Abstract
The main focus of the study was to detect circulating tumor cells (CTCs) in ovarian cancer (OC) patients using a new methodological approach (MetaCellTM) which is based on size-dependent separation of CTCs and subsequent cytomorphological evaluation. Cytomorphological evaluation using vital fluorescence microscopy approach enables to use the captured cells for further RNA/DNA analysis. The cytomorphological analysis is then completed by gene expression analysis (GEA). GEA showed that relative expression of EPCAM is elevated in CTC-enriched fractions in comparison to the whole peripheral blood sample and that the expression grows with in vitro cultivation time. Comparison of the relative gene expression level in the group of peripheral blood samples and CTC-fraction samples confirmed a statistically significant difference for the following genes (p < 0.02): KRT7, WT1, EPCAM, MUC16, MUC1, KRT18 and KRT19. Thus, we suggest that the combination of the above listed genes could confirm CTCs presence in OC patients with higher specificity than when GEA tests are performed for one marker only. The GEA revealed two separate clusters identifying patients with or without CTCs.
Keywords: CTCs, circulating tumor cells, ovarian cancer, cultivation, in vitro, MetaCell, gene expression
Introduction
Circulating tumor cells (CTCs) are tumor cells shed from primary and metastatic sites that circulate in the peripheral blood and can be detected by many advanced methods. The cells are present not only in patients with distant metastases, but also in patients with early, localized tumors. It is important to develop and optimize CTCs detection methods for future management of malignant diseases, especially to enable real-time monitoring of treatment efficacy and identification of new therapy targets and resistance mechanisms.
Recent studies have indicated the presence of a CTCs subpopulation that shows features of Epithelial-mesenchymal transition (EMT) in patients with epithelial origin tumors [1,2] and the importance of detecting new markers which are not downregulated by the induction of EMT, enabling CTCs capture.
The main focus of the study was to detect CTCs in ovarian cancer (OC) patients using a new methodological approach which is based on size-dependent separation of CTCs and subsequent cytomorphological evaluation. Cytomorphological evaluation using vital fluorescence microscopy approach enables to use the captured cells for further RNA/DNA analysis. The cytomorphological analysis is then completed by gene expression analysis.
Materials and methods
Patients
To date 40 patients with diagnosed OC have been enrolled in the gene expression study in accordance with the Declaration of Helsinki. All patients were candidates for surgery or surgical diagnostics. Based on informed consent, clinical data were collected from all participating patients. For each patient, approximately 8 mL of venous blood was drawn from the antecubital veins and placed into S-Monovette tubes (Sarstedt AG & Co., Numbrecht, Germany) containing 1.6 mg EDTA/mL blood as an anticoagulant. The samples were processed at room temperature using an isolation procedure completed within 24 hours after the blood draw.
CTCs enrichment and culture
A size-based separation method for viable CTCs enrichment from peripheral blood has recently been introduced (MetaCell®, MetaCell s.r.o., Ostrava, Czech Republic) [3,4]. The size-based enrichment process is based on the filtration of peripheral blood through a porous polycarbonate membrane (with pores of 8 μm diameter). The minimum and maximum volume of the filtered peripheral blood may be adjusted up to 50 mL with fluid. The standard 8 mL peripheral blood sample from patients suffering from OC was transferred into the filtration tube. Gradual transfer of the blood in several steps is preferred to prevent blood clotting on the membrane filter. The peripheral blood flow is supported by capillary action of the absorbent touching the membrane filter. The filtered CTCs were observed immediately after filtration on the membrane. The control and presence of filtered CTCs immediately after isolation eliminates false negative results of the examination. The membrane filter is kept in a plastic ring that is transferred into the 6-well cultivation plate, 4 mL RPMI media is added to the filter top and CTCs are cultured on the membrane in vitro under standard cell-culture conditions (37°C, 5% atmospheric CO2) and observed by inverted microscope. The CTCs were grown in FBS-enriched RPMI medium (10%) for a minimum of 3-6 days on the membrane. Alternatively, the enriched CTCs fraction can be transferred from the membrane and cultured directly on any plastic surface or a microscopic slide, or the separation membrane may be translocated on a microscopic slide. Microscopic slide is preferred if immunohistochemistry/immunofluorescence analysis is planned. If an intermediate CTCs-analysis is awaited, the CTCs-fraction is transferred in PBS (1.5 mL) to a cytospin slide. The slide is then dried for 24 hours and analyzed by histochemistry (May-Grünwald staining) and/or by automated immunohistochemistry protocols (Ventana, Benchmark Ultra, Roche) using standard differential diagnostic antibodies in the pathological evaluation process.
