Abstract
Introduction
The incidence of multidrug resistant microorganisms worldwide is increasing. The aim of the study was to present institutional experience with the multidrug resistant microorganism colonization patterns observed in children with congenital heart diseases hospitalized in a hybrid pediatric cardiac surgery center.
Material and methods
Microbiological samples were routinely collected in all children admitted to our department. All microbiological samples were analyzed with regard to multidrug resistant microorganisms: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), Gram-negative rods producing extended-spectrum beta-lactamases (ESBL), multidrug resistant Gram-negative rods (MDR-GNRs), carbapenemase-producing Klebsiella pneumoniae (KPC), carbapenem-resistant Acinetobacter baumannii (CRAB) and Pseudomonas aeruginosa (CRPA).
Results
In 30 (9%) swabs ‘alert’ pathogens from the above group of listed microorganisms were found. All positive swabs were isolated in 19 (16.1%) children. Multidrug resistant pathogen colonization was statistically significantly more often observed in children admitted from other medical facilities than in children admitted from home (38% vs. 10%, p = 0.0089). In the group of children younger than 6 months ‘alert’ pathogen were more often observed than in older children (34.1% vs. 5.4%, p < 0.001).
Conclusions
Preoperative multidrug resistant pathogen screening in children admitted and referred for congenital heart disease procedures may be of great importance since many of these patients are colonized with resistant bacteria. Knowledge of the patient's microbiome is important in local epidemiological control along with tailoring the most effective preoperative prophylactic antibiotic for each patient. The impact of preoperative screening on postoperative infections and other complications requires further analysis.
Keywords: alert pathogen colonization, congenital heart defects, cardiac surgery, surgical infections
Introduction
Despite great progress in prevention and treatment of infections in recent years, postoperative complications continue to occur in a significant percentage of pediatric cardiac patients [1, 2]. Major infections can severely compromise the final results of comprehensive treatment for congenital heart defects undertaken in experienced institutions following appropriate protocols. Factors such as extracorporeal circulation (ECC), hypothermia, peripheral tissue hypoperfusion and foreign implantable materials may constitute fundamental risks for increased perioperative infection and complication rates [3]. Additionally, there is reported increased incidence of multidrug resistant microorganisms, particularly in hospital settings [4, 5]. Children with congenital heart disease are usually hospitalized and treated in a number of different institutions prior to the final cardiac procedure. Exposure to multidrug resistant pathogens (‘alert’ pathogens that are commonly regarded as ‘residents’ in hospital environments) increases the risk of asymptomatic colonization of patients. This is the reason that many centers screen all patients before or at the time of hospital admission, to determine the patient's initial microbiological status [6]. This protocol helps to determine optimal preoperative prophylaxis, as well as first line antibiotic therapy in the case of postoperative infections, with consideration of the patient's natural microbiome and its resistance patterns.
After a meticulous analysis of our routine microbiological examinations performed on admission, we found a significant percentage of alert pathogens diagnosed as a colonization flora in children with congenital heart defects. Therefore, we initiated this study to better define this problem in order to diminish the risk of hospital acquired infections which can be life-threatening in cardiac surgery patients.
The aim of this study was to present the experience of a hybrid pediatric cardiac surgery center with defining the patient's microbiome along with multidrug resistant microorganism colonization patterns as a first-step attempt to minimize the risk of opportunistic infections that could potentially compromise their treatment.
Material and methods
Microbiological samples were routinely collected in all children admitted to the Department of Pediatric Cardiac Surgery of Copernicus Hospital in Gdansk from November 2012 to May 2013 as part of the standard protocol. In our department there are 10 patient beds (5 intensive care; 5 normal cardiosurgical beds) from 650 beds in the hospital and more than 300 cardiosurgical operations are carried out yearly. The subjects for further analysis were all microbiological swabs from the nose, pharynx and anus (in children younger than 15 years) or groin skin (≥ 15-year-old children) taken routinely during patients’ admission. We established a very strict program of preoperative screening, guided by highly qualified nurses in regular wards, and then implemented for children who were admitted directly into the intensive care unit in cardiac emergency settings.
