approach would also be useful in secondary prevention. Additionally, because it is a cross sectional study, we cannot confirm prediction of events; a prospective study with follow-up to events would be necessary to confirm these results.
In conclusion, there is a critical need to refine predictive models or to develop them de novo to predict events. As described above, physical and blood-based constituents are dynamic and can participate in atherosclerosis, in the development of vulnerable plaques, and in plaque rupture. A blood-based profile should yield significant predictive information for the near term, and if this biomarker approach could be supplemented by TPA analyses it would be more relevant for near-term cardiovascular risk prediction.
Acknowledgments
We wish to acknowledge the invaluable help of Paul Atkins, for his careful review and editing of the manuscript.
This study was supported by an unrestricted institutional grant from Blossom DMO, Córdoba, Argentina.
Conflict of interest
Drs. Perez and García have no conflicts of interest with regard to the content of this article. J. David Spence and Luis Armando are principals in Vascularis Inc.
References
- 1.McDermott MM. The international pandemic of chronic cardiovascular disease. JAMA. 2007;297:1253–5. doi: 10.1001/jama.297.11.1253. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics--2007 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lauer JA, Hutubessy RC, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003;361:717–25. doi: 10.1016/S0140-6736(03)12655-4. [DOI] [PubMed] [Google Scholar]
- 4.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–76. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 5.Kivimaki M, Tabak AG, Batty GD, et al. Incremental predictive value of adding past blood pressure measurements to the Framingham Hypertension Risk equation: the Whitehall II Study. Hypertension. 2010;55:1058–62. doi: 10.1161/HYPERTENSIONAHA.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo AB, Hall SA, Ganz P, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol. 2010;55:350–6. doi: 10.1016/j.jacc.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA. 2009;302:2345–52. doi: 10.1001/jama.2009.1757. [DOI] [PubMed] [Google Scholar]
- 8.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 9.Park ST, Kim JK, Yoon KH, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. AJNR Am J Neuroradiol. 2010;31:1106–12. doi: 10.3174/ajnr.A2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–33. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis. 2012;220:34–5. doi: 10.1016/j.atherosclerosis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Vera-Cala LM, Orostegui M, Valencia-Angel LI, Lopez N, Bautista LE. Accuracy of the Omron HEM-705 CP for blood pressure measurement in large epidemiologic studies. Arq Bras Cardiol. 2011;96:393–8. doi: 10.1590/s0066-782x2011005000038. [DOI] [PubMed] [Google Scholar]
- 15.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the TromsØ Study. Stroke. 2007;38:2873–80. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 17.Romanens M, Ackermann F, Spence JD, et al. Improvement of cardiovascular risk prediction: time to review current knowledge, debates, and fundamentals on how to assess test characteristics. Eur J Cardiovasc Prevent Rehabil. 2010;17:18–23. doi: 10.1097/HJR.0b013e3283347059. [DOI] [PubMed] [Google Scholar]
- 18.García NH, Pérez HA, Spence JD, Armando LJ. Risk of vascular disease in premenopausal women with diabetes mellitus. Clin Ther. 2014;36:1924–34. doi: 10.1016/j.clinthera.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Gordon T, Garcia-Palmieri MR, Kagan A, Kannel WB, Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974;27:329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- 20.Spence JD. Point: uses of carotid plaque measurement as a predictor of cardiovascular events. Prev Cardiol. 2005;8:118–21. doi: 10.1111/j.1520-037x.2005.03908.x. [DOI] [PubMed] [Google Scholar]
- 21.Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 2010;41:1193–9. doi: 10.1161/STROKEAHA.110.577973. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 23.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–21. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 25.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 27.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 28.Ulmer H, Kollerits B, Kelleher C, Diem G, Concin H. Predictive accuracy of the SCORE risk function for cardiovascular disease in clinical practice: a prospective evaluation of 44 649 Austrian men and women. Eur J Cardiovasc Prevent Rehabil. 2005;12:433–41. doi: 10.1097/01.hjr.0000174791.47059.80. [DOI] [PubMed] [Google Scholar]