Cytomorphological analysis
The stained cells captured on the membrane were examined using fluorescence microscopy in two steps: (i) screening at x20 magnification to locate the cells; (ii) observation at x40/x60 magnification for detailed cytomorphological analysis. Isolated cells and/or clusters of cells of interest (immunostained or not) were selected, digitized, and the images were then examined by an experienced researcher and/or pathologist. CTCs were defined as cells with the following characteristics: (i) with a nuclear size ≥10 μm); (ii) irregular nuclear contour; (iii) visible cytoplasm, cells size over 15 μm; (iv) prominent nucleoli; (v) high nuclear-cytoplasmic ratio; (vi) proliferation, (vii) actively invading cells creating 2D or 3D cell groups.
Gene expression analysis (GEA)
The key purpose of GEA is to compare gene expression of tumor-associated markers in the CTC-enriched fractions to that in the whole blood (PK) and subsequently to confirm the origin of the cells on the separation membrane.
GEA allows up to 20 tumor-associated markers in RNA from different cell fractions to be tested within a single quantitative polymerase chain reaction (qPCR) run. Differential diagnostics markers for qPCR test are chosen in accordance with the suspected diagnosis.
RNA is isolated from the whole blood and CTC-enriched fraction on the membrane. There are two CTC-enriched fractions: the CTC enriched fraction of cells stored immediately after separation process (the so-called “virgin CTCs” - PK IZO) and/or the CTC enriched fraction of cells grown on the separation membrane in vitro (the so-called “membrane fraction” - PK SK). Some of the cells grown on the membrane in vitro may overgrow the membrane and set up a new cell culture on the culture-well bottom. These cells are analyzed as the “bottom fraction” (PK DK).
Finally, the CTC-gene expression analysis allows identification of the relative amount of tumor-associated markers in the whole blood and in CTC-enriched fractions. If the tumor-associated genes are highly expressed in the CTC fraction, a subsequent analysis of chemoresistance-associated genes is performed. Molecular analysis allows identification of which type of the chemotherapeutic agents may be of use in tumor therapy and assigned as personalized cancer therapy based on CTC.
The cells captured on the membrane are lysed by RLT-buffer with beta-mercapto-ethanol (Qiagen). RNA is then isolated using the RNeasy Mini Kit (Qiagen). The RNA from the whole blood is isolated with a modified procedure and the quality/concentration of RNA is measured by NanoDrop (ThermoScientific). As there are only a few hundred cells on the membrane, the median concentration of RNA is quite low (5-10 ng/µl). High Capacity cDNA Reverse Transcription Kit (Life Technologies) was used for cDNA production. GEA was performed using Taqman chemistry with Taqman MGB-probes for all the tested genes (Life Technologies).
The following genes associated with tumorigenic character and therapeutic potential in ovarian cancer were chosen for the multimarker GEA panel: EPCAM, MUC1, MUC16, KRT18, KRT19, WT1, VEGFA, HER2. Additionally, genes associated with chemoresistance were tested (MRP1-10, MDR1, ERCC1, RRM1, RRM2).
Statistical analysis
All analyses were performed using clinicopathological information transformed into variables 0 and 1 if applicable for tested characteristics. Chi-squared test, t-tests, cluster analysis andcorrelation analysis were outperformed using GeneX (MultiD, SE) and GraphPadPrism vs. 5 (Graphpad, US). P-value of less than 0.05 was considered as statistically significant.