All swabs were collected using commercially available transport packs (Amies). Afterwards they were plated on three kinds of agar with the special selection of microorganisms: the first one was the Cocosel chromogenic agar (Graso) with a paper disc impregnated with 5 µg of vancomycin (OXOID), the second one was the ESBL chromogenic agar (Graso), and the third was the MRSA chromogenic agar (Graso). The isolated microorganisms were tested for susceptibility of crucial antibiotics – Staphylococcus aureus – cefoxitin (30 µg), Enterococcus – vancomycin (5 µg). For Gram-negative rods ESBL production was confirmed with a paper disc of cefotaxime (30 µg), ceftazidime (30 µg) and amoxicillin/clavulanic acid (20/10 µg) (DDST) and KPC production with a paper disc of ertapenem (10 µg) (in the case of resistance, the strain was tested with a paper disc impregnated with 300 µg of boronic acid). For nonfermenting rods, there was confirmed resistance to imipenem and meropenem and MBL production (by a test with 0.5 M EDTA). All strains were identified to the species level using a MALDI TOF mass spectrometer.
Patient's age and type of implemented treatment (surgical management with ECC, surgical management without ECC, hybrid treatment, percutaneous cardiac interventions and diagnostic inter-stage hospitalization without invasive management) were analyzed. In addition, the type of admission directly from a patient's home versus from another hospital or medical facility was noted. Admissions within a time period of less than 14 days after hospital discharge from another center was considered as an admission from a medical facility. The patient was regarded as admitted from home if the patient had no contacts with any institutional medical service for more than 14 days prior to admission. The incidence of postoperative infections in all children treated either surgically or with various percutaneous or hybrid interventions was also analyzed.
Statistical analysis
Distribution of relevant characteristics of the patients was described using mean and percentage or standard deviation (SD) and range for continuous and categorical variables, respectively. Quantitative data, defined as ratio and interval measurements, were reported in terms of absolute frequencies and percentages. Pearson goodness-of-fit χ2 test was used to analyze associations between independent variables [9]. P-value < 0.05 was chosen as the cut-off point for significance. Statistical analysis was performed using SPSS v. 13.0 (SPSS Inc, USA).
Results
In the analyzed seven-month period, 118 children with congenital heart diseases (61 girls and 57 boys; mean age of 28.8 months (SD 46.9 months; minimum 1 day; maximum 18 years) were admitted to our Department of Pediatric Cardiac Surgery. Eighty-two patients were referred in emergency settings, while in 36 cases there were elective admissions. There were 61 infants with a mean age of 3.8 ±3.1 months admitted from home, and another 38 (32.2%) children were admitted from other medical facilities according to the definition described above.
In several cases, we were initially informed by referring centers (10 patients – 8.4%), or we obtained the information upon the analysis of the patient's medical documents (20 patients – 16.9%), that the children were colonized with an alert pathogen prior to admission to our institution. None of the children exposed to an alert pathogen received any dedicated decolonization therapy before the admission, despite their having indications for surgery, cardiac interventions or hybrid treatment. Data regarding alert pathogen colonization in patient family members or a family history of alert pathogens were not collected.
Sixteen (13.6%) children were admitted only for diagnostic hospitalizations without any invasive treatment because of the need to perform imaging examinations and laboratory tests to complete preoperative qualification protocols. There were also patients with complex cardiac malformations admitted for routine inter-stage controls, as well as individuals diagnosed before the final definitive disqualification from any further surgical treatment. In the analyzed time, cardiac interventions (percutaneous implantation of coil or Amplatzer device), surgical management with ECC, surgical management without ECC and hybrid treatment were performed in 43 (36.4%) patients, 35 (29.7%) patients, 22 (18.6%) patients and 2 (1.7%) patients, respectively.
Three hundred and thirty-five swabs for microbiological examinations were taken during the study's 7-month time frame (mean 2.8 swabs per child); 118, 108, 97 and 12 swabs from nose, pharynx, anus and groin skin, respectively. In 30 (9%) swabs there were found alert pathogens from the group of above-described microorganisms. All positive swabs were obtained in 19 (16.1%) children. In the analyzed period, there were no multidrug resistant Gram-positive bacteria. Among Gram-negative microorganisms the most common isolated pathogen was Klebsiella pneumoniae ESBL (+) – 13 positive swabs in 7 children; then Escherichia coli ESBL (+) – 11 positive swabs in 8 patients, Enterobacter cloacae ESBL (+) – 6 positive swabs in 4 children and Enterobacter kobei ESBL (+) – 1 positive swab in 1 patient. In 1 anus swab Escherichia coli ESBL (+) and Enterobacter cloacae ESBL (+) were isolated.