- 29.Neuhauser HK, Ellert U, Kurth BM. A comparison of Framingham and SCORE-based cardiovascular risk estimates in participants of the German National Health Interview and Examination Survey 1998. Eur J Cardiovasc Prevent Rehabil. 2005;12:442–50. doi: 10.1097/01.hjr.0000183909.52118.9f. [DOI] [PubMed] [Google Scholar]
- 30.Bhopal R, Fischbacher C, Vartiainen E, Unwin N, White M, Alberti G. Predicted and observed cardiovascular disease in South Asians: application of FINRISK, Framingham and SCORE models to Newcastle Heart Project data. J Public Health. 2005;27:93–100. doi: 10.1093/pubmed/fdh202. [DOI] [PubMed] [Google Scholar]
- 31.Kivimäki M, Nyberg ST, Batty GD, et al. Does adding information on job strain improve risk prediction for coronary heart disease beyond the standard Framingham risk score? The Whitehall II study. Int J Epidemiol. 2011;40:1577–84. doi: 10.1093/ije/dyr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheltens T, Verschuren WMM, Boshuizen HC, et al. Estimation of cardiovascular risk: a comparison between the Framingham and the SCORE model in people under 60 years of age. Eur J Cardiovasc Prevent Rehabil. 2008;15:562–6. doi: 10.1097/HJR.0b013e3283063a65. [DOI] [PubMed] [Google Scholar]
- 33.de Ruijter W, Westendorp RGJ, Assendelft WJJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szadkowska I, Pawlicki L, Kowalski J, Banach M, Goch JH, Chizynski K. Left ventricular dysfunction and NT-pro-BNP levels in patients with one-vessel disease after first ST-elevation myocardial infarction treated with primary coronary angioplasty. Kardiol Pol. 2009;67:1201–6. [PubMed] [Google Scholar]
- 35.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Rogowicz F, Araszkiewicz A, Pilacinski S, Zozulinska Z, Wykretowicz A, Wierusz-Wysocka B. Carotid intima-media thickness and arterial stiffness in type 1 diabetic patients with and without microangiopathy. Arch Med Sci. 2012;8:484–90. doi: 10.5114/aoms.2012.29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–7. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22:1674–9. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 43.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias-Smale SE, Proenca RV, Koller MT, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56:1407–14. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157:111–7. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Estruch-Perez MJ, Plaza-Martinez A, Hernandez-Cadiz MJ, Soliveres-Ripoll J, Solaz-Roldan C, Morales SV. Interaction of cerebrovascular disease and contralateral carotid occlusion in prediction of shunt insertion during carotid endarterectomy. Arch Med Sci. 2012;8:236–43. doi: 10.5114/aoms.2012.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Athyros VG, Katsiki N, Tziomalos K, et al. Statins and cardiovascular outcomes in elderly and younger patients with coronary artery disease: a post hoc analysis of the GREACE study. Arch Med Sci. 2013;9:418–26. doi: 10.5114/aoms.2013.35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercando AD, Lai HM, Aronow WS, et al. Reduction in atherosclerotic events: a retrospective study in an outpatient cardiology practice. Arch Med Sci. 2012;8:57–62. doi: 10.5114/aoms.2012.27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sillesen H, Falk E. Why not screen for subclinical atherosclerosis? Lancet. 2011;378:645–6. doi: 10.1016/S0140-6736(11)60059-7. [DOI] [PubMed] [Google Scholar]
- 50.Puz P, Lasek-Bal A, Ziaja D, Kazibutowska Z, Ziaja K. Inflammatory markers in patients with internal carotid artery stenosis. Arch Med Sci. 2013;9:254–60. doi: 10.5114/aoms.2013.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecht HS. The deadly double standard (the saga of screening for subclinical atherosclerosis) Am J Cardiol. 2008;101:1805–7. doi: 10.1016/j.amjcard.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 52.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 53.Spence JD, Coates V, Li H, et al. Effects of Intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010;67:180–6. doi: 10.1001/archneurol.2009.289. [DOI] [PubMed] [Google Scholar]