Results
The main focus of the study was to detect CTCs in OC patients by a new methodological approach which is based on size-dependent separation of CTCs and subsequent cytomorphological evaluation. Cytomorphological evaluation using vital fluorescence microscopy approach (Figure 1) enables the use of the viable captured cells for further RNA/DNA analysis.
Figure 1.
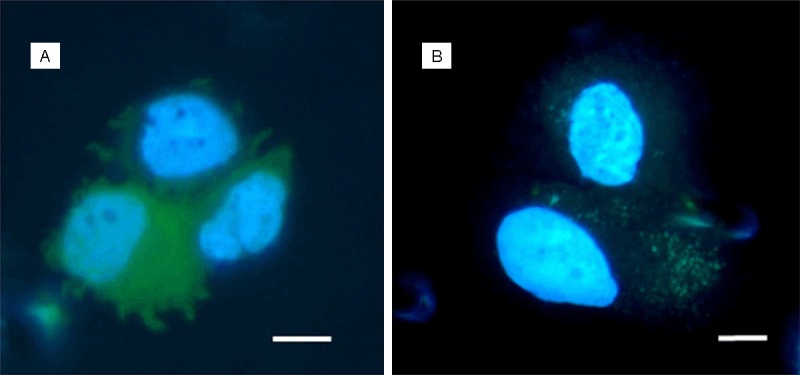
CTCs isolated from patients with ovarian carcinoma, captured on the separation membrane (vital fluorescent staining - NucBlue® and Celltracker®). Bar represents 10 µm.
Patients diagnosed with different stages of ovarian cancer (OC) (n=56) were included in the study in 2014-2015. Cytomorphological analysis revealed that 58% were CTC-positive patients and 42% were CTC-negative patients. The results were confirmed by gene expression analysis with a slightly different ratio of CTC-positive and CTC-negative patients. The results of cytomorphological analysis were in agreement with multimarker gene expression analysis results in 92% of tested samples. The remaining 8-10% of “misdiagnosed patient samples” were re-analyzed by independent researchers.
There is a high potential hidden in the combination of cytomorphological and molecular analysis of CTCs in OC, especially due to the chemoresistance-gene analysis and automated immunohistochemistry protocols (Figure 2). Automation of immunohistochemistry staining could be a step needed for standardization of CTC-testing in clinical diagnostics.
Figure 2.
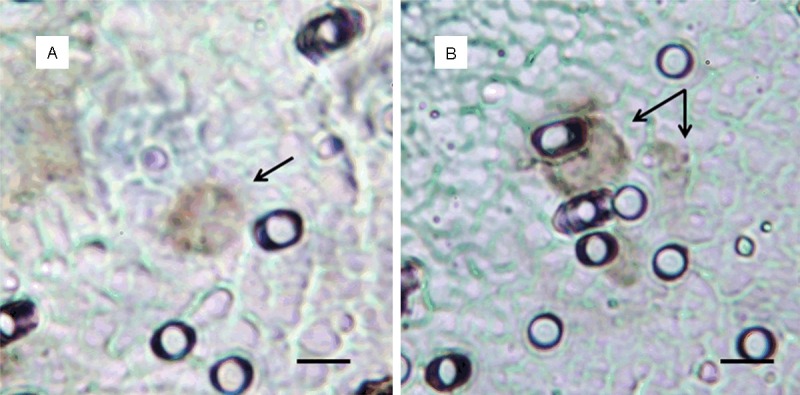
CTCs captured on the separation membrane after immunohistochemistry staining (WT-1 antibody) on Benchmark Ultra automat (Ventana, Roche). Bar represents 10 µm.
Some patients were tested longitudinally so that we were able to analyze their CTCs throughout the whole year and demonstrate how CTCs evolve over time and during disease recurrence.