The most common location of swabs positive for alert pathogens was the anus (20 positive swabs – 20.6%), then the skin in the groin area, the pharynx and the nose – 1 (8.3%) swab, 7 (6.5%) swabs and 2 swabs (1.7%), respectively. The ‘anatomic’ incidence of alert pathogen colonization with regard to the site of isolation is presented in Table I.
Table I.
Multidrug resistant pathogens in analyzed group of patients depending on the place of pathogen isolation
| Place of pathogen isolation | Number of isolates (%) |
|---|---|
| Nose: | 118 (100) |
| Klebsiella pneumonia ESBL | 1 (0.85) |
| Enterobacter kobei ESBL | 1 (0.85) |
| Pharynx: | 108 (100) |
| Klebsiella pneumoniae ESBL | 3 (2.78) |
| Enterobacter cloacae ESBL | 3 (2.78) |
| Escherichia coli ESBL | 1 (0.92) |
| Anus: | 97 (100) |
| Klebsiella pneumoniae ESBL | 9 (9.3) |
| Escherichia coli ESBL | 9* (9.3) |
| Enterobacter cloacae ESBL | 3* (3.1) |
| Groin skin: | 12 (100) |
| Escherichia coli ESBL | 1 (8.3) |
In 1 patient there was anus colonization with Escherichia coli ESBL and Enterobacter cloacae ESBL; ESBL – extended-spectrum β-lactamase.
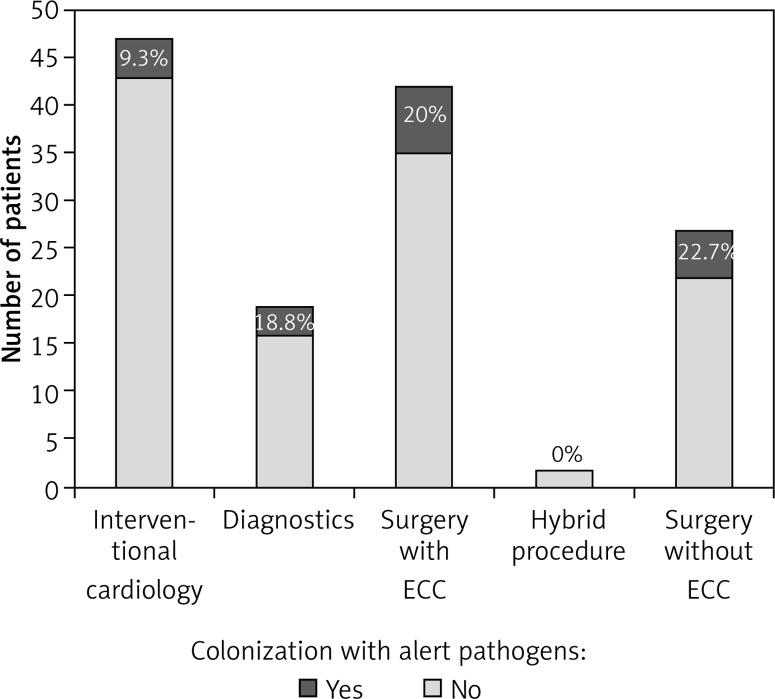
Among children who were treated surgically with ECC the incidence of alert pathogen colonization was 20%, while in children operated without ECC it was 22.7%. The incidence of alert pathogen colonization in the analyzed group of children according to the type of implemented treatment is presented in Figure 1.
Figure 1.
Colonization of alert pathogen incidence in children with congenital heart disease according to the type of implemented treatment
Alert pathogen colonization was observed statistically significantly more frequently in children admitted from other medical facilities (accordingly the definition described above) than in children admitted from home – 11 (38%) of 38 children and 8 (10%) of 80 children, respectively (p = 0.0089). Likewise, in the group of children younger than 6 months, alert pathogen colonization was more often observed than in older children – 15 (34.1%) of 44 children and 4 (5.4%) of 74 children, respectively (p < 0.001).