GEA showed that relative expression of EPCAM is elevated in CTC-enriched fractions in comparison to the whole peripheral blood sample and that the expression grows with in vitro cultivation time (Figure 3). Similarly, the increase in relative gene expression in the CTC-enriched fractions has been observed for KRT7, KRT18, MUC16 and WT1 in addition to EPCAM (Figure 4). Comparison of the relative gene expression level in the group of peripheral blood samples (Sample type 1) and CTC-fraction samples (3 days of in vitro culture - Sample type 3) confirmed a statistically significant difference for the following genes (p < 0.02): KRT7, WT1, EPCAM, MUC16, MUC1, KRT18 and KRT19 (Figure 5). Thus, we suggest that the combination of the above listed genes should confirm CTCs presence in OC patients with higher specificity than when GEA tests are performed for one marker only.
Figure 3.
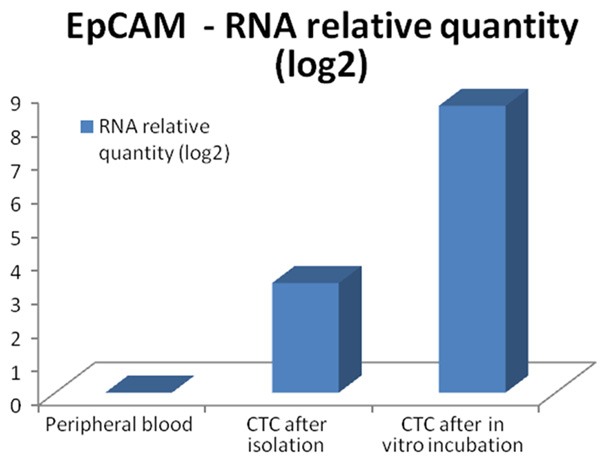
Relative expression of EPCAM RNA in peripheral blood and CTC fractions compared after qPCR analysis.
Figure 4.
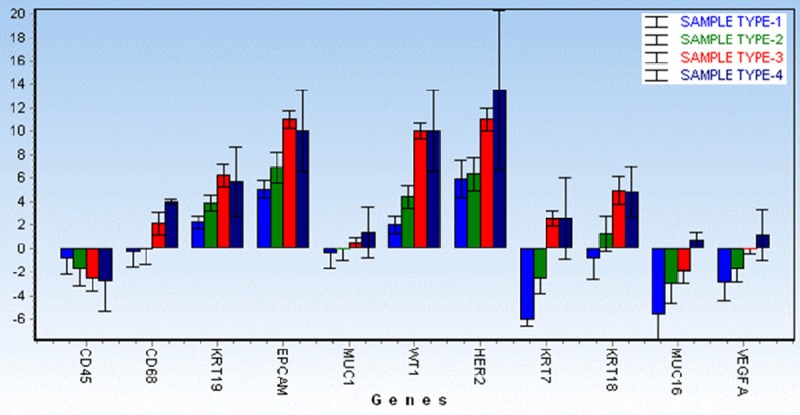
Comparison of averaged relative RNA expression for the genes, shown for all sample types (1-4). Sample type 1 (Peripheral Blood), Sample type 2 (CTC fraction stored immediately after separation process), Sample type 3 (CTC fraction after in vitro culture), Sample type 4 (bottom fraction - cells overgrowing the membrane).
Figure 5.
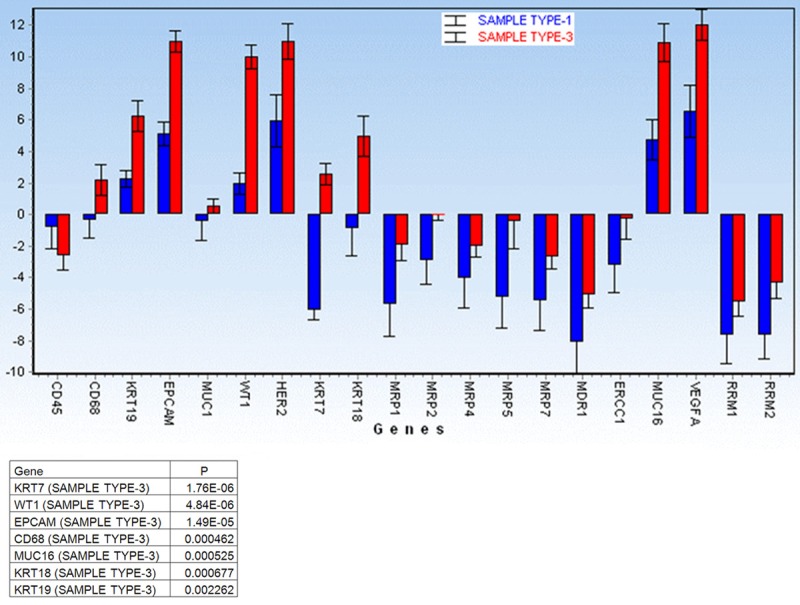
Comparison of the relative gene expression level for the listed genes in the group of peripheral blood (Sample type 1) and CTC-fraction (after 3 days of in vitro culture - Sample type 3). Gene expression levels are relative to the whole peripheral blood data averaged for the patients group. A significant difference was noted for the following genes (p < 0.02): KRT7, WT1, EPCAM, CD68, MUC16, MUC1, KRT18 and KRT19. Thus, we suggest that the combination of the above listed genes should confirm CTCs presence in OC patients with higher specificity than when tests are performed for one marker only.
If evaluated in an individual patient case, the GEA-cluster analysis shows that the highest EPCAM expression has been confirmed for the “membrane fraction” (sample type 3) (Figure 6). That is why all the “membrane fractions” (cells captured on the membrane and cultured in vitro) were compared together. The analysis revealed two separate clusters identifying patients with or without CTCs (Figure 7).
Figure 6.
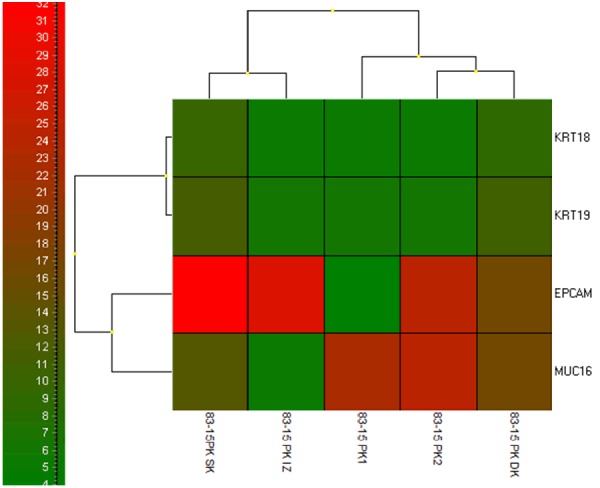
Cluster analysis for patient - RNA relative amount (log2) of tested genes in all of the tested fracions is shown in colours (please refer to the colour scale on the left). Tested fractions (peripheral blood PK), CTC after isolation (PK IZO), CTC after culture (PK SK), CTC overgrowing the membrane (PK DK). The highest expression of EPCAM was detected in PK SK fraction CTCs on the separation membrane.
Figure 7.
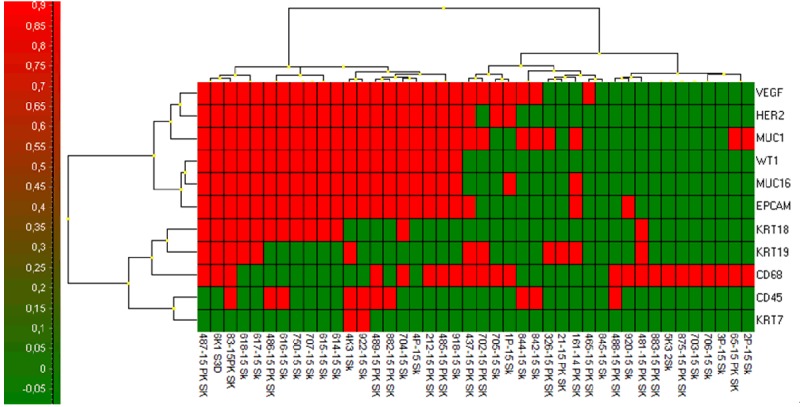
Cluster analysis of gene expression data RNA relative amount (log2) for CTC-membrane fractions (PK SK) of all tested patients clearly identifies CTC-positive and CTC-negative group of patients (Higher expression is shown in red).