In all 59 children treated surgically the standard perioperative antibiotic prophylaxis was applied (the first dose just before surgical intervention repeated after the operation but no longer than for 48 h). In 2 children due to the diagnosis of pneumonia, antibiotic treatment was applied postoperatively. No sepsis or surgical site infection was diagnosed in the analyzed group of patients during the period of observation.
Discussion
The occurrence of alert pathogens has been reported previously, but data concerning the prevalence of multidrug resistant microorganisms in children treated in pediatric cardiac departments in a non-outbreak setting are limited [5]. Dedeić-Ljubović and Hukić [7] reported that 44.4% of children were colonized with multidrug resistant organisms upon admission to an intensive care unit. More importantly, the authors observed that infections occur more frequently in children who had previously been colonized (78%) than in those who had not (22%).
To the best of our knowledge, this is the first study describing the prevalence of alert pathogens in patients with congenital heart diseases admitted to a hybrid pediatric cardiac surgery department in Poland. Children who develop postoperative infections after advanced cardiac procedures have increased morbidity and mortality and inflated financial costs of the extended length of therapy that can be expected. Sparling et al. [8] reported that the average increase in cost for children with surgical site infections (SSI) could reach up to $28,000 per child [8].
Over the analyzed 22-week period the prevalence rate of alert pathogens among 118 patients admitted to our department was 16.1%. The most common pathogens found in our patients were ESBL-producing microorganisms. Alert pathogen colonization was detected in every group of patients referred for various cardiac treatments available in our institution (Figure 1). Colonization with alert pathogens was more often observed in children who were transferred from other medical facilities (‘borderline’ hybrid treatment, neonatal and infant cardiovascular emergencies) than in children admitted from home. This finding may suggest that transfer between various healthcare facilities is a risk factor for colonization with alert pathogens, but the issue requires further examination and multivariate analysis.
There are many studies suggesting that ESBL-producing Klebsiella pneumoniae and Escherichia coli are very common in hospital environments, in some settings regarded as ‘residents’ of healthcare facilities [5, 6, 9, 10]. Screening for alert pathogens from this group seems to be helpful in optimal empirical antibiotic therapy in children with clinical symptoms of infection. It is well known that an inadequate empirical antibiotic therapy for serious infections caused by organisms producing ESBL is independently associated with increased mortality [11].
It should be emphasized that current universal practice followed by advanced comprehensive cardiothoracic programs to prevent perioperative infections in children is unknown. However, hand hygiene together with adequate antibiotic prophylaxis seems to be the most important strategy for preventing healthcare-associated infections [12, 13]. The question arises how should we proper prepare children with congenital heart diseases for surgical interventions regarding antibiotic prophylaxis? Common standard prophylaxis in pediatric cardiac surgery is based on cephalosporins [14]. The main reasons for modification of this standard prophylaxis before cardiac surgery are the patient's allergy to cephalosporin (or its history) and colonization with MRSA. Colonization with resistant pathogens other than MRSA may be problematic – in our data all alert pathogens were ESBL-producing microorganisms. Local antibiotic policy creators should have their professional skills, microbiological experience and adequate on-time information required to make recommendations for specific drug regimens. The institutional policy should be based on an epidemiological assessment of evidence of resistant microorganisms, patient alert bacteria colonization status, and finally the costs of drug therapies.
With regard to our daily clinical practice, in the choice of antibiotics, despite the focus on basic cardiac problems and therapeutic strategies, we take into consideration local resistance patterns [5]. In our opinion screening is very important, since one would like to adjust and match local antibiotic prophylaxis with the presence of resistance patterns in individual patients.
We found that screening protocols are not commonly used in pediatric cardiac surgery departments. Woodward et al. reported that in 37 pediatric cardiac surgery departments only in 9 (24.3%) centers do children undergo preoperative screening of their nose for carriage of MRSA and only 4 prescribed topical antibiotic ointment to eradicate nasal MRSA [15]. No recommendation for nasal decolonization with mupirocin due to the risk of MRSA surgical site infections (SSI) in children has been published. However, Kavanagh et al. reported that there is sufficient evidence demonstrating a beneficial effect of surveillance and eradication prior to surgery to recommend its use on an expanded basis [16]. Ruef et al. reported six children with SSI infections with Staphylococcus aureus after cardiac surgery and concluded that preoperative decontamination for children might be indicated [17]. A randomized, controlled study in this group of patients is warranted.
Major infections after pediatrics cardiac surgery may be a serious life-threatening complication, with greater importance in babies with complex cardiac malformations. This concerns not only general bacterial, but also fungal and viral infections [3]. In addition, surgical site and sternal wound infections have an incidence in pediatric cardiac surgery departments estimated at around 1.53% [15]. In 2010, Barker et al. described a risk estimation model for major infections after pediatric cardiac surgery that was externally validated in 2012 by Kansy et al. [18, 19]. In both studies, independent variables associated with increased infection risk were age, previous cardiac operation, preoperative length of stay, preoperative ventilator support or tracheostomy, any genetic abnormality and the complexity of procedure score. The authors emphasized that preoperative antibiotic protocols were not recorded in the databases. In addition, these studies did not address practices to prevent major infections in this group of children.
It should be emphasized that pediatric programs do not consistently follow adult preventive guidelines and multicenter randomized trials. Thus, there is a need to formulate preventive guidelines to reduce the incidence of infections in pediatric cardiac patients [15]. The most important problem, the prevention of healthcare-associated infections, is probably not only addressed by the proper antibiotics policy, but also with proper hand hygiene and protocols to address other epidemiological issues [13, 17].
The successful control of multidrug resistant microorganism has been documented before. Necessary activities include improvements in hand hygiene, the use of contact precautions until patients are culture-negative for alarm pathogens, and active surveillance cultures with pathogens resistance to drugs monitoring [20]. We emphasize education programs, enhanced operative suite cleaning and improvements in communication within and between different healthcare facilities regarding alert pathogens [1].
Molecular methods should be the gold standard for surveillance, yielding higher sensitivity than slower culture-based methods. Baron and Tenover concluded that the newer molecular methods in MRSA colonization detection, along with rapid and accurate Staphylococcus aureus identification in culture systems, have revolutionized patient care, enabling rapid interventions leading to better outcomes, such as fewer postsurgical site infections and better overall institutional infection control [21].
In conclusion, preoperative multidrug resistant pathogen screening in children admitted to a pediatric cardiac surgery center with congenital heart disease may be of great importance, since many of these patients are colonized with resistant bacteria. In our opinion, patient's microbiome screening should be performed in all pediatric cardiac surgery departments. The epidemiology of the facility microbiome along with knowledge of the patient's microbiological flora enables the adjustment of perioperative antibiotic prophylaxis, and proper empirical drug therapy. The relationship between preoperative multidrug resistant pathogen colonization and the incidence of serious infections after comprehensive congenital heart defect treatment in children requires further analysis.
Acknowledgments
The authors thank the staff of the Department of Pediatric Cardiac Surgery, COPERNICUS Hospital in Gdansk, especially Aneta Szofer-Sendrowska, Jacek Juscinski and Konrad Paczkowski for their great support in collection of the data for the purpose of this study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Woodward CS, Son M, Taylor R, Husain SA. Prevention of sternal wound infection in pediatric cardiac surgery: a protocolized approach. World J Pediatr Congenit Heart Surg. 2012;3:463–9. doi: 10.1177/2150135112454145. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ami E, Levy I, Katz J, Dagan O, Shalit I. Risk factors for sternal wound infection in children undergoing cardiac surgery: a case-control study. J Hosp Infect. 2008;70:335–40. doi: 10.1016/j.jhin.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Jaworski R, Irga N, Haponiuk I, et al. Candidemia in children after complex congenital heart defects surgery treated with caspofungin: our own experience and a review of literature. Med Sci Monit. 2011;17:PH35–39. doi: 10.12659/MSM.881751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benenson S, Levin PD, Block C, et al. Continuous surveillance to reduce extended-spectrum beta-lactamase Klebsiella pneumoniae colonization in the neonatal intensive care unit. Neonatology. 2013;103:155–60. doi: 10.1159/000343150. [DOI] [PubMed] [Google Scholar]