Discussion
The development of personalized treatment for patients with cancer depends on the specification of the molecular character of their disease. Therefore, there is a need to monitor tumor evolution and mechanism of treatment resistance. One of the approaches complementing traditional biopsy sampling could be the detection and analysis of CTCs.
The two most used enrichment techniques are the size-based filtration method and immuno-magnetic antigen-dependent method. The immuno-magnetic method is dependent on epithelial cell adhesion molecule (EpCAM), a tumor antigen highly expressed in epithelial cancers and at lower levels in normal epithelia [5]. EpCAM has always beenconsidered the ideal marker for the detection of CTCs in the peripheral blood of cancer patients.
The changes in CTCs phenotype are increasingly being recognized and it is clear that CTCs represent a heterogeneous entity. Indeed, a large amount of data demonstrates that in cancer the expression of epithelial surface markers might be transiently lost during the EMT process, to enable tumor cells to detach from the primary tumor and circulate into the bloodstream [6,7].
Several lines of evidence have recently demonstrated that CTCs may adopt different strategies to protect themselves from the cell death fate, changing their phenotype from epithelial to mesenchymal, grouping into cell clusters or switching between the cancer stem cell state and the differentiated state of cancer cells [9]. Further studies widely demonstrated that EpCAM negative CTCs with mesenchymal cell like phenotype and downregulation of epithelial markers are frequently derived from EpCAM-positive primary tumors [10].
In contrast to the above, filtration methods are based on physical properties that allow size-based isolation of CTCs. The size-based method is an easy process which makes use of classic cytopathology evaluation criteria. Further gene-expression studies in CTCs are essential to determine tumor heterogeneity linking phenotypic differences. However, it is more difficult to preserve RNA than DNA, and the presence of wild-type DNA or RNA from WBC is a technical hurdle. As reported above, the method of isolating viable CTCs followed by three days’ incubation was used. The methodology protocol presented in this paper ensures a straightforward CTC-identification by combining cytomorphology, gene expression analysis and automated immunohistochemistry. It is very likely to be implemented into clinical routine, especially in the accredited pathology laboratories using automated immunohistochemistry staining.
GEA enables the researchers to detect changes in CTCs on a molecular level and thus to create an individual profile for each patient and to personalize the treatment.
Acknowledgements
This study was supported by the grant of the Czech Ministry of Health: IGA NT14441-3/2013.
References
- 1.Josse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- 2.Ning Y, Gerger A, Zhang W, Hanna DL, Yang D, Winder T, Wakatsuki T, Labonte MJ, Stintzing S, Volz N, Sunakawa Y, Stremitzer S, El-Khoueiry R, Lenz HJ. Plastin polymorphisms predict genderand stage-specific colon cancer recurrence after adjuvant chemotherapy. Mol Cancer Ther. 2014;13:528–39. doi: 10.1158/1535-7163.MCT-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolostova K, Matkowski R, Jędryka M, Soter K, Cegan M, Pinkas M, Jakabova A, Pavlasek J, Spicka J, Bobek V. The added value of circulating tumor cells examination in ovarian cancer staging. Am J Cancer Res. 2015;5:3363–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Herlyn M, Steplewski Z, Herlyn D, Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979;76:1438–42. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, Lackner MR. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010;5:e12517. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M, Dittrich C. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011;50:700–10. doi: 10.3109/0284186X.2010.549151. [DOI] [PubMed] [Google Scholar]
- 9.Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–31. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 10.Driemel C, Kremling H, Schumacher S, Will D, Wolters J, Lindenlauf N, Mack B, Baldus SA, Hoya V, Pietsch JM, Panagiotidou P, Raba K, Vay C, Vallböhmer D, Harréus U, Knoefel WT, Stoecklein NH, Gires O. Context-dependent adaption of EpCAM expression in early systemic esophageal cancer. Oncogene. 2014;33:4904–15. doi: 10.1038/onc.2013.441. [DOI] [PubMed] [Google Scholar]