- 5.Paluchowska P, Skalkowska M, Spelak A, Budak A. Occurrence of alert pathogens in hospital environment. Part I. ESBL-producing enterobacteriaceae strains. Med Dosw Mikrobiol. 2012;64:35–43. [PubMed] [Google Scholar]
- 6.Pessoa-Silva CL, Meurer Moreira B, Câmara Almeida V, et al. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit: risk factors for infection and colonization. J Hosp Infect. 2003;53:198–206. doi: 10.1053/jhin.2002.1373. [DOI] [PubMed] [Google Scholar]
- 7.Dedeić-Ljubović A, Hukić M. Occurrence of colonization and infection with multidrug-resistant organisms in a neonatal intensive care unit. Med Glas (Zenica) 2012;9:304–10. [PubMed] [Google Scholar]
- 8.Sparling KW, Ryckman FC, Schoettker PJ, et al. Financial impact of failing to prevent surgical site infections. Qual Manag Health Care. 2007;16:219–25. doi: 10.1097/01.QMH.0000281058.99929.ea. [DOI] [PubMed] [Google Scholar]
- 9.Goulenok T, Ferroni A, Bille E, et al. Risk factors for developing ESBL E. coli: can clinicians predict infection in patients with prior colonization? J Hosp Infect. 2013;84:294–9. doi: 10.1016/j.jhin.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Sadowska-Krawczenko I, Jankowska A, Kurylak A. Healthcare-associated infections in a neonatal intensive care unit. Arch Med Sci. 2012;8:854–8. doi: 10.5114/aoms.2012.31412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther. 2008;6:671–83. doi: 10.1586/14787210.6.5.671. [DOI] [PubMed] [Google Scholar]
- 12.Ryckman FC, Schoettker PJ, Hays KR, et al. Reducing surgical site infections at a pediatric academic medical center. Jt Comm J Qual Patient Saf. 2009;35:192–8. doi: 10.1016/s1553-7250(09)35026-6. [DOI] [PubMed] [Google Scholar]
- 13.Jaworski R, Haponiuk I, Chojnicki M, et al. Programme to improve hand hygiene in a paediatric cardiac surgery department. Kardiochir Torakochir Pol. 2012;12:278–82. [Google Scholar]
- 14.Bratzler DW, Dellinger EP, Olsen KM, et al. American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 15.Woodward CS, Son M, Calhoon J, Michalek J, Husain SA. Sternal wound infections in pediatric congenital cardiac surgery: a survey of incidence and preventative practice. Ann Thorac Surg. 2011;91:799–804. doi: 10.1016/j.athoracsur.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh KT, Calderon LE, Saman DM, Abusalem SK. The use of surveillance and preventative measures for methicillin-resistant staphylococcus aureus infections in surgical patients. Antimicrob Resist Infect Control. 2014;3:18. doi: 10.1186/2047-2994-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruef C, Fanconi S, Nadal D. Sternal wound infection after heart operations in pediatric patients associated with nasal carriage of Staphylococcus aureus. J Thorac Cardiovasc Surg. 1996;112:681–6. doi: 10.1016/S0022-5223(96)70052-1. [DOI] [PubMed] [Google Scholar]
- 18.Barker GM, O'Brien SM, Welke KF, et al. Major infection after pediatric cardiac surgery: a risk estimation model. Ann Thorac Surg. 2010;89:843–50. doi: 10.1016/j.athoracsur.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kansy A, Jacobs JP, Pastuszko A, et al. Major infection after pediatric cardiac surgery: external validation of risk estimation model. Ann Thorac Surg. 2012;94:2091–5. doi: 10.1016/j.athoracsur.2012.07.079. [DOI] [PubMed] [Google Scholar]
- 20.Biernat MM, Poniewierka E, Blaszczuk J, et al. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Arch Med Sci. 2014;10:505–9. doi: 10.5114/aoms.2013.36917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron EJ, Tenover FC. Methicillin-resistant Staphylococcus aureus diagnostics: state of the art. Expert Opin Med Diagn. 2012;6:585–92. doi: 10.1517/17530059.2012.709233. [DOI] [PubMed] [Google Scholar]